Abstract
Background
Muscle atrophy in end-stage renal disease (ESRD) may be due to the activation of apoptotic and proteolytic pathways.
Objective
We hypothesized that activation of caspase-3 in the skeletal muscle mediates apoptosis and proteolysis during hemodialysis (HD).
Materials and Methods
Eight ESRD patients were studied before (pre-HD) and during HD and the finding were compared with those from six healthy volunteers. Protein kinetics was determined by primed constant infusion of L-(ring 13C6) Phenylalanine.
Results
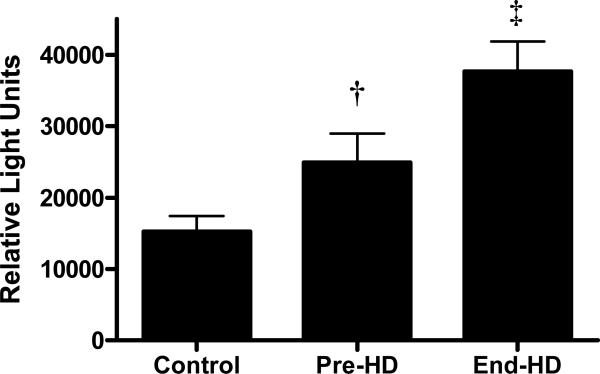
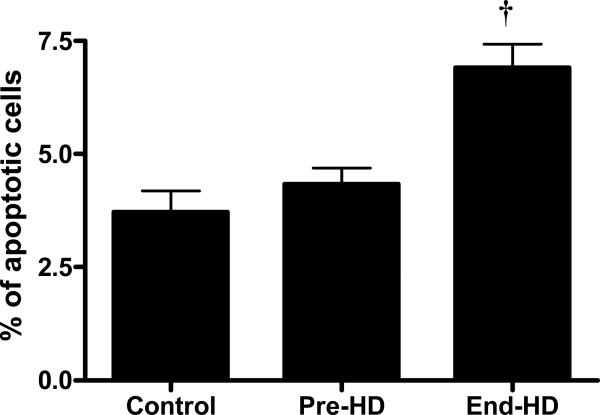
Caspase-3 activity in the skeletal muscle was higher in ESRD patients pre-HD than in controls (24966.0±4023.9 vs. 15293.3±2120.0 units, p<0.01) and increased further during HD (end-HD) (37666.6±4208.3 units) (p<0.001). 14 kDa actin fragments generated by caspase-3 mediated cleavage of actinomyosin was higher in the skeletal muscle pre-HD (68%) and during HD (164%) compared to controls. The abundance of ubiquitinized carboxy-terminal actin fragment was also significantly increased during HD. Skeletal muscle biopsies obtained at the end of HD exhibited augmented apoptosis, which was higher than that observed in pre-HD and control samples (p<0.001). IL-6 content in the soluble fraction of the muscle skeletal muscle was increased significantly during HD. Protein kinetic studies showed that catabolism was higher in ESRD patients during HD compared to pre-HD and control subjects. Muscle protein catabolism was positively associated with caspase-3 activity and skeletal muscle IL-6 content.
Conclusion
Muscle atrophy in ESRD may be due to IL-6 induced activation of caspase-3 resulting in apoptosis as well as muscle proteolysis during HD.
Keywords: End-stage renal disease, Apoptosis, Proteolysis, Ubiquitin, Inflammation, Interleukin-6
INTRODUCTION
Altered skeletal muscle metabolism and muscle wasting are common in patients with end-stage renal disease (ESRD). [1-3] Muscle atrophy in ESRD may be due to the activation of apoptotic and proteolytic pathways. Apoptosis is a tightly regulated process in which cell death follows a programmed sequence of events. In mononucleated cells apoptosis results in cell death, but in multinucleated cells such as myocytes, it causes cell atrophy.[4] Caspases are the principal enzymes involved in the initiation and execution of apoptosis, as well as myofibrillar proteolysis in skeletal muscle. Mitch's laboratory identified the ATP-dependent, ubiquitin-proteasome (Ub-P) system as the primary proteolytic pathway of muscle in uremia.[5;6] Activated caspase-3 cleaves actomyosin to generate substrate for the Ub-P system to degrade.[7;8] Although investigators have demonstrated that hemodialysis (HD) induces muscle proteolysis,[9] the role of apoptosis in uremic muscle wasting has not been well studied in human subjects.
We hypothesized that cytokine activation during HD induces skeletal muscle apoptosis and protein breakdown through activation of the caspase cascade and the Ub-P pathway. Our results show that caspase-3 activity and skeletal muscle apoptosis are elevated in ESRD patients at baseline (Pre-HD) compared to control subjects and increases further at end of HD (End-HD). Muscle protein catabolism was positively associated with interleukin-6 (IL-6) and caspase-3 activity.
MATERIALS AND METHODS
The study was approved by the Human Research Review committee at the University of New Mexico Health Sciences Center and it followed the ethical standards of the institution. The study population included eight ESRD patients and six control subjects. ESRD patients were studied pre-HD and during HD. Participants with ESRD were placed on a 35 kcal. kg-1 day-1 and 1.2 g.kg-1day-1 protein diet. Control subjects were recommended a minimum protein intake of 1.2 g. kg-1 day-1. The participants consumed the recommended diet at home for at least for 14 days before the study. Dietary intake was confirmed by a three day dietary history. In ESRD patients, plasma bicarbonate was maintained at ≥22 meq/L by oral sodium bicarbonate supplementation. Dual Energy X-ray Absorptiometry (Lunar DPX™ dual energy X-ray absorptiometer, Lunar Radiation Corp., Madison, WI) was used to measure lean body mass. The study was performed at mid-week on a non-dialysis day.
