Abstract
Background and Objectives
Reactive oxygen species (ROS) mediate various signaling pathways that underlie vascular inflammation in atherogenesis and cardiovascular diseases. Cardiac rehabilitation (CR) has a variety of multiple beneficial effects, including anti-inflammatory effects. The purpose of the present study was to investigate the effects of CR on ROS in patients with cardiovascular diseases.
Subjects and Methods
The serum level of derivatives of reactive oxidative metabolites, an index of oxidative stress, was measured in 100 patients with cardiovascular diseases before, and, subsequently, 3 and 6 months after, CR. A biological antioxidant potential (BAP) test was applied to assess the antioxidant power of the serum.
Results
The resting reactive oxidative metabolite levels decreased 3-6 months after CR {pre: 351±97 Carratelli unit (CARR U), 3 months: 329±77 CARR U, 6 months: 325±63 CARR U, all p<0.01} with the increase of the percentage of the predicted values of V̇O2 peak and the percentage of the predicted values of V̇O2 at the anaerobic threshold (V̇O2 AT) and the decrease of the B-type natriuretic peptide (BNP). The BAP test and antioxidative/oxidative stress ratio increased 6 months after CR. The % changes of the antioxidative/oxidative stress ratio was positively correlated with the % changes of V̇O2 AT, and negatively correlated with the % changes of the BNP.
Conclusion
These results suggest that intensive supervised CR significantly improved exercise capacity, which may be attributable to an adaptive response involving more efficient oxidative metabolites or the increased capacity of endogenous anti-oxidative systems in patients with cardiovascular diseases.
Keywords: Reactive oxygen species, Antioxidants, Exercise therapy, Oxygen consumption, Cardiovascular diseases
Introduction
Cardiovascular risk factors, such as hypertension, obesity, hypercholesterolemia, diabetes mellitus, and chronic smoking, stimulate the production of reactive oxygen species (ROS) in the vascular wall.1) Additionally, increases in ROS, such as superoxide and hydrogen peroxide (H2O2), have been reported in patients with cardiovascular diseases2) and chronic heart failure (CHF).3),4)
Decreased nitric oxide (NO) production due to changes in the expression and activity of endothelial NO synthase and increased degradation of NO, through a reaction with superoxide, accounts for the reduction in endothelium-dependent vascular relaxation.5) Furthermore, although the activation of the renin-angiotensin-aldosterone system occurs in cardiovascular diseases, including hypertension, CHF, and coronary artery diseases, angiotensin II has been shown to induce the activity of nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase, and to increase local ROS production.6) Thus, the pathophysiologic causes of oxidative stress in cardiovascular diseases are considered likely to involve changes in different oxidative enzyme systems. On the other hand, oxidative stress is a balance between ROS and antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase. While a small amount of superoxide is normally produced as a byproduct of the use of molecular oxygen during mitochondrial oxidative phosphorylation,7) superoxide is inactivated by either NO or SOD, and SOD rapidly converts superoxide to H2O2 (which is, itself, broken down by GPX and catalase to water).8) However, studies in relation to oxidant stress and antioxidant defense mechanisms in patients with cardiovascular diseases remain quite scarce.
Exercise training is associated with a decreased risk of many of the diseases linked to excessive oxidative stress.9-11) Studies of experimental animals also suggested that long-term voluntary exercise can reduce mitochondrial ROS production in the heart of old rats.12) Linke et al.13) showed that anti-oxidative enzymes in skeletal muscles were lower among patients with CHF than in normal subjects, and were improved 6 months after aerobic training compared with control groups. However, the effects of cardiac rehabilitation (CR) on oxidative stress in patients with cardiovascular diseases remain unclear. The purpose of the present study was to investigate the effects of CR on ROS in patients with cardiovascular diseases.
