Introduction
Asthma is a chronic disease with high morbidity that affects an estimated 7.1 million children in the United States(1). The disease is a notable healthcare burden that is associated with an estimated 5.6% of all annual pediatric hospitalizations and 2% of all pediatric physicians’ office visits(2). Not only are children bothered by the physical symptoms of asthma, including chest tightness, cough, wheeze, and shortness of breath, but their quality of life is also affected. One study found that 45% of adolescents with asthma reported feeling depressed while another noted a significant difference in frequency of participation in physical activities between healthy children and those with asthma(3,4). In 2008 alone, 10.5 million missed school days were attributed to asthma(1). Asthma also affects the quality of life of patients’ families and primary caregivers, resulting in days missed from work and interference with family activities. In addition, caregivers of children with asthma often worry about their child’s well-being(5). Despite increasing knowledge of disease pathophysiology and new intervention and management strategies, pediatric asthma continues to be a national problem(2).
Asthma is particularly concerning in the pediatric population since the disease prevalence is higher in children than adults(6). Investigating the effect of asthma on children is difficult and presents unique challenges. The “4 D’s of childhood” - “developmental change, dependence on adults for accessing care and implementing treatments, different disease epidemiology from adults, and demographic characteristics unique to childhood” are variables that increase the complexity of asthma outcomes research in the pediatric population(7). Given these challenges, researchers have sought to determine appropriate and valid methods of assessing a child’s burden of disease. Health related quality of life questionnaires are potential tools to monitor disease status and help guide clinical management.
Health related quality of life (HRQOL) is patient-specific and has been defined as “the functional effects of an illness and its consequent therapy upon a patient, as perceived by the patient”(8). Evaluation of HRQOL in asthma is a vital component of patient assessment as quality of life and measurements of clinical asthma control have been shown to be only moderately correlated at best(5,9-11). Accordingly, most experts agree that quality of life (QOL) instruments and clinical measures of asthma control measure two independent aspects of health status, such that each should be assessed individually(5,12-13).
Evaluation of health status in pediatric asthma is challenging. In the pediatric population, health care providers must rely on the caregiver, the child or both to gauge symptoms and disease effect on QOL. Although caregivers can provide a proxy-report, children as young as 7 have been shown to be dependable reporters of their health status with acceptable reliability and validity(14). However, previous studies have generally found only low to moderate correlation between caregiver and child responses to various HRQOL questionnaires(5,9,15-17). As there is currently no “gold standard” to evaluate QOL in children with asthma, it is important to understand the relationship between child and caregiver responses on HRQOL questionnaires.
We studied children with asthma and their primary caregivers to assess their overall QOL, as well as disease effect on activity limitation and emotional function. The purpose of this study was to analyze the relationship between caregiver and child responses and to assess their agreement in each of the domains (activity limitation, emotional function, and overall QOL).
Methods
Study Participants
We used enrollment and first follow-up visit data from a single-center prospective cohort study that assessed the frequency of Mycoplasma pneumoniae (Mp) in pediatric patients with asthma and healthy children(18). Data were collected between December 2010 and August 2012. We enrolled 79 children with asthma (5-17 years). All caregivers and children 7 years of age and older completed QOL assessments; a few children under 7 years of age (3 children age 5 years and 4 children age 6 years) completed the questionnaire.
We enrolled two groups of children with asthma: children with a known diagnosis of asthma who were hospitalized for an acute asthma exacerbation (53 children) and children with refractory or difficult to control asthma (moderate to severe persistent asthma) who were recruited during routine outpatient clinic visits (26 children). The “refractory asthma” group represented children with ongoing asthma symptoms after appropriate medical management, including mitigation of asthma triggers, exclusion of alternative diagnoses, confirmation of treatment compliance, and optimal treatment of other co-morbidities(19). The diagnosis of asthma was based on physician assessment according to national guidelines(20). Exclusion criteria included a diagnosis of pulmonary disease other than asthma (i.e. interstitial lung disease, pulmonary hypertension, bronchopulmonary dysplasia) or other significant medical conditions (e.g., cerebral palsy, acute hypertension, active heart failure, cardiac arrhythmias, pregnancy, cancer requiring treatment, HIV, etc).
The Institutional Review Board of the University of Texas Health Science Center at San Antonio approved this study. After the study was explained to them, all caregivers of participants signed an IRB-approved consent form in English or Spanish, depending on language preference.
