Abstract
Background and Objectives
It is widely known that both bone loss and vascular calcification are age-related processes. The purpose of this study was to investigate the relationship between coronary artery calcium (CAC) score or bone mineral density (BMD) with age and whether there is a gender difference factoring in the two conditions among healthy subjects.
Subjects and Methods
Between March 2009 and August 2011, participants included 1727 subjects (mean age: 55±10 years, M : F=914 : 813) with routine health check-ups. After being categorized into three groups (normal, osteopenia, and osteoporosis) according to the World Health Organization (WHO) diagnostic classification, we estimated BMD by dual energy X-ray absorptiometry (DEXA) and CAC score by dual-source CT (DSCT).
Results
There was a significant gender difference among the risk factors, including total-lumbar spine (1.213±0.176 g/cm2 : 1.087±0.168 g/cm2, p<0.001) and femur (1.024±0.131 g/cm2 : 0.910±0.127 g/cm2, p<0.001) in BMD by DEXA, and CAC score (68±227 : 27±116, p<0.001) in coronary artery calcification by DSCT. Age in male [odds ratio (OR): 1.138 {95% confidence interval (CI): 1.088-1.190}, p<0.001] and menopause in female subjects {OR: 12.370 (95% CI: 3.120-49.047), p<0.001} were, respectively, independently associated with osteopenia.
Conclusion
Although our results do not demonstrate a direct association between CAC score and BMD in both genders, there is a gender difference of CAC score in normal and osteopenia groups according to the WHO diagnostic classification. Additionally, we suggest that more specific therapeutic strategies be considered during any early bone loss period, especially in female subjects.
Keywords: Gender, Osteoporosis, Coronary vessels, Calcium, Bone density
Introduction
It is widely known that both bone loss and vascular calcification are age-related processes. Worldwide, osteoporosis is the most common bone disease in humans, characterized by the consequence of continued bone loss and structural deterioration of bone tissue and disruption of bone architecture, compromised bone strength, and an increase in the risk of fracture. It represents a major public health problem that affects both men and women, usually as they grow older. According to the World Health Organization (WHO) diagnostic classification, osteoporosis is defined by bone mineral density (BMD) at the hip or spine of less than or equal to 2.5 standard deviations (SD) below the young normal mean reference population.1),2)
Until recently, several clinical data series have reported a relationship between osteoporosis and vascular calcification, including coronary artery disease (CAD), aortic calcification, carotid artery plaque, arterial stiffness, or cardiovascular outcomes.3-7) However, while most previous studies have considered osteoporosis a disease of older women, accelerated bone loss also occurs in older men.
Previous hypotheses have implied estrogen deficiency, abnormalities of vitamin D metabolism, and lipid oxidation in a common pathogenesis for these two conditions.8-10) Although a shift of calcium from the skeleton towards the arterial wall can account for both bone loss and vascular calcification, the underlying pathophysiologic mechanisms that are identified in bone metabolism and vascular homoeostasis have not been defined.
Vascular calcification is widely used as a clinical indicator of atherosclerosis and related to the major cause of morbidity and mortality in older men and women. Since coronary artery calcification can be visualized and quantified by electron beam computed tomography by moving the X-ray source-point electronically, recent multidetector computed tomography technology, using higher spatial resolution, has been shown to play an important role in the diagnosis and prognosis of CAD. Nevertheless, a few studies have demonstrated the relationship between osteoporosis and coronary artery calcification, and it remains a matter of debate.11),12)
The purpose of this study was to investigate the relationship between coronary artery calcium (CAC) score using dual-source computed tomography (DSCT) and BMD with age, and whether there is a gender difference involved in the two conditions in healthy subjects.
Subjects and Methods
Study population
Between March 2009 and August 2011, the participants included 1727 subjects (mean age: 54.8±10.0 years, M : F=914 : 813) who visited the Heath Promotion Center of the Catholic University Seoul St. Mary's Hospital for a routine health check-up.
Criteria for exclusion included: patients 1) who had undergone previous percutaneous coronary intervention or previous coronary artery bypass graft; 2) patients with a disorder that could possibly influence bone and calcium metabolism, such as thyrotoxicosis, hyperparathyroidism, or chronic renal failure; and 3) patients that were consuming drugs that might interact with bone and calcium metabolism, such as glucocorticosteroid, estrogen, and bisphosphonates. Before the health check-up began, all clinical information, such as information on hypertension, diabetes mellitus, dyslipidemia, personal history of CAD, personal history of cerebrovascular accidents (CVA), familial history of cardiovascular risk, and current smoking status were collected through self-reporting.
This study was approved by the Institutional Review Committee of St. Mary's Hospital, The Catholic University of Korea. The participants were informed of the investigative nature of the study and written informed consent was obtained prior to enrollment (XC11RIMI0091S).
Anthropometric parameters measurement
Each participant also underwent a complete physical examination, including anthropometric measurements. Heights were measured to the nearest 0.1 cm with a portable stadiometer (InBody 720; Biospace Ltd., Seoul, Korea) and body weights were measured to the nearest 0.1 kg using a digital scale wearing a standardized health check-up gown. Body mass index (BMI) was calculated as weight in kilograms, divided by height in meters squared. Waist circumference was measured using a standardized tape by the same well-trained staff. The tape was calibrated before use. The waist circumference was measured 1 inch above the umbilicus in the standing position. The hip circumference was measured at the site of the largest circumference between the waist and thighs. Systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate were measured using an automatic sphygmomanometer (BP203RV-II; Nippon Colin, Komaki, Japan), with subjects in a seated position after having rested quietly for 10 minutes.
Bone mineral density
Bone mineral density for bone loss was evaluated by dual energy X-ray absorptiometry (DEXA; Lunar Prodigy, GE Healthcare, Waukesha, WI, USA) in the lumbar spine (L1-L4) and femur neck according to the WHO diagnostic criteria.