Subjects were admitted to the General Clinical Research Center one day before the experiment. All the studies were performed in a post-absorptive state after overnight fast. Leg volume was estimated using anthropometic formula as described previously.[10] After obtaining blood samples for background amino acid enrichment, a primed (2 μmol.kg-1) continuous (0.1 μmol.kg-1. min-1) infusion of L-(ring 13C6) Phenylalanine was initiated through the forearm vein. Tracer infusion was continued throughout the experiment. Blood samples for enrichment were collected as previously described.[10] All patients had 4 hours of hemodialysis treatment. Patient's usual blood and dialysate flow rates were used. A new polysulfone membrane (F70, Fresenius, Hemoflow) was used. Arterio-venous (A-V) balance studies were performed pre-HD and during the last 10 minutes of HD (End-HD). Blood flow to the lower extremity was measured by dye dilution technique.[11] Muscle biopsies (~40-60 mg) were taken from lateral portion of vastus lateralis ~ 20 cm above the knee using a Bergstrom biopsy needle.
Blood samples were obtained pre- and end-HD for biochemical analysis. Albumin was measured by bromcresol green method, glucagon by radioimmunoassay, insulin, cortisol and high sensitive C-reactive protein (hsCRP) by Immulite assay.
Analytical methods
Free amino acid concentrations were determined by HPLC (Waters 2960 system, Milford, MA). The enrichment of free amino acids in the plasma sample was determined by gas-chromatography mass-spectrometry (GC HP 5890, MSD HP 5989, Hewlett Packard, Palo Alto, CA) by selected ion (mass to charge ratio, m/z) monitoring. Chemical ionization was used for nitrogen-acetyl-n-propyl esters derivatives of phenylalanine (m/z 336, 342 and 346). Data are expressed as tracer/tracee ratio.
Calculations
Net phenylalanine balance (NB) in the leg was calculated using the Fick's principle as:
Where Ca and Cv are concentration of amino acid in the artery and vein and PF is plasma flow rate.
The rate of disposal of arterial phenylalanine (Rd) is an index of absolute amount of protein synthesis. Rd was calculated as
Where Ea is enrichment in the artery and Ev is enrichment in the vein
The rate of appearance of phenylalanine (Ra) is an estimate of protein degradation. Ra was calculated as
Caspase-3 activity
Caspase-3 enzyme activity was determined using the Caspase Glo assay (Promega, Madison, WI) according to the manufacturer's protocol. Using western blotting we determined that the caspase-3 enzyme was present in the soluble fraction of the skeletal muscle homogenates. Equal protein amounts (1.5μg) were loaded into a 96-well opaque luminometer plate. Each sample (and appropriate negative and positive controls) had 100ul of deionized water and 100μl of Caspase-glo reagent added. The plates were sealed, mixed on rotating platform for 30 minutes. The enzyme activity was read on a plate luminometer (Turner Biosystems, Sunnyvale, CA).
Western Blotting for carboxy-terminal of 14 KDa Actin fragments
Skeletal muscle samples were dissected free of connective tissue and the 14 KDa actin fragment content determined as described by Du et al. [7] Equal loading was confirmed by stripping the membranes with mercaptoethanol/SDS solution at 60 degrees centigrade for 45 minutes, and reprobing with anti GAPDH antibody (Santa Cruz Biotiech) and developed in a similar manner. Densitometry of the proteins of interest was measured using adobe photoshop 7.0 (Adobe Systems, San Jose CA)
Detection of ubiquitinized actin (Ub-Actin)
Co-Immunoprecipitation of the insoluble fractions was performed using anti C-terminal Actin antibody bound to protein G-sepharose (Amersham Biosciences, Uppsla, Sweden). In short, insoluble fractions were pre-cleared with sepharose beads and then combined with anti actin-bound sepharose beads and tumbled end over end overnight at 4 degrees centigrade. Immunoprecipitated actin was released by adding 2X Laemmli buffer and boiling for 7 minutes. The samples were then loaded and run on a polyacrylamide gel. The proteins were transferred to a 0.2 μm PVDF membrane and incubated with anti ubiquitin (Santa Cruz Biotechnologies) antibodies and developed as described above.
TUNEL Assay
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) was carried out to detect apoptotic DNA damage using the Apo-Brdu-IHC In Situ DNA fragmentation assay kit (BioVision,Mountain View, CA). Positive staining was indicated as brown and background staining as blue-green. For semiquantitative measurement of apoptosis, the numbers of TUNEL-positive and - negative cells were counted. A pathologist who was blinded to the treatment group systematically and randomly counted the cells and the values are expressed as percentage of apoptotic cells. TUNEL assay detects the presence of 3'hydroxyl-free DNA ends associated with the DNA fragmentation that characterizes the late phase of apoptotic pathway.[12]
IL-6 content in the soluble fraction of the muscle skeletal muscle
IL-6 levels in skeletal muscle extracts were quantified using the R&D systems Quantikine high sensitivity (Minneapolis, MN) ELISA kit.[13] Equal amounts of cytoplasmic fractions were loaded and incubated with the anti IL-6 sandwich antibody according to the manufacturer's specifications. Colorometric analysis of signal intensity was performed at 490 nm and compared to standard concentrations.