Quality of Life Assessment Instruments
PAQLQ [S]
The Pediatric Asthma Quality of Life Questionnaire with Standardized Activities (PAQLQ [S]) is a 23-item, self-reported assessment of asthma effect on QOL and is designed for children ages 7 and above(11). In general, research assistants read the questions to children, while older children were allowed to complete the questionnaire alone. Children and caregivers were interviewed in separate rooms so that respondents would not influence one another. The PAQLQ [S] is divided into three domains that contribute to an overall QOL score: activity limitation (5 items), emotional function (8 items), and symptoms (10 items). Seven point Likert scales are used to assess limitations in normal daily activities, the degree to which the child felt bothered by his disease during the past week and the frequency of symptoms (e.g. 1= all of the time and 7=none of the time). The total score for items in a specific domain and for the entire questionnaire is divided by the number of items to create a domain score or total score, respectively; scores range from 1-7 with higher scores indicating better QOL. The minimal clinically important difference in scores (0.5) is defined as “the smallest difference in score in the domain of interest which patients perceive as beneficial and which would mandate, in the absence of troublesome side-effects and excessive cost, a change in the patient’s management”(21). We calculated scores for each domain as well as for overall quality of life. The PAQLQ[S] is a good evaluative and discriminative instrument with high reliability(10,11).
PACQLQ
The Pediatric Asthma Caregiver’s Quality of Life Questionnaire (PACQLQ) is a 13-item assessment of disease effect on the QOL of caregivers of children ages 7-17 with asthma(22). The PACQLQ is divided into two domains which contribute to an overall QOL score: activity limitation (4 items) and emotional function (9 items). Seven point Likert scales are used to assess the frequency of disruption of family or caregiver activities attributed to the child’s asthma during the past week (activity limitation) as well as the degree to which caregivers feel that their child’s asthma has worried or concerned them (emotional function) (e.g. 1= all of the time, 7= none of the time). Scores range from 1-7 with higher scores indicating better QOL. The minimal clinically important difference in scores is 0.5(21). The PACQLQ is a good evaluative and discriminative instrument(22,23).
Statistical Analysis
We used SPSS (version 20.0) software to analyze the data. We calculated the PAQLQ[S] and PACQLQ scores at enrollment for each domain (e.g. activity limitation and emotional function) as well as overall QOL for the acute asthma group, the refractory asthma group, and both groups combined (all subjects). We examined data for normality as well as for other specific test assumptions, such as homogeneity of variance. Chi-Squares or Fisher’s Exact Tests were used to analyze frequencies. Two-tailed paired t-tests were used to examine differences between child and caregiver responses as well as comparisons between enrollment and follow-up visit. We used Pearson’s correlation coefficients to examine the pattern of relationship between child and caregiver responses. We tested for differences in the degree of correlation between child and caregiver responses across QOL domains with Steiger’s Z-test(24-26).
Pearson’s correlation coefficients were calculated to assess the relationship of child age to differences between child/caregiver responses. Similarly, point-biserial correlations were calculated to analyze the relationship of dichotomous variables (i.e. gender and ethnicity) to these differences. We then used step-wise regression analysis to determine the effect of child age, gender, and ethnicity on differences in child/caregiver responses. We interpreted correlation coefficients of <.36 as “low”; 0.36-0.67 as “moderate”; and >0.67 as “strong”(27). A P value ≤ 0.05 was considered statistically significant.
Results
Baseline Demographics
We enrolled 79 children and their caregivers; 53 had acute asthma and 26 had refractory asthma. In the acute asthma group, 9 children did not complete questionnaires (due to age < 7 years) and 2 caregivers failed to complete questionnaires, resulting in 42 pairs. In the refractory asthma group, one child did not complete a questionnaire (age < 7 years), resulting in 25 pairs. Twenty-nine child/caregiver pairs (11 acute asthma; 18 refractory asthma) completed at least one follow-up visit 3-12 months following enrollment.
Fifty-eight (73%) of the children enrolled were males; 39 (49%) of the children were white; 59 (75%) identified themselves as Hispanic (predominantly Mexican American). Seventy-six (96%) caregivers were female and 78 (99%) were English speaking. All continuous variables, including age, BMI and QOL scores, were distributed normally. Baseline demographic information was analyzed and no significant difference in child gender, average age, race, or ethnicity was found between asthma groups. A significantly higher BMI percentile was noted in the refractory asthma group (79.14 ± 28.4) when compared to subjects in the acute asthma group (62.81 ±36.8) (P=0.047) (Table 1).
Table 1.