In postmenopausal women and men aged 50 years and over, the WHO diagnostic T-score criteria (normal, osteopenia, and osteoporosis) was applied to the BMD measurement by a central DEXA at the lumbar spine (L1-L4) and femoral neck: normal was defined when BMD was within 1 SD of the average peak bone mass in young normal adults (T-score at -1.0 and above); osteopenia was defined as BMD being between 1.0 and 2.5 SD below that of young normal adults (T-score between -1.0 and -2.5); osteoporosis was defined as a BMD of 2.5 SD or more below that of a young normal adult (T-score at or below -2.5); and severe or established osteoporosis was defined for patients in this group as those who had already experienced one or more fractures.2)
In premenopausal women and men less than 50 years of age, an ethnic or race adjusted Z-score instead of a T-score was recommended by the International Society for Clinical Densitometry13) and applied to the BMD measurement: a Z-score above -2.0 was defined as "within the expected range for the age" (classified as normal); a Z-score of -2.0 or lower was defined as either a "low BMD for chrono-logical age" or "below the expected range for the age" (classified as osteopenia).
Coronary artery calcium score
We measured coronary artery calcification with a DSCT (SOMATOM Definition; Siemens Healthcare, Forchheim, Germany). The heart rate during CT acquisition ranged from 43-75 bpm (mean 67 bpm). The participants did not receive additional premedications, such as β-blockers, for heart rate control.
The DSCT parameters were as follows: tube voltage=120 kVp, gantry rotation time=0.33 seconds, slice collimation=64×0.6 mm, reconstruction slice width=0.75 mm, reconstruction slice interval=0.4 mm, kernel=B26f, field of view=25 cm. Eighty mL of contrast agent (Iohexol, IOBRIX INJ 300; Tae Joon Pharm. Ind. Co., Ltd, Seoul, Korea) using a dual-head power injector (CT Stellant; Medrad Inc., Indianola, PA, USA) was injected intravenously at 5 mL/s for 16 seconds. Fifty mL of saline solution chaser at 5 mL/s was also injected, over 10 seconds.
All post-processing examinations were performed using retrospective electrocardiography-gating. Scans were analyzed by the consensus of two observers with more than two years of experience. CAC score for vascular calcification was analyzed using software (syngo.CT CaScoring; Siemens Healthcare; Forcheim, Germany); DSCT angiograms were analyzed on a three-dimensional workstation (Advantage Windows Workstation 4.3, GE Healthcare, Milwaukee, WI, USA), using software (Card IQ; GE Healthcare, Milwaukee, WI, USA).
For defining the quantity of coronary calcium, the Agatston score, a standard parameter, was used as the product of the area of calcification per coronary tomographic segment, and a factor rated 1 though 4 dictated by the maximal calcium X-ray density in that segment, as described elsewhere.14) The sum of all lesion scores for each major coronary artery including left main (LM), left anterior descending artery, left circumflex artery, and right coronary artery, was used to generate the total CAC score. Additionally, volume score in cubic millimeters was used as a continuous parameter.
Assays biochemical and hematologic parameter analysis
Blood samples were taken during the routine health check-up day. A fasted blood sample of 10 mL was taken and mixed with ethylenediaminetetraacetic acid to prevent clotting. Plasma was obtained by centrifugation, frozen in liquid nitrogen, and stored at -80℃ until further analysis was required.
The lipid profile, including total cholesterol, low density lipoprotein-cholesterol, high density lipoprotein-cholesterol (HDL-C), and triglyceride levels, were measured using the enzymatic method by an automatic analyzer (7600-210; Hitachi Medical Corp., Tokyo, Japan). The complete blood count, including white blood cells, hemoglobin, hematocrit, and platelet, was measured using a hematology analyzer (Sysmex XE-2100; Sysmex Corporation, Kobe, Japan). HbA1c was measured by a G8 HbA1c analyzer (Tosoh Corporation, Tokyo, Japan). Biochemistry levels, including serum calcium, serum phosphorus, uric acid, fasting blood sugar, bone-specific alkaline phosphates (bsALP), thyroid-stimulating hormone, free thyroxin, and Creactive protein from blood samples, were measured by a biochemistry analyzer (7600-210; Hitachi Medical Corp., Tokyo, Japan). The value of an intact parathyroid hormone was determined by an immunometric assay (ADVIA Centaur; Siemens Healthcare, Munich, Germany). The levels of insulin and calcitonin were measured by a gamma counter (Beckman Instruments Inc., Fullerton, CA, USA). The active form of vitamin D, 1,25-dihydroxy vitamin D3 was measured by a rapid chemiluminescent assay (LIAISON® automated analyzer; DiaSorin Inc., Stillwater, MN 55082, USA). C-terminal telopeptide of Type I collagen (CTX-I) was measured by an electrochemiluminescence immunoassay (Modular Analytics E170; Roche Diagnostics, Penzberg, Germany).
Statistical analysis
All data are expressed as mean±SD for continuous variables and as a frequency percentage for categorical variables. Statistical analysis was performed using the SAS statistical software version 9.1 (SAS Institute, Cary, NC, USA). The parameters related to CAC score or BMD were assessed using Spearman's correlation coefficient by rank. The analysis of gender difference was performed using an unpaired t-test for continuous variables and a chi-squared test for categorical data. The analysis of the three categories according to the WHO diagnostic classification: normal, osteopenia, and osteoporosis, was performed using analysis of variance test for continuous variables and Tukey's b-test as a post-hoc t-test for categorical data. To identify independent factors associated with osteopenia or osteoporosis, we used multiple logistic regression analysis and calculated odds ratios (OR) and 95% confidence intervals (95% CI). All statistical tests were 2-tailed and p<0.05 was considered statistically significant.