Subjects and Methods
Subjects
One hundred patients with cardiovascular diseases who had been referred to CR {Male/Female: 88/12; age: 63±10 years; height: 166±8 cm; weight: 67.5±13.9 kg; body mass index (weight/height2): 24.4±3.5 kg/m2} participated in the present study. Patients were enrolled in the present study if they had visited the hospital for CR as a new patient between July 2009 and March 2012. The underlying cardiovascular diseases included ischemic heart diseases in 90 patients, dilated cardiomyopathy in seven patients, a dilated phase of hypertrophic cardiomyopathy in one patient, idiopathic ventricular tachycardia in one patient, and a complete atrioventricular blockage in one patient. According to the New York Heart Association classification of functional capacity, nine patients were in class I, 72 patients were in class II, and 19 patients were in class III (Table 1). Left anterior descending artery, left circumflex artery, and right coronary artery lesions were observed in 79 (88%), 39 (43%), and 47 (52%) of coronary patients, suggesting that many patients with ischemic heart diseases had multiple coronary lesions. Myocardial infarction before 2 months was identified in 24 (24%) patients. 12 patients had had coronary artery bypass grafting surgery, especially among those with left anterior descending artery involvement. Percutaneous coronary intervention, using drug-eluting stents in 68 (76%) patients, was performed for not less than 75% of stenosis in 80 (89%) patients. On the other hand, seven patients with dilated cardiomyopathy were not ischemic. Hypertension, diabetes mellitus, and past smoking were identified in 83 (83%), 46 (46%), and 64 (64%) patients, respectively. More than half of the patients used statins, and almost half of the patients used angiotensin converting enzyme inhibitors and angiotensin receptor blockers. The treatment regimen had not been changed during the 6-month follow-up period, indicating no treatment had interfered with the patients' inflammatory status. Although the proportion of smokers was relative high (64%), a large majority had quit smoking before they were enrolled in this study. The B-type natriuretic peptide (BNP) level before CR was 142.2±213.8 pg/mL. The present study was approved by the Ethics Committee of the University of Tokyo. All subjects were informed of the methods, procedures, and risks of the study, and signed an informed consent document prior to their participation.
Table 1.
Clinical characteristics of 100 patients with cardiovascular diseases
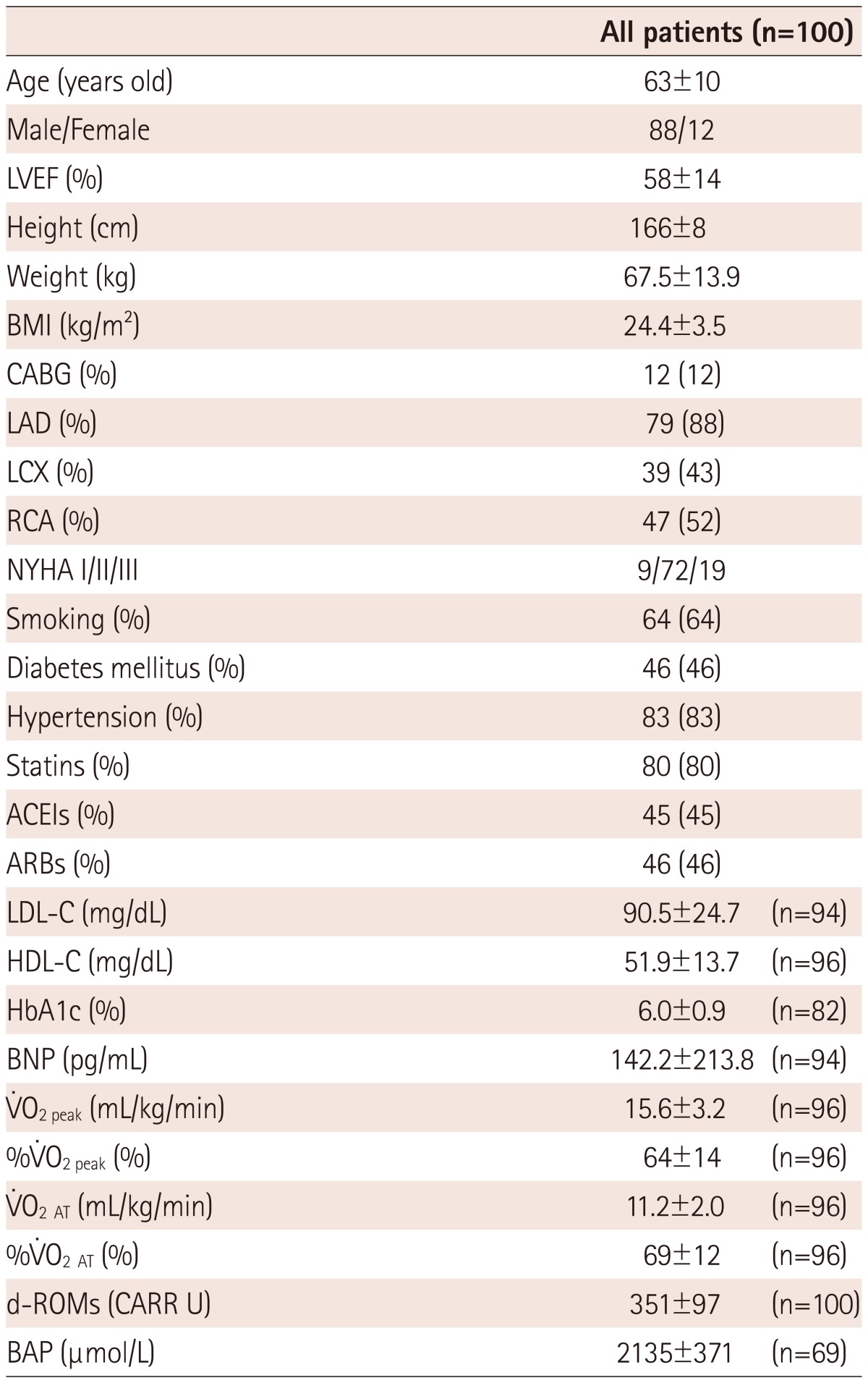