Study Population Baseline Characteristics
| Acute Asthma (n =53) | Refractory Asthma (n = 26) | P value1 | |
|---|---|---|---|
| Age (years) 2 | 9.5 ± 3.0 | 10.6 ± 2.6 | 0.134 |
| Male Gender, no (%) | 38 (71.7) | 20 (76.9) | 0.621 |
| Race | 0.329 | ||
| White | 29 (54.7) | 10 (38.5) | |
| Black | 10 (18.9) | 6 (23.1) | |
| American Indian | 1 (1.9) | 0 (0) | |
| Asian | 2 (3.8) | 0 (0) | |
| Other | 11 (20.8) | 10 (38.5) | |
| Hispanic Ethnicity, no (%) | 40 (75.5) | 19 (73.1) | 0.818 |
| BMI Percentile | 62.8 ± 36.8 | 79.1 ± 28.4 | 0.047 |
| Spanish Speaking Caregiver | 1 (1.9) | 0 (0) | 1.000 |
Abbreviations: BMI Percentile (body mass index) percentile (calculated for age and gender)
P value for difference between groups
Numbers represent mean ± standard deviation unless otherwise specified
Child/Caregiver Response Agreement
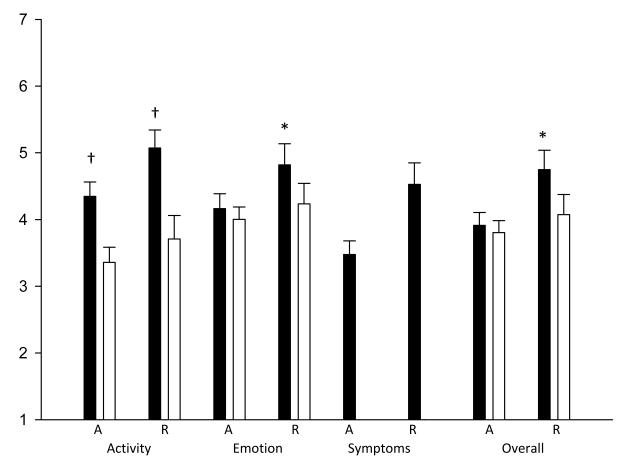
Children and their caregivers had similar scores for overall QOL in both the acute group and in the combined groups. Children reported a slightly higher mean score than their caregivers, but the difference was not significant (3.91 vs. 3.80, P=0.615 for the acute group and 4.23 vs. 3.91, P= 0.073 for combined groups). Results for the emotional function domain were similar (4.16 vs. 4.00, P=0.483 for the acute group and 4.41 vs. 4.09, P=0.075 for combined groups). In contrast, for the activity limitation domain, children and their caregivers differed significantly in their responses. Children reported a significantly higher mean score, indicating better QOL, for activity limitation compared to their caregivers. This was true in the acute and refractory groups as well as in the combined group (4.35 vs. 3.36, P=0.001, 5.07 vs. 3.71, P=0.002, and 4.62 vs. 3.49, P<0.001 respectively). In addition to this observed difference in child/caregiver scores for activity limitation, the refractory asthma group also demonstrated significant child/caregiver differences for overall QOL and emotional function (Table 2 and Figure 1).
Table 2.
PAQLQ and PACQLQ Scores at Enrollment
| Acute Asthma Group | ||||
| Child Mean Score | Caregiver Mean Score |
Two-Tailed Paired t-test1 |
Pearson’s Correlation2 |
|
| Activity Limitation (n= 42) | 4.35 | 3.36 | P = 0.001 | r = 0.20 (P=.203) |
| Emotional Function (n=42) | 4.16 | 4.00 | P = 0.483 | r = 0.40 (P=.009) |
| Symptoms (n = 44) | 3.48 | |||
| Overall Quality of Life (n= 42) | 3.91 | 3.80 | P =0.615 | r = 0.33 (P= .032) |
| Refractory Asthma Group | ||||
| Child Mean Score | Caregiver Mean Score |
Two-Tailed Paired t-test |
Pearson’s Correlation |
|
| Activity Limitation (n= 25) | 5.07 | 3.71 | P = 0.002 | r = 0.24 (P=0.246) |
| Emotional Function (n= 25) | 4.82 | 4.24 | P = 0.046 | r = 0.60 (P=0.001) |
| Symptoms (n= 25) | 4.53 | |||
| Overall Quality of Life (n= 25) | 4.75 | 4.07 | P = 0.030 | r = 0.51 (P=0.009) |
| Combined Groups | ||||
| Child Mean Score | Caregiver Mean Score |
Two-Tailed Paired t-test |
Pearson’s Correlation |
|
| Activity Limitation (n = 67) | 4.62 | 3.49 | P = 0.000 | r = 0.24 (P = 0.056) |
| Emotional Function (n = 67) | 4.41 | 4.09 | P = 0.075 | r = 0.50 (P = 0.000) |
| Symptoms (n= 69) | 3.86 | |||
| Overall Quality of Life (n= 67) | 4.23 | 3.91 | P = 0.073 | r = 0.43 (P = 0.000) |
Difference between child and caregiver score
Correlation between child and caregiver score
Figure 1.
QOL Child and Caregiver Responses by group
Mean child (dark bars) and caregiver (light bars) responses in acute (A) and refractory (R) asthma groups, by domain (activity, emotional, symptoms, overall). P value for child-caregiver difference: *P≤0.05; †P≤0.01.