Results
Clinical characteristics
The mean age of the total participants was 55±10 years; there were more male (n=914) than female (n=813) subjects. A total of 642 out of 813 (78.8%) female subjects were postmenopausal. The prevalence of hypertension, diabetes mellitus, dyslipidemia, personal history of CAD, personal history of CVA, familial history of cardiovascular risk, and current smoking status, were 32.5%, 12.8%, 16.0%, 6.0%, 1.6%, 84.2%, and 20.3%, respectively.
Coronary artery disease was defined as an obstruction of more than 50% of the diameter of the vessel based on the DSCT angiographic finding, reported by three radiologists and categorized as; normal (93.3%); LM-(0.1%); 1-(4.9%); 2-(1.2%); 3-vessel disease (0.3%); and LM with 1-vessel disease (0.1%), respectively. 1028 (59.5%) subjects showed no calcium deposit in the coronary artery (0 of total CAC score; no identifiable atherosclerotic plaque). Base-line clinical characteristics according to gender are presented in Table 1.
Table 1.
Baseline clinical characteristics
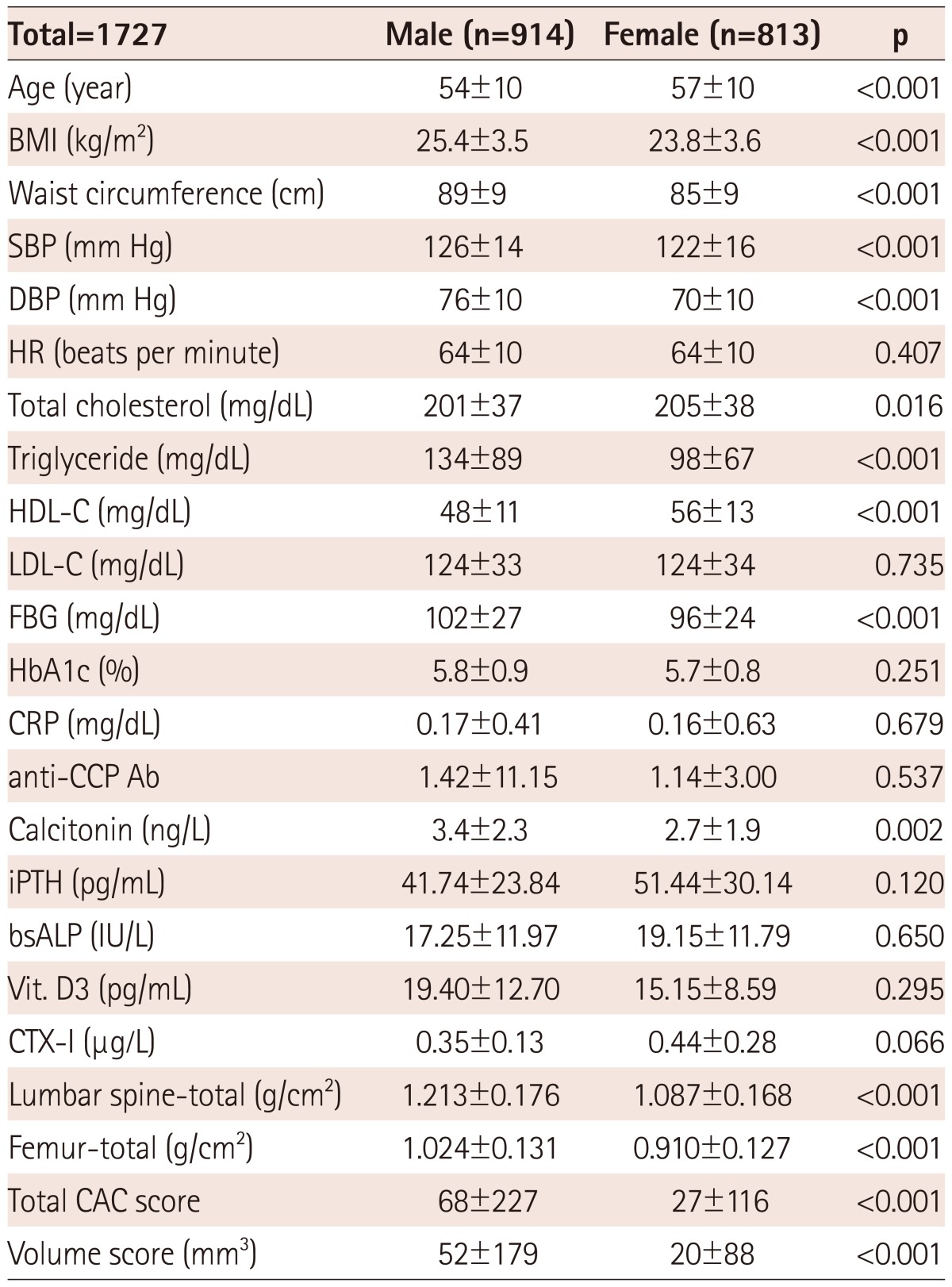
BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, HR: heart rate, HDL-C: high density lipoprotein-cholesterol, LDLC: low density lipoprotein-cholesterol, FBG: fasting blood sugar, CRP: C reactive protein, iPTH: intact parathyroid hormone, anti-CCP Ab: anti-cyclic citrullinated protein antibodies, bsALP: bone specific alkaline phosphatase, Vit. D3: 1,25-dihydroxy vitamin D3, CTX-I: C-terminal telopeptide of type I collagen, CAC: coronary artery calcium
There was a certain gender difference among the risk factors, including age (M : F=54±10 years : 57±10 years, p<0.001), BMI (25.4±3.5 kg/m2 : 23.8±3.6 kg/m2, p<0.001), waist circumference (89±9 cm : 85±9 cm, p<0.001), SBP (126±14 mm Hg : 122±16 mm Hg, p<0.001), and DBP (76±10 mm Hg : 70±10 mm Hg, p<0.001) in demographic data, total cholesterol (201±37 mg/dL : 205±38 mg/dL, p=0.016), triglyceride (134±89 mg/dL : 98±67 mg/dL, p<0.001), HDL-C (48±11 mg/dL : 56±13 mg/dL, p<0.001), fasting blood sugar (102±27 mg/dL : 96±24 mg/dL, p<0.001), and calcitonin (3.4±2.3 ng/L : 2.7±1.9 ng/L, p=0.002) in laboratory findings, BMD at the level of lumbar spine (-total: 1.213±0.176 g/cm2 : 1.087±0.168 g/cm2, p<0.001), and femur (-total: 1.024±0.131 g/cm2 : 0.910±0.127 g/cm2, p<0.001), total CAC score (68±227 : 27±116, p<0.001), and volume score (52±179 mm3 : 20±88 mm3, p<0.001) for coronary artery calcification, respectively.
