Abstract
Background and Purpose
Caliceal diverticula are rare congenital abnormalities that can become symptomatic if associated with a calculus or infection. We review percutaneous management of caliceal diverticula.
Methods
Pathogenesis, clinical evaluation, management options, and recommended follow-up for symptomatic caliceal diverticula are reviewed. We present our single-stage and prepercutaneous nephrolithotomy opacification techniques for the management of caliceal diverticula. This involves complete extraction of all stone particles and ablation of the diverticular cavity without infundibular identification or dilation. Comparison of outcomes between our current ablative technique and our previous dilation technique is evaluated.
Results
Percutaneous management of caliceal diverticula offers the highest symptomatic relief and stone-free rate of available management options. We identified 106 patients with caliceal diverticula who were treated with a percutaneous approach. Review of 85 of these patients demonstrated that most procedures can be performed with a small nephrostomy tube in place for 24 hours and an overnight hospital stay. Minimal complication and stone recurrence rates were observed. Patients treated with caliceal diverticular ablation experienced a shorter hospital stay, fewer complications, and a higher stone-free status than those patients who were treated with dilation of the diverticular infundibulum.
Conclusions
Percutaneous management of caliceal diverticula using cavity ablation is a minimally invasive technique that offers long-term symptomatic relief with minimal complications.
Introduction
Caliceal diverticula are congenital smooth-walled, nonsecretory urothelium-lined cavities within the renal parenchyma that communicate with the caliceal fornix through a diverticular neck. Urine is received by the diverticulum by passive retrograde filling from the adjacent collecting system, which can include the renal pelvis. Failure of small ureteral buds to degenerate is thought to be the origin for the diverticular anatomic variation.
Caliceal diverticula are uncommon and have been noted in 0.21% to 0.6% of patients who are undergoing renal imaging.1–4 Bilateral occurrence is observed in only 3% of patients with diverticula.5 Calculi occur in 9.5% to 50% of diverticula. Although many of these cavities occur without symptoms, they can be associated with pain, hematuria, recurrent urinary tract infections, and even damage to surrounding parenchyma.2,4,6,7 Furthermore, complete obstruction of the diverticular neck can be associated with sepsis, abscess formation, or hypertension.8
The etiology of caliceal diverticular calculi is controversial, with both urinary stasis and underlying metabolic abnormalities implicated as factors.9–12 Previous investigators have suggested that particle retention time, especially in the setting of a diverticulum, could be the cause of stone formation.12 Liatsikos and colleagues10 further suggested stasis as a cause of diverticular calculi formation, because they noted a low incidence of metabolic abnormalities in their cohort (25% in caliceal diverticulum cohort vs 77.3% in other stone formers). Other studies, however, have noted metabolic derangements in 50% to 100% of patients with diverticular stone disease.9,13
Finally, Matlaga and colleagues11 suggested that it is both metabolic and stasis factors. In their comparison of 24-hour urine studies of 29 patients with caliceal diverticula with 245 calcium oxalate stone formers and 162 normal patients, they found the urinary stone risk parameters of patients with caliceal diverticular stones were similar to those of the calcium oxalate stone forming group. Furthermore, when compared with the normal cohort, the caliceal diverticulum and calcium oxalate stone formers were significantly more hypercalciuric and their urine was significantly more supersaturated with calcium oxalate. Of interest, the urine aspirated from the diverticulum of three patients in the series from Matlaga and colleagues11 demonstrated a significantly lower supersaturate of calcium oxalate compared with urine that was obtained from the renal pelves of the same patient. The authors hypothesize that, although these patients have baseline metabolic derangements, the urinary stasis in the diverticulum allows for incorporation of ions into a stone nidus, thus propagating stone formation and lowering local calcium oxalate supersaturation.
Diagnosis and Evaluation
The differential diagnosis of caliceal diverticulum includes: Renal cyst, malignancy, solitary abscess, and, most commonly, hydrocalicosis secondary to infundibular stenosis. In geographic locations where tuberculosis is prevalent, the differential diagnosis of cortical cavitation secondary to renal tuberculosis should be considered, because the radiologic appearances of these two conditions may be similar.8 Most patients with calculi in a caliceal diverticulum present with flank pain, hematuria, recurrent urinary tract infections, and, less frequently, an incidentally noted stone. The work-up for a calculi in a caliceal diverticula follows the same pathway for the above presenting symptoms and includes urinalysis, complete blood cell count, basic metabolic panel, and imaging of the abdomen. The urine study should include both microscopy and a urine dipstick test for leukocytes, erythrocytes, protein, nitrites, and pH.