Mean±SD. LVEF: left ventricular ejection fraction, BMI: body mass index, CABG: coronary artery bypass grafting, LAD: left anterior descending artery, LCX: left circumflex artery, RCA: right coronary artery, NYHA: New York Heart Association, ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, LDL-C: low density lipoprotein-cholesterol, HDL-C: high density lipoprotein-cholesterol, HbA1c: hemoglobin A1c, BNP: B-type natriuretic peptide, %V̇O2peak: percentage of the predicted values of V̇O2 peak, V̇O2 AT: V̇O2 at the anaerobic threshold, %V̇O2 AT: percentage of the predicted values of V̇O2 AT, d-ROMs: derivatives of reactive oxidative metabolites, BAP: biological antioxidant potential, CARR U: carratelli unit
Study protocol
Before CR, a symptom-limited exercise test on an electromagnetically braked upright cycle ergometer (Corival, Load, Holland) at least 2 hours after a meal was performed to determine peak V̇O2 (V̇O2peak) and anaerobic threshold (AT). After a 4 minutes rest on the cycle ergometer, exercising was commenced at 20 W for a 4 minutes warm up, and the work rate was then increased in 1-W increments every 6 seconds. Blood pressure was measured by an automatic indirect cuff manometer (FB-300, Fukuda Denshi, Tokyo, Japan) every minute. Subjects stopped exercising because of leg fatigue or dyspnea. Expired gases were measured continuously in all subjects on a breath-by-breath basis, using an expired gas analyzer (AE-300S, Minato Medical Science, Osaka, Japan). Ventilatory parameters, including V̇O2, were calculated. AT was determined by gas exchange criteria as the point of nonlinear increase in the ventilatory equivalent for oxygen. Electrocardiogram (ECG) was monitored throughout the test to detect any possible ECG signal abnormalities (ML-9000, Fukuda Denshi, Tokyo, Japan). Subsequently, each subject visited the laboratory to receive CR for 40 minutes using aerobic bicycle exercise of 70-75% AT levels or 1 minute before AT levels, 2 or 3 times per week, for 3-6 months. The number of scheduled exercise sessions attended was very high. On average, each subject attended 87% of the scheduled exercise sessions. Only two subjects attended less than 50% of the scheduled sessions and 16 attended all of the scheduled sessions. As the results indicate, adherence to the exercise protocol was high in this study, with 87% of all sessions attended. Drug compliance was good and diet change during CR was not evident. At 3 and 6 months, a symptom-limited exercise test was again performed to determine V̇O2peak and AT. A mean value of V̇O2peak was found, as shown in Table 1. The percentage of the predicted values of V̇O2peak for healthy Japanese of the same age and sex was 64±14%, suggesting that the subjects exhibited mild exercise intolerance. An echocardiography was performed inside of two months of enrollment. Systolic dysfunction, left ventricular ejection fraction of 45% or less, was observed in 29 (29%) patients. Also, at the baseline (pre-CR), 3 and 6 months after CR, blood samples were obtained to measure the resting levels of the derivatives of reactive oxidative metabolites (d-ROMs), biological antioxidant potential (BAP), and plasma levels of the BNP.
