Abstract
Objective
The objective of this study was to compare the clinical characteristics and outcomes of preeclamptic women presenting with a normal plasma angiogenic profile with those subjects that are characterized by an abnormal angiogenic profile.
Methods
This was a secondary analysis of a prospective cohort study in women presenting to obstetrical triage at <37 weeks of gestation and diagnosed with preeclampsia within 2 weeks of enrollment and in whom angiogenic factors (sFlt1 and PlGF) measurements were available. Patients were divided into two groups based on their circulating levels of these factors described as a ratio the sFlt1/PlGF ratio, non-angiogenic preeclampsia (sFlt1/PlGF ratio <85) and angiogenic preeclampsia (sFlt1/PlGF ratio ≥85). The data are presented by sFlt1/PlGF category using median and quartile 1-quartile 3 for continuous variables and by frequency and sample sizes for categorical variables.
Results
In our cohort the patients with non-angiogenic preeclampsia (N=46) were more obese {BMI - 35.2 (31.6,38.7) vs. 31.1(28.0,39.0), p=0.04}, more likely to have preexisting diabetes (21.7% vs. 2.0%, p=0.002) and presented at a later gestational age {35 (32,37) vs. 32 (29,34) weeks, p<0.0001} as compared to women with angiogenic preeclampsia (N=51). Women with non-angiogenic preeclampsia had no serious adverse outcomes {Elevated LFT's/Low platelets: 0% vs. 23.5%, abruption: 0% vs. 9.8%, pulmonary edema: 0% vs. 3.9%, eclampsia: 0% vs. 2.0 %, small for gestational age: 0% vs. 17.7% and fetal/neonatal death: 0% vs. 5.9%} as compared to women with angiogenic preeclampsia. The rate of preterm delivery <34 weeks was 8.7% in non-angiogenic preeclampsia compared to 64.7% in angiogenic preeclampsia (p<0.0001). Interestingly, delivery between 34-37 weeks and resource utilization (hospital admission days) were similar in the two groups.
Conclusion
In contrast to the angiogenic form, the non-angiogenic form of preeclampsia is characterized by little to no risk of preeclampsia related adverse outcomes, other than iatrogenic prematurity. Incorporation of angiogenic biomarkers in the evaluation of preeclampsia may allow accurate and early identification of severe disease.
Keywords: non-angiogenic preeclampsia, angiogenic factors, adverse outcomes
Introduction
Preeclampsia is a common hypertensive disorder specific to pregnancy occurring in 3-5% of all pregnant women that is diagnosed by new onset hypertension and proteinuria occurring after 20 weeks of gestation.(1) Since preeclampsia is a multi-systemic disease that is characterized by adverse maternal and fetal outcomes, the emphasis for the current clinical diagnostic criteria is not to miss any cases of true preeclampsia so as to avoid morbidity and mortality and in some countries legal consequences. However, neither hypertension nor proteinuria is specific to the pathophysiology and disturbed histology of preeclampsia. (2-5) As a result, women with other conditions such as diabetes, lupus and/or obesity that present with proteinuria and hypertension during pregnancy are often incorrectly classified as preeclampsia. (2-5)
In 1980, Fisher et al reviewed renal biopsies at their institution performed on women with a clinical diagnosis of preeclampsia. Glomerular endotheliosis (the renal lesion associated with preeclampsia) was not seen in 15% of primiparous and 40% of multiparous women thus raising the question that clinical diagnosis of preeclampsia may be imprecise especially in multiparous women. Other studies have suggested an elevated rate of underlying renal disease in women with clinical diagnosis of preeclampsia, suggesting that the appearance of hypertension and proteinuria may also be due to exacerbation of the underlying renal disease rather than preeclampsia.(6, 7) Thus, it is no surprise that when clinical diagnosis alone is utilized many now label preeclampsia a heterogeneous disease. Data suggest that, in some women, preeclampsia and even eclampsia may be atypical and develop in the absence of either hypertension and/or proteinuria.(8)
Recently, angiogenic factors have been associated with preeclampsia phenotypes. Serum levels of fms like tyrosine kinase 1(sFlt1) and soluble endoglin (sEng) are elevated, and placental growth factors (PlGF) decreased in women with preeclampsia.(9-14) Over expression of sFlt1 is associated with preeclampsia-like phenotypes in rodents,(15) and the symptoms appear to diminish with supplementation of pro-angiogenic factors such as placenta-like growth factor (PlGF) or vascular endothelial growth factor (VEGF).(16, 17) This provides a biological plausibility for imbalance of angiogenic factors in the pathogenesis of preeclampsia. Recently, a small pilot clinical study suggested that extracorporeal removal of sFlt1 seems to have beneficial effect on pregnancy prolongation in women with severe early-onset preeclampsia.(18) Angiogenic factors have been shown to have high sensitivity and specificity not only for diagnosis of preterm preeclampsia but also for preeclampsia-related adverse outcomes.(19-21) While the improved test characteristics of angiogenic markers indicate a potential for improving maternal and fetal health, there is a debate in the literature whether a true form of preeclampsia that is not associated with an “angiogenic imbalance” exists.(22) In particular when preeclampsia presents at term, a substantial number of the patients do not have angiogenic imbalance.(12, 23) There may be several reasons for this. One is that in much of the early literature, angiogenic factors were evaluated in the prediction of the diagnosis based upon clinical criteria – which we know is imprecise. A second is that many of the samples were drawn weeks prior to clinical onset of disease resulting in an increased false negative rate. A third possibility is that non-angiogenic pathways may mediate the disease pathology in this subgroup of patients with preeclampsia.
