Abstract
We have encountered numerous cases of rhabdomyolysis associated with acute pesticide intoxication; however, the cause, incidence, and treatment outcomes of rhabdomyolysis have not been studied. The current study involved 2,125 patients hospitalized with acute chemical poisoning. Based on clinical and laboratory parameters and treatment outcomes, we found that overall incidence of rhabdomyolysis in our hospital was 0.06% (93 of 143,830 patients admitted), but the incidence associated with acute pesticide intoxication was 1.8% (33 of 1,793 cases). The incidence of rhabdomyolysis after pesticide intoxication was significantly higher in men than in women (P = 0.010). The amount of pesticide ingested was significantly higher in rhabdomyolysis patients than that in those who did not develop rhabdomyolysis (mean ± SD, 114.1 ± 79.5 mL vs 74.1 ± 94.2 mL, P = 0.010). Our results show that pesticide intoxication is a frequent cause of rhabdomyolysis and is more common among men than women. The volume of pesticide ingested, and not the degree of human toxicity, is the main factor influencing the incidence of rhabdomyolysis.
Keywords: Acute Kidney Injury, Intoxication, Pesticides, Rhabdomyolysis, Surfactant
INTRODUCTION
Various causes of rhabdomyolysis have been described, such as exertion, crush injuries, ischemia, metabolic disorders, abnormal body temperatures, infection, autoimmune muscle damage, and drugs and/or toxins (1). Among the drugs known to induce rhabdomyolysis, the most common examples are statins (2) and fibrates (3). Antipsychotic medications (4) and neuromuscular blocking agents (5) are also known to cause rhabdomyolysis. In addition, substance abuse (6, 7) including alcohol, amphetamine, cocaine, heroin, and ketamine may induce the condition. Poisons linked to rhabdomyolysis include heavy metals (8) and venoms from insects or snakes (9, 10).
Acute pesticide intoxication, a method commonly used by people commiting suicide, is unique in that a specific toxic substance may be associated with the development of rhabdomyolysis (11-14). We have encountered many patients with rhabdomyolysis associated with acute pesticide intoxication at our hospital, accounting for approximately 500 cases per year (15, 16). The current study was designed to assess the etiology, incidence, and treatment outcomes of rhabdomyolysis at our hospital over a 5-yr period.
MATERIALS AND METHODS
Study design and data collection
This cross-sectional study was performed by reviewing the medical records of rhabdomyolysis patients admitted to the Department of Nephrotoxicology, Soonchunhyang Cheonan Hospital, Korea, between July 2006 and June 2011 (Fig. 1). The parent population included all patients admitted during the investigation period. We screened patients with rhabdomyolysis on the basis of the current consensus definition of rhabdomyolysis (creatine kinase [CK] level higher than 1,000 U/L), using an order communication system and electronic medical records. We documented patient age, sex, hospitalization period, the amount of pesticide ingested, the active toxic compounds, the time lag to hospital admission, and laboratory findings. We also scored acute renal failure (ARF) and mortality to assess the complications and outcomes of rhabdomyolysis.
Fig. 1.
Causes of rhabdomyolysis in the current study. The numbers of cases are shown in parentheses. CNS, central nervous system.
Definition of ARF
ARF was diagnosed according to the Risk, Injury, Failure, Loss, and End-Stage Renal Disease criteria for ARF (17). In brief, ARF was diagnosed when the serum creatinine level increased 3 times that of the lowest level during the observation period retrospectively, when the glomerular filtration rate decreased >75% of the lowest level during the admission, when the serum creatinine level increased over 4 mg/dL without a history of renal disease, when there was an acute rise of serum creatinine ≥0.5 mg/dL, and when the urine output decreased to <0.3 mL/[kg·h], or if anuria persisted for >12 hr.
Causes of rhabdomyolysis
Patients were divided into 2 groups based on the cause of rhabdomyolysis (the chemical-induced group and the non-chemical group). The chemical-induced group was further divided into 3 classes based on the type of chemical agent (pesticide, medicine, and other chemicals) (Fig. 1). The pesticide class was further divided into 3 subclasses (fungicides, herbicides, and insecticides). The medicine class, which included cases of acute overdose rather than actual intoxication, was categorized into 5 subclasses according to the World Health Organization Anatomical Therapeutic Chemical Classification System, as follows: analgesics (N02 and M01), antihistamines (D04), central nervous system (CNS)-acting agents (N03, N05, and N06), mixed medications, and others. For all pesticides, the amount ingested was estimated based on the number of swallows (1 mouthful was equivalent to 20 mL). The non-chemical group was categorized into physical and non-physical subclasses (Fig. 1).
Statistical analysis
Continuous variables are shown as the mean±SD, with or without the median value and range, and categorical variables are shown as the frequency (the number of cases and percentage). The differences between groups were analyzed using Student's t-test or the Mann-Whitney U test for continuous variables and the chi-square test or Fisher's exact test for categorical variables. To analyze the differences among >3 groups, we used the Kruskal-Wallis test. Statistical analyses were performed using SPSS software, version 14.0 (SPSS, Chicago, IL, USA). P values less than 0.05 were considered statistically significant. Because of the limited sample size, laboratory measurements of muscle enzyme levels were compared among the pesticide, chemical (other than pesticide), and physical classes.