Initial imaging studies depend on the physician's practice preference and can be renal ultrasonography (US), plain abdominal radiography of the kidneys, ureters, and bladder (KUB), or noncontrast CT. Renal US will accurately make a diagnosis of a caliceal diverticulum in 80% of cases,14 with the classic US finding of milk of calcium.15 When echogenic material is present in the affected kidney, the patient is scanned in different positions to demonstrate gravitational changes of the content, which is diagnostic of a caliceal diverticulum. KUB radiography can also point to a diagnosis of a caliceal diverticulum if milk of calcium is present. The milk of calcium will appear as a meniscus-like, half-moon–shaped calcification. Caliceal diverticula are often easily identified on noncontrast CT as a dilated outpouching from the collecting system containing a stone.
Once the diagnosis of caliceal diverticulum is suspected, imaging of the collecting system should be performed to assess location of the diverticulum and communication with other elements of the kidney. With intravenous urography (IVU) or CT urography, most caliceal diverticula will opacify if they have significant communication with the collecting system. The opacification will occur later in the examination, because the diverticulum is filled in a retrograde fashion from the calix or renal pelvis and thus delayed images are necessary.16 A retrograde pyelogram can be helpful in determining where the neck of the diverticulum is located. If the neck or infundibulum is obstructed, the diverticulum will not opacify. The presence of an obstructed infundibulum can dictate how access to the caliceal diverticulum is achieved, which will be covered in detail.
Surgical Treatment Options
Patients with caliceal diverticula can present a treatment challenge. Historically, patients with caliceal diverticula were treated by open surgical nephrostomy with closure of the infundibulum, marsupialization and fulguration of the diverticular cavity, or partial nephrectomy. With technologic advancements, the treatment has become progressively less invasive. Currently used minimally invasive treatment for patients with symptomatic caliceal diverticula includes shockwave lithotripsy (SWL),17–19 ureteroscopy (URS),20–23 percutaneous nephrolithotomy (PCNL),16,17,20,24–30 and laparoscopy.31–36
SWL for the management of caliceal diverticula is an attractive but controversial minimally invasive treatment option. Although at short-term follow-up SWL can provide symptomatic pain relief in 36% to 70% of patients, stone-free rates are low, ranging from 4% to 20%.17,18 Jones and associates17 have further demonstrated that some patients who are initially rendered symptom free with SWL will subsequently become symptomatic with longer observation and need re-treatment.
The highest stone-free rate reported for SWL of caliceal diverticular calculi remains suboptimal at 58%.19 Thus, SWL is rarely considered as monotherapy for caliceal diverticula because most investigators agree that to prevent stone recurrence, eradication of the diverticulum should accompany stone removal, a goal that is rarely achieved with SWL.37,38
An advantage of URS over SWL is that while both are minimally invasive, URS allows for simultaneous ablation of the diverticular cavity. Retrograde URS is a reasonable option for certain patients with diverticula in the upper or middle portions of the kidney when the stone burden is small and the diverticular neck is short and easily accessible.21,37 Stone-free rates for URS range from 19% to 73%, but diverticular obliteration is as low as 18%; a significant number of patients demonstrate residual symptoms and need re-treatment.23,24,38 Furthermore, identification of the diverticular neck can be difficult from a retrograde approach and may account for the lengthy operative times (1.25 to 4 hours) reported in some URS series.21,23
Laparoscopic management of caliceal diverticula has been described, but the indications for such an approach are limited. It has been suggested that anterior diverticula, especially if there is a thin layer of parenchyma overlying a large anterior cavity, are appropriate for laparoscopy.31,32 The laparoscopic approach, however, is more invasive than PCNL, with lengthy operative times (up to 2.5 hours) even in experienced hands,32 thus limiting its widespread application. In general, laparoscopic management is reserved for diverticula that are extremely anterior or in ectopic kidneys where a percutaneous approach will be hampered.
Percutaneous management of caliceal diverticular calculi achieves excellent stone-free (87.5%–100%) and obliteration rates of the diverticular cavity (76%–100%).16,17,25,26,28–30 More than 90% of patients report symptomatic relief with percutaneous treatment,17,20,25–28 and long-term studies have demonstrated these results to be durable.28 In most cases, percutaneous access is directly onto the caliceal diverticulum, because it allows use of a rigid nephroscope.37 Direct puncture can be difficult if the cavity is small or if the caliceal diverticulum is located in the upper pole of the kidney. If direct puncture into the calix fails, a neighboring calix can be punctured and the diverticulum entered indirectly by perforating the wall of the diverticulum or by entering in a retrograde fashion, through the diverticular neck.39 The indirect access technique, however, is associated with inferior results and thus should be reserved as a secondary measure.40
Biplanar fluoroscopy is the imaging modality most commonly used for percutaneous access when subsequent PCNL is planned41; this technique needs a radiopaque target for which to aim. Kim and coworkers30 have described a single-stage technique in which percutaneous access is directed straight onto the stone. If the targeted diverticular calculi are not visible with fluoroscopic imaging, contrast can be instilled into the diverticular cavity via a ureteral catheter placed retrograde.28 If the diverticular communication with the intrarenal collecting system is attenuated, however, the diverticulum may not readily fill with contrast. In such circumstances, US guidance can be used.42 Unfortunately, it is difficult to monitor the guidewire manipulation with US imaging. Matlaga and associates16 have described CT or US guided pre-PCNL opacification of the caliceal diverticulum as an alternative approach. Opacification of the diverticulum by interventional radiology provides the necessary target for percutaneous access in the operating room.
