Abstract
Uveitis is a challenging disease to treat. Corticosteroids have been used in the treatment of uveitis for many years. Immunosuppressives are gaining momentum in recent years in the treatment of uveitis. In this article we present an overview of current treatment of uveitis and the major breakthroughs and advances in drugs and ocular drug delivery systems in the treatment of uveitis.
Keywords: Corticosteroids, immunosuppressives, medical management, uveitis
Uveitis is a potentially sight threatening disease. It may occur due to an infection or may be due to an autoimmune etiology. Specific antimicrobial therapies with or without corticosteroids are used in cases of infectious uveitis. Several drugs are available for the management of noninfectious uveitis including corticosteroids, immunosuppressive agents, and more recently biologics. The treatment of uveitis is evolving with newer drugs and innovative advances in ocular drug delivery. This is due to better understanding of the pathophysiology of disease and barriers in drug delivery. In this article, we present an overview of current treatment of uveitis and the major breakthroughs and advances in drugs and ocular drug delivery systems in the treatment of uveitis. The following sections will be discussed in detail in the manuscript:
I. Corticosteroids:
-
Local delivery of corticosteroids:
- Topical corticosteroids
- Iontophoresis
- Periocular injections
- Intravitreal injections and inserts
Systemic oral steroids (oral and intravenous)
II. Immunosuppressants
III. Biologics
IV. Adjuvant therapy:
Cycloplegics
Newer nonsteroidal anti-inflammatory agents
Anti-vascular endothelial growth factor (anti-VEGF) therapy
V. Current concepts in infectious uveitis management
Corticosteroids are the first line of therapy in patients with noninfectious ocular inflammatory diseases as these drugs are inexpensive, potent, and act fast. They may be administered either topically, periocular injections, or systemically (oral, intramuscular or intravenous routes). The mechanism of action of corticosteroids is complex and incompletely understood. They act at the cellular and molecular level.[1,2]
Topical Corticosteroids
Topical corticosteroids are used in the treatment of anterior uveitis. Concentrations of these steroids in the aqueous, depends on the rate of their diffusion across the cornea.[2,3] Among the topical corticosteroids, prednisolone acetate eye drops provides greater theoretical anti-inflammatory effect than either dexamethasone or betamethasone. This is due to two reasons: Firstly, the concentration of betamethasone and dexamethasone eye preparations is 0.1% as compared to 1% for prednisolone acetate and secondly, though prednisolone acetate is six times less potent on a molar basis than betamethasone or dexamethasone, the penetration into the cornea of prednisolone acetate is much more than betamethasone or dexamethasone. Dosing frequency and the length of time the medication stays in contact with ocular surface also influences efficacy. Suspensions have a higher degree of anti-inflammatory effect.[2,3]
Other corticosteroid eye preparations include fluorometholone, rimexolone, and loteprednol etabonate. These are called “soft steroids” due to the lesser propensity of increasing the intraocular pressure (IOP)[2,4,5] Another recently approved steroid is difluprednate (0.05%) (difluoroprednisolone butyrate acetate). It is a synthetic fluorinated prednisolone derivative. This has greater glucocorticosteroid receptor binding activity than prednisolone acetate. This is due to fluorination at C6 and C9 positions and replacement of C-17 hydroxyl group with butyrate ester, which increases its specificity for the glucocorticoid receptor. Greater corneal penetration is achieved by the addition of acetate ester at position C-21. Stringer et al., have reported that consistent dose uniformity is achieved with difluprednate ophthalmic emulsion 0.05% when compared with branded and generic prednisolone acetate ophthalmic suspensions 1%.[6] Foster et al., have inferred from their studies that a qid dosing of difluprednate ophthalmic emulsion 0.05% is as effective as eight times dosing with prednisolone acetate 1% ophthalmic suspension in the treatment of endogenous anterior uveitis.[7] Reports on its use in post cataract surgery show potency equivalent to prednisolone acetate.[8,9] Elevation of IOP in patients with uveitis especially in children treated with topical difluprednate have been reported in the literature. One needs to exert caution while using this preparation in children.[10,11] Table 1 outlines the list of commonly available topical corticosteroid preparations.[2]
Table 1.
Commonly available topical corticosteroid preparations
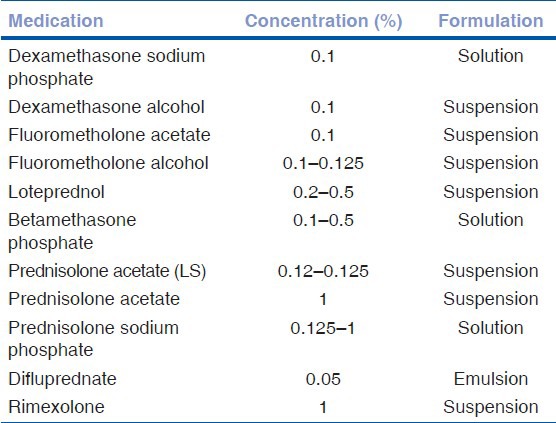
Side effects of topical administration of steroids: Elevation of IOP, susceptibility to infections, impaired corneal or scleral wound healing, corneal epithelial toxicity, and crystalline keratopathy are reported with the use of topical administration of corticosteroids.