Eleven child/caregiver pairs in the acute group and 18 child/caregiver pairs in the refractory group completed QOL questionnaires at both enrollment and follow-up visits. The difference between child and caregiver scores within pairs at the follow-up visit was not statistically different from enrollment. This was true in all three domains for both the acute asthma group (mean child/caregiver difference at enrollment vs. follow-up 1.65 vs. 1.79, P= 0.733 for activity limitation; 1.16 vs. 1.80, P=0.086 for emotional function; 0.93 vs. 1.30, P=0.460 for overall QOL) and for the refractory asthma group (mean child/caregiver difference at enrollment vs. follow-up 1.39 vs. 1.33, P=0.897 for activity limitation; 0.74 vs. 0.55, P=0.624 for emotional function; 0.81 vs. 0.72, P=0.812 for overall QOL).
The difference in child and caregiver scores was calculated for each pair of respondents in each domain. We calculated how often this difference in scores was “clinically important” (a difference of ≥0.5). In the combined asthma group, 44 (66%) of the child/caregiver pairs differed by ≥0.5; this was true in all domains. The frequency of clinically important differences in child/caregiver scores was highest in the activity limitation domain and lowest in overall quality of life (60 pairs (89.6%) vs. 45 pairs (67.2%) respectively, P=0.009). The acute and refractory asthma groups were similar in the frequency of minimal clinically important difference between child/caregiver scores.
Another way to analyze child/caregiver responses is to look at the pattern of correlation between their responses, regardless of the score. Child and caregiver responses demonstrated low to moderate positive and statistically significant correlation in overall QOL(r=0.33, P=0.032 and r=0.51, P=0.009 in acute and refractory subgroups, respectively). Similarly, child/caregiver responses were moderately correlated in the emotional function domain. This pattern was seen in both asthma groups, and the correlation was highest in the refractory asthma group (r=0.40, P=0.009 and r=0.60, P=0.001; acute and refractory).
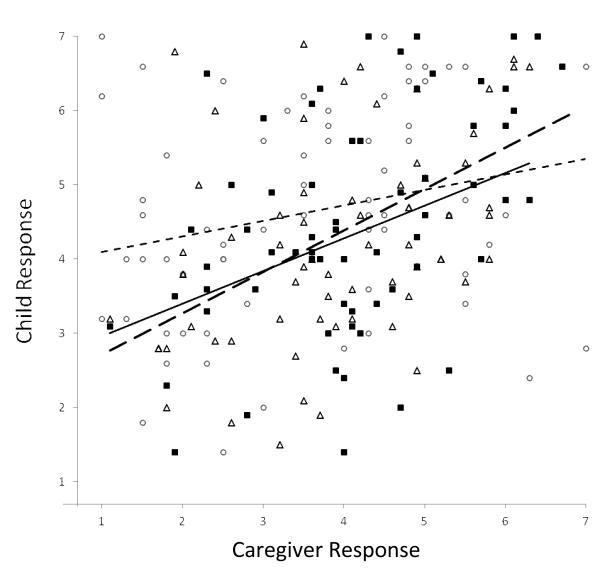
Child/caregiver responses were not significantly correlated for activity limitation; this was true in both asthma groups. The degree of correlation was significantly lower in the activity limitation domain compared to the emotional function and overall QOL domains (z=-2.31, P=0.02; z=-2.26, P=0.02, respectively for pairwise differences). The degree of correlation in child/caregiver responses was similar in the emotional function and overall QOL domains (z= -1.27, P= 0.20) (Figure 2).
Figure 2.
Child and Caregiver QOL Responses by Domain
Child/caregiver pairs and fitted line by domain: activity (solid circles, dotted line, r=0.23*), emotional (open squares, dashed line, r=0.50†), overall (solid triangles, solid line, r=0.43†). P=0.02 for * vs. †.
Effect of Gender on Child/Caregiver Agreement
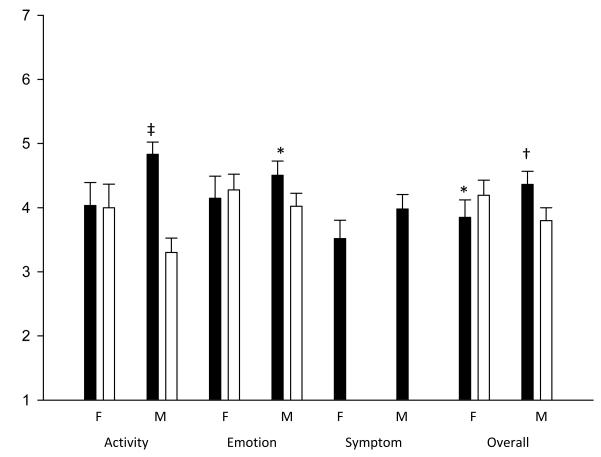
We found differences in child/caregiver agreement based on gender. In general, male children were more likely to differ from their caregivers than female children. Male children reported higher scores than their caregivers in all domains, indicating better QOL. This difference was significant in the activity limitation domain (P<0.001) as well as for emotional function and overall QOL in the combined groups (P=0.022 and P=0.009 respectively). In contrast, female children did not differ significantly from their caregivers in reported activity limitation. In addition, female children reported lower scores than their caregivers in all domains in the acute asthma group as well as in the emotional function and overall QOL domains in both groups combined, although most differences were not statistically significant (Figure 3).