Ethics statement
The study protocol and design were reviewed and approved by the institutional review board (IRB) of Soonchunhyang University Cheonan Hospital (IRB approval number: 2013-38). Informed consent was not obtained because of retrospective design of the study.
RESULTS
The overall incidence of rhabdomyolysis in our hospital was 0.06% (93 cases of 143,830 total admissions) (Fig. 1), whereas the incidence of rhabdomyolysis in patients with acute pesticide intoxication was much higher (1.8% [33 of 1,793 cases]) (Table 1). The frequency of rhabdomyolysis caused by different active ingredients varied (Tables 2 and 3). Not all pesticides that may cause severe human toxicity triggered rhabdomyolysis. For example, among the 1,420 patients with acute paraquat intoxication, none had rhabdomyolysis. In contrast, the frequency of rhabdomyolysis was high in patients poisoned with certain pesticides, as shown in Table 3.
Table 1.
Demographic characteristics of patients in the chemical-induced group
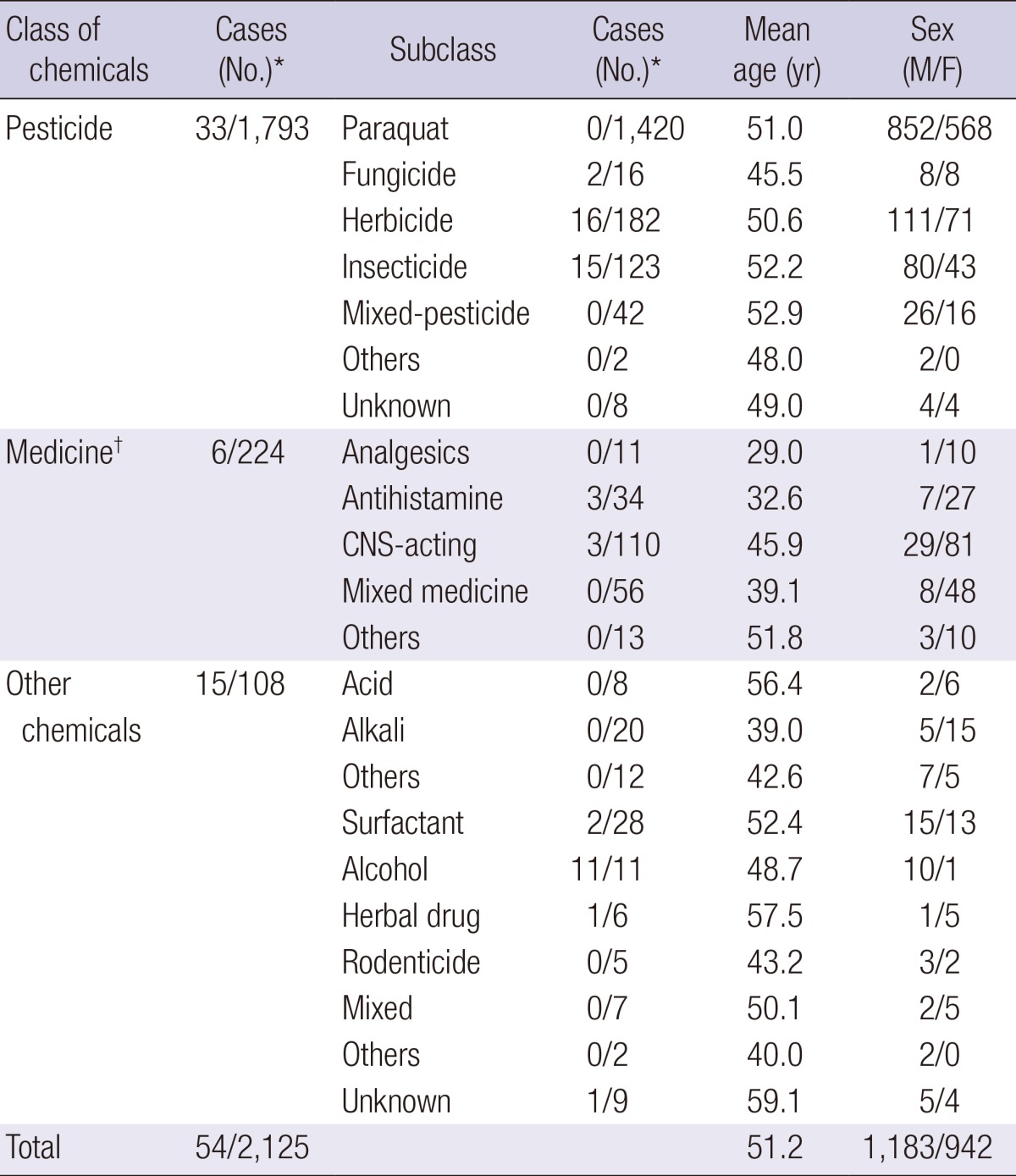
*The number of rhabdomyolysis patients and total admitted patients; †The medicine class was categorized into 5 subclasses according to the World Health Organization Anatomical Therapeutic Chemical Classification System: analgesics (N02 and M01), antihistamines (D04), CNS-acting agents (N03, N05, and N06), mixed medications, and others. M, male; F, female; CNS, central nervous system.