Parameters associated with an age-related process
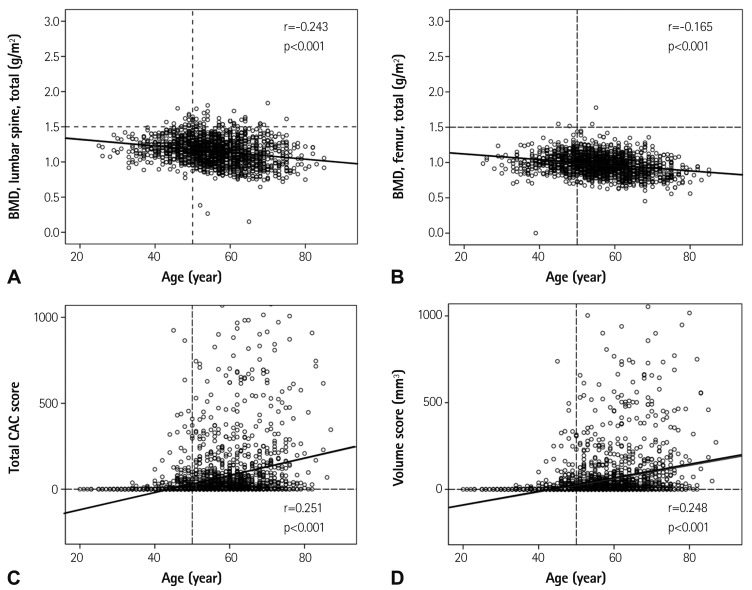
For bone loss, there was an inverse correlation between BMD and age {lumbar spine, total: r=-0.243, p<0.001 (Fig. 1A) femur, total: r= -0.165, p<0.001 (Fig. 1B) respectively}. For coronary artery calcification, there was a positive correlation between total CAC score (r= 0.251, p<0.001) (Fig. 1C), volume score {r=0.248, p<0.001 (Fig. 1D) respectively}, and age.
Fig. 1.
Association with age in bone loss (A and B) and vascular calcification (C and D). BMD: bone mineral density, CAC: coronary artery calcium.
In the subgroup analysis according to gender, there was an inverse correlation between BMD at the level of femur and age (femur, total: r=-0.157, p<0.001) and there was a positive correlation between total CAC score (r=0.277, p<0.001), volume score (r=0.275, p<0.001; respectively), and age in 914 male subjects. In the 813 female subjects, there was an inverse correlation between BMD and age (lumbar spine, total: r=-0.477, p<0.001; femur, total: r=-0.401, p<0.001), and a positive correlation between total CAC score (r=0.284, p<0.001), volume score (r=0.280, p<0.001; respectively), and age.
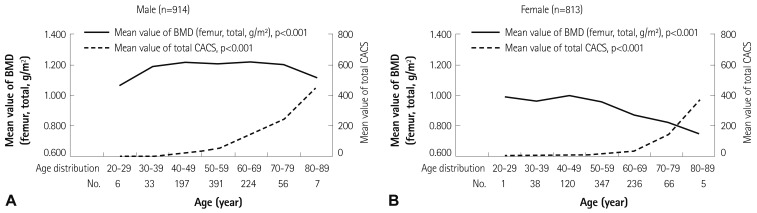
In the subgroup analysis according to age distribution, the mean value of total BMD at the level of femur and total CAC score were significantly different in both male (total BMD at the level of femur: p<0.001; total CAC: p<0.001, respectively) (Fig. 3A) and female {total BMD at the level of femur: p<0.001; total CAC: p<0.001 (Fig. 3B) respectively} groups.
Fig. 3.
Comparison of mean value of total BMD at the level of femur and total CAC score according to age distribution. The mean value of BMD at the level of femur decreases progressively, but total CAC score for vascular calcification increases sequentially through all ages in male subjects (A), while females aged 50 to 59 was a turning point for the abrupt decrease of BMD, but the sudden increase of total CAC score (B). p<0.05, the analysis for mean value of BMD and total CAC score according to age distribution was performed using analysis of variance test. BMD: bone mineral density, CAC: coronary artery calcium.
Relationship between bone mineral density and coronary artery calcium score
There was no significant correlation between BMD and total CAC score in the 813 female subjects (lumbar spine, total: r=-0.094, p=0.008; femur, total: r=-0.088, p=0.013; respectively), or the 914 male subjects (lumbar spine, total: r=-0.008, p=0.801; femur, total: r=-0.044, p=0.188; respectively).