Figure 3.
QOL Child and Caregiver Responses by Gender Mean child (dark bars) and caregiver (light bars) responses by domain. M= male child respondent; F=female child. P value for child-caregiver difference: *P≤0.05. †P≤0.01. ‡P≤0.001.
Although more likely to significantly differ from their caregivers in their responses, male respondents were also more likely to correlate with their caregivers in their response patterns. For combined groups, male children and their caregivers showed moderate correlation in overall QOL and emotional function (r=0.466, P=0.001 and r=0.541, P<0.001 respectively). Lower correlation was seen in the activity limitation domain (r=0.365, P=0.010). In contrast to the male children, female child/caregiver responses did not correlate significantly in any of the domains.
Multivariate Analysis of Differences in Child/Caregiver Responses
We initially performed Pearson’s correlation and point biserial correlations to examine the effect of child age, gender, and ethnicity on the difference in child/caregiver responses. As expected, only gender correlated significantly with the difference in responses in that male children were more likely than females to differ from their caregivers. This was true for the activity limitation domain and overall QOL (r=0.360, P=0.003 and r=0.283, P=0.020 respectively). Subsequently, we used stepwise regression to further characterize the effect of gender, child age, and ethnicity (independent variables) on the difference in child/caregiver responses (dependent variable). Male children were more likely than female children to differ from their caregiver in activity limitation and overall QOL (β= 0.360, t(3.113), P=0.003 and β=0.283, t(2.377), P=0.020 respectively). Gender explained a significant proportion of variance in child/caregiver differences in activity limitation and overall QOL (R2=0.130, F(1,65) = 9.69, P=0.003 and R2=0.080, F(1,65) =5.651, P=0.020 respectively).
Child/Caregiver Response Styles
We evaluated the frequency of Likert scale response choices of child and caregiver respondents for each specific question to determine selection patterns. We used these data to evaluate whether children and their caregivers displayed a tendency to select extreme responses, such as “none of the time” or “all of the time,” rather than using the full range of options available on the Likert scale. Approximately 30-50% of subjects and caregivers responded to specific questions with either a 1 or a 7 (extremes of the scale) and the remainder rated their QOL somewhere in-between these extremes (i.e. they selected a response between 2 and 6). Children and caregivers in both asthma groups demonstrated this response pattern. Thus, we did not find a tendency for either children or their caregivers to favor selection of extreme responses in either direction.
Discussion
This study demonstrates that providers should ask both children and their caregivers about the effects of asthma on a child’s QOL, especially with regard to disease effect on activity limitation. Our data further contribute to increasing evidence that caregiver reports cannot be used in place of a child’s report about disease-specific QOL(5,16,28,29). We showed that children and their caregivers were most likely to differ significantly on the activity limitation subscale. Child/caregiver pairs showed less difference in scores and higher levels of correlation for emotional function and overall QOL. Thus, our data agree with previous literature that the degree of child/caregiver agreement on HRQOL questionnaires depends upon the specific domain studied(17,28). Our study is unique in that we enrolled children with an acute asthma exacerbation, as well as children with chronic asthma and showed similar patterns of child/caregiver responses in these two groups.
In the activity limitation domain, we found that children generally felt less limited and reported higher scores than did their caregivers. However, caregivers were asked to report on the impact of asthma on family activities, rather than on the child’s physical activities. Thus, the two questionnaires provide information on related, but not identical constructs. A previous study by Erikson et. al. using the PAQLQ and PACQLQ in children ages 9-17 found that children rated their activity limitation lower than did their caregivers(30). These opposite results could be attributed to differences in study population. Our study included a higher proportion of young children and males than did Erikson’s study. Asthma in young children may have a greater impact on the activities of the family while older children may function more independently, and thus, caregivers may be less aware of their child’s limitations. Our study included a high proportion of male children, who were more likely to report higher scores in activity limitation than female respondents, as discussed below. Our cohort may represent a group of less active children, given the high BMI percentiles noted in our enrolled subjects. These children might report less activity limitation because they are sedentary, while their caregivers’ ratings are based on the disease effect on family activities. Erikson showed that caregivers had higher scores in the activity limitation domain (indicating better QOL) when household income was higher, the child had longer enrollment in a specialty clinic, and when the caregiver felt that seeing the child’s physician was convenient(30). It is possible that our cohort includes families with little access to asthma care, especially within the acute asthma group, such that caregiver scores in the activity domain are lower than those reported in previous studies. Without an independent “gold standard” for assessment, it is difficult to know if children or their caregivers are more accurate in assessing QOL.