We have recently published our experience with the use of the sFlt1/PlGF ratio in prediction of adverse outcomes among patients undergoing evaluation for suspected preeclampsia. The test's sensitivity and specificity for adverse outcomes were excellent, specifically for women presenting at a gestational age less than 34 weeks.(20) We believe that the use of highly specific tests, such as sFlt1 and PlGF, in the evaluation, risk stratification, and management of patients with suspected preeclampsia will reduce the number of unnecessary evaluations, inductions, and possibly even preterm births while more appropriately focusing resources towards patients truly at increased risk for adverse outcomes. However, we remain intrigued by the notion of “non-angiogenic” preeclampsia (22) and we decided to perform a secondary analysis of the previously published study (20) focusing on details (clinical characteristics, maternal and fetal outcomes and resource utilization) of patients who carried a diagnosis of preeclampsia but had a normal angiogenic profile.
Materials and methods
Ethics Statement
The study was approved by the Beth Israel Deaconess Medical Center Institutional Review Board (IRB), and all patients provided written informed consent.(20)
Study Population
This is part of an ongoing prospective cohort study on pregnant patients who presented to obstetrical triage at Beth Israel Deaconess Medical Center (BIDMC), Boston, MA, as described elsewhere.(20) It involves pregnant women who were either referred by their obstetric provider for signs of preeclampsia or self-presented with symptoms of preeclampsia. Patients were included if the triage care provider deemed an evaluation of preeclampsia necessary. Discarded plasma samples sent from triage were collected, processed and stored at −80°C for analysis. In the current analysis, women presenting at <37 weeks of gestation (between July 2009-October 2010) with a diagnosed as of being preeclamptic were included.
Clinical diagnosis of preeclampsia
Patients were diagnosed with preeclampsia within 2 weeks of enrollment and sample collection, based on American College of Obstetrics and Gynecology (ACOG).(1) Preeclampsia was defined as new onset hypertension (a blood pressure (BP) ≥140/90 mmHg) and proteinuria of ≥ 300mg/24 hour or spot urine protein to creatinine (P/C) ratio of ≥ 0.3 after 20 weeks of gestation. For patients with chronic hypertension, superimposed preeclampsia was diagnosed if there was a significant increase in blood pressures compared to baseline (≥30 mmHg systolic, ≥ 15 mmHg diastolic) in association with new onset proteinuria. (24) If proteinuria was present at baseline, superimposed preeclampsia was diagnosed if there was a doubling of urinary protein along with an increase in blood pressure (as defined above).(24) Superimposed preeclampsia was also diagnosed if there were elevated blood pressures and elevated levels of aspartate aminotransferase (AST) or alanine aminotransferase (ALT) (≥ 80 U/L) and thrombocytopenia (platelet count ≤100 K/uL. (24) We included patients at gestational ages (GA) <37 weeks as patients presenting after 37 weeks at our institution are frequently delivered for suspected preeclampsia with little attempt to verify the diagnosis. A sFlt1/PlGF ratio of 85 was chosen as the value that divides our populations based on previously published data in relation to predicting adverse outcomes. (20, 25-27) Thus for the analysis patients were divided and divided patients into two groups- “non-angiogenic preeclampsia” (sFlt1/PlGF ratio <85) and “angiogenic preeclampsia” (sFlt1/PlGF ratio ≥ 85). Each participant's chart was reviewed to determine clinical diagnosis within two weeks of the initial preeclampsia evaluation. All clinical data were collected during the two weeks after the initial preeclampsia evaluation including age, race, height, weight, smoking status, GA at the time of triage visit, clinical findings, BP, and the results of laboratory tests and fetal scans were included. All pregnancy outcomes including complications and delivery characteristics such as route of delivery, birth weight and diagnosis of hypertensive disorder were recorded.(20)
Resource utilization for evaluation and management of preeclampsia
Participant re-enrollment was permitted among the cohort, however resource determination was restricted to participants’ initial study encounter. Antepartum, delivery-associated and postpartum resources were determined by review of participants’ medical records and hospital administrative data. All triage evaluations, maternal radiologic studies, tests of fetal wellbeing (any combination of biophysical profiles, non-stress tests, obstetric ultrasounds, and Doppler ultrasounds), laboratory evaluations, admissions, consultations, deliveries, and miscellaneous charges were tallied over the two week period following enrollment.