Comparisons among the three groups according to the World Health Organization diagnostic classification
To evaluate the gender difference of BMD measurement, the total 1727 participants were divided into two groups: male (n=914) and female (n=813) subjects and then categorized into three groups according to the WHO diagnostic classification: 1) normal (M : F=401 : 183); 2) osteopenia (M : F=404 : 325); and 3) osteoporosis (M : F=109 : 305) (Table 2). Of the 914 male subjects, there was a significant difference am-ong the three groups in BMI (25.9±3.3 kg/m2 vs. 24.8±3.2 kg/m2 vs. 23.4±2.6 kg/m2; p<0.001), and waist circumference (90±8 cm vs. 88±9 cm vs. 86±7 cm; p<0.001, respectively) in demographic data, as parameters related to obesity, serum HDL-C concentration (48±10 mg/dL : 48±11 mg/dL : 52±12 mg/dL; p=0.010) in laboratory findings, total CAC score (48±144 : 93±288 : 77±198; p=0.018), and volume score (37±115 mm3 : 73±227 mm3 : 59±155 mm3; p=0.019, respectively) (Table 2).
Table 2.
Parameters associated with BMD in both genders
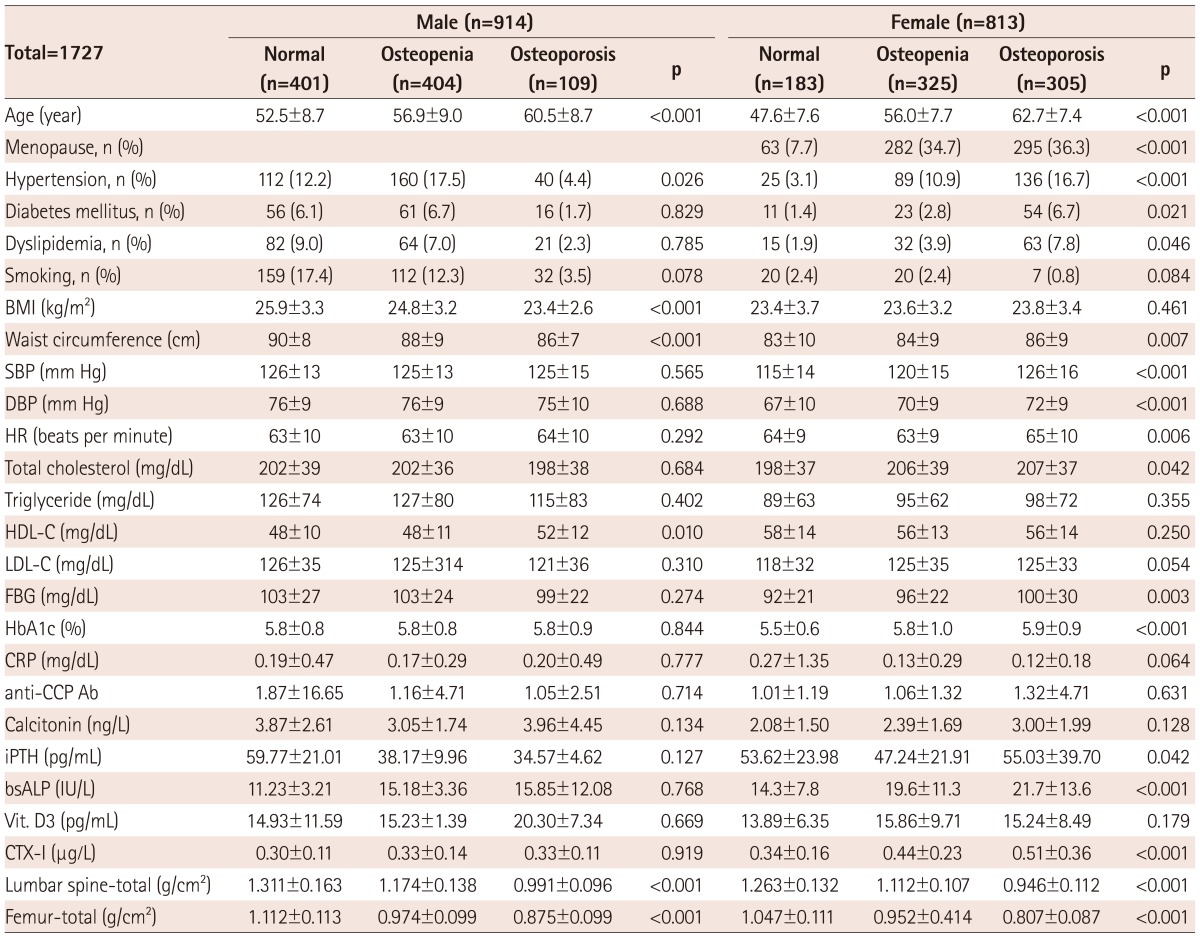
BMD: bone mineral density, BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, HR: heart rate, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, FBG: fasting blood sugar, CRP: C reactive protein, iPTH: intact parathyroid hormone, anti-CCP Ab: anti-cyclic citrullinated protein antibodies, bsALP: bone specific alkaline phosphatase, Vit. D3: 1,25-dihydroxy vitamin D3, CTX-I: C-terminal telopeptide of type I collagen, CT: computed tomography, LM: left main, LAD: left anterior descending artery, LCX: left circumflex artery, RCA: right coronary artery, CAC: coronary artery calcium
Of the 813 female subjects, there was a significant difference among the three groups in waist circumference (83±10 : 84±9 : 86±9; p=0.007), SBP (115±14 mm Hg : 120±15 mm Hg : 126±16 mm Hg; p<0.001), and DBP (67±10 mm Hg : 70±9 mm Hg : 72±9 mm Hg; p<0.001, respectively) in demographic data, serum total cholesterol concentration (198±37 mg/dL : 206±39 mg/dL : 207±37 mg/dL; p=0.042), and fasting blood sugar (92±21 mg/dL : 96±22 mg/dL : 100±30 mg/dL; p=0.003), HbA1c (5.5±0.6 % : 5.8±1.0% : 5.9±0.9%; p<0.001), bsALP (14.3±7.8 IU/L : 19.6±11.3 IU/L : 21.7±13.6 IU/L; p<0.001), and CTX-I level (0.34±0.16 µg/L : 0.44±0.23 µg/L : 0.51±0.36 µg/L; p<0.001, respectively) in laboratory findings, total CAC score (9±54 : 18±99 : 45±161; p=0.002), and volume score (8±49 : 13±70 : 34±124; p=0.002, respectively) (Table 2).