Table 2.
Details of active ingredients causing rhabdomyolysis for each class in the chemical-induced group
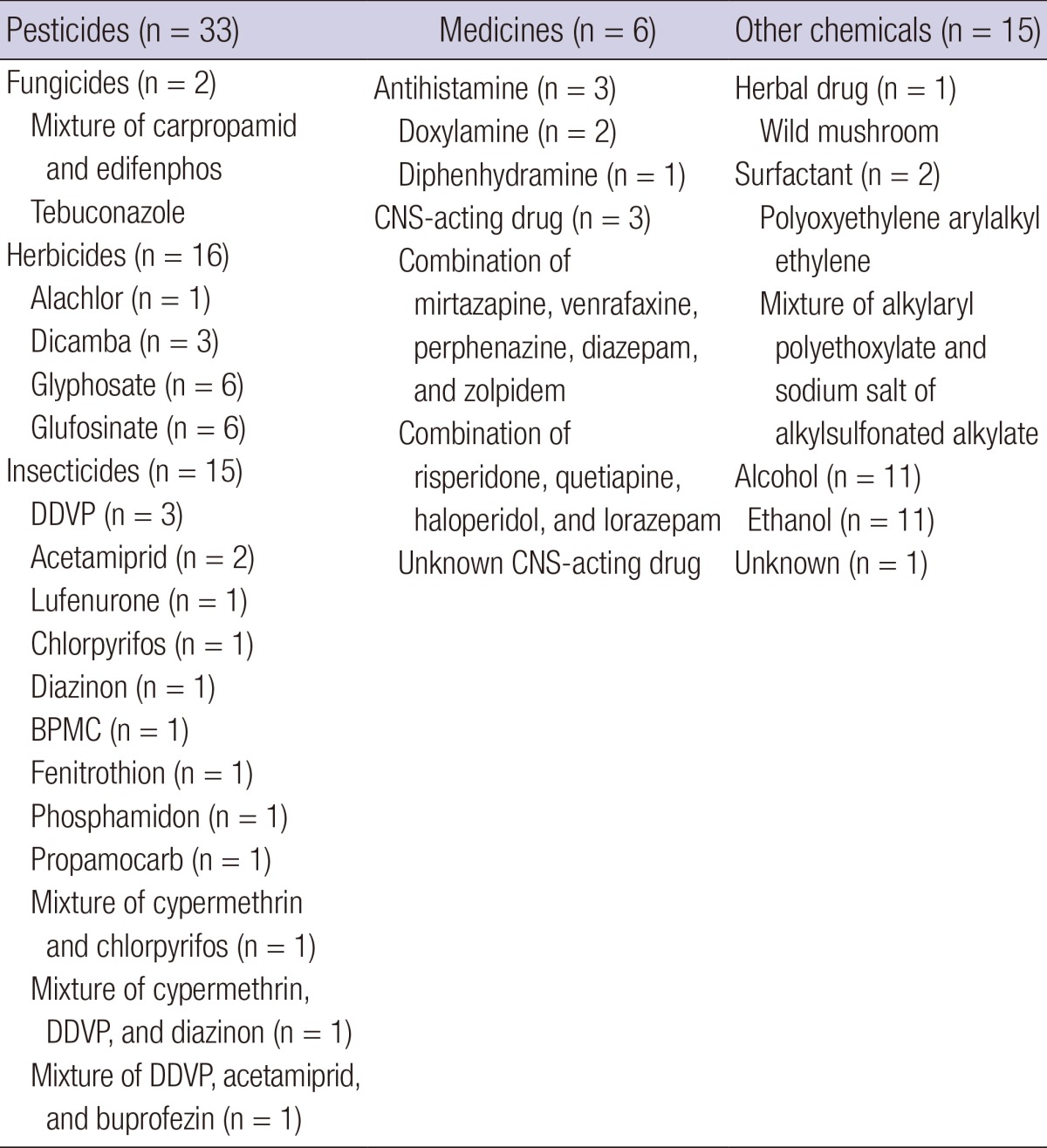
DDVP, dichlorvos or 2,2-dichlorovinyl dimethyl phosphate; BPMC, fenobucarb; CNS, central nervous system.
Table 3.