Primary Outcome
Among this group of proteinuric hypertensive women adverse maternal outcomes were defined as one of the following: abnormal Liver function test and platelets (the combination of elevated elevated aspartate aminotransferase (AST) or alanine aminotransferase (ALT) (≥ 80 U/L) and thrombocytopenia (platelet count ≤150 K/uL), abruption (clinical and/or pathological), pulmonary edema, cerebral hemorrhage, seizure (in the absence of an underlying seizure disorder), acute renal failure (creatinine >1.5 mg/dL), or maternal death.(28) The adverse fetal/neonatal outcomes included iatrogenic delivery indicated for hypertensive complications of pregnancy as reported by the primary obstetrician, small for gestational age neonate (≤10th percentile for gestational age), fetal death, and neonatal death.(28) The presence of adverse outcomes was determined by two study staff members without knowledge of the assay results. Diagnosis and adverse outcomes were ascertained within 2 weeks of presentation.
sFlt1 , PlGF and soluble endoglin assays
Automated assays for sFlt1 and PlGF in this cohort were performed at clinical laboratory of Charite Hospital (Berlin, Germany) using the commercially available assays on Elecsys platform (Roche Diagnostics, Penzberg, Germany) and have been previously published. (20) The inter-assay coefficient of variance for sFlt1 and PlGF immunoassays ranged from 2.6-3.0% and 2.0-2.4% respectively. Enzyme-linked immunosorbent assay (ELISA) for sEng was performed with commercially available kits (R&D systems, Minneapolis, MN) as described previously.(29) Assays were performed in duplicate and values averaged. The correlation coefficient between duplicate results was 0.97. The intra-assay and inter-assay coefficients of variation were 3.0 and 6.3%. The assay operators were blinded to the clinical information of the participants. All analysis was done after the diagnosis and outcomes were already achieved by the patients and the treating physicians were unaware of the test results of sFlt1, PlGF and sEng levels.
Statistical analysis
Characteristics of the study population, resource utilization, and adverse outcomes were summarized using median and quartile 1-quartile 3 for continuous variables and frequency and sample sizes for categorical variables separately by sFlt1/PIGF category (<85 vs. ≥85). Subjects with angiogenic and non-angiogenic preeclampsia were compared using the Mann-Whitney-U test and chi-square test as appropriate. Spearman rank correlation coefficient was utilized to examine the association between sFlt1/PIGF and birth weight. The distributions of all continuous variables were explored. Based on previous literature sFlt1/PlGF cutoff of 85 was used and sFlt1/PIGF was analyzed as binary variable using this cut-off. (20, 25-27) Non-parametric methods were employed due to the non-normality of many for the relevant laboratory values. For resource utilization, variables were summarized as the proportion of patients receiving a given service in each group unless otherwise noted. A two-sided P value of 0.05 or less was interpreted as a statistically significant result. All analyses were performed using SAS version 9.2 (Cary, NC).
Since this was secondary analysis, no formal power calculation was done. However, based on retrospective power calculations, we are more than adequately powered for the current analysis. For a comparison of two independent binomial proportions with a two-sided significance level of 0.05, a total sample size of 97 assuming an allocation ratio of 46 to 51 has an approximate power of >99% when the proportions are 0.53 and 0.00 (as seen in the data). A 16% difference in the rate of adverse outcomes would have adequately powered (80%) for the current study.
Results
Preeclampsia diagnosis and sFlt1/PlGF ratio
Among the entire cohort of 616 patients, 97 women presented at GA <37 weeks in triage and developed preeclampsia within two weeks. Forty six of the 97 women had non-angiogenic preeclampsia and 51 had angiogenic preeclampsia based on plasma sFlt1/PlGF ratio at presentation.
The demographic characteristics of these two groups are depicted in Table 1. Women with non-angiogenic preeclampsia presented to triage at a later gestational age and had higher BMI. A greater proportion of these women had a history of pre-gestational diabetes. These women also had significantly lower systolic and diastolic BP's, ALT, creatinine, uric acid and higher platelet counts. The median time to delivery from enrollment was longer for the women with non-angiogenic preeclampsia, with higher average GA of delivery and higher fetal weights at delivery (all P<0.05). We found that sFlt1/PlGF ratio was inversely correlated to birth weight (r= −0.62, p<0.0001). In addition, the percentage of infants with macrosomia (>90th centile birth weight) was higher among women with non-angiogenic preeclampsia compared to women with angiogenic preeclampsia (p=0.008). The median (25th-75th IQR) sFlt1/PlGF ratio between the two groups was 29.7(9.9, 52.1) versus 248.5 (143.1, 563.67), p<0.0001. The patients with non-angiogenic preeclampsia also had lower levels of soluble endoglin 9.5 (6.9, 15.9) compared to women with angiogenic preeclampsia 38.6 (26.2, 66.1), p<0.0001(Table 1).