It is important that patients on topical corticosteroids be regularly monitored to assess the response to therapy as well as development of side effects.
Iontophoresis
Iontophoresis is a noninvasive method of application of low current to an ionizable substance (drug) to increase its mobility across a surface by electrochemical repulsion. Phase 2 trials have been conducted on delivery of dexamethasone phosphate by ocular iontophoresis in noninfectious anterior uveitis. Dexamethasone phosphate (40 mg/ml, EGP-437) is a prodrug and is a good candidate for iontophoresis delivery, as it possesses two acidic protons (pK values of 1.9 and 6.4), making it highly water-soluble formulation with a high buffering capacity. In addition, it has a well-characterized safety and efficacy profiles for ophthalmic use. To deliver dexamethasone phosphate, which is an anion at physiologic pH, a cathodic delivery is used to produce hydroxide ions, which drive the dexamethasone phosphate anions into the ocular tissues, by electrochemical repulsion. Also, these hydroxyl ions (OH) increase the pH of the drug solution, shifting the equilibrium towards ionized state, and hence increasing the efficiency while buffering the formulation.
The Eye Gate II Delivery System (EGDS, Eye gate Pharmaceuticals, Inc., Waltham, MA) is a novel ocular iontophoresis system designed to deliver substantial levels of drug noninvasively into the anterior segments of the eye while minimizing systemic distribution.
In this trial, 40 eyes were randomly assigned, to receive one of the four iontophoresis dose levels of EGP-437 for duration of 4 min (1.6 mA-min at 0.4 mA; 4.8 mA-min at 1.2 mA; 10 mA-min at 2.5 mA; and 14 mA-min at 3.5 mA). The results show effectivity and fewer adverse events at lower doses (1.6 mA-min group).[12]
Periocular Steroids
They are indicated in moderate to severe chronic or recurrent uveitis, cystoid macular edema, and in cases with anterior chamber inflammation not responding adequately to topical corticosteroids. Periocular steroids offer the benefit of higher, local, and sustained drug to the eye with greater posterior segment penetration. Posterior injections are typically given via the posterior subtenon approach using the Smith and Nozik technique.[1,2,13,14] Orbital floor injections provide an alternative to posterior subtenon injection. Anterior injections are often given subconjunctivally on the inferior or superior bulbar surface. Table 2 outlines the list of injectable corticosteroid preparations.
Table 2.
Injectable corticosteroid preparations
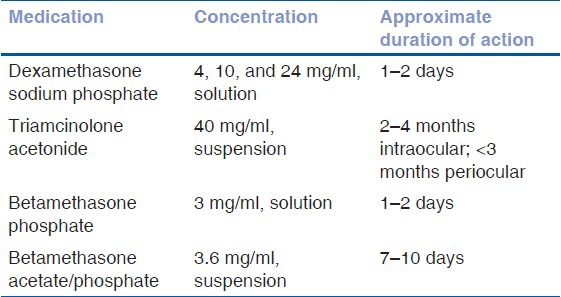
The duration of action differs for various injectable corticosteroid preparations.
Side effects of periocular steroids: Periocular administration may cause a variety of side effects, which include increased IOP and glaucoma, ptosis, cataract, and inadvertent globe perforation.[2,3]
Intravitreal Steroids
Intravitreal corticosteroids, such as triamcinolone acetonide are often used to manage noninfectious intermediate and posterior uveitis and its complications such as cystoid macular edema due to the direct action and hence greater efficacy. It is administered as 4 mg in 0.1 ml. The effects are usually short-lived and may last for 6–8 weeks.[15] Sustained release corticosteroid intraocular implants may be considered in place of repeated injections. Retisert[16] (Bausch and Lomb, Rochester, NY) contains fluocinolone acetonide 0.59 mg (requires a surgical procedure to suture the implant to the scleral wall) that achieves sustained release of approximately 2.5 years. Another implant for treatment of noninfectious posterior uveitis is a dexamethasone intravitreal implant[17] (Ozurdex, Allergan, Inc., Irvine, CA). It is a 0.7 mg biodegradable implant that delivers a sustained release of dexamethasone over 3–6 months through the Novadur solid polymer delivery system, which is given intravitreally via an injector.
Serious side effects include cataract, increased IOP, glaucoma, retinal detachment, vitreous hemorrhage, and endophthalmitis.