In our study, caregiver and child emotional function responses tended to be moderately correlated. Correlation was generally highest in the emotional function domain in the refractory asthma group. Children with chronic asthma symptoms and their caregivers have had the opportunity to share the day-to-day experience of asthma over time and thus may exhibit stronger agreement in their assessment of the impact of asthma on emotional function. A previous systematic review showed different findings, with generally low correlations (r<0.3) for domains that were “non-observable” (i.e. social or emotional)(28). This previous review, however, included children with a number of different chronic diseases, not just asthma. Accordingly, our results demonstrate that children with the specific diagnosis of asthma may communicate better with their caregivers and are thus more in-sync in recognizing the disease’s effect on the child’s QOL.
We found that child/caregiver agreement was similar at enrollment and follow-up in both asthma groups across all domains. It is not surprising that there was no difference in agreement over time in the refractory group, since these children and their caregivers had likely become accustomed to the chronic effect of the disease on the child’s QOL, such that their pattern of agreement remained similar. However, child/caregiver agreement in the acute group was also similar over time, despite enrollment during an acute exacerbation and follow-up when their asthma was more stable. These findings bear further exploration in future studies with a larger sample.
Although one previous study found no effect of gender on PAQLQ scores (30), our study results show that gender had a significant effect on child/caregiver response agreement. Female respondents typically reported lower scores than their caregivers (although not statistically different) and their responses did not correlate significantly with caregiver responses. Male respondents, on the other hand, differed significantly from their caregivers in all domains, especially in the activity limitation domain where male child scores were higher than their caregivers. In our study, lower female respondent scores on the symptoms subscale likely lowered the overall QOL scores reported by female children. It is also possible that male children were downplaying the effect of their illness, so as not to seem weak or vulnerable, especially with regard to physical activity.
Our study did not show a significant effect of age on child/caregiver agreement across any of the domains studied. In contrast, a previous study showed that child/caregiver agreement improved with increasing age of the child(23). Similarly, another study demonstrated that children 11 years of age and older were good reporters of asthma health status and QOL; whereas caregiver reports provided additional information for younger children(31). Our study may have lacked sufficient statistical power to demonstrate the effect of age on child/caregiver agreement.
The differences we found between child and caregiver responses were not explained by a tendency of either group to respond more frequently on the extremes of the Likert scale (1 or 7). Both child and caregiver respondents generally used the full scale across almost all questions on the PAQLQ and PACQLQ, respectively. Previous studies have shown conflicting results regarding response patterns. Davis demonstrated that children were more likely to give extreme scores than their caregivers(32) while Theunissen showed that child scores tended to be less extreme than caregiver scores(33). In contrast to the two previous studies cited, our study was limited to children with asthma and included children over the age of 12. It is possible that older children utilized the full scale, and children with asthma were more accustomed to rating their health status than healthy children or children with other illnesses.
Our study had a few limitations. The PAQLQ is validated for children age 7 and above; however, we administered the questionnaire to a total of 7 children who were 5 or 6 years old based on the research assistant’s subjective evaluation of the child’s capability. This could have affected our outcomes if these young children actually were not able to comprehend the questions. In addition, the caregivers enrolled in our study were overwhelmingly female. This may have skewed our data on caregiver responses and our interpretation of the agreement between children with asthma and their caregivers. One prior study reported that fathers and children had greater agreement than mothers and their children(16). Because our study population included few female subjects (a reflection of the male predominance in pediatric asthma), we were unable to fully assess the effect of gender on child/caregiver agreement. Similarly, few African American children were enrolled in our study, so we were unable to fully examine child/caregiver patterns in this group of children, who have a higher prevalence, hospitalization rate, number of emergency department visits, and mortality rate compared to other races(34). Finally, although PAQLQ and PACQLQ questionnaires are designed to address the most common concerns of children with asthma and their caregivers, these instruments may not necessarily measure all of the problems that every individual would find to be important(35).
Caregivers of children with asthma can provide useful proxy information about asthma-related QOL. However children and their caregivers do not always agree, particularly in their assessment of activity limitation due to asthma. Thus, it is critical to obtain information from both parties in the evaluation of children with asthma. Future research is needed to determine the role of language, caregiver gender, and disease severity on differences between child and caregiver responses to QOL questionnaires.
Acknowledgments
The authors would like to thank Dana Word, RN for study subject recruitment and management, Joel Baseman, PhD for support and advice in analysis and manuscript preparation, Brad Smith, PhD and Mercedes Vaughn, M.S. for assistance with analyses, and Monica McCall for assistance in manuscript preparation.