Statistical analysis
Data is given as mean and standard error of mean. α was set at 0.05. Student's paired and unpaired “t” tests and Mann-Whitney Rank Sum test were used as appropriate. A repeated measure of analysis of variance with a post-hoc Tukey test was used when comparing more than two variables. The relationship between muscle protein breakdown to precursor variables was examined in each group (control, pre-HD and end-HD) by univariate and multivariate analysis. The multivariate analysis included stepwise and “best subsets” regression.
RESULTS
Patient characteristics are described in Table 1. Etiology of renal failure was glomerulonephritis (N=2), hypertension (N=2), tubulointerstitial nephropathy (N=1) and unknown (N=3). Mean blood and dialysate flow rates during HD were 371.8±19.0 ml.min-1 and 800 ml.min-1 respectively.
Table 1.
Patient characteristics and biochemistry and laboratory variable
| Control | Pre-HD | End-HD | |
|---|---|---|---|
| Age | 44.6 ± 4.7 | 43±5.9 | |
| Weight (kg) | 78.3 ± 16.4 | 75.2±3.5 | |
| Lean body mass (kg) | 42.4±2.4 | 40.0±2.8 | |
| Protein g• kg-1•day-1 | 1.37±0.05 | 1.45 ± 0.09 | |
| Calories kcal•kg-1•day-1 | 35.2±1.21 | 33.2±3.7 | |
| Hemoglobin (g/dL) | 14.6±0.5a | 12.1±0.3 | 12.3±0.5 |
| BUN (mg/dL) | 14.4±1.1 | 67.2±6.0b | 18.1±3.7 |
| Creatinine (mg/dL) | 1.1±0.2 | 9.6±1.4b | 4.8±0.7 |
| Glucose (mg/dL) | 84.6±9.6 | 89.8±10.1 | 105.2±9.7 |
| Bicarbonate (meq/L) | 23.2±0.5 | 23.6±0.8 | 27.3±2.4c |
| Albumin (g/dL) | 4.2±0.2a | 3.3±0.2 | 3.4±0.2 |
| Insulin (μU/dL) | 11.6±4.1 | 22.9±4.4d | 17.5±4.2 |
| Glucagon (pg/dL) | 66.2±8.2 | 147.4±14.8e | 90.2±8.5 |
| IGF-1 (μg/L) | 198.6±9.4 | 192.9±24.8 | |
| Cortisol (μg/dL) | 14.4±3.1 | 10.3±1.2 | 17.7±2.4c |
| hsCRP (mg/dL) | 1.01±0.19A | 3.72±0.89 | 3.81±0.76 |
p<0.01 Control vs. Pre-HD and End-HD
p<0.001 Pre-HD vs. control and End-HD
p<0.01 End-HD vs. control
p<0.05 Pre-HD vs. Control
p<0.01 Pre-HD vs. Controls
Caspase-3 activity was elevated in ESRD patients at pre-HD compared to controls, and increased further with dialysis (Figure 1). Apoptosis was quantitated by TUNEL assay. The percentage of apoptotic cells in muscle biopsies obtained at end-HD (6.9%) was significantly higher than that observed pre-HD (4.3%) and in control subjects (3.7%) (p<0.001) (Figure 2).
Figure 1.
Caspase-3 activity in muscle was increased in patients with ESRD at baseline (pre-HD) compared to controls and was further augmented by hemodialysis (End-HD)
† p<0.01Pre-HD vs. Control; ‡ p<0.001 End-HD vs. Pre-HD and control.
Figure 2.
TUNEL assay shows that the percentage of apoptotic cells in the skeletal muscle is higher at end-HD in ESRD patients