Gender difference among the three groups according to the World Health Organization diagnostic T-score criteria
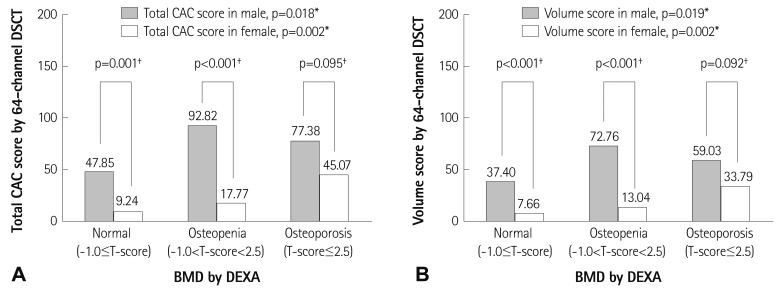
Total CAC score showed a significant gender difference in normal (M : F=48±144 : 9±54, p<0.001) and osteopenia groups (M : F= 93±288 : 18±99, p<0.001, respectively), but not in the osteoporosis group (M : F=77±198 : 45±161, p=0.095) (Fig. 2A). Volume score showed a significant gender difference in the normal (M : F=37±115 mm3 : 8±49 mm3, p<0.001) and osteopenia groups (M : F=73±227 mm3 : 13±70 mm3, p<0.001, respectively), but not in the osteoporosis group (M : F=59±155 mm3 : 34±124 mm3, p=0.095) (Fig. 2B).
Fig. 2.
Gender difference of each measurement for the three categorical groups according to the WHO diagnostic T-score criteria. Total CAC score (A) and volume score (B) by 64-channel DSCT. *The analysis for the three categorical groups according to the WHO diagnostic classification: normal, osteopenia and osteoporosis, was performed using analysis of variance test for continuous variables, †The analysis of gender difference was performed using an unpaired t-test for continuous variables. WHO: World Health Organization, CAC: coronary artery calcium, DSCT: dual source computed tomography, BMD: bone mineral density, DEXA: dual energy X-ray absorptiometry.
Independent factor assoiated with osteopenia and osteoporosis
Age in male (OR: 1.138, 95% CI: 1.088-1.190, p<0.001) and menopause in female subjects (OR: 12.370, 95% CI: 3.120-49.047, p<0.001) were independently associated with osteopenia (-1.0≤T-score<-2.5) in multiple logistic regression analysis (Table 3).
Table 3.
Multivariate logistic regression analysis
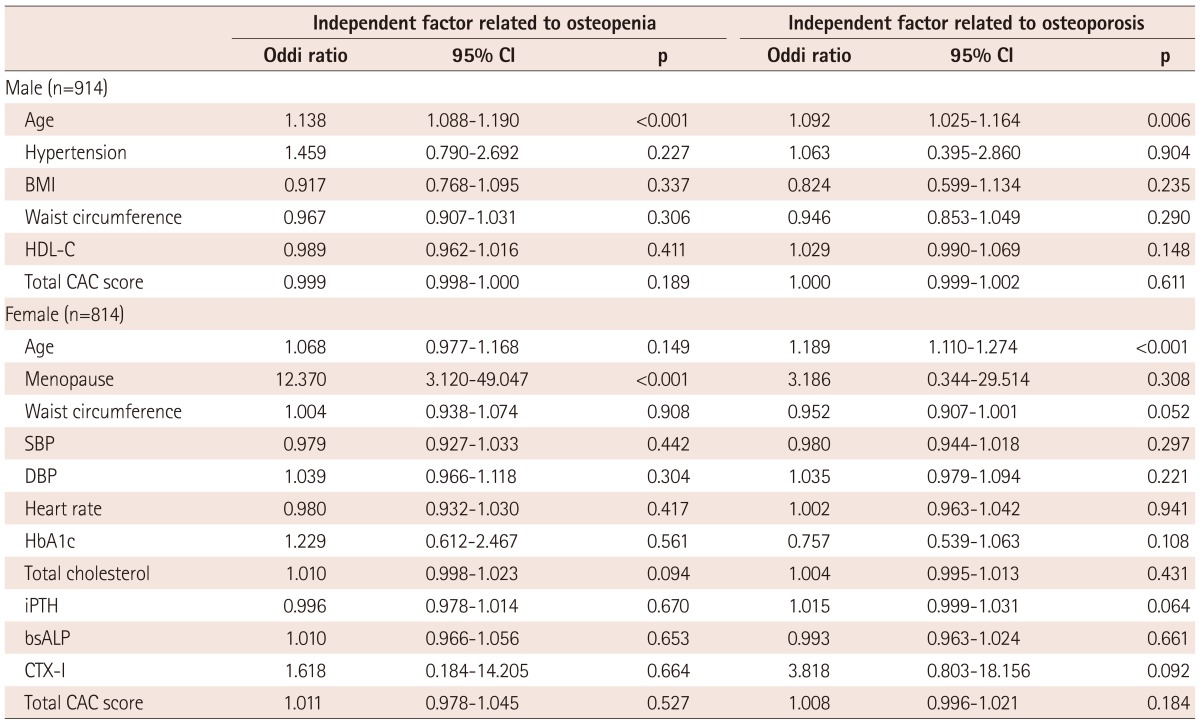
CI: confidence interval, BMI: body mass index, HDL-C: high density lipoprotein-cholesterol, CAC: coronary artery calcium, SBP: systolic blood pressure, DBP: diastolic blood pressure, iPTH: intact parathyroid hormone, bsALP: bone specific alkaline phosphatase, CTX-I: C-terminal telopeptide of type I collagen
Age in both male (OR: 1.092, 95% CI: 1.025-1.164, p=0.006) and female subjects (OR: 1.189, 95% CI: 1.110-1.274, p<0.001) was independently associated with osteoporosis (T-score≤-2.5) in multiple logistic regression analysis (Table 3).