Systemic Steroids
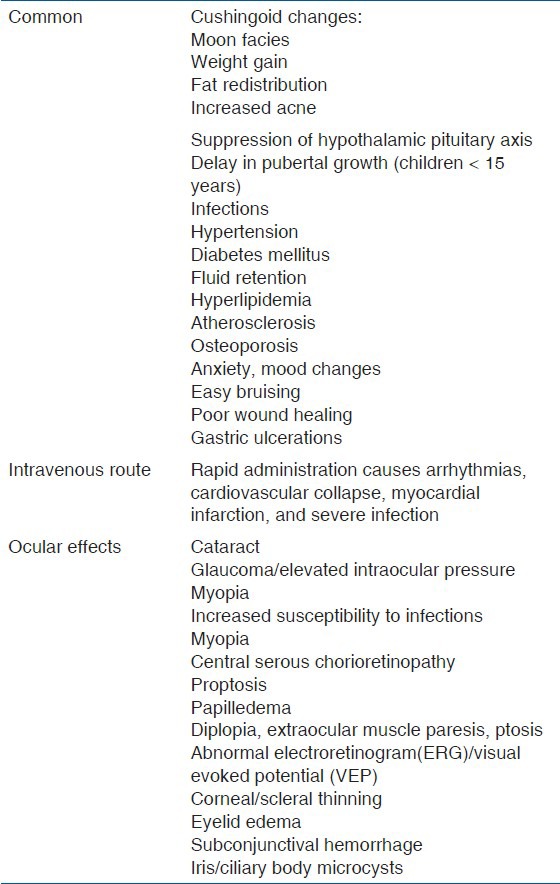
Systemic corticosteroids play an important role in the management of intraocular inflammation.[18] Indications include inflammation; which is moderate to severe, bilateral, beyond anterior segment, resistant to local therapy, or associated with systemic disease. Cortisone, hydrocortisone, prednisone, and fludrocortisone are the different steroids available for oral administration. Prednisone is the most common corticosteroid used orally in the treatment of uveitis. The initiation of therapy is typically 0.5–1 mg/kg daily followed by a slow taper once the inflammation is under control.[3] Optimally, the dose should be less than 0.1 mg/kg daily within 3 months of initiating therapy. For immediate control of vision threatening diseases (necrotizing scleritis, bilateral serous detachments), methyl prednisolone sodium succinate can be given intravenously over a period of more than 30 min. The usual regimen consists of 250–1,000 mg/day (pulse dose) intravenously on 3 consecutive days.[3] Table 3 outlines the guidelines for tapering and monitoring while on systemic corticosteroids. Systemic corticosteroids can cause a variety of systemic side effects. Table 4 outlines the adverse effects of systemic corticosteroids.
Table 3.
Suggested guidelines for the use of prednisone for chronic ocular inflammation[4]
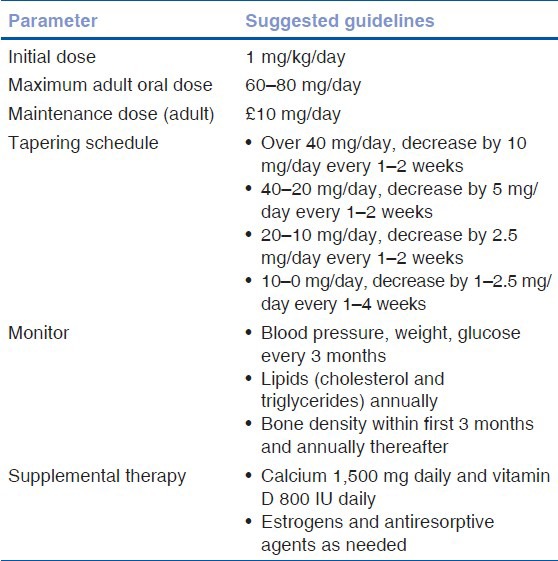
Table 4.
Outlines the adverse events of systemic corticosteroids
Immunosuppressive Agents
Immunosuppressive drugs can be classified as antimetabolites, T cell inhibitors, and alkylating agents.[4] The antimetabolites include methotrexate, azathioprine, and mycophenolate mofetil.[4,18] The T cell inhibitors include cyclosporine, tacrolimus, voclosporin,[19] and sirolimus.[20,21] The alkylating agents include cyclophosphamide and chlorambucil.[4]
The mechanism of action, required dosage, and side effects of various immunosuppressives are listed in Table 5. These drugs take many weeks to have an effect, so initial therapy of ocular inflammation typically include high dose of systemic steroids. Immunosuppressive therapy can be started simultaneously with corticosteroids in severe cases or during the tapering of oral corticosteroids 4–8 weeks later in cases of chronic uveitis.
Table 5.