Frequency of rhabdomyolysis and WHO toxicity of pesticides
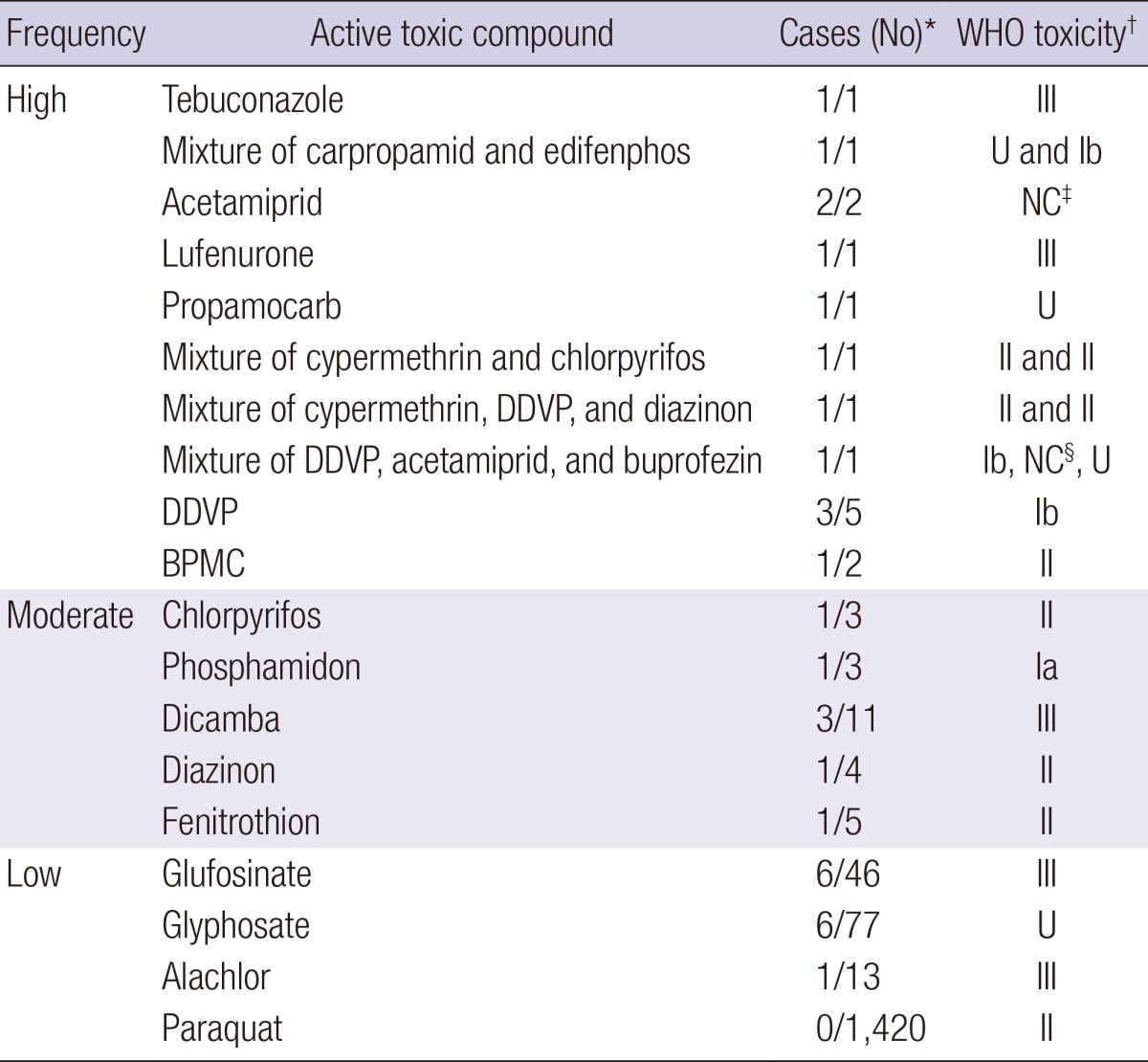
*The number of rhabdomyolysis patients and total patients in each subclass; †WHO toxicity classes; ‡Not classified; §WHO toxicity class of acetamiprid is currently "not classified." However, the acute oral LD50 for male rats is 217 mg/kg. WHO, World Health Organization; DDVP, dichlorvos or 2,2-dichlorovinyl dimethyl phosphate; BPMC, fenobucarb; Ia, extremely hazardous; Ib, highly hazardous; II, moderately hazardous; III, slightly hazardous; U, unlikely to present acute hazard in normal use.