†p<0.001 End-HD vs. control and Pre-HD.
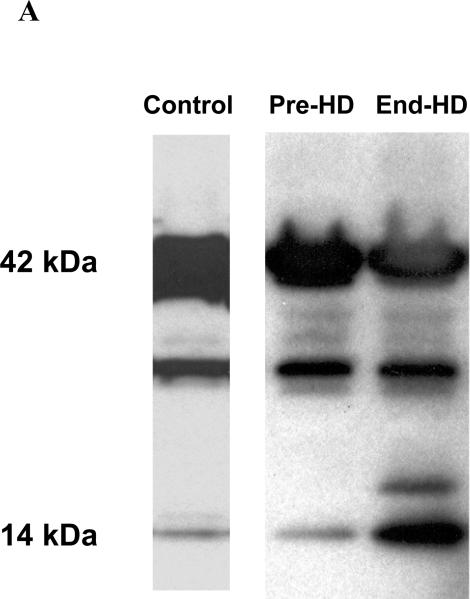
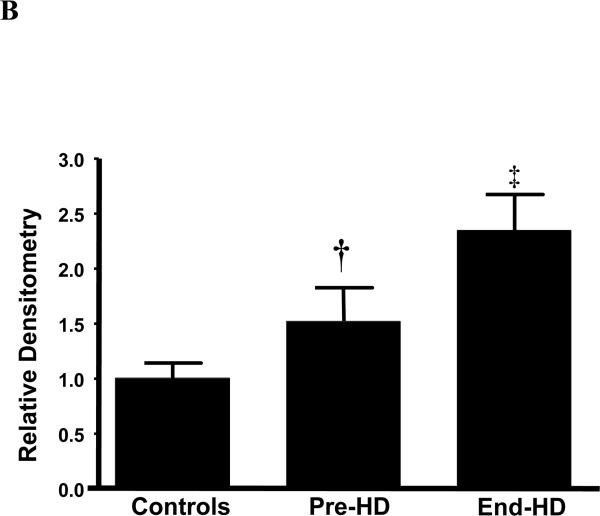
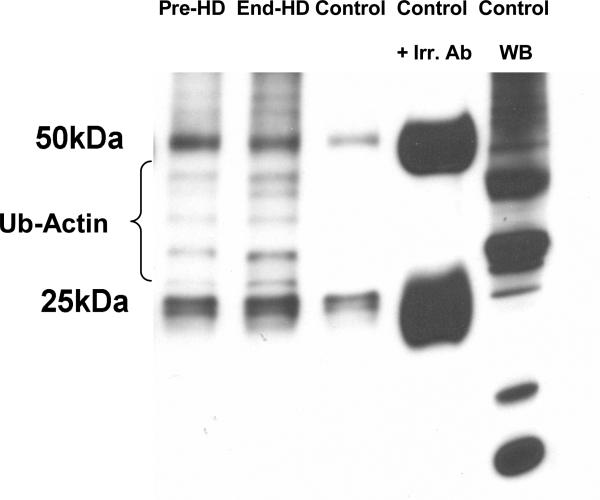
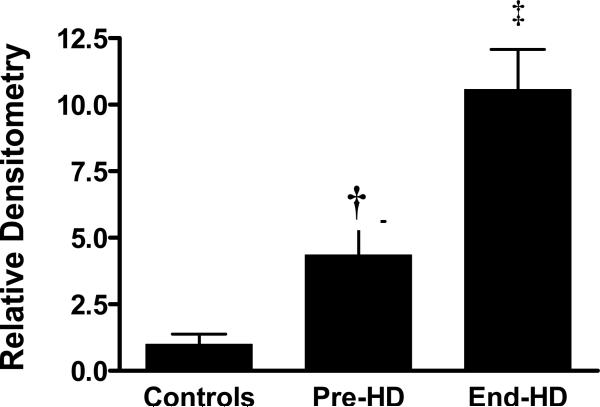
ESRD subjects had evidence of caspase induced actomyosin cleavage as measured by abundance of 14 kDa carboxy-terminal actin fragments in skeletal muscle. The abundance of 14 kDa actin fragments was significantly higher in ESRD patients pre-HD (68%) and end-HD (164%) compared to controls. (Figures 3-A & B). Targeting of actin fragment for degradation by the Ub-P system was assessed by co-immunoprecipitation of carboxy-terminal actin fragment with Ubiquitin, demonstrating the presence of ubiquitin-bound actin. The level of Ubiquitinized carboxy-terminal actin (Ub-Actin) fragments was increased in ESRD subjects pre-HD (5-fold) and at end-HD (12-fold) compared to controls (Figure 4-A & B).
Figure 3 A.
Representative Western blots of a Control, and an ESRD subject (Pre-HD and End-HD) of carboxy-terminal actin. The 2 blots demonstrate an increase in the 14kDa fragment in the ESRD patient Pre-HD and a greater increase at End-HD. All blots were loaded with the same control patient and reprobed for GAPDH to ensure equal loading across blots.
Figure 3 B.
Abundance of 14 kDa actin fragment was higher in ESRD patients at baseline (Pre-HD) compared to controls and it increased further during hemodialysis (End-HD).
† p<0.05 Pre-HD vs. Control; ‡ p<0.01 End-HD vs. Control and Pre-HD.
Figure 4 A.
Co-immunoprecipitation of C-terminal actin with ubiquitin. Density of ubiquitin bands measured between 25-50 kDa representing carboxy-terminal fragments (14 kDa) with bound Ub (multiples of 8kDa, as fragments can be polyubiqiutinated). Fragments above 50 kDa were not measured as may represent ubiquitinated whole actin. Samples represent Pre-HD, End-HD, Control, Control + irrelevant ab (to demonstrate antibody bands at 25 and 50 kDa, and specificity of bands) and western blot of non-immunoprecipitated control representing all Ub.
Figure 4 B.
Densitometry for all samples measuring intensity of ubiquinized actin fragments between 25-50 kDa. ESRD subjects had significantly greater ubiquitinzed actin fragments than control, which increased even further at End-HD
† p<0.01 pre-HD vs. control; ‡ p<0.01 compared to control and Pre-HD
The precursor, phenylalanine reached an isotopic plateau at 240 min and remained stable throughout the experiment. Both muscle protein synthesis and catabolism increased significantly during HD (Table 2). However, increase in muscle breakdown (107%) was significantly higher than synthesis (38%) during HD, resulting in net negative protein balance.
Table 2.