Immunosuppressives in uveitis
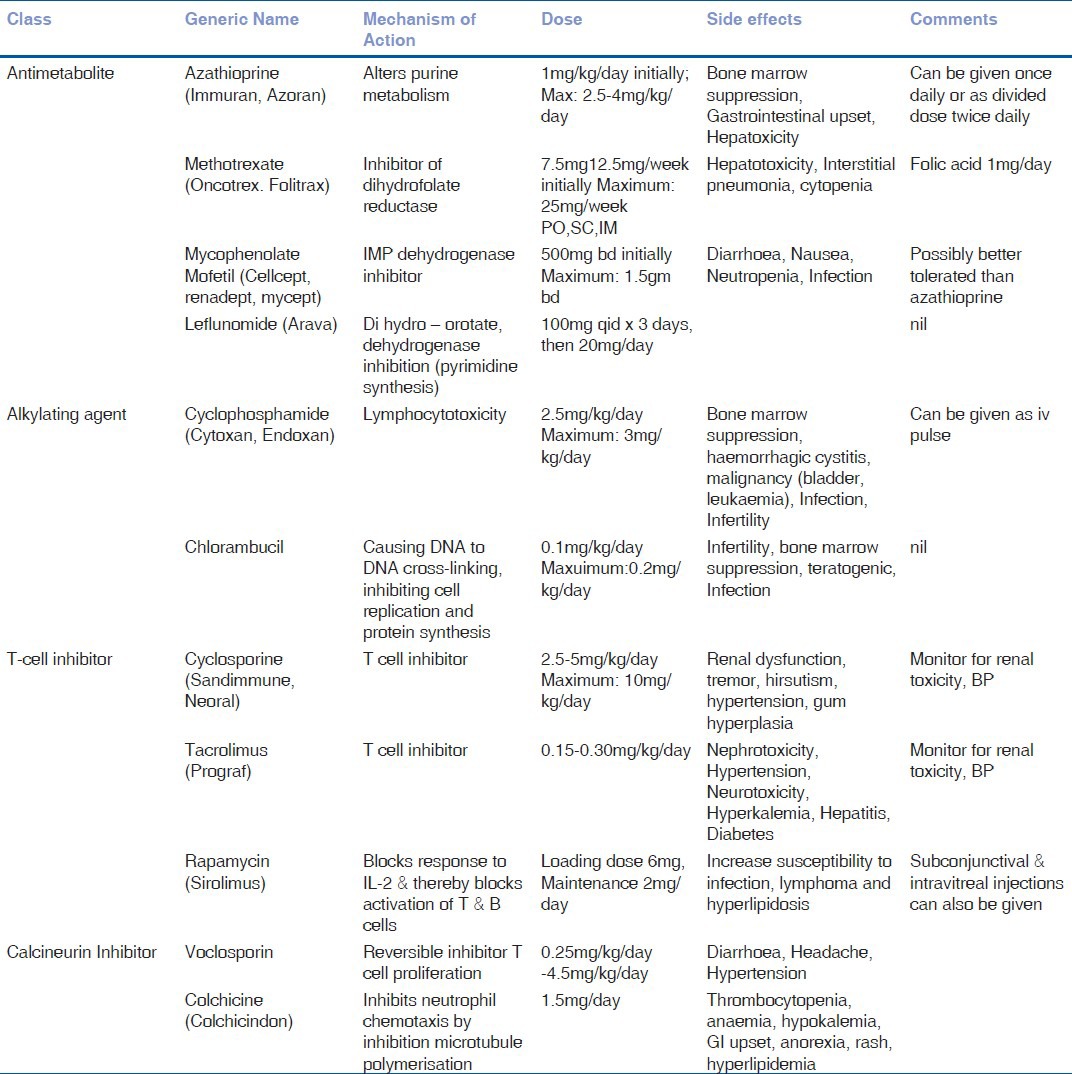
Tuberculosis and HIV needs to be ruled out prior to starting immunosuppressive therapy. Complete blood counts and platelet count need to be performed every 4 weeks. Liver function tests such as aspartate aminotransferase and alanine aminotransferase need to be performed every 12 weeks.[4] If the total white blood cell (WBC) count falls less than 3,500 cells/mm3, platelet count less than 100,000 cells/mm3, or the liver enzymes greater than five times of normal level; the immunosuppressive therapy needs to be discontinued.[18,20] Methotrexate and azathioprine are the commonly used drugs in uveitis. Recently, mycophenolate mofetil is gaining popularity. Voclosporin[19] and sirolimus[20,21] are the recently introduced T cell inhibitors with good efficacy.