Assay of oxidative stress and antioxidant potential
Oxidative status was studied by measuring hydrogen peroxide (H2O2) concentration in the serum, in accordance with an automated method (d-ROMs test; Diacron srl, Italy) using a free radical elective evaluator (F.R.E.E.; Diacron srl, Italy).14),15) H2O2 were converted into radicals that oxidize N,N-diethyl-para-phenylenediamine and can be detected spectrophotometrically using F.R.E.E. The results of the d-ROMs levels were expressed in an arbitrary unit called a Carratelli unit (CARR U), where 1 CARR U corresponds to 0.8 mg/L H2O2. The BAP level was measured to determine antioxidant potential and was also measured using F.R.E.E. This test examines the blood concentration of antioxidants as agents that can reduce iron from a ferric (Fe3+) to a ferrous form (Fe2+). We estimated the intensity of this chromatic change photometrically. The results are expressed as µmol/L. The BAP test was performed in 69 patients, because we started to measure d-ROMs only. The antioxidative/oxidative stress ratio was also calculated using the equation: BAP/d-ROMs.
Data analysis
Statistically significant differences among the three groups (pre-CR, 3 months, and 6 months) were determined using repeated measure one-way analysis of variance (ANOVA), followed by Tukey's honestly significant difference test. Spearman's rank correlation coefficient (r) was used to examine the relationship between two parameters. The effect of potential covariates on d-ROMs and BAP during the CR program was evaluated with two-way ANOVA for repeated measures. We tested the following covariates: age more than 63 years old (yes, no), gender (male, female), left ventricular ejection fraction less than 55% (yes, no), New York Heart Association class III (yes, no), and ischemic heart disease (yes, no). Differences were considered significant if the p were less than 0.05. All values were expressed as a mean±SD. Statistical analyses were performed using JMP 9.0.1 software (SAS Institute, Cary, NC, USA).
Results
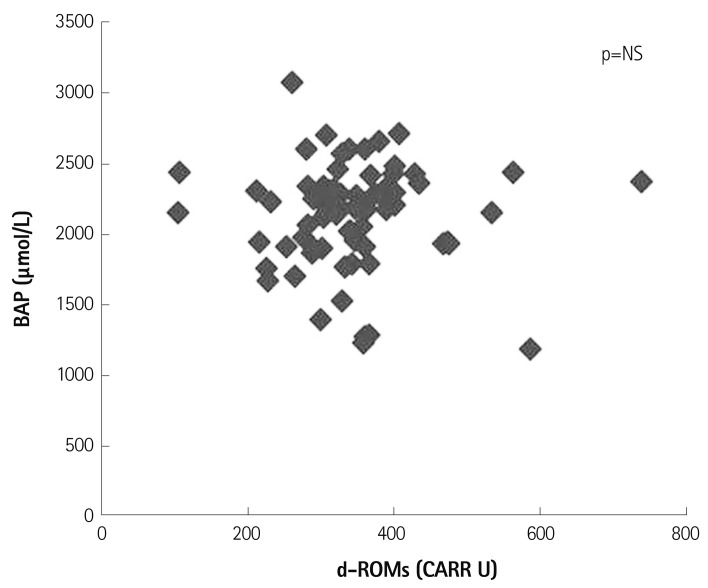
Firstly, we investigated the relationship between rest d-ROMs and BAP in patients with cardiovascular diseases receiving CR. Fig. 1 shows that there was no significant relationship between d-ROMs and BAP at rest.