Muscle protein kinetics studies during hemodialysis
| Control | Pre-HD | End-HD | |
|---|---|---|---|
| Phenylalanine concentration in artery (Ca; μmol/L) | 89.8±4.5 | 84.5±7.8a | 65.9±5.8 |
| Phenylalanine concentration in vein (Cv; μmol/L) | 92.0±4.6 | 85.2±7.9a | 74.6±5.4b |
| Leg muscle protein synthesis (Rd; nmol•100 ml-1•min-1) | 42.62±5.78 | 41.19±3.03 | 55.15±4.48c |
| Leg muscle proteolysis (Ra; nmol•100 ml-1•min-1) | 50.47±7.69 | 41.63±2.47 | 84.61±3.65de |
| Net balance (nmol•100 ml-1•min-1) | -7.85±5.47 | -2.28±1.93 | -29.47±6.03e |
p<0.05 Pre-HD vs. End-HD
Ca vs. Cv p<0.01
p<0.02 End-HD vs. Pre-HD
Ra vs. Rd p<0.05
p<0.001 End-HD vs. Control and Pre-HD
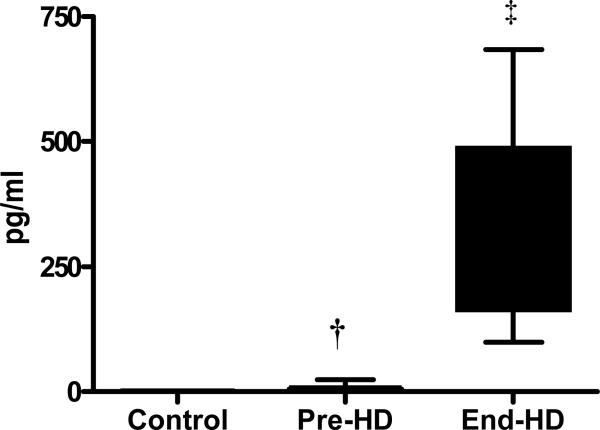
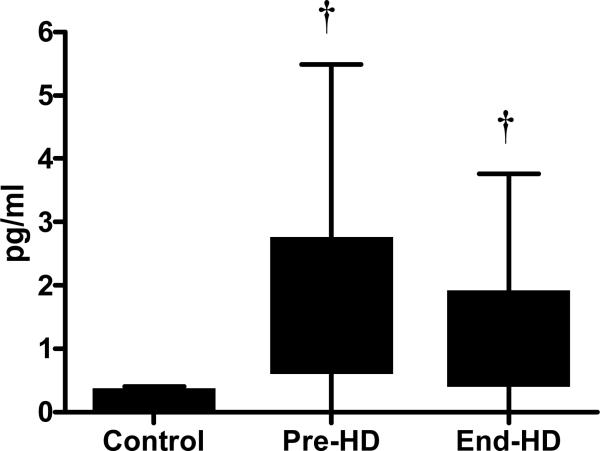
The content of IL-6 measured in the soluble fraction of the muscle was higher in ESRD patients (pre-HD) compared to controls (p<0.05), which increased significantly more at end-HD. (p<0.001).(Figure 5-A) TNF-α level in the soluble fraction of skeletal muscle from ESRD patients was significantly higher than that in control subjects. (Figure 5-B) Plasma cortisol levels increased significantly during HD. Plasma insulin and glucagon levels were higher pre-HD compared to controls, and showed a tendency to decrease at end-HD.
Figure 5 A.
IL-6 in the soluble fraction of the skeletal muscle was elevated in ESRD patients pre-HD (7.74±8.1 pg/ml) compared to controls (0.62±0.49 pg/ml) and increased further at end-HD (347.0±209.2 pg/ml).
† p<0.05 Pre-HD vs. Control; ‡ p<0.001 End-HD vs. Control and Pre-HD
Figure 5 B.
TNF-α content in the soluble fraction of the skeletal muscle was higher in ESRD patients compared to controls
† p<0.05 vs. Control
Caspase-3 activity, 14 kDa actin fragment, Ub-Actin, measures of apoptosis and IL-6 content in the muscle were inter-correlated. Univariate and multivariate analysis showed that caspase-3 is the best predictor of muscle proteolysis in controls. In ESRD patients both caspase-3 and IL-6 expression in skeletal muscle emerged as predictors of muscle protein catabolism, with caspase-3 activity being the stronger of the two factors. Concentration of amino acid in the artery was not significantly associated with apoptosis or proteolysis.
DISCUSSION
Muscle wasting is common in patients with ESRD and is an important cause of mortality and morbidity in this highly vulnerable population.[14] Amplified muscle proteolysis as a cause of decreased lean body mass in ESRD has been the focus of intense investigation so far. Muscle atrophy, however results not only from augmented proteolysis but also from accelerated apoptosis.[15] We observed that increased caspase-3 activity during HD was associated with augmented muscle protein catabolism and enhanced apoptosis of skeletal muscle cells. To our knowledge this is the first study showing increased apoptosis of skeletal muscle in ESRD patients.
Apoptosis is a physiological, conserved program of cellular death, characterized by nuclear condensation, DNA-fragmentation and release of mitochondrial cytochrome c into the cytoplasm. Induction of cells to commit suicide is required for proper organismal development, to remove cells with damaged DNA and also for self preservation by the process of eliminating cells that pose a threat to the organism. Cells undergoing apoptosis are eventually removed by phagocytosis. The role of skeletal muscle apoptosis in the pathogenesis of in muscle wasting with ageing and in chronic diseases is increasingly being recognized.[16;17] Muscle atrophy has been shown to correlate with the magnitude of apoptosis in patients with cardiac cachexia and chronic obstructive pulmonary disease. [18;19] We reported previously that apoptosis of peripheral blood mononuclear cells is increased during HD.[20] Results from this study show that HD induces apoptosis in the skeletal muscle as well and could contribute to muscle wasting in ESRD patients.