Table 1.
Characteristics of patients with non-angiogenic and angiogenic preeclampsia
| Variable | Non-Angiogenic PE (S/P Ratio <85) | Angiogenic PE (S/P Ratio ≥85) | p-value |
|---|---|---|---|
| N | 46 | 51 | |
| Baseline | |||
| Gestational Age (weeks) | 35 (32, 37) | 32 (29, 34) | 0.0001* |
| Age (years) | 32 (29, 35) | 31 (24, 35) | 0.30 |
| Body Mass Index (kg/m2) | 35.2 (31.6, 38.7) | 31.1 (28.0, 39.0) | 0.04* |
| Nulliparous (%) | 33 (71.7) | 35 (68.6) | 0.74 |
| Smoker (%) | 4 (8.7) | 2 (3.9) | 0.33 |
| Race (%) | 0.87 | ||
| White/Caucasian | 32 (69.6) | 34 (66.7) | |
| Black/African American | 6 (13.0) | 5 (9.8) | |
| Asian | 3 (6.5) | 4 (7.8) | |
| Other | 5 (10.9) | 8 (15.7) | |
| History of Preeclampsia (%) | 8 (17.4) | 7 (13.7) | 0.62 |
| History of Chronic Hypertension (%) | 15 (32.6) | 9 (17.7) | 0.09 |
| History of Diabetes (%) | 10 (21.7) | 1 (2.0) | 0.002* |
| Presentation | |||
| Highest SBP (mmHg) | 147 (132, 155) | 154 (143, 167) | 0.02* |
| Highest DBP (mmHg) | 90 (82, 99) | 97 (86, 103) | 0.04* |
| Proteinuria (%) | 38 (82.6) | 46 (90.2) | 0.27 |
| ALT (U/L) | 17 (13, 21) | 21 (14, 40) | 0.03* |
| Creatinine (mg/dl) | 0.6 (0.5, 0.7) | 0.7 (0.6, 0.8) | 0.02* |
| Uric Acid (mg/dL) | 4.6 (4.1, 5.7) | 6.3 (5.2, 7.0) | <0.0001* |
| Platelet Count (K/uL) | 258 (204, 296) | 211 (143, 253) | 0.003* |
| sFlt1/PlGF Ratio | 29.7 (9.9, 52.1) | 248.5 (143.1, 563.6) | <0.0001* |
| Soluble Endoglin (ng/ml) | 9.5 (6.9, 15.9) | 38.6 (26.2, 66.1) | <0.0001* |
| Delivery | |||
| GA at Delivery (weeks) | 36 (34, 37) | 32 (29, 34) | <0.0001* |
| Birthweight (grams) | 2,789 (2,495, 3,315) | 1,555 (1,090, 2,100) | <0.0001* |
| Macrosomia (%) | 10 (21.7) | 2(3.9) | 0.008* |
| Indicated deliveries (%) | 17 (37.0) | 47 (92.2) | <0.0001* |
Data is shown as median (quartile 1, quartile 3) or n, (%) where appropriate.
Significant at p<0.05
PE= preeclampsia, S/P= sFlt1/PlGF ratio, ALT= alanine transamninases, GA= gestational age
sFlt1/PlGF ratio and adverse outcomes
Excluding obstetric indicated deliveries, no adverse outcomes were recorded in women with non-angiogenic preeclampsia. All of the adverse maternal and neonatal outcomes occurred exclusively in women with angiogenic preeclampsia. Using a composite outcome, the proportion of patients experiencing any adverse outcome was 0.0% (0 out of 46) in patients with non-angiogenic preeclampsia vs. 52.9% (27 out of 51) in patients with angiogenic preeclampsia (p<0.0001), (Table 2). Patients enrolled at <34 weeks had similar results (p=0.0001).
Table 2.
Distribution of adverse outcomes in subjects with non-angiogenic and angiogenic preeclampsia
| Outcome | Non-Angiogenic PE (S/P Ratio <85) | Angiogenic PE (S/P Ratio ≥85) | p- value |
|---|---|---|---|
| N | 46 | 51 | |
| Elevated LFT's & Low Plts | 0.0 (0) | 12 (23.5) | 0.0004* |
| Abruption | 0.0 (0) | 5 (9.8) | 0.03* |
| Pulmonary Edema | 0.0 (0) | 2 (3.9) | 0.17 |
| Eclampsia | 0.0 (0) | 1 (2.0) | 0.34 |
| SGA | 0.0 (0) | 9 (17.7) | 0.003* |
| Fetal/Neonatal Death | 0.0 (0) | 3 (5.9) | 0.09 |
| Composite Adverse outcome | 0.0 (0) | 27 (52.9) | <0.0001* |
Significant at p<0.05. Data shown as n (%)
PE= preeclampsia, S/P= sFlt1/PlGF ratio, LFT's= Liver function tests, low plts= low platelets, SGA= Small for Gestational Age. Composite outcome was defined as any adverse outcome occurring in each group.