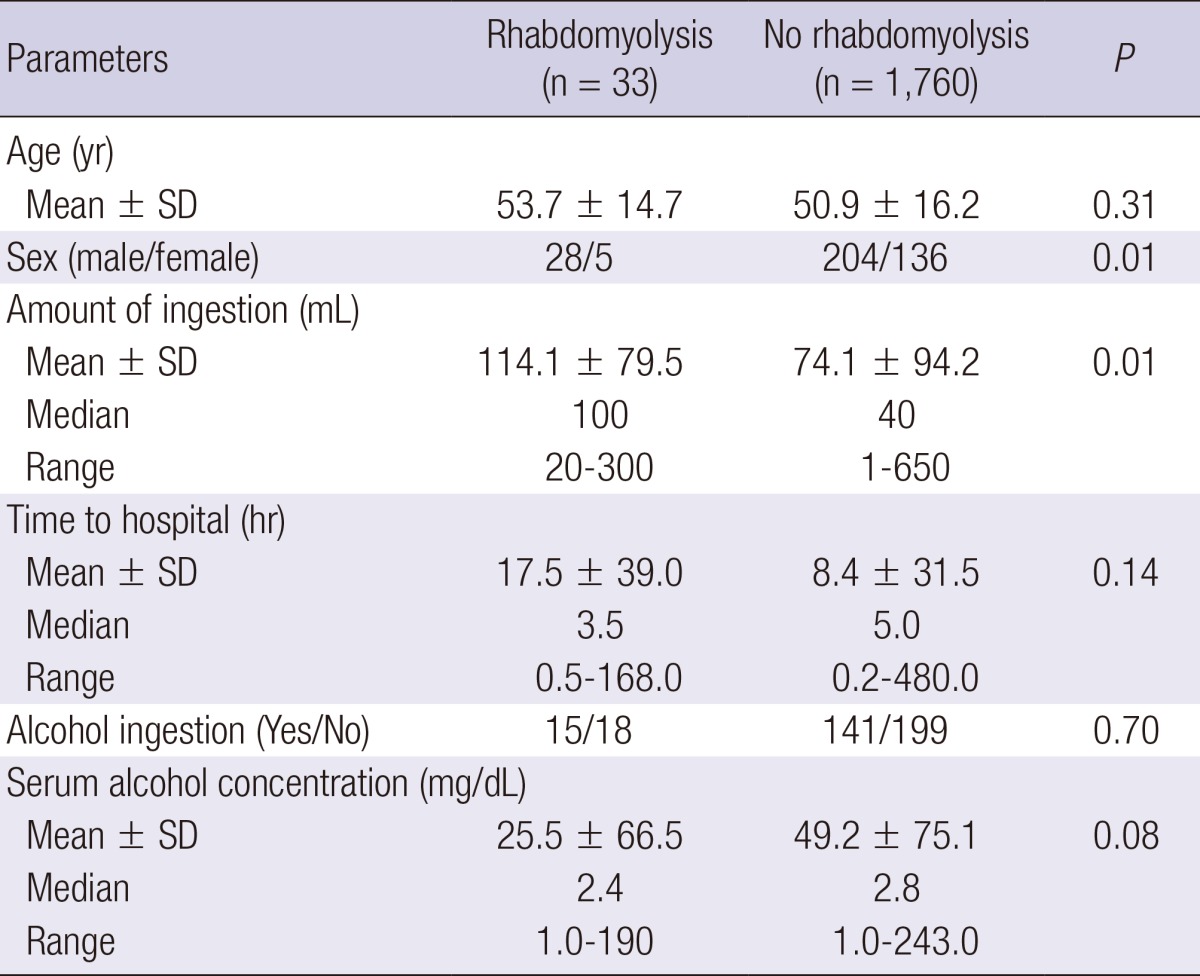
The incidence of rhabdomyolysis associated with pesticides was significantly higher among men than that among women (Table 4). However, this sex difference was not observed for the other classes of chemicals. The amount of pesticide ingested was significantly higher among rhabdomyolysis patients than among non-rhabdomyolysis patients (mean±SD, 114.1±79.5 mL vs 74.1±94.2 mL, P = 0.010); however, among the rhabdomyolysis and non-rhabdomyolysis patients who ingested pesticides, there were no differences in age, time lag to hospital admission, or the incidence of alcohol coingestion.
Table 4.
Age, sex, time lag to hospital admission, amount of ingestion, and alcohol coingestion of patients in the pesticide class
There was no difference in the incidence of ARF or mortality between the chemical-induced group and the non-chemical group. A binary logistic regression analysis showed that the risk factor most closely associated with ARF was the peak level of serum myoglobin (P = 0.04), but the odds ratio (1.001) was not significant; none of the examined risk factors was significantly associated with mortality.
Peak CK levels were significantly lower in the pesticide class than those in the non-pesticide chemical classes (P = 0.01) or those in the physical class (P = 0.01). Peak serum myoglobin levels in the pesticide class were also lower than those in the physical class (P = 0.01) (Table 5).
Table 5.