Caspases are a family of cysteine proteases that cleave proteins after aspartic acid residues and are the main effectors of apoptosis. Initiator caspases (caspase-2, 8, 9 and 10) cleave and activate the effector caspases (caspase-3, 6 and 7), which cleave, degrade or activate other cellular proteins. In vivo studies suggest that caspase-3 activity is increased in the presence of uremic serum.[21] Caspase-3 expression is often used as a surrogate for apoptosis, but caspases also have non-apoptotic roles such as amplifying the inflammatory response, immune cell proliferation, cell differentiation and protein degradation. [7;22] Caspase-3 mediated cleavage of actomyosin generates 14 kDa actin fragments, substrate for Ub-P system.[7] Workeneh et al[23] showed that 14kDa carboxy-terminal actin fragments accumulates in diverse catabolic states in skeletal muscle. We found that the abundance of 14 kDa actin fragments was higher pre-HD in ESRD patients and increased significantly more at end-HD. Furthermore, we demonstrated that these carboxy-actin fragments are ubiquitinized, thus targeted for proteosomal degradation in the skeletal muscle of ESRD patients.
Consistent with previous studies, we observed that muscle protein breakdown is increased during HD. We noted a positive and significant inter-correlation between muscle protein breakdown and caspase-3 activity, 14 kDa actin fragment and Ub-Actin fragment. We also noted a positive association between muscle IL-6 content and these markers of apoptosis and proteolysis. However, there was no association between concentration of amino acids in the artery and apoptosis or muscle proteolysis.
IL-6 content in skeletal muscle was elevated in ESRD subjects pre-HD (~10 fold) compared to control and to a much greater extent at end-HD (~300 fold). Increase in TNF-α content was not as impressive as that of IL-6. A difference in metabolic turnover, clearance, rate of shedding and sensitivity of assays may partially explain the disparity between circulating levels of the cytokines. While IL-6 has metabolic functions besides the immune regulation, the biological system tends to regulate excessive increase in TNF in order to attenuate excessive inflammation through generation of neutralizing endogenous inhibitors.[24;25] Furthermore, soluble TNF-α receptors have been shown to interfere with TNF-α when measuring TNF-α with certain ELISA kits, but is less frequent with the ELISA kits used in this study.[26] Intra-dialytic activation of apoptotic-proteolytic pathways may be related to amino acid depletion and activation of pro-inflammatory cytokines during HD. [27] We previously demonstrated that HD activates expression of genes involved in inflammatory and apoptotic cascade in the skeletal muscle [28] and that IL-6 is released from skeletal muscle during HD and may mediate muscle protein breakdown and hepatic acute phase protein synthesis.[27;29] IL-6 is known to mediate protein breakdown through activation of the Ub-P pathway.[30] IL-6 has also been shown to be associated with activation of caspase-3 and apoptosis in rat skeletal muscle. [31] In the present study we show that IL-6 content in the soluble fraction of skeletal muscle and caspase-3 activity are key determinants of muscle protein breakdown in ESRD. TNF-α has been shown to induce DNA fragmentation in skeletal muscle.[32] Preliminary evidence suggests that TNF-α activity alone may not insufficient to cause muscle protein degradation.[33] It is speculated that TNF-α functions in concert with other pro-inflammatory cytokines such as IL-6 to induce apoptosis muscle wasting [34-36].
Plasma cortisol level increased during HD, with a concomitant decrease in insulin level. Impaired glucose induced insulin secretion is described in chronic kidney disease.[37] Investigators have observed that plasma cortisol level, insulin resistance and inflammation are inter-linked [38;39] Siew et al [40] showed that the Homeostasis Model Assessment (HOMA) index is positively associated with skeletal muscle breakdown. Capsapse-3 activity is modulated by insulin signaling [41] and inflammation.[22] Furthermore, IL-6 and TNF-α may also impair insulin/ IGF-1 signaling.[42;43] Thus, protein breakdown during HD could be due to the concerted effect of pro-inflammatory cytokines, elevated stress hormones and impaired anabolic response to insulin and IGF-1.[44]
Thus, patients with ESRD show molecular evidence of activation of the apoptotic and proteolytic pathways pre-HD. Skeletal muscle apoptosis was increased in ESRD patients pre-HD, but without evidence of overt muscle proteolysis. On the other hand, HD induces profound increase in apoptosis as well as net protein loss. It is likely that the micro-inflammatory state in uremia sustains these pathways in a primed state. The robust increase in IL-6 expression in skeletal muscle and amino acid depletion during HD result in overt activation of proteolytic and apoptotic pathways resulting in net protein loss and muscle atrophy.
Acknowledgements
We express our sincere gratitude to the patients, who participated in the study.
We are grateful to Prof. Clifford Qualls for the statistical analysis of the data.
We specially acknowledge Dr. Bill Mitch in assisting us with establishing the 14 kD actin assay.
Grants and support
This study was supported in part by the Norman Coplan Satellite Research Grant, National Institute of Health Grants AG21560 and R01DK073665-01A1 and Young Investigator Award from National Kidney Foundation.