Once access is obtained into the diverticular cavity, stone removal is performed with either forceps or ultrasonic lithotripsy. Regardless of the method used, it is imperative that the patient is rendered stone free. Kim and associates30 noted that successful obliteration of the diverticular cavity was closely associated with rendering the patient stone free, and that successful obliteration of diverticula ranging from 5 to 44 mm in diameter was possible when the cavity was completely cleared of stone material; however, residual fragments increased the risk of incomplete diverticular resolution. After all stone material is extracted, the urothelium should be inspected to identify a flattened renal papilla, the presence of which would indicate an obstructed calix rather than a caliceal diverticulum.
Once the cavity is confirmed to be a true diverticulum, treatment can include creating a large communication to the collecting system to promote drainage and prevent urinary stasis. When the infundibular connection to the renal collecting system can be found, dilation of this communication can be performed and the area “stented” with a nephrostomy tube.17,26,29 If the infundibular connection cannot be found or traversed with a wire, some have advocated creation of a neoinfundibulum into the calix or the renal pelvis.24,27 Both techniques require the placement of a nephrostomy tube for a prolonged period to ensure the channel will remain open. Furthermore, dilation of the infundibulum and creation of a neoinfundibulum have the potential to create significant bleeding. Neoinfundibulotomy should not be performed when the diverticulum is located anteriorly and normal parenchyma is traversed and dilated to form a connection to the collecting system.27
Another treatment strategy is to fulgurate the diverticulum. Although the need for cavity ablation or destruction is a controversial issue,23 because the caliceal diverticulum is lined by a nonsecretory endothelium, most authors advocate fulguration at the time of PCNL.37 Hulbert and associates,25 however, suggested that trauma to the wall of the diverticulum that is caused by the percutaneous dilation process is sufficient to ablate the diverticular lumen. Conversely, others have reported that dilation or incision of the diverticular neck without fulguration results in complete ablation of the diverticulum in only 30% of cases43 as opposed to the 76% to 100% ablation rate when fulguration is performed.17,26,28,44
If fulguration is to be performed, the infundibulum of the diverticulum should not be dilated to maximize the chance of diverticular obliteration. Diverticular fulguration can be performed with28 or without30 ureteral catheterization using a direct percutaneous access technique. Monga and colleagues28 performed fulguration of the caliceal diverticulum lining without any attempt at cannulation of the infundibular communication. All patients were left with a ureteral stent for 2 to 4 weeks, and the nephrostomy tube was removed at 24 to 48 hours. The authors reported a 100% diverticular ablation rate, and all patients were symptom free at 38 months follow-up. Kim and associates30 also presented their series of percutaneous diverticular ablation without identification of the infundibulum; however, ureteral catheters were not placed in their series, and 20 of 21 patients were sent home on postoperative day 1 tubeless. Operative times for their series were less than 60 minutes. At 3 months follow-up, all diverticula had decreased in size, and 87.5% had completely resolved.
Technique for Percutaneous Management of Caliceal Diverticula
In our single-stage PCNL technique, patients are placed in the prone position with the side containing the caliceal diverticulum elevated 30 degrees. A C-arm is used to visualize the diverticular calculi. In all cases possible, a direct infracostal puncture is performed using an 18-gauge, diamond-tipped needle and a biplanar fluoroscopic triangulation technique as previously described.37 When access is achieved, a 0.035 inch J-tipped removable core guidewire is coiled inside the diverticular cavity (Fig. 1A). The main advantage of the removable core J-wire is the flexible distal end of the wire can be adapted to the size of the diverticulum, while the wire proximal to the removed core remains stiff enough to function as the working wire.