Methotrexate may also be given as intravitreal injections (400 mg in 0.1 ml).[22] Sirolimus can be used as subconjunctival or intravitreal injections and are undoing phase 3 clinical trials in noninfectious uveitis.[23] Alkylating agents are highly toxic with good efficacy and these agents are used in severe cases of necrotizing scleritis and systemic diseases like Wegener's granulomatosis, etc. In severe or refractory cases of uveitis, more than one drug (e.g., triple immunosuppressive therapy in serpiginous choroditis) can be used to control the inflammation.
Biologics
Table 6 shows the types of biologics available.
Table 6.
Classification of Biologics
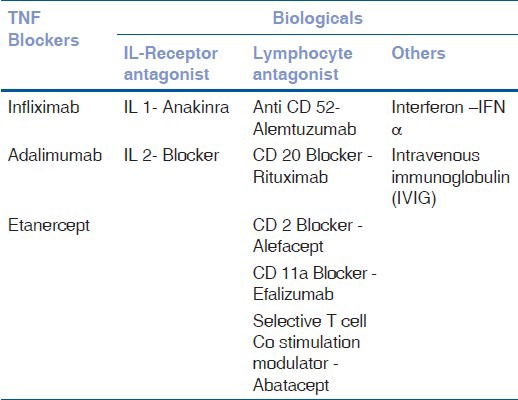
Anti-tumor Necrosis Factor-alpha (Anti TNFα) Therapies
TNF-α is a key cytokine involved in the pathogenesis of noninfectious uveitis. Anti TNFα agents used in uveitis include etanercept, infliximab, and adalimumab. Their mechanism of action, recommended dosage, and side effects of Anti TNFα agents are listed in Table 7.[24,25,26] Infliximab and adalimumab seem to be more effective than etanercept for the treatment of ocular inflammation. Reactivation of tuberculosis is a serious complication of anti-TNF therapy. Infliximab is administered intravenously, at a usual dose of 3–10 mg/kg, but doses as high as 10–20 mg/kg have been reported with success and few side effects. Infliximab causes good initial response to therapy in uveitis; however, the effect is temporary and repeated infusions are necessary every 4–8 weeks to maintain remission. Infliximab causes the development of antibodies against the murine component of the molecule. For this reason, we need to use concomitant therapy with an antimetabolite or glucocorticoid to suppress antibody production.
Table 7.
Mechanism of action, dosage, and side effects of cytokine inhibitors
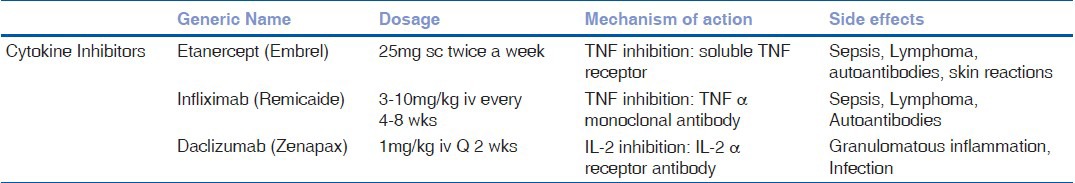
Adalimumab, a recombinant immunoglobulin (Ig)G1 monoclonal antibody targeting TNF-α, showed efficacy both as monotherapy and in combination with other disease-modifying antirheumatic drugs (DMARDs). It has the advantage of self-subcutaneous administration and the recommended dose is 40 mg at 2-week intervals.
Interferons (IFN)
IFN-α is a naturally occurring cytokine secreted in response to viral infections. It causes enhancement of suppressor T cell activity, reduction of proinflammatory cytokine production, and enhancement of phagocytic activity. IFN-α is given as subcutaneous injection daily 6 million units per day initially and subsequently tapered to 3 million units two to three times a week. Side effects include redness at the site of injection, flu-like symptoms, depression and suicidal behavior, severe cytopenias, cotton wool spots, and retinal hemorrhages in the retina.[27]
Intravenous Immunoglobulin
Intravenous immunoglobulin has multiple mechanisms of action to enhance the immunity. Main advantage is that it is not associated with systemic immune suppression. Usual dose is 1–2.5 g/kg/cycle every 2-4 weeks until inflammation has subsided. Side effects include thromboembolism, aseptic meningitis, and risk of transmitting blood-borne infections.
Anti-interleukin Therapies
Daclizumab is a humanized monoclonal antibody against the interleukin (IL)-2 receptor.