Sources of financial support: This work is supported by the National Institute of Allergy and Infectious Disease (NIAID; 3U19AI070412-04S1; 5U19AI070412), and by the University of Texas Health Science Center San Antonio Medical Student Research Program, and by Award Number 8UL1TR000149 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Margaret L Burks, Department of Pediatrics, University of Texas Health Science Center at San Antonio (UTHSCSA).
Edward G Brooks, Department of Pediatrics, UTHSCSA Vanessa L Hill, MD; Department of Pediatrics, Baylor College of Medicine.
Jay I Peters, Department of Medicine, UTHSCSA Pamela R Wood, MD; Department of Pediatrics, UTHSCSA.
References
- 1.Akinbami LJ, Moorman JE, Liu X. National Health Statistics Reports; no 32. National Center for Health Statistics; Hyattsville, MD: 2011. Asthma Prevalence, Health Care Use, and Mortality: United States, 2005-2009. [PubMed] [Google Scholar]
- 2.Akinbami LJ, Morrman JE, Garbe PL, Sondik EJ. Status of Childhood Asthma in the United States, 1980-2007. Pediatrics. 2009;123:S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 3.Okelo SO, Wu AW, Krishnan JA, Rand CS, Skinner EA, Diette GB. Emotional Quality-of-Life and Outcomes in Adolescents with Asthma. J Pediatr. 2004;145:523–529. doi: 10.1016/j.jpeds.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 4.Glazebrook C, McPherson AC, Macdonald IA, et al. Asthma as a Barrier to Children’s Physical Activity: Implications for Body Mass Index and Mental Health. Pediatrics. 2006;118(6):2443–2449. doi: 10.1542/peds.2006-1846. [DOI] [PubMed] [Google Scholar]
- 5.Farnik M, Pierzchala W, BroŜek G, Zejda JE, Skrzypek M. Quality of Life Protocol in the Early Asthma Diagnosis in Children. Pediatr Pulmonol. 2010;45:1095–1102. doi: 10.1002/ppul.21293. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention National Surveillance for Asthma – United States 1980-2004. Surveillance Summaries. MMWR 2007. 2007 Oct 19;56(No. SS-8) [PubMed] [Google Scholar]
- 7.Forrest CB, Shipman SA, Dougherty D, Miller MR. Outcomes Research in Pediatric Settings: Recent Trends and Future Directions. Pediatrics. 2003;111(1):171–8. doi: 10.1542/peds.111.1.171. [DOI] [PubMed] [Google Scholar]
- 8.Schipper H, Clinch J, Powell V. Definitions and Conceptual Issues. In: Spiker B, editor. Quality of Life Assessment in Clinical Trials. Raven Press; New York: 1990. pp. 11–24. [Google Scholar]
- 9.Stelmach I, Podlecka D, Smejda K, et al. Pediatric Asthma Caregiver’s Quality of Life Questionnaire is a Useful Tool for Monitoring Asthma in Children. Qual Life Res. 2012;21:1639–1642. doi: 10.1007/s11136-011-0070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stelmach I, Podlecka D, Majak P, et al. Validity of the Pediatric Asthma Quality of Life Questionnaire in Polish Children. Pediatr Allergy Immunol. 2011;22:660–666. doi: 10.1111/j.1399-3038.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 11.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring Quality of Life in Children with Asthma. Qual Life Res. 1996;5:35–46. doi: 10.1007/BF00435967. [DOI] [PubMed] [Google Scholar]
- 12.Halterman JS, Yoos HL, Conn KM, et al. The Impact of Childhood Asthma on Parental Quality of Life. J Asthma. 2004;41(6):645–653. doi: 10.1081/jas-200026410. [DOI] [PubMed] [Google Scholar]
- 13.Wilson SR, Rand CS, Cabana MD, et al. Asthma Outcomes: Quality of Life. J Allergy Clin Immunol. 2012;129(3):S88–123. doi: 10.1016/j.jaci.2011.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olson LM, Radecki L, Frintner MP, Weiss KB, Korfmacher J, Siegel RM. At What Age Can Children Report Dependably on Their Asthma Health Status? Pediatrics. 2007;119(1):e93–102. doi: 10.1542/peds.2005-3211. [DOI] [PubMed] [Google Scholar]
- 15.Walker J, Winkelstein M, Land C, et al. Factors That Influence Quality of Life in Rural Children with Asthma and their Parents. Journal of Pediatric Health Care. 2008;22:343–350. doi: 10.1016/j.pedhc.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petsios K, Priftis KN, Tsoumakas C, et al. Level of Parent-Asthmatic Child Agreement on Health-Related Quality of Life. J Asthma. 2011;48:286–297. doi: 10.3109/02770903.2011.555031. [DOI] [PubMed] [Google Scholar]