Fig. 1.
Relationship between derivatives of reactive oxidative metabolites (d-ROMs) and biological antioxidant potential (BAP) before cardiac rehabilitation in patients with cardiovascular diseases (n=69). CARR U: carratelli unit, NS: not significant.
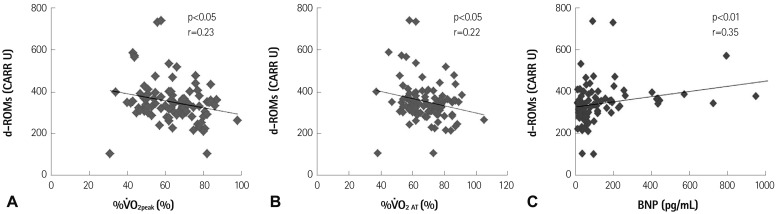
Fig. 2 depicts the relationships between d-ROMs and exercise capacity or plasma BNP before CR. Resting d-ROMs levels were negatively correlated with the percentage of the predicted values of V̇O2peak (%V̇O2peak) (Fig. 2A) and the percentage of the predicted values of V̇O2 at AT (%V̇O2 AT) (Fig. 2B) (all p<0.05), and were positively correlated with BNP (p<0.01) (Fig. 2C), whereas resting BAP test levels were not correlated with either %V̇O2peak, %V̇O2 AT or BNP (data not shown).
Fig. 2.
Relationships between derivatives of reactive oxidative metabolites (d-ROMs), and the percentage of the predicted values of V̇O2peak (%V̇O2peak; n=96) (A), the percentage of the predicted values of V̇O2 at the anaerobic threshold (%V̇O2 AT; n=96) (B), and plasma B-type natriuretic peptide (BNP; n=94) (C) before cardiac rehabilitation in patients with cardiovascular diseases. CARR U: carratelli unit.
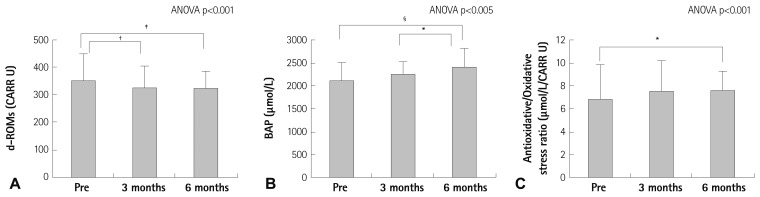
Fig. 3 shows that d-ROMs significantly decreased time-dependently (3 months vs. pre-CR, 329±77 vs. 351±97 CARR U; p<0.01, 6 months vs. pre-CR, 325±63 vs. 351±97 CARR U, p<0.005) (Fig. 3A). The BAP level increased after 6 months compared with pre-CR (6 months vs. pre-CR, 2424±404 vs. 2135±371 µmol/L, p=0.001) (Fig. 3B). The antioxidative/oxidative stress ratio also increased after 6 months compared with pre-CR levels (6 months vs. pre-CR, 7.6±1.7 vs. 6.8±3.1 µmol/L/CARR U, p<0.05) (Fig. 3C).
Fig. 3.
Time-dependent effects of cardiac rehabilitation on the derivatives of reactive oxidative metabolites (d-ROMs) (A), biological antioxidant potential (BAP) (B), and BAP/d-ROMs, the antioxidative/oxidative stress ratio (C). *p<0.05, †p<0.01, ‡p<0.005, §p=0.001. CARR U: carratelli unit, ANOVA: analysis of variance.