Nussenblatt et al., described the use of 4-weekly intravenous infusions of daclizumab for treatment of severe sight threatening noninfectious uveitis.[28] IL-1 receptor antagonist (IL-1RA) is a naturally occurring inhibitor of IL-1. Anakinra is a recombinant human IL-1RA, administered at a daily dose of 100 mg subcutaneously in adults.[29]
Other Biologic Agents
Alemtuzumab is also known as Campath-1H, is a humanized B monoclonal antibody against the pan lymphocyte antigen CD52. Dick et al., used for 5 consecutive days by intravenous infusion and seems to be an effective treatment for uveitis, leading to long-term remission.[30]
Rituximab is a mouse-human chimeric monoclonal IgG1 antibody against CD20, expressed by B cells. It is effective against predominantly T cell mediated autoimmune diseases, such as rheumatoid arthritis and Wegener's granulomatosis. Heiligenhaus et al., used rituximab in juvenile rheumatoid arthritis (JRA) associated uveitis, which was refractory to traditional immunosuppressives and TNF-α inhibitors.[31]
Biologic therapy provides new options for the treatment of refractory uveitis, showing a favorable safety and efficacy profile. However, lack of evidence from randomized controlled studies limits our understanding as to when to start the treatment, which drug to choose, and how long to continue therapy. In addition; high cost, lack of long-term usage, and reactivation of latent tuberculosis limits its use in India.
Adjuvant Therapy
To provide symptomatic relief for pain and discomfort and break the posterior synechiae, the following cycloplegics can be used:
-
Short acting cycloplegics:
- Tropicamide (0.5 and 1%) has a duration of 6 hours
- Cyclopentolate (0.5 and 1%) has a duration of 24 hours
-
Long acting cycloplegics:
- Homatropine 2% has a duration of up to 2 days
- Atropine 1% is the most powerful cycloplegic and mydriatic with duration of up to 2 weeks.
Newer Nonsteroidal Anti-Inflammatory Agents: Bromfenac, Nepafenac[32]
They are used for reduction of ocular pain and inflammation following cataract surgery and in scleral inflammation. It is a potent inhibitor of the cyclooxygenase (COX)-2 enzyme and has a highly lipophilic molecule that rapidly penetrates to produce early and sustained drug levels in all ocular tissues.
Bromfenac ophthalmic solution 0.09%: It can be used (twice daily dosage) as either monotherapy or as an adjunct therapy to steroids.
Nepafenac 0.1%: It is a prodrug. It penetrates the cornea six times faster than diclofenac. It is converted to amfenac in ocular tissues. It has been approved for thrice daily dosage beginning 1 day before cataract surgery.
Current Trends in Infectious Uveitis Management
Toxoplasmosis is the commonest cause of infectious posterior uveitis. Ocular toxoplasmosis is usually managed with systemic antitoxoplasma drugs (Cotrimoxazole-sulphamethoxazole trimethoprim, clindamycin, pyrimethamine, azithromycin, atavoquone, spiramycin) and systemic steroids. Soheilian et al., used intravitreal clindamycin (1 mg/0.1ml) along with dexamethasone (0.4 mg/0.1ml) to control the ocular inflammation.[33]
In tropical countries, fungal infection is commonly seen. Recently voriconazole (oral dose 200 mg twice a day) and intravitreal voriconazole (50–100 g in 0.1 ml) can be used to treat the fungal infection in the eyes.[34]
In ocular tuberculosis, there may be paradoxical worsening of inflammation following antitubercular therapy and systemic steroids. If the inflammation is progressing inspite of addition of oral steroids, immunosuppressives may be added with close monitoring of liver function.[35]
In viral uveitis, ganciclovir gel (0.15%) is used topically to control the cytomegalovirus anterior uveitis[36] and intravitreal ganciclovir implants can be used to treat cytomegalovirus retinitis.[37]
Anti-vascular Endothelial Growth Factor (anti-VEGF) Therapy
The use of intravitreal anti-VEGF therapies, namely bevacizumab (1.25 mg in 0.05 ml) and ranibizumab (5 mg/0.05 ml) has been described in the treatment of uveitic complications such as cystoid macular edema, choroidal neovascularization, and retinal neovascularization.[38]
Conclusion
Corticosteroids still remain the mainstay of treatment in uveitis. Immunosuppressives have revolutionized the treatment in chronic uveitis. Better understanding of immunology and uveitic diseases help providing more targeted treatment in uveitis. The future thus holds great promise for uveitis with continuing development of newer drugs.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Nussenblatt RB, Whitcupp SM. Fundamentals and Clinical Practice. 4th ed. Philadelphia, PA: Mosby-Elsevier; 2010. Philosophy, goals and approach to medical therapy. Uveitis; pp. 76–113. [Google Scholar]