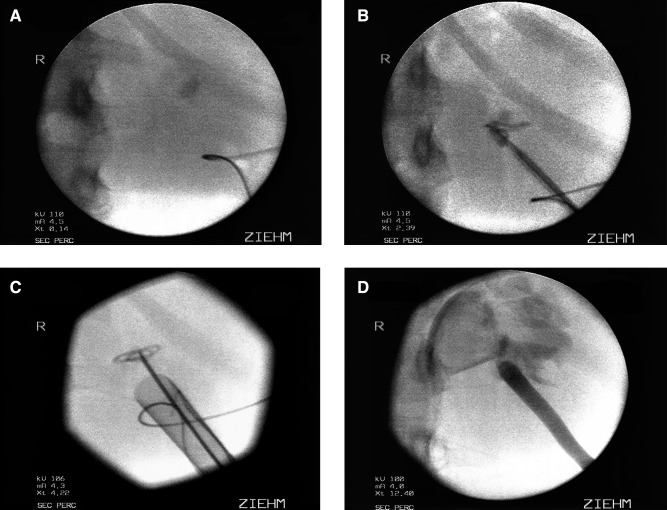
FIG. 1.
Fluoroscopic images of percutaneous management of a caliceal diverticulum. (A) After needle access is obtained to the caliceal diverticulum, a 0.035 J wire is coiled in the diverticular cavity. (B) An 8/10 fascial dilator is used to placed a second 0.035 J wire as a safety wire. (C) The Amplatz sheath is advanced over the balloon dilator to the edge of the diverticulum. Care is taken to avoid perforation of the diverticular back wall. (D) After the diverticular cavity has been fulgurated, a red rubber catheter is left in place.
With the J-wire in place, an 8/10F coaxial dilator is passed over the J-wire in a sequential fashion. The 8F dilator is removed, and a second 0.035 inch J-tipped removable core wire is coiled inside the diverticulum and used as the safety wire (Fig. 1B). The tract is balloon dilated over the working wire. Special attention is given to the placement of the balloon dilator and the wires to prevent any trauma to or perforation of the back wall of the diverticulum.
A 30F Amplatz sheath is then passed over the balloon dilator under fluoroscopic guidance. The balloon dilator has a taper on the distal end that precludes placement of the sheath directly into the diverticular space unless the diverticulum is large (Fig. 1C). Next, a 24.5F rigid offset Wolf nephroscope without the external sheath is placed inside the sheath using normal saline irrigant. An 11F alligator forceps is used to manually dilate the part of the tract immediately adjacent to the diverticulum, allowing for advancement of the scope and subsequently the sheath into the diverticular lumen.
Once the offset nephroscope is gently guided into the diverticular cavity, ultrasonic lithotripsy or forceps graspers are used to remove the stone burden. After removal of all stone material, the irrigant is switched to 1.5% glycine, and a 24F resectoscope with a rollerball electrode is used to fulgurate the diverticular lining at 30 W pure coagulation. The infundibular communication is neither assessed nor dilated. An 18F red rubber catheter or an 8.5F loop catheter is placed in the cavity at the conclusion of the procedure (Fig. 1D). The Cope loop is only used for caliceal diverticula that are large enough to house the entire loop. Proper placement of the nephrostomy tube is confirmed by contrast instillation under fluoroscopy. If the diverticulum is small, the red rubber catheter acts as a perinephric drain, because it usually becomes dislodged from the diverticular cavity.
For patients in whom the stone cannot be visualized fluoroscopically, either because it is too small or is radiolucent, the direct puncture technique cannot be performed unless the diverticulum is first opacified. In such cases, a retrograde pyelogram is first performed via a ureteral catheter. If the diverticulum does not readily opacify because of a narrow infundibulum, we will perform percutaneous opacification with either CT or US guidance. The patient is transported to the radiology suite, where radiographic evaluation of the caliceal diverticulum is performed with the patient in the prone position. CT or US is used to identify the diverticulum, and a puncture site overlying the posterior aspect of the kidney is selected. Local anesthesia is injected, and a 20-gauge spinal needle is manipulated under radiographic guidance into the caliceal diverticulum. Iodinated contrast is gently instilled until resistance is encountered, and KUB imaging is performed to confirm opacification of the diverticulum. Care must be taken to not inadvertently overfill the diverticular cavity by instilling contrast material under too great pressure, because extravasation may occur, which will make subsequent fluoroscopic targeting difficult. The patient is then transported directly to the operating room. Renal access and PCNL are performed as outlined above. It is imperative that little time elapses from the injection of contrast to the initiation of the procedure, because too late a delay may permit the absorption of the contrast material and the loss of the target.
Results
We have previously presented our results for single-stage PCNL of caliceal diverticula as well as pre-PCNL opacification.16,30 We currently present our updated series using both techniques and compare outcomes with those of patients who were treated with our old technique in which we located the communication between the diverticulum and the collecting system and dilated the infundibulum.