- 17.Cremeens J, Eiser C, Blades M. Factors Influencing Agreement Between Child Self-Report and Parent Proxy-Reports on the Pediatric Quality of Life Inventory 4.0 (PedsQL) Generic Core Scales. Health and Quality of Life Outcomes. 2006;4(58) doi: 10.1186/1477-7525-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood PR, Hill VL, Burks ML. Mycoplasma pneumoniae in children with acute and refractory asthma. Ann Allergy Asthma Immunol. 2012 doi: 10.1016/j.anai.2013.01.022. et.al. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bel EH, Sousa A, Fleming L, et al. Diagnosis and Definition of Severe Refractory Asthma: An International Consensus Statement from the Innovative Medicine Initiative (IMI) Thorax. 2011;66:910–917. doi: 10.1136/thx.2010.153643. [DOI] [PubMed] [Google Scholar]
- 20.National Asthma Education and Prevention Program . Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute (US); Bethesda, MD: 2007. [NIH Publication no. 07-4051] [Google Scholar]
- 21.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a Minimal Important Change in a Disease-Specific Quality of Life Questionnaire. J Clin Epidemiol. 1994;47(1):81–87. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 22.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring Quality of Life in the Parents of Children with Asthma. Qual Life Res. 1996;5:27–34. doi: 10.1007/BF00435966. [DOI] [PubMed] [Google Scholar]
- 23.Annett RD, Bender BG, DuHamel TR, Lapidus J. Factors Influencing Parent Reports on Quality of Life for Children with Asthma. J Asthma. 2003;40(5):577–587. doi: 10.1081/jas-120019030. [DOI] [PubMed] [Google Scholar]
- 24.Steiger JH. Tests for Comparing Elements of a Correlation Matrix. Psychol Bull. 1980;87(2):245–251. [Google Scholar]
- 25.Revelle W. [Retrieved Feb 8, 2013];Procedures for Psychological, Psychometric, and Personality Research. Package “psych”. 2012 Dec 31; from http://personality-project.org/r/psych.manual.pdf.
- 26.R Core Team [Retrieved Feb 8, 2013];R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2012 ISBN 3-900051-07-0. from http://www.R-project.org.
- 27.Taylor R. Interpretation of the Correlation Coefficient: A Basic Review. Journal of Diagnostic Medical Sonography. 1990;6:35–39. [Google Scholar]
- 28.Eiser C, Morse R. Can Parents Rate Their Child’s Health-Related Quality of Life? Results of a Systematic Review. Qual Life Res. 2001;10(4):347–357. doi: 10.1023/a:1012253723272. [DOI] [PubMed] [Google Scholar]
- 29.Callery P, Milnes L, Verduyn C, Couriel J. Qualitative Study of Young People’s and Parents’ Beliefs About Childhood Asthma. Br J Gen Pract. 2003;53:185–190. [PMC free article] [PubMed] [Google Scholar]
- 30.Erickson SR, Munzenberger PJ, Plante MJ, Kirking DM, Hurwitz ME, Vanuya RZ. Influence of Sociodemographics on the Health-Related Quality of Life of Pediatric Patients with Asthma and Their Caregivers. J Asthma. 2002;39(2):107–117. doi: 10.1081/jas-120002192. [DOI] [PubMed] [Google Scholar]
- 31.Guyatt GH, Juniper EF, Griffith LE, Feeny DH, Ferrie PJ. Children and Adult Perceptions of Childhood Asthma. Pediatrics. 1997;99:165–168. doi: 10.1542/peds.99.2.165. [DOI] [PubMed] [Google Scholar]
- 32.Davis E, Nicolas C, Waters E, et al. Parent-Proxy and Child Self-Reported Health-Related Quality of Life: Using Qualitative Methods to Explain the Discordance. Qual Life Res. 2007;16(5):863–871. doi: 10.1007/s11136-007-9187-3. [DOI] [PubMed] [Google Scholar]
- 33.Theunissen NCM, Vogels TGC, Koopman HM, et al. The Proxy Problem: Child Report Versus Parent Report in Health-Related Quality of Life Research. Qual Life Res. 1998;7(5):387–397. doi: 10.1023/a:1008801802877. [DOI] [PubMed] [Google Scholar]
- 34.Akinbami LJ, Schoendorf KC. Trends in Childhood Asthma: Prevalence, Health Care Utilization, and Mortality. Pediatrics. 2002;110:315–322. doi: 10.1542/peds.110.2.315. [DOI] [PubMed] [Google Scholar]
- 35.Juniper EF. Health-Related Quality of Life in Asthma. Curr Opin Pulm Med. 1999;5(2):105–110. doi: 10.1097/00063198-199903000-00005. [DOI] [PubMed] [Google Scholar]