- 2.Cunningham ET, Jr, Wender JD. Practical approach to the use of corticosteroids in patients with uveitis. Can J Ophthalmol. 2010;45:352–8. doi: 10.3129/i10-081. [DOI] [PubMed] [Google Scholar]
- 3.McGhee CN. Pharmacokinetics of ophthalmic steroids. Br J Ophthalmol. 1992;76:681–4. doi: 10.1136/bjo.76.11.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jabs DA, Rosenbaum JT, Foster CS, Holland GN, Jaffe GJ, Louie JS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: Recommendations of an expert panel. Am J Ophthalmol. 2000;130:492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 5.The loteprednol etabonate US Uveitis Study Group. Controlled Evaluation of loteprednol etabonate and prednisolone acetate in the treatment of acute anterior uveitis. Am J Ophthalmol. 1999;127:537–44. doi: 10.1016/s0002-9394(99)00034-3. [DOI] [PubMed] [Google Scholar]
- 6.Stringer W, Bryant R. Dose uniformity of topical corticosteroid preparations: Difluprednate ophthalmic emulsion 0.05% versus branded and generic prednisolone acetate ophthalmic suspension 1% Clin Ophthalmol. 2010;5:1119–24. doi: 10.2147/OPTH.S12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster CS, Davanzo R, Flynn TE, McLeod K, Vogel R, Crockett RS. Durezol (Difluprednate Ophthalmic Emulsion 0.05%) compared with Pred Forte 1% ophthalmic suspension in the treatment of endogenous anterior uveitis. J Ocul Pharmacol Ther. 2010;26:475–83. doi: 10.1089/jop.2010.0059. [DOI] [PubMed] [Google Scholar]
- 8.Korenfeld MS, Silverstein SM, Cooke DL, Vogel R, Crockett RS Difluprednate Ophthalmic Emulsion 0.05% (Durezol) Study Group. Difluprednate ophthalmic emulsion 005% for postoperative inflammation and pain. J Cataract Refract Surg. 2009;35:26–34. doi: 10.1016/j.jcrs.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Mulki L, Foster CS. Difluprednate for inflammatory eye disorders. Drugs Today (Barc) 2011;47:327. doi: 10.1358/dot.2011.47.5.1590791. [DOI] [PubMed] [Google Scholar]
- 10.Birnbaum AD, Jiang Yi, Tessler HH, Goldstein DA. Elevation of Intraocular pressure in patients with uveitis treated with topical difluprednate. Arch Ophthalmol. 2011;129:667–8. doi: 10.1001/archophthalmol.2011.82. [DOI] [PubMed] [Google Scholar]
- 11.Slabaugh MA, Herlihy E, Ongchin S, van Gelder RN. Efficacy and potential complications of difluprednate use for pediatric uveitis. Am J Ophthalmol. 2012;153:932–8. doi: 10.1016/j.ajo.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Cohen AE, Assang C, Patane MA, From S, Korenfeld M Avion Study Investigators. Evaluation of dexamethasone phosphate delivered by ocular iontophoresis for treating noninfectious anterior uveitis. Ophthalmology. 2012;119:66–73. doi: 10.1016/j.ophtha.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Kempen JH, Altaweel MM, Holbrook JT, Jabs DA, Sugar EA Multicenter Uveitis Steroid Treatment Trial Research Group. The multicenter uveitis steroid treatment trial: Rationale, design and baseline characteristics. Am J Ophthalmol. 2010;149:550–61. doi: 10.1016/j.ajo.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nozik RA. Periocular administration of steroids. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:695–705. [PubMed] [Google Scholar]
- 15.Cunningham MA, Edelman JL, Kaushal S. Intravitreal steroids for macular edema: The past, the present and the future. Surv Ophthalmol. 2008;53:139–49. doi: 10.1016/j.survophthal.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Jaffe GJ, Ben-Nun J, Guo H, Dunn JP, Ashton P. Fluocinolone acetonide sustained drug delivery device to treat severe uveitis. Ophthalmology. 2000;107:2024–33. doi: 10.1016/s0161-6420(00)00466-8. [DOI] [PubMed] [Google Scholar]
- 17.Haller JA, Bandello F, Belfort R, Jr, Blumenkranz MS, Gillies M, Heier J, et al. Ozurdex Geneva group. Randomized, sham controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–46. doi: 10.1016/j.ophtha.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 18.Biswas J. Practical concepts in the management of uveitis. Indian J Ophthalmol. 1993;41:133–41. [PubMed] [Google Scholar]
- 19.Anglade E, Aspeslet LJ, Weiss SL. A new agent for the treatment of noninfectious uveitis: Rationale and design of three LUMINATE (Lux Uveitis Multicenter Investigation of a New Approach to Treatment) trials of steroid-sparing voclosporin. Clin Ophthalmol. 2008;2:693–702. doi: 10.2147/opth.s2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomes Bittencourt M, Sepah YJ, Do DV, Agbedia O, Akhtar A, Liu H, et al. New treatment options for noninfectious uveitis. Dev Ophthalmol. 2012;51:134–61. doi: 10.1159/000336338. [DOI] [PubMed] [Google Scholar]