After Institutional Review Board approval, our prospectively collected PCNL database was reviewed to identify all patients with caliceal diverticula who were treated with PCNL. From February 2001 to November 2008, we have treated 106 patients with percutaneous stone extraction and ablation of caliceal diverticulum. To limit confounding variables, we focused just on those patients who were treated by a single surgeon (JEL). Our old technique was used in 28 patients and the new technique of diverticular fulguration in 57 patients. In the old technique cohort, the mean age was 44 years (range 18–84 yrs), and there 18 females and 10 males. In the new technique cohort, the mean age at treatment was 42 years (range 10–78 yrs), and there were 37 females and 20 males. Of the 45 patients with location of the diverticula documented, 21 (46.7%) were upper pole, 18 (40%) midpolar, and 6 (13.3%) lower pole. Mean diverticular diameter was 15.8 mm (range 5–44 mm).
A single-stage approach was used in 82 patients. Pre-PCNL opacification using CT or US guidance was needed in three patients (all in the new technique cohort). Mean operative time for the old technique cohort was 96.4 minutes (range 30–210 min) and for the new technique cohort, 67.3 minutes (range 30–150 min). Overall, of 78 nephrostomy tubes placed, 43 (55.1%) were red rubber catheters and 35 (44.9%) were Cope loops (Table 1). A postoperative noncontrast CT scan was performed on 74 (87.1%) patients. Of the 74 patients, 66 (89.2%) were stone free after the initial procedure. Of the patients who were undergoing the old technique, 22 had a CT scan on postoperative day 1 of whom 17 (77.3%) were stone free and 5 had residual stones.
Table 1.
Nephrostomy Tubes Used for Patients Undergoing Percutaneous Nephrolithotomy for Calculi in a Caliceal Diverticulum
| |
Old technique |
New technique |
||
|---|---|---|---|---|
| Type of nephrostomy tube | n = 28 | % | n = 57 | % |
| Cope loop | ||||
| 8.5F Cope loop | 6 | 21.4 | 3 | 5.3 |
| 10F Cope loop | 17 | 60.1 | 9 | 15.8 |
| Red rubber catheter | ||||
| 14F red rubber catheter | 0 | 1 | 1.8 | |
| 16F red rubber catheter | 0 | 1 | 1.8 | |
| 18F red rubber catheter | 0 | 23 | 40.4 | |
| 20F red rubber catheter | 3 | 10.7 | 13 | 22.8 |
| 22F red rubber catheter | 0 | 1 | 1.8 | |
| 24F red rubber catheter | 1 | 3.6 | 0 | |
Secondary PCNL was performed on four of the five patients with residual calculi, and four of the patients without a postoperative CT scan had negative KUB radiography and US by 2 months postoperative follow-up. Of the patients who were undergoing the new technique, 52 had a CT scan on postoperative day 1 of whom 49 (94.2%) were stone free and 3 had residual calculi. A secondary PCNL was performed on two of the three patients with residual calculi, and two of the patients without a postoperative CT scan had negative KUB radiography and US by 6 months postoperative follow-up. Final stone-free status after primary and secondary PCNL and using short-term follow-up data for the entire cohort was 78 of 80 (97.5%).
Mean length of hospitalization was 2.32 days (range 1–8 d) for the old technique and 1.12 days (range 18 hrs–2 d) for the new technique. Of the 79 patients who did not undergo a secondary PCNL, the nephrostomy tube was removed on postoperative day 1 in 75 patients. Postoperative complications are listed in Table 2. In total, eight (9.4%) patients experienced some time of postoperative complication. Overall, blood transfusions were necessary in three patients, and two patients experienced a pulmonary complication.
Table 2.
Postoperative Complications in Patients Undergoing Percutaneous Nephrolithotomy for Calculi in Caliceal Diverticulum
| Postoperative complication | Old technique n = 5* | New technique n = 3 |
|---|---|---|
| Pulmonary | ||
| Pleural effusion | 0 | 1 |
| Hemothorax | 1 | 0 |
| Pneumothorax | 0 | 0 |
| Hematologic | ||
| Perirenal hematoma | 1 | 1 |
| Hematuria | 2 | 0 |
| Arrhythmia | 1 | 0 |
| Acute renal failure | 1 | 0 |
| Ureteral edema/ pain after nephrostomy tube removed | 1 | 1 |
Two patients had more than one complication.
Stone material was retrieved from 77 patients, 26 in the old technique cohort and 51 in the new technique cohort. Of the stones analyzed, all were composed of calcium, including 24 hydroxyapatite, 49 calcium oxalate, 4 brushite, and 1 calcium carbonate phosphate. A positive stone culture was noted in four stones: Two with Proteus mirabilis and two with Escherichia coli. There were 26 real units with the new ablation technique that had follow-up IVU at 3 months. Only seven patients had the diverticulum visible on follow-up imaging, of whom four had a noted decrease in size, while three were not commented on by the radiologist. Over the follow-up period, no patients experienced recurrent stone events in the area of the caliceal diverticulum.
When the old infundibular dilation technique was compared with the new percutaneous diverticulum dilation technique, we noted a shorter hospitalization time and higher stone-free status with initial procedure with the new technique. Furthermore, none of the patients in whom the new technique was used needed a blood transfusion, and complication rates appeared to be lower compared with the old technique.