sFlt1/PlGF and indicated preterm delivery
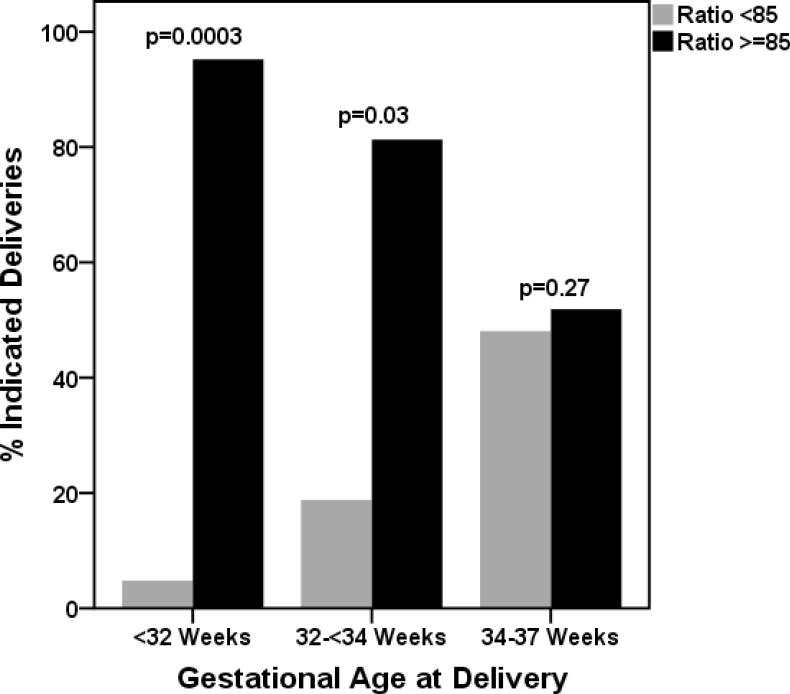
There were total 64 preterm deliveries less than 37 weeks, 37 (57.8%) of them prior to 34 weeks. Among women delivered prior to 34 weeks a majority of patient had angiogenic preeclamspia (89.2% vs 10.8%) compared to non-angiogenic preeclampsia (p=0.0003). However among women delivered between 34-37 weeks the proportions was similar (48.1 % vs. 51.9%, p=0.27). Figure 1.
Figure 1. Indicated preterm delivery within 2 weeks of enrollment at various gestational ages based on sFlt1/PlGF ratio.
Among gestational age group <34 weeks at delivery, the rate of indicated delivery was lower among women with non-angiogenic preeclampsia compared to women with angiogenic preeclampsia, delivery <32 weeks (4.8 vs. 95.2%, p=0.0003) and 32-<34 weeks (18.8% vs. 81.3%, p=0.03). The rate of delivery was similar in both groups between 34-37 weeks (48.1 % vs. 51.9%, p=0.27).
In 3 of the 4 patients with non-angiogenic preeclampsia and preterm delivery at <34 weeks the indication for delivery was headache, without evidence of any other end organ damage. The fourth patient's diagnosis was changed to acute fatty liver of pregnancy. All these patients were obese with average BMI of 43.6 (kg/m2). Clinical characteristics of patients who delivered less than 37 weeks in the group with non-angiogenic preeclampsia ratio are presented in Supplementary Table 1.
sFlt1/PlGF ratio and resource utilization
Resource determination was restricted to participants’ initial study encounter. Thus, 80 individual women met this criteria. At enrollment 38 had non-angiogenic preeclampsia and 42 were found to have angiogenic preeclampsia upon initial enrollment. Although the rates of indicated preterm delivery were less among women with non-angiogenic preeclampsia (36.8% vs. 97.6%; p<0.001), these women required slightly longer length of stay (p=0.05) and had similar rate of overall number of antenatal admissions (Table 3). Women with non-angiogenic preeclampsia demonstrated a higher median outpatient visit total (10 vs. 0; p<0.0001) and more tests for fetal well-being {4 (2, 6) vs. 1 (1, 2); p<0.0001}. 66.5% of all tests for fetal well-being among the cohort were performed on women with non-angiogenic preeclampsia. Similarly, large proportions of the cohort's total laboratory tests (such as complete blood counts, coagulation studies, serum chemistries and urine studies) and imaging studies were performed on women with non-angiogenic preeclampsia (Table 3).
Table 3.