Discussion
Although both calcium depositions in the vasculature and bone loss through bone metabolism are natural aging processes, underlying pathophysiologic mechanisms remain subject to debate. Since a report on the relationship between cardiovascular disease and calcium deposition in the vasculature was first described in the middle of the 19th century,1) the concept of vascular calcification, considered a passive process or a degenerative consequence of aging in the past, has begun to be thought of as an active and regulated process closely related to bone metabolism.2)
Our results show that age in both genders is an independent factor associated with osteoporosis, as well as age in males and menopause in females are independent factors associated with osteopenia.
Compared with the mean value of BMD, bone loss decreases progressively, but the total CAC score for vascular calcification increases sequentially through all ages in male subjects. Females aged 50 to 59 appear to be a turning point for the abrupt decrease of BMD, but a sudden increase of total CAC score. Our results showed that there is a gender difference in terms of bone loss acceleration in the natural aging process, and especially, in female subjects, there were some interrupting factors in the perimenopausal period. Therefore, our investigators considered that menopause in females was an independent factor associated with osteopenia and it should be recognized that more specific therapeutic strategies in the perimenopausal period may play an important role in the prevention of osteoporosis.
Additionally, in this study, there was an inverse correlation between BMD and age. It has been well documented that the effect of advancing age is independent of all other risk factors, with the odds of osteoporosis increasing exponentially, 10-23 times greater for women aged 80 years or over than for those 50-59 years old. This has been identified in several reports.15),16) Moreover, Hernandez et al.17) reported a more detailed study using an experimental computer model of the bone remodeling process to predict the relative influences of peak BMD. In the relationship between coronary arterial calcification and age, Newman et al.18) reported that the extent of coronary artery calcification was strongly associated with age through the ninth decade in men and women, and was associated with cardiovascular disease, (but the risk factor levels were only weakly associated with the extent of coronary artery calcification in these older adults). In contrast, Yoon et al.19) demonstrated that neither sex nor age was a significant predictor of CAC progression and, among traditional risk factors, only hypertension and diabetes were significant independent factors for calcium progression. In our results, there was a positive correlation between total CAC score and age, and total CAC score showed a significant difference between male and female subjects. Additionally, in the subgroup analysis according to the WHO diagnostic classification: in both the normal and osteopenia groups, total CAC score was significantly different between males and females, but not different in the osteoporosis group. To prove not only the CAC progression but also the gender difference according to the subgroup analysis, future long-term follow-up studies are warranted after considering other risk factors affecting coronary artery calcification.
In the subgroup analysis according to the WHO diagnostic classification, our data also found similar results regarding traditional risk factors for osteoporosis-related fractures,20),21) including BMI and HDL-C level in males: SBP, DBP, total cholesterol, fasting blood sugar, HbA1c, bsALP, and CTX-I in females; and height, weight, waist circumference, and total CAC score in both genders. In the present study, there was an inverse correlation in females but not in males. Recently, there were several studies exploring the relationship between BMD and CAC score. In subjects older than 50 years, Kim et al.22) reported age, hypertension, glucose, and male gender were independent factors determining coronary artery calcification, but not BMD. Lee et al.23) reported that BMD was inversely correlated with coronary artery calcification only in patients with metabolic syndrome. Despite the lack of gender difference between BMD and CAC score, Bakhireva et al.24) suggested that the association between coronary and bone calcium might be mediated by hormone therapy, otherwise known as estrogen. Therefore, for further evaluation of the underlying etiologic mechanisms between BMD and CAC score, our investigators suggest that genetic variations as well as hormonal variations may be of some significance.
In addition, in this cross-sectional observational study, although they mentioned that this study population was relatively large, it remains difficult to assess the significance of CAC as a cardiovascular risk factor in each gender in this relatively low risk population. This consideration may be another study limitation to be described.
First, the index of abnormal coronary calcification, total CAC, and volume scores, were generally higher among male participants than female patients. Secondary, female patients with lower BMD had significantly higher CAC, while there was no significant difference in those levels among male participants.
I strongly encourage authors to exclude those participants with advanced atherosclerosis from future analysis, because they might have extremely advanced coronary artery calcification. Similarly, as chronic kidney disease, especially chronic hemodialysis, is known to be one of the major risk factors of coronary calcification, the population suffering from chronic hemodialysis at least should also be excluded. Furthermore, authors should put renal function data (e.g. eGFR, serum creatine) into any analysis.