ARF incidence, hospitalization duration, and peak laboratory parameters of patients in the pesticide, chemical other than pesticide, and physical classes
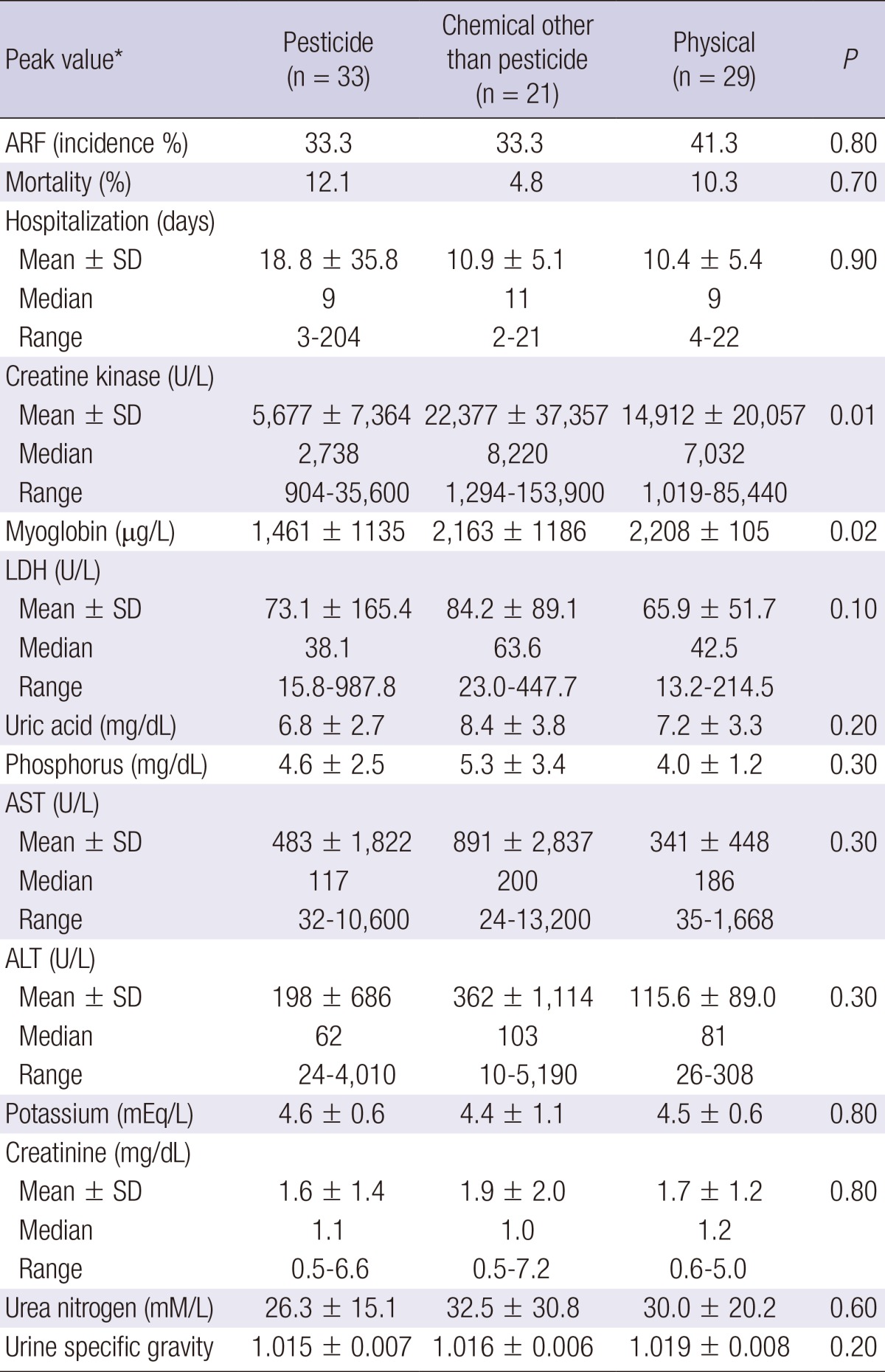
Note: Continuous variables expressed as mean±SD, median, or range. Conversion factors for units: myoglobin in µg/L to mM/L,×0.0571; uric acid in mg/dL to µM/L, ×59.48; phosphorus in mg/dL to mM/L,×0.3229; potassium in mEq/L to mM/L, ×1.0; creatinine in mg/dL to µM/L,×88.4; urea nitrogen in mg/dL to mM/L,×0.357. *The highest value anytime during the measured data that was higher than the upper limit. ARF, acute kidney injury; LDH, lactate dehydrogenase; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
In the herbicide subclass, all 16 rhabdomyolysis patients were men, showing a sex difference in the incidence of rhabdomyolysis. The amount of chemical ingested was significantly higher for the rhabdomyolysis patients than for the non-rhabdomyolysis patients (P = 0.01, data not shown). The time lag to hospital admission was significantly shorter (P = 0.02, data not shown) for the rhabdomyolysis patients than for the non-rhabdomyolysis patients.
Among the 224 patients in the medicine class, rhabdomyolysis developed only in the antihistamine and CNS-acting drug subclasses (Tables 1 and 2 and Fig. 1). During the 5-yr observational period, 8,486,084 tablets of 5 types of statin were prescribed to 27,902 patients. The hydroxymethylglutaryl-coenzyme A reductase inhibitor statin did not result in rhabdomyolysis in our study. Two patients were admitted to our hospital during the observational period, not because of rhabdomyolysis but for observation after statin overdose. Two of the 28 patients with acute surfactant intoxication had rhabdomyolysis (Tables 1 and 2 and Fig. 1).
The non-chemical group, in which rhabdomyolysis was caused by all etiologies other than chemicals, was classified into 3 categories (physical [n=29], infection [n=3], and metabolic [n=7]). Each class was further divided into the following: immobilization (n=16), overactivity (n=9), and trauma (n=4) for the physical class; meningitis (n=2) and unknown focus (n=1) for the infection subclass; and hypernatremia (n=1), hyponatremia (n=1), hyperthermia (n=2), and hypoglycemia (n=3) for the metabolic subclass (Fig. 1).
There were no differences between the patients surviving rhabdomyolysis and those succumbing to the disease with respect to the incidence of ARF or rhabdomyolysis-related laboratory parameters.
DISCUSSION
The principal finding of the current study is that the incidence of rhabdomyolysis was 0.06% (93 of 143,830 admitted patients) in our hospital, which was considerably lower than the incidence (1.8%) in patients with acute pesticide intoxication. Among the 93 cases of rhabdomyolysis, the chemical-induced group accounted for 58% (n=54) and the non-chemical group for 42% (n=39). Pesticide intoxication was the leading cause of rhabdomyolysis (35.5%) in these patients.