Resource utilization in patients with diagnosis of preeclampsia based on S/P ratio
| Resource utilization | Non-Angiogenic PE (S/P Ratio <85) N=38 | Angiogenic PE (S/P Ratio ≥85) N=42 | p-value |
|---|---|---|---|
| Triage Visits | 1.5 (1, 2) | 1.0 (1, 1) | <0.001* |
| Antepartum Admissions (%) | 20 (52.6) | 23 (54.8) | 1.0 |
| Duration of Antepartum Admissions (days) | 5 (3, 10) | 3 (3, 4) | 0.05 |
| Outpatient Visits (%) | 10 (26.3) | 0 (0.0) | <0.001* |
| Tests for Fetal Well Being | 4 (2, 6) | 1 (1, 2) | <0.001* |
| Magnetic Resonance Imaging (%) | 2 (5.3) | 1 (2.4) | 0.60 |
| Magnetic Resonance Angiogram (%) | 1 (2.6) | 2 (4.8) | 0.62 |
| Chest X Ray (%) | 2 (5.3) | 8 (19.0) | 0.06 |
| Complete Blood Count | 5 (3, 6) | 7 (5, 10) | <0.001* |
| Coagulation Studies | 2 (0, 4) | 8 (4, 14) | <0.001* |
| Serum Chemistries | 21 (14, 28) | 35 (21, 60) | 0.002* |
| Urine studies | 3 (1, 4) | 1 (1, 2) | <0.001* |
| Consultations (%) | 17 (44.7) | 26 (61.9) | 0.12 |
| Indicated preterm delivery <37 weeks (%) | 14 (36.8) | 41 (97.6) | <0.001* |
Significant at p<0.05.
Data is shown as median (quartile 1, quartile 3) or n (%) where appropriate PE= preeclampsia, S/P= sFlt1/PlGF ratio. Tests for fetal well being include all combinations of biophysical profiles, non-stress tests, obstetric ultrasounds, and Doppler ultrasounds.
Discussion
The results of this secondary analysis clearly indicate that women with clinical diagnosis of preeclampsia and low sFlt1/PlGF ratio (non-angiogenic preeclampsia) have lower risks of preeclampsia related adverse outcomes, other than iatrogenic preterm delivery. In contrast, preeclampsia characterized by high sFlt1/PlGF ratio (angiogenic preeclampsia) defines a subset of patients with preeclampsia who develop a more aggressive disease at early gestational ages, and are at high risk for preeclampsia related adverse maternal and neonatal outcomes. About 47.4% of the women who received a clinical diagnosis of preeclampsia had low sFlt1/PlGF ratio, consistent with recent literature suggesting that there is a form of preeclampsia, which is not associated with angiogenic imbalance.(22) However, our data suggests that non-angiogenic preeclampsia is a benign variant, which is not associated with the same risk of short-term adverse outcomes as angiogenic preeclampsia. Our study is also in line with prior studies suggesting that measurement of angiogenic factors may help to distinguish preeclampsia from hypertension and/or proteinuria due to other conditions.(24, 27, 30, 31)
Clinicians primarily seek to identify women with preeclampsia in order to prevent adverse maternal and fetal outcomes including eclampsia, end organ damage, and restricted fetal growth. If there is a subset of women who are not at risk for these adverse outcomes, the presence of hypertension and proteinuria alone would not necessitate intervention. We observed that women with non-angiogenic preeclampsia had no other adverse outcomes except for iatrogenic delivery, often occurring prior to 37 weeks gestation. Common indications for delivery included isolated elevated LFTs, headache, or reaching a certain gestational age (~36-37 weeks). Our data suggests that a low sFlt1/PlGF ratio identifies low risk preeclampsia in whom the risks of preterm delivery may outweigh the benefits of prolongation of pregnancy. It can be conceived that managing patients aided by sFlt1/PlGF ratio would reduce the rate of iatrogenic preterm delivery; our data suggests that such a strategy would not put women at risk of adverse outcomes in the short-term.
Although the women with non-angiogenic preeclampsia had very low risk of adverse outcomes, they utilized significant quantities of resources. Given that more of more of these women extended their pregnancy beyond the diagnosis, this was expected but we suggest these costs would have been less then neonatal complications associated with prematurity, including neonatal ICU and nursery costs. Clinicians are charged with the duty to identify the subset of patients who will ultimately develop severe disease, however our current standard clinical tests perform poorly for risk stratification.(32) Although current standard diagnostic tests are readily available and used in routine practice, they lead to significant misclassification and over-testing/treating of many women. Correct identification of women at risk by measurement of sFlt1 and PlGF for evaluation of preeclampsia has the potential to reduce antepartum admissions, hospital evaluations, and iatrogenic preterm births in patients with non-angiogenic preeclampsia. This will enable a focused cost and resource expenditure that will lead to reductions over time.
We speculate that a high sFlt1/PlGF ratio corresponds to the specific pathology of the multi-systemic disease noted in human autopsy and biopsy studies from preeclamptic subjects. (2, 33). We also speculate that lack of adverse outcomes in preeclamptic patients with low sFlt1/PlGF may be due to the misclassification of the diagnosis. Indeed when we study the baseline characteristics, number of these patients had other diseases such as chronic hypertension, diabetes or obesity – conditions that mimic preeclampsia in the clinical presentation. However, we cannot rule out the possibility that patients with non-angiogenic preeclampsia may not develop complications if they were not delivered prematurely.