Postsurgical Evaluation and Follow-up
All retrieved stone material should be sent for stone analysis to assess stone composition. Abdominal imaging should be obtained to assess stone-free status after surgical intervention. We prefer a noncontrast CT scan on postoperative day 1 before removal of the nephrostomy tube. To assess resolution of the caliceal diverticulum, imaging of the collecting system should be performed at 3 months postoperatively either with an IVU or CT urography.
Metabolic evaluation should also be performed 4 to 6 weeks after surgery or before surgical intervention. Current literature supports metabolic evaluation for patients with calculi-containing caliceal diverticula. All patients with a caliceal diverticulum evaluated in one study had at least one metabolic abnormality, including hypercalciuria, hyperuricosuria, hypocitraturia, and hyperoxaluria.13 The most common abnormality noted was low urine volume. Matlaga and associates16 noted patients with caliceal diverticula had similar stone risk parameters as a cohort of patients with calcium oxalate stones without caliceal diverticula. Both the caliceal diverticula and the calcium oxalate stone cohorts had significantly higher stone risk parameters when compared with a group of normal patients. Based on these studies, we perform metabolic evaluation for all patients who are treated for a symptomatic caliceal diverticulum.
Conclusions
Caliceal diverticula are rare congenital anatomic malformations that can cause significant symptoms when they contain calculi or become infected. Treatment includes removal of the stone material and management of the cavity. Percutaneous management of the caliceal diverticulum offers the highest stone-free rate of available treatment options. We prefer and have found the technique of diverticulum ablation without dilation of the infundibulum to be highly successful. In our comparison of diverticulum ablation with infundibular dilation, we found that ablation resulted in faster operative times, fewer complications, higher stone-free status, and shorter hospitalization. For this reason, we perform a single-stage or pre-PCNL opacification technique for all symptomatic caliceal diverticula we manage with a subcostal approach. Removal of all stone fragments in the diverticula is necessary for maximal ablation of the diverticular cavity.
Although stasis most likely plays a role in stone formation in a caliceal diverticulum, we and others have noted that metabolic stone risks are also present in many of these patients, and thus we recommend that patients undergo 24-hour urine metabolic evaluation.
Abbreviations Used
- CT
computed tomography
- IVU
intravenous urography
- KUB
kidneys, ureters, and bladder
- PCNL
percutaneous nephrolithotomy
- SWL
shockwave lithotripsy
- URS
ureteroscopy
- US
ultrasonography
Disclosure Statement
Dr. James E. Lingeman: Cook—Consultant/Advisor; BSC—Consultant/Advisor, Meeting Participant/Lecturer, and Scientific Study/Trial; Olympus—Scientific Study Trial.
References
- 1.Timmons JW., Jr Malek RS. Hattery RR. Deweerd JH. Caliceal diverticulum. J Urol. 1975;114:6–9. doi: 10.1016/s0022-5347(17)66930-1. [DOI] [PubMed] [Google Scholar]
- 2.Middleton AW., Jr Pfister RC. Stone-containing pyelocaliceal diverticulum: Embryogenic, anatomic, radiologic and clinical characteristics. J Urol. 1974;111:2–6. doi: 10.1016/s0022-5347(17)59872-9. [DOI] [PubMed] [Google Scholar]
- 3.Michel W. Finker PJ. Tunn UW. Senge T. Pyelocalyceal diverticula. Int Urol Nephrol. 1985;17:225–230. doi: 10.1007/BF02085408. [DOI] [PubMed] [Google Scholar]
- 4.Wulfsohn MA. Pyelocaliceal diverticula. J Urol. 1980;123:1–8. doi: 10.1016/s0022-5347(17)55748-1. [DOI] [PubMed] [Google Scholar]
- 5.Gross AJ. Herrmann TR. Management of stones in caliceal diverticulum. Curr Opin Urol. 2007;17:136–140. doi: 10.1097/MOU.0b013e328011bcd3. [DOI] [PubMed] [Google Scholar]