Footnotes
Disclosure: None
REFERENCES
- 1.Floyd M, Ayyar DR, Barwick DD, Hudgson P, Weightman D. Myopathy in chronic renal failure. Q J Med. 1974;43:509–524. [PubMed] [Google Scholar]
- 2.Guarnieri G, Toigo G, Situlin R, Faccini L, Coli U, Landini S, et al. Muscle biopsy studies in chronically uremic patients: evidence for malnutrition. Kidney Int Suppl. 1983;16:S187–S193. [PubMed] [Google Scholar]
- 3.Carrero JJ, Chmielewski M, Axelsson J, Snaedal S, Heimburger O, Barany P, et al. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr. 2008;27:557–564. doi: 10.1016/j.clnu.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, et al. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol. 1997;273:C579–C587. doi: 10.1152/ajpcell.1997.273.2.C579. [DOI] [PubMed] [Google Scholar]
- 5.Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17:1807–1819. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- 6.Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol. 2004;15:1537–1545. doi: 10.1097/01.asn.0000127211.86206.e1. [DOI] [PubMed] [Google Scholar]
- 7.Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113:115–123. doi: 10.1172/JCI200418330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Workeneh BT, Rondon-Berrios H, Zhang L, Hu Z, Ayehu G, Ferrando A, et al. Development of a Diagnostic Method for Detecting Increased Muscle Protein Degradation in Patients with Catabolic Conditions. J Am Soc Nephrol. 2006;17:3233–3339. doi: 10.1681/ASN.2006020131. [DOI] [PubMed] [Google Scholar]
- 9.Ikizler TA, Pupim LB, Brouillette JR, Levenhagen DK, Farmer K, Hakim RM, et al. Hemodialysis stimulates muscle and whole body protein loss and alters substrate oxidation. Am J Physiol. 2002;282:E107–E116. doi: 10.1152/ajpendo.2002.282.1.E107. [DOI] [PubMed] [Google Scholar]
- 10.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol. 1995;268:E75–E84. doi: 10.1152/ajpendo.1995.268.1.E75. [DOI] [PubMed] [Google Scholar]
- 11.Jorfeld L, Waren J. Leg blood flow during excercise in man. Clin Sci Lond. 1971;41:459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- 12.Vescovo G, Volterrani M, Zennaro R, Sandri M, Ceconi C, Lorusso R, et al. Apoptosis in the skeletal muscle of patients with heart failure: investigation of clinical and biochemical changes. Heart. 2000;84:431–437. doi: 10.1136/heart.84.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hacham M, White RM, Argov S, Segal S, Apte RN. Interleukin-6 and interleukin-10 are expressed in organs of normal young and old mice. Eur Cytokine Netw. 2004;15:37–46. [PubMed] [Google Scholar]
- 14.Carrero JJ, Chmielewski M, Axelsson J, Snaedal S, Heimburger O, Barany P, et al. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr. 2008;27:557–564. doi: 10.1016/j.clnu.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Vazeille E, Codran-Raison A, Claustre A, Averous J, Listrat A, Bechet D, et al. The ubiquitin-proteasome and the mitochondria-associated apoptotic pathways are sequentially down-regulated during recovery after immobilization-induced atrophy. Am J Physiol. 2008;295:E1181–E1190. doi: 10.1152/ajpendo.90532.2008. [DOI] [PubMed] [Google Scholar]
- 16.Busquets S, Deans C, Figueras M, Moore-Carrasco R, Lopez-Soriano FJ, Fearon KC, et al. Apoptosis is present in skeletal muscle of cachectic gastro-intestinal cancer patients. Clin Nutr. 2007;26:614–618. doi: 10.1016/j.clnu.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Dirks AJ, Leeuwenburgh C. The role of apoptosis in age-related skeletal muscle atrophy. Sports Med. 2005;35:473–483. doi: 10.2165/00007256-200535060-00002. [DOI] [PubMed] [Google Scholar]
- 18.Vescovo G, Volterrani M, Zennaro R, Sandri M, Ceconi C, Lorusso R, et al. Apoptosis in the skeletal muscle of patients with heart failure: investigation of clinical and biochemical changes. Heart. 2000;84:431–437. doi: 10.1136/heart.84.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agusti AG, Sauleda J, Miralles C, Gomez C, Togores B, Sala E, et al. Skeletal muscle apoptosis and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:485–489. doi: 10.1164/rccm.2108013. [DOI] [PubMed] [Google Scholar]
- 20.Raj DS, Boivin MA, Dominic EA, Boyd A, Roy PK, Rihani T, et al. Haemodialysis induces mitochondrial dysfunction and apoptosis. Eur J Clin Invest. 2007;37:971–977. doi: 10.1111/j.1365-2362.2007.01886.x. [DOI] [PubMed] [Google Scholar]
- 21.Harwood SM, Allen DA, Chesser AM, New DI, Raftery MJ, Yaqoob MM. Calpain is activated in experimental uremia: is calpain a mediator of uremia-induced myocardial injury? Kidney Int. 2003;63:866–877. doi: 10.1046/j.1523-1755.2003.00823.x. [DOI] [PubMed] [Google Scholar]
- 22.Launay S, Hermine O, Fontenay M, Kroemer G, Solary E, Garrido C. Vital functions for lethal caspases. Oncogene. 2005;24:5137–5148. doi: 10.1038/sj.onc.1208524. [DOI] [PubMed] [Google Scholar]