The two way ANOVA with repeated measures on d-ROMs during the CR program showed significant main effects (for age more than 63 years old, F=3.79 and p<0.05; for New York Heart Association class III, F=8.34 and p<0.005), and no significant interaction effects (for gender; for ischemic heart disease; for left ventricular ejection fraction less than 55%). The two way ANOVA with repeated measures on BAP during the CR program also indicated significant main effects (for age more than 63 years old, F=5.03 and p<0.01; for New York Heart Association class III, F=7.08 and p<0.01), and no significant interaction effects (for gender; for ischemic heart disease; for left ventricular ejection fraction less than 55%). These multivariate analysis results suggested that both age and functional class may be potential covariates of CR on oxidative stress and antioxidant potential persistence.
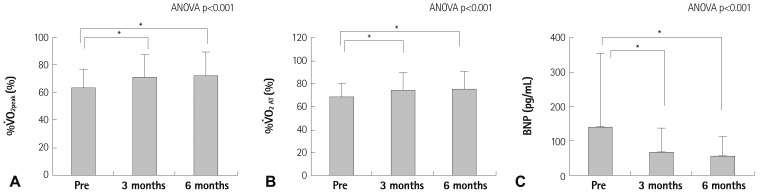
The effects of CR on %V̇O2peak (Fig. 4A), %V̇O2 AT (Fig. 4B), and plasma BNP (Fig. 4C) are as shown in Fig. 4. V̇O2peak and V̇O2 AT increased after 3-6 months (V̇O2peak: 3 months vs. pre-CR, 17.5±4.3 vs. 15.6±3.2 mL/kg/min, p<0.001, 6 months vs. pre-CR, 17.8±4.5 vs. 15.6±3.2 mL/kg/min, p<0.001). %V̇O2peak and %V̇O2 AT also increased after 3-6 months (%V̇O2peak: 3 months vs. pre-CR, 71±17 vs. 64±14%, p<0.001, 6 months vs. pre-CR, 73±17 vs. 64±14%, p<0.001), and plasma BNP decreased 3-6 months after CR.
Fig. 4.
Time-dependent effects of cardiac rehabilitation on the percentage of the predicted values of V̇O2peak (%V̇O2peak) (A), the percentage of the predicted values of V̇O2 at the anaerobic threshold (%V̇O2 AT) (B), and plasma B-type natriuretic peptide (BNP) (C). *p<0.001. ANOVA: analysis of variance.
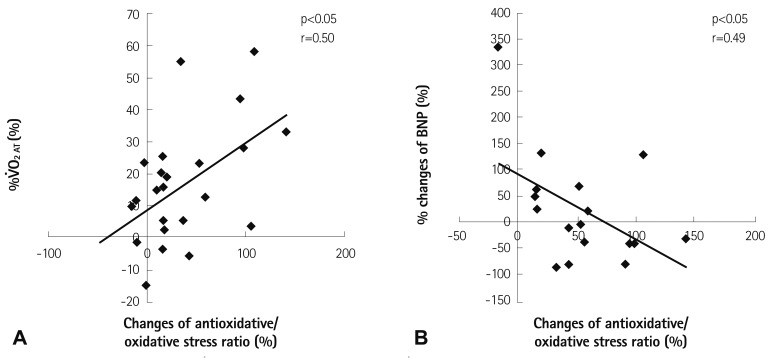
Fig. 5 illustrates that the % changes of V̇O2 AT from pre-CR to 6 months after CR were positively correlated with the % changes of the antioxidative/oxidative stress ratio from pre-CR to 6 months after CR (p<0.05) (Fig. 5A). In addition, the % changes of BNP from pre-CR to 6 months after CR were negatively correlated with the % changes of the antioxidative/oxidative stress ratio from pre-CR to 6 months after CR (p<0.05) (Fig. 5B).
Fig. 5.
Relationship between the % changes of V̇O2 at the anaerobic threshold (V̇O2 AT) and the % changes of biological antioxidant potential/derivatives of reactive oxidative metabolites, the antioxidative/oxidative stress ratio (n=23) (A), and the relationship between the % changes of B-type natriuretic peptide (BNP) and the % changes of the antioxidative/oxidative stress ratio (n=17) (B) from pre-CR to 6 months after CR, respectively. CB: cardiac rehanilitation.