- 21.Larson T, Nussenblatt RB. Emerging drugs for uveitis. Expert Opin Emerg Drugs. 2011;16:309–22. doi: 10.1517/14728214.2011.537824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor SR, Habot-Wilner Z, Pacheco P, Lightman SL. Intraocular methotrexate in the treatment of uveitis and uveitic cystoid macular edema. Ophthalmology. 2009;116:797–801. doi: 10.1016/j.ophtha.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 23.Shanmuganathan VA, Casely EM, Raj D, Powell RJ, Joseph A, Amoaku WM, et al. The efficacy of sirolimus in the treatment of patients with refractory uveitis. Br J Ophthalmol. 2005;89:666–9. doi: 10.1136/bjo.2004.048199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Posarelli C, Arapi I, Figus M, Neri P. Biologic agents in inflammatory eye disease. J Ophthalmic Vis Res. 2011;6:309–16. [PMC free article] [PubMed] [Google Scholar]
- 25.Servat JJ, Mears KA, Black EH, Huang JJ. Biological agents for the treatment of uveitis. Expert Opin Biol Ther. 2012;12:311–28. doi: 10.1517/14712598.2012.658366. [DOI] [PubMed] [Google Scholar]
- 26.Lindstedt EW, Baarsma GS, Kuijpers RW, van Hagen PM. Anti-TNF-alpha therapy for sight threatening uveitis. Br J Ophthalmol. 2005;89:533–6. doi: 10.1136/bjo.2003.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bodaghi B, Gendron G, Wechsler B, Terrada C, Cassoux N, Huong du LT, et al. Efficacy of interferon alpha in the treatment of refractory and sight threatening uveitis: A retrospective monocentric study of 45 patients. Br J Ophthalmol. 2007;91:335–9. doi: 10.1136/bjo.2006.101550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nussenblatt RB, Peterson JS, Foster CS, Rao NA, See RF, Letko E, et al. Initial evaluation of subcutaneous daclizumab treatments for noninfectious uveitis: A multicenter no comparative interventional case series. Ophthalmology. 2005;112:764–70. doi: 10.1016/j.ophtha.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 29.Teoh SC, Sharma S, Hogan A, Lee R, Ramanan AV, Dick AD. Tailoring biological treatment: Anakinra treatment of posterior uveitis associated with the CINCA syndrome. Br J Ophthalmol. 2007;91:263–4. doi: 10.1136/bjo.2006.0101477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dick A, Meyer P, James T, Forrester JV, Hale G, Waldmann H, et al. Campath-1H therapy in refractory ocular inflammatory diseases. Br J Ophthalmol. 2000;84:107–9. doi: 10.1136/bjo.84.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heiligenhaus A, Miserocchi E, Heinz C, Gerloni V, Kotaniemi K. Treatment of severe uveitis associated with juvenile idiopathic arthritis with anti-CD20 monoclonal antibody (rituximab) Rheumatology (Oxford) 2011;50:1390–4. doi: 10.1093/rheumatology/ker107. [DOI] [PubMed] [Google Scholar]
- 32.Cho H, Wolf KJ, Wolf EJ. Management of ocular inflammation and pain following cataract surgery: Focus on bromfenac ophthalmic solution. Clin Ophthalmol. 2009;3:199–210. doi: 10.2147/opth.s4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soheilian M, Ramezani A, Azimzadeh A, Sadoughi MM, Dehghan MH, Shahghadami R, et al. Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisolone in treatment of ocular toxoplasmosis. Ophthalmology. 2011;118:134–41. doi: 10.1016/j.ophtha.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 34.Riddell J, 4th, Comer GM, Kauffman CA. Treatment of endogenous fungal endophthalmitis: Focus on new antifungal agents. Clin Infect Dis. 2011;52:648–53. doi: 10.1093/cid/ciq204. [DOI] [PubMed] [Google Scholar]
- 35.Gupta V, Bansal R, Gupta A. Continuous progression of tubercular serpiginous-like choroiditis after initiating antituberculosis treatment. Am J Ophthalmol. 2011;152:857–63. doi: 10.1016/j.ajo.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Chee SP, Jap A. Cytomegalovirus anterior uveitis: Outcome of treatment. Br J Ophthalmol. 2010;94:1648–52. doi: 10.1136/bjo.2009.167767. [DOI] [PubMed] [Google Scholar]
- 37.Hatton MP, Duker JS, Reichel E, Morley MG, Puliafito CA. Treatment of relapsed cytomegalovirus retinitis with the sustained-release ganciclovir implant. Retina. 1998:1850–5. doi: 10.1097/00006982-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Gulati N, Forooghian F, Lieberman R, Jabs DA. Vascular endothelial growth factor inhibition in uveitis: A systematic review. Br J Ophthalmol. 2011;95:162–5. doi: 10.1136/bjo.2009.177279. [DOI] [PubMed] [Google Scholar]