- 6.Yow RM. Bunts RC. Calyceal diverticulum. J Urol. 1955;73:663–670. doi: 10.1016/S0022-5347(17)67451-2. [DOI] [PubMed] [Google Scholar]
- 7.Williams G. Blandy JP. Tresidder GC. Communicating cysts and diverticula of the renal pelvis. Br J Urol. 1969;41:163–170. doi: 10.1111/j.1464-410x.1969.tb09918.x. [DOI] [PubMed] [Google Scholar]
- 8.Canales B. Monga M. Surgical management of the calyceal diverticulum. Curr Opin Urol. 2003;13:255–260. doi: 10.1097/00042307-200305000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Hsu TH. Streem SB. Metabolic abnormalities in patients with caliceal diverticular calculi. J Urol. 1998;160:1640–1642. [PubMed] [Google Scholar]
- 10.Liatsikos EN. Bernardo NO. Dinlenc CZ, et al. Caliceal diverticular calculi: Is there a role for metabolic evaluation? J Urol. 2000;164:18–20. [PubMed] [Google Scholar]
- 11.Matlaga BR. Miller NL. Terry C, et al. The pathogenesis of calyceal diverticular calculi. Urol Res. 2007;35:35–40. doi: 10.1007/s00240-007-0080-x. [DOI] [PubMed] [Google Scholar]
- 12.Burns JR. Finlayson B. Gauthier J. Calcium oxalate retention in subjects with crystalluria. Urol Int. 1984;39:36–39. doi: 10.1159/000280941. [DOI] [PubMed] [Google Scholar]
- 13.Auge BK. Maloney ME. Mathias BJ, et al. Metabolic abnormalities associated with calyceal diverticular stones. BJU Int. 2006;97:1053–1056. doi: 10.1111/j.1464-410X.2006.06134.x. [DOI] [PubMed] [Google Scholar]
- 14.Rathaus V. Konen O. Werner M, et al. Pyelocalyceal diverticulum: The imaging spectrum with emphasis on the ultrasound features. Br J Radiol. 2001;74:595–601. doi: 10.1259/bjr.74.883.740595. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs RP. Kane RA. Sonographic appearance of calculi in renal calyceal diverticula. J Clin Ultrasound. 1984;12:289–291. doi: 10.1002/jcu.1870120512. [DOI] [PubMed] [Google Scholar]
- 16.Matlaga BR. Kim SC. Watkins SL, et al. Pre-percutaneous nephrolithotomy opacification for caliceal diverticular calculi. J Endourol. 2006;20:175–178. doi: 10.1089/end.2006.20.175. [DOI] [PubMed] [Google Scholar]
- 17.Jones JA. Lingeman JE. Steidle CP. The roles of extracorporeal shock wave lithotripsy and percutaneous nephrostolithotomy in the management of pyelocaliceal diverticula. J Urol. 1991;146:724–727. doi: 10.1016/s0022-5347(17)37906-5. [DOI] [PubMed] [Google Scholar]
- 18.Psihramis KE. Dretler SP. Extracorporeal shock wave lithotripsy of caliceal diverticula calculi. J Urol. 1987;138:707–711. doi: 10.1016/s0022-5347(17)43349-0. [DOI] [PubMed] [Google Scholar]
- 19.Streem SB. Yost A. Treatment of caliceal diverticular calculi with extracorporeal shock wave lithotripsy: Patient selection and extended follow-up. J Urol. 1992;148:1043–1046. doi: 10.1016/s0022-5347(17)36812-x. [DOI] [PubMed] [Google Scholar]
- 20.Auge BK. Munver R. Kourambas J, et al. Endoscopic management of symptomatic caliceal diverticula: A retrospective comparison of percutaneous nephrolithotripsy and ureteroscopy. J Endourol. 2002;16:557–563. doi: 10.1089/089277902320913233. [DOI] [PubMed] [Google Scholar]
- 21.Grasso M. Lang G. Loisides P, et al. Endoscopic management of the symptomatic caliceal diverticular calculus. J Urol. 1995;153:1878–1881. [PubMed] [Google Scholar]
- 22.Fuchs GJ. Fuchs AM. [Flexible endoscopy of the upper urinary tract. A new minimally invasive method for diagnosis and treatment.] (Ger) Urologe A. 1990;29:313–320. [PubMed] [Google Scholar]
- 23.Batter SJ. Dretler SP. Ureterorenoscopic approach to the symptomatic caliceal diverticulum. J Urol. 1997;158:709–713. doi: 10.1097/00005392-199709000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Auge BK. Munver R. Kourambas J, et al. Neoinfundibulotomy for the management of symptomatic caliceal diverticula. J Urol. 2002;167:1616–1620. [PubMed] [Google Scholar]
- 25.Hulbert JC. Reddy PK. Hunter DW, et al. Percutaneous techniques for the management of caliceal diverticula containing calculi. J Urol. 1986;135:225–227. doi: 10.1016/s0022-5347(17)45590-x. [DOI] [PubMed] [Google Scholar]