Study limitations
There are several limitations in our present study. First, many organ systems show age-related functional decline, though this does not necessarily imply a direct or causal relationship between them. The present data is from a relatively small observational study with retrospective design, but acquired and dynamic processes associated with aging cannot be directly assessed. Second, there was a methodological problem in that there is no clear guidance for the diagnosis of osteoporosis as to how to proceed with or without DEXA in this study. Bone mineral measurement by DEXA has high specificity but low sensitivity.1) In other words, the risk of fractures is very high when osteoporosis is present, but by no means negligible when BMD is normal. Indeed, the majority of osteoporotic fractures will occur in individuals with a negative test. Thus, the potential impact of widespread testing of BMD on the burden of fractures is less than optimal, and this is one of the reasons why many agencies do not recommend population screening for BMD.1),25)
In conclusion, although the present data do not show a direct association between CAC score and BMD and age in both genders, there is a gender difference of CAC score in the normal and osteopenia groups according to the WHO diagnostic classification. Additionally, we suggest that more specific therapeutic strategies be considered during early the bone loss period, especially among female subjects.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Kanis JA WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. Osteoporos Int. 1994;4:368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 2.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 3.Farhat GN, Newman AB, Sutton-Tyrrell K, et al. The association of bone mineral density measures with incident cardiovascular disease in older adults. Osteoporos Int. 2007;18:999–1008. doi: 10.1007/s00198-007-0338-8. [DOI] [PubMed] [Google Scholar]
- 4.Samelson EJ, Cupples LA, Broe KE, Hannan MT, O'Donnell CJ, Kiel DP. Vascular calcification in middle age and long-term risk of hip fracture: the Framingham Study. J Bone Miner Res. 2007;22:1449–1454. doi: 10.1359/jbmr.070519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jørgensen L, Joakimsen O, Rosvold Berntsen GK, Heuch I, Jacobsen BK. Low bone mineral density is related to echogenic carotid artery plaques: a population-based study. Am J Epidemiol. 2004;160:549–556. doi: 10.1093/aje/kwh252. [DOI] [PubMed] [Google Scholar]
- 6.Frost ML, Grella R, Millasseau SC, et al. Relationship of calcification of atherosclerotic plaque and arterial stiffness to bone mineral density and osteoprotegerin in postmenopausal women referred for osteoporosis screening. Calcif Tissue Int. 2008;83:112–120. doi: 10.1007/s00223-008-9153-2. [DOI] [PubMed] [Google Scholar]
- 7.Tankó LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20:1912–1920. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- 8.Christian RC, Harrington S, Edwards WD, Oberg AL, Fitzpatrick LA. Estrogen status correlates with the calcium content of coronary atherosclerotic plaques in women. J Clin Endocrinol Metab. 2002;87:1062–1067. doi: 10.1210/jcem.87.3.8354. [DOI] [PubMed] [Google Scholar]
- 9.Watson KE, Abrolat ML, Malone LL, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–1760. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 10.Parhami F, Morrow AD, Balucan J, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 11.Lin T, Liu JC, Chang LY, Shen CW. Association between coronary artery calcification using low-dose MDCT coronary angiography and bone mineral density in middle-aged men and women. Osteoporos Int. 2011;22:627–634. doi: 10.1007/s00198-010-1303-5. [DOI] [PubMed] [Google Scholar]
- 12.Choi SH, An JH, Lim S, et al. Lower bone mineral density is associated with higher coronary calcification and coronary plaque burdens by multidetector row coronary computed tomography in pre- and postmenopausal women. Clin Endocrinol (Oxf) 2009;71:644–651. doi: 10.1111/j.1365-2265.2009.03535.x. [DOI] [PubMed] [Google Scholar]
- 13.International Society for Clinical Densitometry Official Positions. [updated 2007; cited 2008 July]. Available from: http://www.iscd.org.
- 14.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 15.Looker AC, Johnston CC, Jr, Wahner HW, et al. Prevalence of low femoral bone density in older U.S. women from NHANES III. J Bone Miner Res. 1995;10:796–802. doi: 10.1002/jbmr.5650100517. [DOI] [PubMed] [Google Scholar]
- 16.Siris ES, Miller PD, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez CJ, Beaupré GS, Carter DR. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int. 2003;14:843–847. doi: 10.1007/s00198-003-1454-8. [DOI] [PubMed] [Google Scholar]
- 18.Newman AB, Naydeck BL, Sutton-Tyrrell K, Feldman A, Edmundowicz D, Kuller LH. Coronary artery calcification in older adults to age 99: prevalence and risk factors. Circulation. 2001;104:2679–2684. doi: 10.1161/hc4601.099464. [DOI] [PubMed] [Google Scholar]
- 19.Yoon HC, Emerick AM, Hill JA, Gjertson DW, Goldin JG. Calcium begets calcium: progression of coronary artery calcification in asymptomatic subjects. Radiology. 2002;224:236–241. doi: 10.1148/radiol.2241011191. [DOI] [PubMed] [Google Scholar]
- 20.Dawson-Hughes B, Tosteson AN, Melton LJ, 3rd, et al. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19:449–458. doi: 10.1007/s00198-008-0559-5. [DOI] [PubMed] [Google Scholar]
- 21.Kanis JA, McCloskey EV, Johansson H, et al. Case finding for the management of osteoporosis with FRAX--assessment and intervention thresholds for the UK. Osteoporos Int. 2008;19:1395–1408. doi: 10.1007/s00198-008-0712-1. [DOI] [PubMed] [Google Scholar]
- 22.Kim KI, Suh JW, Choi SY, et al. Is reduced bone mineral density independently associated with coronary artery calcification in subjects older than 50 years? J Bone Miner Metab. 2011;29:369–376. doi: 10.1007/s00774-010-0229-5. [DOI] [PubMed] [Google Scholar]
- 23.Lee HT, Shin J, Lim YH, et al. The relationship between coronary artery calcification and bone mineral density in patients according to their metabolic syndrome status. Korean Circ J. 2011;41:76–82. doi: 10.4070/kcj.2011.41.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakhireva LN, Barrett-Connor EL, Laughlin GA, Kritz-Silverstein D. Differences in association of bone mineral density with coronary artery calcification in men and women: the Rancho Bernardo Study. Menopause. 2005;12:691–698. doi: 10.1097/01.gme.0000184422.50696.ef. [DOI] [PubMed] [Google Scholar]
- 25.Compston JE, Papapoulos SE, Blanchard F. Report on osteoporosis in the European Community: current status and recommendations for the future. Working Party from European Union Member States. Osteoporos Int. 1998;8:531–534. doi: 10.1007/s001980050094. [DOI] [PubMed] [Google Scholar]