The incidence and causes of rhabdomyolysis may differ from country to country (18, 19) on the basis of social and environmental factors. The association of rhabdomyolysis with pesticide intoxication was reported in some case reports (11-14), but the current study provides epidemiologic data on this subject. The clinical course of pesticide-mediated rhabdomyolysis was characterized by the early onset. In all of the rhabdomyolysis patients, the diagnosis was confirmed by serum levels of muscle enzymes, which were high enough to suspect rhabdomyolysis in the emergency room. This suggests that the initiation of rhabdomyolysis by pesticide intoxication occurs soon after ingestion.
In our cases of rhabdomyolysis, the levels of myoglobin and muscle enzymes such as CK and lactate dehydrogenase were very high, while the levels of electrolytes such as phosphate, calcium, and potassium were in the normal range. As an initial treatment modality for acute chemical intoxication, we used a conventional blood purification technique using hemodialysis (AK 95S Hemodialysis Machine [Gambro, Lund, Sweden]; blood flow, 250 mL/min and dialysate flow, 500 mL/min). We believe that the discordance in rhabdomyolysis-related laboratory results for the plasma tests was caused by hemodialysis.
Pesticide-induced rhabdomyolysis may manifest in 3 distinct ways: active toxic compound-induced cellular toxicity, cellular toxicity of additives, and synergistic toxicity from both the active toxic compound and the additives. Our study included 28 patients with acute surfactant intoxication. Among them, 2 patients (7.1%) had rhabdomyolysis (Tables 1 and 2). We have previously described the cell membrane toxicity of surfactants that are frequently included in pesticide formulations (20) and the synergistic toxicity between these surfactants and the actual herbicides (21). On the basis of these findings, we believe that the combined toxicity of surfactants and the chief pesticide ingredient is responsible for the high incidence of rhabdomyolysis in acute pesticide intoxication. Furthermore, the observation that men are more prone to have rhabdomyolysis after pesticide intoxication than women may be related to the differences in sex hormones and muscle mass between men and women.
Some patients died during the progression of rhabdomyolysis. In such situations, we could not determine the extent to which rhabdomyolysis was responsible for the deaths. The deaths may have been the result of synergistic or additive effects of rhabdomyolysis and the pesticide poisoning. Ethanol itself may cause rhabdomyolysis, as is shown in Table 2. In our study, most of the patients who developed rhabdomyolysis in the pesticide class also ingested ethanol. However, there was no difference in the serum alcohol levels measured in the emergency room between the patients with pesticide intoxication who did and did not have rhabdomyolysis. This result suggests that ethanol itself was not responsible for causing rhabdomyolysis in the patients with pesticide intoxication.
The lack of rhabdomyolysis among patients taking statins was notable. During the observation period, 8,486,084 statin tablets were prescribed to 27,902 patients in our hospital. None of these patients developed rhabdomyolysis during their treatment with statins. Although not a focus of the current study, we suspect that pharmacogenetic differences between races may explain this unexpected observation.
Recently, Heyne et al. (22) described high cutoff renal replacement therapy (molecular cutoff at 45 kDa) as a novel approach for the extracorporeal elimination of myoglobin in rhabdomyolysis-associated ARF. However, conservative treatment including hydration with lactated ringer solution, alkalization of urine with bicarbonate, and the addition of mannitol is generally recommended, and nephrotoxic drugs should be avoided.
Our study has some limitations. We could not discern whether rhabdomyolysis or the chemical poisoning was more responsible for ARF because ARF occurred in patients with acute pesticide poisoning and coincident rhabdomyolysis. For similar reasons, we could not determine whether rhabdomyolysis-associated ARF or pesticide poisoning caused death. Despite this limitation, our study determined incidence of rhabdomyolysis in patients with acute pesticide intoxication. In conclusion, pesticide intoxication is a frequent cause of rhabdomyolysis and is more common among men than women. The volume of pesticide ingested, and not the degree of human toxicity, is the main factor influencing the incidence of rhabdomyolysis.
ACKNOWLEDGEMENTS
The authors thank Min-sook Seo, currently a student at the Department of Pharmacology, University of Queensland, Australia, for her contribution to this project during her observationship at the Pesticide Intoxication Institute of Soonchunhyang Cheonan Hospital, Korea.
Footnotes
The work was carried out by the support of the "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ008246)", under the auspices of the Rural Development Administration, Republic of Korea.
All of authors have no conflicts of interest to declare.