Discussion
The major findings of the present study are as follows. 1) There was no relationship between resting d-ROMs and BAP in patients with cardiovascular diseases. 2) The resting levels of d-ROMs were negatively correlated with %V̇O2peak and %V̇O2 AT, and positively correlated with plasma BNP. However, the BAP test did not suggest a correlation with either %V̇O2peak, %V̇O2 AT, or BNP. 3) D-ROMs levels significantly decreased 3-6 months after CR with the increase of %V̇O2peak and %V̇O2 AT and the decrease of BNP. Furthermore, the BAP test significantly increased 6 months after CR. Furthermore, the % changes of the antioxidative/oxidative stress ratio were positively correlated with the % changes of V̇O2 AT, and negatively correlated with the % changes of BNP. These results suggest that CR increased exercise capacity with the reduction of oxidative stress and the increase of antioxidant capacity.
Cardiac rehabilitation has a variety of multiple beneficial effects, including improved exercise capacity and anti-inflammatory effects. Cardiovascular risk factors, such as hypertension, obesity, hypercholesterolemia, diabetes mellitus, and chronic smoking, stimulate the production of ROS in the vascular wall. ROS mediate various signaling pathways that underlie vascular inflammation in atherogenesis and cardiovascular diseases. While statins and angiotensin receptor blockers are useful drugs to help prevent cardiovascular diseases, both drugs reduce superoxide by NADPH oxidase.
The results of the present study have several important clinical implications. First, we demonstrated that, among patients with established cardiovascular diseases, there was no relationship observed between d-ROMs and BAP. There had been only a small number of previous studies investigating the relationship between d-ROMs and BAP. D-ROMs levels were negatively correlated with BAP levels in a relatively small number of unselected outpatients16) and subjects who had undergone a health checkup examination,17) which differed from the present study. These previous data on this issue were obtained from studies of subjects without cardiovascular diseases, suggesting that the relationship between d-ROMs and BAP is different for those with and without cardiovascular diseases, probably due to the higher levels of oxidative stress in patients with cardiovascular diseases.
Second, the present study showed that d-ROMs levels at rest were negatively correlated with %V̇O2peak and %V̇O2 AT, and positively correlated with plasma BNP, yet the BAP test failed to correlate with either %V̇O2 peak, %V̇O2 AT or BNP. To the best of our knowledge, there have been no previous studies investigating the relationship between d-ROMs and/or BAP and exercise capacity in patients with cardiovascular diseases. Thus, the present study for the first time showed that systemic oxidative stress, as measured by d-ROMs, was negatively correlated with exercise capacity. This result suggests that exercise intolerance is related to oxidative stress in patients with cardiovascular diseases.
Third, in the present study, d-ROMs levels significantly decreased 3-6 months after CR with the increase of %V̇O2peak and %V̇O2 AT, and the decrease of BNP. Mercken et al.18) found that patients with chronic obstructive pulmonary disease had increased pulmonary and systemic oxidative stress levels, as measured by urinary malondialdehyde and H2O2, both at rest and during exercise. They also found that pulmonary rehabilitation increased exercise capacity and reduced exercise-induced oxidative stress. However, as far as we are aware, only one previous study has investigated the effect of CR on oxidative stress.6) In that study, using left internal mammary artery rings sampled during bypass surgery, Adams et al.6) showed that exercise training reduced the vascular expression of the NADPH oxidase and angiotensin II receptor type I receptor, resulting in the decreased local ROS generation in patients with coronary artery disease, which is consistent with the present findings.
The present study also showed that the BAP test, an index of the antioxidant power of the serum, was improved 6 months after aerobic training compared with control groups. A previous study found that anti-oxidative enzymes assessed in skeletal muscle biopsies were lower in patients with CHF than in normal subjects, and were also improved 6 months after aerobic training compared with controls.13) These results are also in accordance with our results. Furthermore, the present study showed that the % changes of the antioxidative/oxidative stress ratio were positively correlated with the % changes of V̇O2 AT, and negatively correlated with the % changes of BNP. These results show that CR increased exercise capacity, and the observed improvement may be attributable to an adaptive response involving more efficient oxidative metabolites or an increased capacity of endogenous anti-oxidative systems. The possible mechanisms underlying improved exercise capacity and decreased oxidative stress require further investigations.