- 23.Workeneh BT, Rondon-Berrios H, Zhang L, Hu Z, Ayehu G, Ferrando A, et al. Development of a diagnostic method for detecting increased muscle protein degradation in patients with catabolic conditions. J Am Soc Nephrol. 2006;17:3233–3239. doi: 10.1681/ASN.2006020131. [DOI] [PubMed] [Google Scholar]
- 24.Febbraio MA. Signaling pathways for IL-6 within skeletal muscle. Exerc Immunol Rev. 2003;9:34–39. [PubMed] [Google Scholar]
- 25.Brockhaus M. Soluble TNF receptor: what is the significance? Intensive Care Med. 1997;23:808–809. doi: 10.1007/s001340050416. [DOI] [PubMed] [Google Scholar]
- 26.Kapadia S, Torre-Amione G, Mann DL. Pitfalls in measuring cytokines. Ann Intern Med. 1994;121:149–150. doi: 10.7326/0003-4819-121-2-199407150-00016. [DOI] [PubMed] [Google Scholar]
- 27.Raj DS, Moseley P, Dominic EA, Onime A, Tzamaloukas AH, Boyd A, et al. Interleukin-6 modulates hepatic and muscle protein synthesis during hemodialysis. Kidney Int. 2008;73:1061. doi: 10.1038/ki.2008.21. [DOI] [PubMed] [Google Scholar]
- 28.Raj DSC, Shah H, Shah V, Ferrando A, Bankhurst A, Wolfe R, et al. Markers of inflammation, proteolysis and apoptosis in ESRD. Am J Kidney Dis. 2003;42:1212–1220. doi: 10.1053/j.ajkd.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 29.Raj DS, Adeniyi O, Dominic EA, Boivin MA, McClelland S, Tzamaloukas AH, et al. Amino acid repletion does not decrease muscle protein catabolism during hemodialysis. Am J Physiol. 2007;292:E1534–E1542. doi: 10.1152/ajpendo.00599.2006. [DOI] [PubMed] [Google Scholar]
- 30.Fujita J, Tsujinaka T, Yano M, Ebisui C, Saito H, Katsume A, et al. Anti-interleukin-6 receptor antibody prevents muscle atrophy in colon-26 adenocarcinoma-bearing mice with modulation of lysosomal and ATP-ubiquitin-dependent proteolytic pathways. Int J Cancer. 1996;68:637–643. doi: 10.1002/(SICI)1097-0215(19961127)68:5<637::AID-IJC14>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 31.Kocturk S, Kayatekin BM, Resmi H, Acikgoz O, Kaynak C, Ozer E. The apoptotic response to strenuous exercise of the gastrocnemius and solues muscle fibers in rats. Eur J Appl Physiol. 2008;102:515–524. doi: 10.1007/s00421-007-0612-7. [DOI] [PubMed] [Google Scholar]
- 32.Carbo N, Busquets S, van Royen M, Alvarez B, Lopez-Soriano FJ, Argiles JM. TNF-alpha is involved in activating DNA fragmentation in skeletal muscle. Br J Cancer. 2002;86:1012–1016. doi: 10.1038/sj.bjc.6600167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moldawer LL, Svaninger G, Gelin J, Lundholm KG. Interleukin 1 and tumor necrosis factor do not regulate protein balance in skeletal muscle. Am J Physiol. 1987;253:C766–C773. doi: 10.1152/ajpcell.1987.253.6.C766. [DOI] [PubMed] [Google Scholar]
- 34.Kocturk S, Kayatekin BM, Resmi H, Acikgoz O, Kaynak C, Ozer E. The apoptotic response to strenuous exercise of the gastrocnemius and solues muscle fibers in rats. Eur J Appl Physiol. 2008;102:515–524. doi: 10.1007/s00421-007-0612-7. [DOI] [PubMed] [Google Scholar]
- 35.Spiegelman BM, Hotamisligil GS. Through thick and thin: wasting, obesity, and TNF alpha. Cell. 1993;73:625–627. doi: 10.1016/0092-8674(93)90243-j. [DOI] [PubMed] [Google Scholar]
- 36.Tisdale MJ. Biology of cachexia. J Natl Cancer Inst. 1997;89:1763–1773. doi: 10.1093/jnci/89.23.1763. [DOI] [PubMed] [Google Scholar]
- 37.Fadda GZ, Hajjar SM, Perna AF, Zhou XJ, Lipson LG, Massry SG. On the mechanism of impaired insulin secretion in chronic renal failure. J Clin Invest. 1991;87:255–261. doi: 10.1172/JCI114979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol. 2003;285:E433–E437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 39.Holmang A, Bjorntorp P. The effects of cortisol on insulin sensitivity in muscle. Acta Physiol Scand. 1992;144:425–431. doi: 10.1111/j.1748-1716.1992.tb09316.x. [DOI] [PubMed] [Google Scholar]
- 40.Siew ED, Pupim LB, Majchrzak KM, Shintani A, Flakoll PJ, Ikizler TA. Insulin resistance is associated with skeletal muscle protein breakdown in non-diabetic chronic hemodialysis patients. Kidney Int. 2007;71:146–152. doi: 10.1038/sj.ki.5001984. [DOI] [PubMed] [Google Scholar]
- 41.Gao Y, Ordas R, Klein JD, Price SR. Regulation of caspase-3 activity by insulin in skeletal muscle cells involves both PI3-kinase and MEK-1/2. J Appl Physiol. 2008;105:1772–1778. doi: 10.1152/japplphysiol.90636.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodman MN. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc Soc Exp Biol Med. 1994;205:182–185. doi: 10.3181/00379727-205-43695. [DOI] [PubMed] [Google Scholar]
- 44.Du J, Mitch WE, Wang X, Price SR. Glucocorticoids induce proteasome C3 subunit expression in L6 muscle cells by opposing the suppression of its transcription by NF-kappa B. J Biol Chem. 2000;275:19661–19666. doi: 10.1074/jbc.M907258199. [DOI] [PubMed] [Google Scholar]