References
- 1.Allison RC, Bedsole DL. The other medical causes of rhabdomyolysis. Am J Med Sci. 2003;326:79–88. doi: 10.1097/00000441-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C–60C. doi: 10.1016/j.amjcard.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Song Y, Li H, Chen J. Rhabdomyolysis associated with fibrate therapy: review of 76 published cases and a new case report. Eur J Clin Pharmacol. 2009;65:1169–1174. doi: 10.1007/s00228-009-0723-7. [DOI] [PubMed] [Google Scholar]
- 4.Hadad E, Weinbroum AA, Ben-Abraham R. Drug-induced hyperthermia and muscle rigidity: a practical approach. Eur J Emerg Med. 2003;10:149–154. doi: 10.1097/00063110-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Tritakarn T, Teeratchanan T. Acute rhabdomyolysis and cardiac arrest following succinylcholine in a patient with Parry-Romberg syndrome. Anaesth Intensive Care. 2011;39:135–136. [PubMed] [Google Scholar]
- 6.Riggs JE. Alcohol-associated rhabdomyolysis: ethanol induction of cytochrome P450 may potentiate myotoxicity. Clin Neuropharmacol. 1998;21:363–364. [PubMed] [Google Scholar]
- 7.Richards JR. Rhabdomyolysis and drugs of abuse. J Emerg Med. 2000;19:51–56. doi: 10.1016/s0736-4679(00)00180-3. [DOI] [PubMed] [Google Scholar]
- 8.Propst A, Propst T, Feichtinger H, Judmaier G, Willeit J, Vogel W. Copper-induced acute rhabdomyolysis in Wilson's disease. Gastroenterology. 1995;108:885–887. doi: 10.1016/0016-5085(95)90465-4. [DOI] [PubMed] [Google Scholar]
- 9.Mastaglia FL, Needham M. Update on toxic myopathies. Curr Neurol Neurosci Rep. 2012;12:54–61. doi: 10.1007/s11910-011-0232-9. [DOI] [PubMed] [Google Scholar]
- 10.Sitprija V. Animal toxins and the kidney. Nat Clin Pract Nephrol. 2008;4:616–627. doi: 10.1038/ncpneph0941. [DOI] [PubMed] [Google Scholar]
- 11.Weng SF, Hung DZ, Hu SY, Tsan YT, Wang LM. Rhabdomyolysis from an intramuscular injection of glyphosate-surfactant herbicide. Clin Toxicol (Phila) 2008;46:890–891. doi: 10.1080/15563650802286731. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz BZ, Marquardt K, Swenson E. Calcium polysulfide overdose: a report of two cases. J Toxicol Clin Toxicol. 1997;35:299–303. doi: 10.3109/15563659709001215. [DOI] [PubMed] [Google Scholar]
- 13.Meulenbelt J, Zwaveling JH, van Zoonen P, Notermans NC. Acute MCPP intoxication: report of two cases. Hum Toxicol. 1988;7:289–292. doi: 10.1177/096032718800700312. [DOI] [PubMed] [Google Scholar]
- 14.Roberts DM, Seneviratne R, Mohammed F, Patel R, Senarathna L, Hittarage A, Buckley NA, Dawson AH, Eddleston M. Intentional self-poisoning with the chlorophenoxy herbicide 4-chloro-2-methylphenoxyacetic acid (MCPA) Ann Emerg Med. 2005;46:275–284. doi: 10.1016/j.annemergmed.2005.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong JR, Seok SJ, Jeong DS, Lee SG, Gil HW, Yang JO, Lee EY, Hong SY. Association of the superoxide dismutase (V16A) and catalase (C262T) genetic polymorphisms with the clinical outcome of patients with acute paraquat intoxication. Korean J Intern Med. 2010;25:422–428. doi: 10.3904/kjim.2010.25.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seok SJ, Gil HW, Jeong DS, Yang JO, Lee EY, Hong SY. Paraquat intoxication in subjects who attempt suicide: why they chose paraquat. Korean J Intern Med. 2009;24:247–251. doi: 10.3904/kjim.2009.24.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Englberger L, Suri RM, Li Z, Casey ET, Daly RC, Dearani JA, Schaff HV. Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care. 2011;15:R16. doi: 10.1186/cc9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Floyd JS, Heckbert SR, Weiss NS, Carrell DS, Psaty BM. Use of administrative data to estimate the incidence of statin-related rhabdomyolysis. JAMA. 2012;307:1580–1582. doi: 10.1001/jama.2012.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill OT, Wahi MM, Carter R, 3rd, Kay AB, McKinnon CJ, Wallace RF. Rhabdomyolysis in the US Active Duty Army, 2004-2006. Med Sci Sports Exerc. 2012;44:442–449. doi: 10.1249/MSS.0b013e3182312745. [DOI] [PubMed] [Google Scholar]
- 20.Song HY, Kim YH, Seok SJ, Gil HW, Yang JO, Lee EY, Hong SY. Cellular toxicity of surfactants used as herbicide additives. J Korean Med Sci. 2012;27:3–9. doi: 10.3346/jkms.2012.27.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song HY, Kim YH, Seok SJ, Gil HW, Hong SY. In vitro cytotoxic effect of glyphosate mixture containing surfactants. J Korean Med Sci. 2012;27:711–715. doi: 10.3346/jkms.2012.27.7.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heyne N, Guthoff M, Krieger J, Haap M, Häring HU. High cut-off renal replacement therapy for removal of myoglobin in severe rhabdomyolysis and acute kidney injury: a case series. Nephron Clin Pract. 2012;121:c159–c164. doi: 10.1159/000343564. [DOI] [PubMed] [Google Scholar]