Notwithstanding these findings, the present study is subject to several limitations. First, our research did not have a control group. Thus, the results were unable to differentiate the effects of CR itself and possible nonspecific changes over time. In addition, about one quarter of the subjects had had recent myocardial infarctions, and many patients had undergone a percutaneous coronary intervention, which may also contribute to the improvement of a patient's condition over time in addition to the CR. Second, the present study was performed among patients with cardiovascular diseases primarily involving ischemic heart diseases. Therefore, further research is required to examine the effect of CR on the ROS and antioxidant potential among patients with other cardiovascular disease types, such as dilated cardiomyopathy and valvular heart disease.
In conclusion, the present study for the first time revealed that oxidative stress was related to exercise intolerance, and CR reduced oxidative stress and increased antioxidant capacity in patients with cardiovascular diseases. Furthermore, the % changes of exercise capacity were positively correlated with the % changes of the antioxidative/oxidative stress ratio. These results suggest that CR may increase exercise capacity with the reduction of oxidative stress and the increase of antioxidant capacity.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Tousoulis D, Briasoulis A, Papageorgiou N, et al. Oxidative stress and endothelial function: therapeutic interventions. Recent Pat Cardiovasc Drug Discov. 2011;6:103–114. doi: 10.2174/157489011795933819. [DOI] [PubMed] [Google Scholar]
- 2.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 3.Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93:903–907. doi: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 6.Adams V, Linke A, Kränkel N, et al. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation. 2005;111:555–562. doi: 10.1161/01.CIR.0000154560.88933.7E. [DOI] [PubMed] [Google Scholar]
- 7.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 8.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–H2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 9.Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 10.Church TS, Barlow CE, Earnest CP, Kampert JB, Priest EL, Blair SN. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol. 2002;22:1869–1876. doi: 10.1161/01.atv.0000036611.77940.f8. [DOI] [PubMed] [Google Scholar]
- 11.LaMonte MJ, Blair SN, Church TS. Physical activity and diabetes prevention. J Appl Physiol. 2005;99:1205–1213. doi: 10.1152/japplphysiol.00193.2005. [DOI] [PubMed] [Google Scholar]
- 12.Judge S, Jang YM, Smith A, et al. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1564–R1572. doi: 10.1152/ajpregu.00396.2005. [DOI] [PubMed] [Google Scholar]
- 13.Linke A, Adams V, Schulze PC, et al. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation. 2005;111:1763–1770. doi: 10.1161/01.CIR.0000165503.08661.E5. [DOI] [PubMed] [Google Scholar]
- 14.Cesarone MR, Belcaro G, Carratelli M, et al. A simple test to monitor oxidative stress. Int Angiol. 1999;18:127–130. [PubMed] [Google Scholar]
- 15.Gerardi G, Usberti M, Martini G, et al. Plasma total antioxidant capacity in hemodialyzed patients and its relationships to other biomarkers of oxidative stress and lipid peroxidation. Clin Chem Lab Med. 2002;40:104–110. doi: 10.1515/CCLM.2002.019. [DOI] [PubMed] [Google Scholar]
- 16.Lubrano V, Vassalle C, L'Abbate A, Zucchelli GC. A new method to evaluate oxidative stress in humans. Immunoanal Biol Spec. 2002;17:172–175. [Google Scholar]
- 17.Fukui T, Yamauchi K, Maruyama M, Yasuda T, Kohno M, Abe Y. Significance of measuring oxidative stress in lifestyle-related diseases from the viewpoint of correlation between d-ROMs and BAP in Japanese subjects. Hypertens Res. 2011;34:1041–1045. doi: 10.1038/hr.2011.76. [DOI] [PubMed] [Google Scholar]
- 18.Mercken EM, Hageman GJ, Schols AM, Akkermans MA, Bast A, Wouters EF. Rehabilitation decreases exercise-induced oxidative stress in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:994–1001. doi: 10.1164/rccm.200411-1580OC. [DOI] [PubMed] [Google Scholar]