Our findings have important implications for the design of clinical trials for prediction and treatment of preeclampsia. Identification of women with angiogenic preeclampsia may help target therapeutic trials for preeclampsia. Women with angiogenic preeclampsia may benefit from sFlt1 based therapy (such as extracorporeal removal of sFlt1 or drugs that inhibit sFlt1 production), where as those with non-angiogenic preeclampsia may not. Importantly, prediction studies being carried out in early pregnancy should focus on identifying angiogenic preeclampsia and its related complications rather than the clinical diagnosis of preeclampsia.
Our study has several strengths. First this was a secondary analysis of a well designed prospective study with well defined clinical outcomes, the population selected relevant to clinical practice. However, our study was limited by the inability to assess whether the non-angiogenic preeclamptic subjects who were delivered early would have had adverse outcomes had their pregnancies been allowed to progress. Although our data suggests that adverse outcomes other than iatrogenic preterm delivery would not occur in this group, we are not able to accurately classify these subjects and in particular to assess whether delivery prevented adverse outcomes. An additional limitation was sample size and no formal power calculation was done since this was a secondary analysis. More studies among larger cohorts are required to verify our conclusions, particularly with respect to some of the rare adverse outcomes such as eclampsia and fetal deaths. Future studies should also evaluate whether angiogenic factor measurements can not only predict short-term adverse outcomes, but also long term adverse outcomes including post-partum complications of preeclampsia.
In conclusion, if these observations are confirmed by others, serious thought should be given to incorporation of sFlt1 and PlGF into the definition of severe preeclampsia, to more accurately reflect the morbidity of true disease and the risk of adverse outcomes. Larger prospective studies using measurement of these factors (sFlt1/PlGF) should be conducted to confirm whether use of angiogenic factors in clinical decision making can decrease the incidence of preterm delivery and reduce recourse utilization without increasing the risk of adverse maternal and neonatal outcomes.
Supplementary Material
Precis.
Preeclampsia when associated with normal levels of circulating angiogenic proteins is a relatively benign variant of the disease, characterized by lower risk of adverse maternal and fetal/neonatal outcomes.
Acknowledgments
S.R. is supported by Harvard Diversity and Community Partnership Faculty Fellowship Award and K08HD068398-01A1 (NIH/NICHD). S.A.K is an investigator of the Howard Hughes Medical Institute. We thank Dawn McCullough, RN for patient recruitment and data collection.
Sources of funding
Harvard Diversity and Community Partnership Faculty Fellowship, NIH/NICHD and Howard Hughes Medical Institute.
Footnotes
Conflict of Interest/Disclosure(s)
Dr. Verlohren has served as a consultant to Roche Diagnostics. Dr. Thadhani is a co-inventor on patents related to the prediction of preeclampsia that has been out licensed to diagnostic companies and has financial interest in Aggamin LLC. Dr. Karumanchi is a co-inventor of multiple patents related to angiogenic proteins for the diagnosis and therapy of preeclampsia. These patents have been licensed to multiple companies. Dr. Karumanchi reports having served as a consultant to Roche and Beckman Coulter and has financial interest in Aggamin LLC. The remaining authors report no conflicts.
References
- 1.ACOG practice bulletin Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002 Jan;99(1):159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 2.Fisher KA, Luger A, Spargo BH, Lindheimer MD. Hypertension in pregnancy: clinical-pathological correlations and remote prognosis. Medicine (Baltimore) 1981 Jul;60(4):267–76. [PubMed] [Google Scholar]
- 3.Germain S, Nelson-Piercy C. Lupus nephritis and renal disease in pregnancy. Lupus. 2006;15(3):148–55. doi: 10.1191/0961203306lu2281rr. [DOI] [PubMed] [Google Scholar]
- 4.Catalano PM. Management of obesity in pregnancy. Obstet Gynecol. 2007 Feb;109(2Pt 1):419–33. doi: 10.1097/01.AOG.0000253311.44696.85. [DOI] [PubMed] [Google Scholar]
- 5.Powe CE, Thadhani R. Diabetes and the kidney in pregnancy. Semin Nephrol. 2011 Jan;31(1):59–69. doi: 10.1016/j.semnephrol.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Ihle BU, Long P, Oats J. Early onset pre-eclampsia: recognition of underlying renal disease. Br Med J (Clin Res Ed) 1987 Jan 10;294(6564):79–81. doi: 10.1136/bmj.294.6564.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nochy D, Birembaut P, Hinglais N, Freund M, Idatte JM, Jacquot C, et al. Renal lesions in the hypertensive syndromes of pregnancy: immunomorphological and ultrastructural studies in 114 cases. Clin Nephrol. 1980 Apr;13(4):155–62. [PubMed] [Google Scholar]
- 8.Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol. 2009 May;200(5):481, e1–7. doi: 10.1016/j.ajog.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 9.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003 Mar;111(5):649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004 Feb 12;350(7):672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 11.Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009 Nov;22(11):1021–38. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010 Aug 3;122(5):478–87. doi: 10.1161/CIRCULATIONAHA.109.895458. [DOI] [PubMed] [Google Scholar]
- 13.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008 Jan;21(1):9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005 Jan;17(1):3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 15.Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, et al. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol. 2007 Apr;196(4):396, e1–7. doi: 10.1016/j.ajog.2006.12.024. discussion e7. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Zhang Y, Ying Ma J, Kapoun AM, Shao Q, Kerr I, et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007 Oct;50(4):686–92. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- 17.Kumasawa K, Ikawa M, Kidoya H, Hasuwa H, Saito-Fujita T, Morioka Y, et al. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci U S A. 2011 Jan 25;108(4):1451–5. doi: 10.1073/pnas.1011293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thadhani R, Kisner T, Hagmann H, Bossung V, Noack S, Schaarschmidt W, et al. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation. 2011 Aug 23;124(8):940–50. doi: 10.1161/CIRCULATIONAHA.111.034793. [DOI] [PubMed] [Google Scholar]
- 19.Chaiworapongsa T, Romero R, Savasan ZA, Kusanovic JP, Ogge G, Soto E, et al. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011 Oct;24(10):1187–207. doi: 10.3109/14767058.2011.589932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012 Feb 21;125(7):911–9. doi: 10.1161/CIRCULATIONAHA.111.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore AG, Young H, Keller JM, Ojo LR, Yan J, Simas TA, et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med. 2012 Aug 22; doi: 10.3109/14767058.2012.713055. [DOI] [PubMed] [Google Scholar]
- 22.Powers RW, Roberts JM, Plymire DA, Pucci D, Datwyler SA, Laird DM, et al. Low placental growth factor across pregnancy identifies a subset of women with preterm preeclampsia: type 1 versus type 2 preeclampsia? Hypertension. 2012 Jul;60(1):239–46. doi: 10.1161/HYPERTENSIONAHA.112.191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006 Sep 7;355(10):992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 24.Perni U, Sison C, Sharma V, Helseth G, Hawfield A, Suthanthiran M, et al. Angiogenic factors in superimposed preeclampsia: a longitudinal study of women with chronic hypertension during pregnancy. Hypertension. 2012 Mar;59(3):740–6. doi: 10.1161/HYPERTENSIONAHA.111.181735. [DOI] [PubMed] [Google Scholar]
- 25.Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010 Feb;202(2):161, e1–e11. doi: 10.1016/j.ajog.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Stepan H, Schaarschmidt W, Jank A, Verlohren S, Kratzsch J. [Use of angiogenic factors (sFlt-1/PlGF ratio) to confirm the diagnosis of preeclampsia in clinical routine: first experience]. Z Geburtshilfe Neonatol. 2010 Dec;214(6):234–8. doi: 10.1055/s-0030-1262827. [DOI] [PubMed] [Google Scholar]
- 27.Verdonk K, Visser W, Russcher H, Danser AH, Steegers EA, van den Meiracker AH. Differential diagnosis of preeclampsia: remember the soluble fms-like tyrosine kinase 1/placental growth factor ratio. Hypertension. 2012 Oct;60(4):884–90. doi: 10.1161/HYPERTENSIONAHA.112.201459. [DOI] [PubMed] [Google Scholar]
- 28.Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010 Apr 8;362(14):1282–91. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rana S, Cerdeira AS, Wenger J, Salahuddin S, Lim KH, Ralston SJ, et al. Plasma Concentrations of Soluble Endoglin versus Standard Evaluation in Patients with Suspected Preeclampsia. PLoS One. 2012;7(10):e48259. doi: 10.1371/journal.pone.0048259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qazi U, Lam C, Karumanchi SA, Petri M. Soluble Fms-like tyrosine kinase associated with preeclampsia in pregnancy in systemic lupus erythematosus. J Rheumatol. 2008 Apr;35(4):631–4. [PubMed] [Google Scholar]
- 31.Rolfo A, Attini R, Nuzzo AM, et al. Chronic kidney disease may be differentially diagnosed from preeclampsia by serum biomarkers. Kidney Int. 2013;83(1):177–81. doi: 10.1038/ki.2012.348. [DOI] [PubMed] [Google Scholar]
- 32.Menzies J, Magee LA, Macnab YC, Ansermino JM, Li J, Douglas MJ, et al. Current CHS and NHBPEP criteria for severe preeclampsia do not uniformly predict adverse maternal or perinatal outcomes. Hypertens Pregnancy. 2007;26(4):447–62. doi: 10.1080/10641950701521742. [DOI] [PubMed] [Google Scholar]
- 33.Sheehan HL LJ. Pathology of toxaemia of pregnancy. Williams and Wilkins Co.; Baltimore: 1973. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.