- 26.Shalhav AL. Soble JJ. Nakada SY, et al. Long-term outcome of caliceal diverticula following percutaneous endosurgical management. J Urol. 1998;160:1635–1639. [PubMed] [Google Scholar]
- 27.Al-Basam S. Bennett JD. Layton ZA, et al. Treatment of caliceal diverticular stones: Transdiverticular percutaneous nephrolithotomy with creation of a neoinfundibulum. J Vasc Interv Radiol. 2000;11:885–889. doi: 10.1016/s1051-0443(07)61806-5. [DOI] [PubMed] [Google Scholar]
- 28.Monga M. Smith R. Ferral H. Thomas R. Percutaneous ablation of caliceal diverticulum: Long-term followup. J Urol. 2000;163:28–32. [PubMed] [Google Scholar]
- 29.Bellman GC. Silverstein JI. Blickensderfer S. Smith AD. Technique and follow-up of percutaneous management of caliceal diverticula. Urology. 1993;42:21–25. doi: 10.1016/0090-4295(93)90327-7. [DOI] [PubMed] [Google Scholar]
- 30.Kim SC. Kuo RL. Tinmouth WW, et al. Percutaneous nephrolithotomy for caliceal diverticular calculi: A novel single stage approach. J Urol. 2005;173:1194–1198. doi: 10.1097/01.ju.0000152320.41995.c2. [DOI] [PubMed] [Google Scholar]
- 31.Ruckle HC. Segura JW. Laparoscopic treatment of a stone-filled, caliceal diverticulum: A definitive, minimally invasive therapeutic option. J Urol. 1994;151:122–124. doi: 10.1016/s0022-5347(17)34887-5. [DOI] [PubMed] [Google Scholar]
- 32.Miller SD. Ng CS. Streem SB. Gill IS. Laparoscopic management of caliceal diverticular calculi. J Urol. 2002;167:1248–1252. [PubMed] [Google Scholar]
- 33.Gluckman GR. Stoller M. Irby P. Laparoscopic pyelocaliceal diverticula ablation. J Endourol. 1993;7:315–317. doi: 10.1089/end.1993.7.315. [DOI] [PubMed] [Google Scholar]
- 34.Harewood LM. Agarwal D. Lindsay S, et al. Extraperitoneal laparoscopic caliceal diverticulectomy. J Endourol. 1996;10:425–430. doi: 10.1089/end.1996.10.425. [DOI] [PubMed] [Google Scholar]
- 35.Hoznek A. Herard A. Ogiez N, et al. Symptomatic caliceal diverticula treated with extraperitoneal laparoscopic marsupialization fulguration and gelatin resorcinol formaldehyde glue obliteration. J Urol. 1998;160:352–355. [PubMed] [Google Scholar]
- 36.Curran MJ. Little AF. Bouyounes B, et al. Retroperitoneoscopic technique for treating symptomatic caliceal diverticula. J Endourol. 1999;13:723–725. doi: 10.1089/end.1999.13.723. [DOI] [PubMed] [Google Scholar]
- 37.Lingeman JE. Matlaga BR. Evan AP. Surgical management of upper urinary tract calculi. In: Kavoussi LR, editor; Novick AC, editor; Partin AW, et al., editors. Campbell-Walsh Urology. 9th. Philadelphia: Saunders-Elsevier; 2007. pp. 1431–1507. [Google Scholar]
- 38.Cohen TD. Preminger GM. Management of calyceal calculi. Urol Clin North Am. 1997;24:81–96. doi: 10.1016/s0094-0143(05)70356-6. [DOI] [PubMed] [Google Scholar]
- 39.Hedelin H. Geterud K. Grenabo L, et al. Percutaneous surgery for stones in pyelocaliceal diverticula. Br J Urol. 1988;62:206–208. doi: 10.1111/j.1464-410x.1988.tb04319.x. [DOI] [PubMed] [Google Scholar]
- 40.Jarrett TW. Smith AD. Percutaneous treatment of caliceal diverticula. In: Smith AD, editor. Controversies in Endourology. Philadelphia: WB Saunders; 1986. [Google Scholar]
- 41.Marcovich R. Smith AD. Percutaneous renal access: Tips and tricks. BJU Int. 2005;95(suppl 2):78–84. doi: 10.1111/j.1464-410X.2005.05205.x. [DOI] [PubMed] [Google Scholar]
- 42.vanSonnenberg E. Casola G. Talner LB, et al. Symptomatic renal obstruction or urosepsis during pregnancy: Treatment by sonographically guided percutaneous nephrostomy. AJR Am J Roentgenol. 1992;158:91. doi: 10.2214/ajr.158.1.1727366. [DOI] [PubMed] [Google Scholar]
- 43.Donnellan SM. Harewood LM. Webb DR. Percutaneous management of caliceal diverticular calculi: Technique and outcome. J Endourol. 1999;13:83. doi: 10.1089/end.1999.13.83. [DOI] [PubMed] [Google Scholar]
- 44.Ellis JH. Patterson SK. Sonda LP, et al. Stones and infection in renal caliceal diverticula: Treatment with percutaneous procedures. AJR Am J Roentgenol. 1991;156:995. doi: 10.2214/ajr.156.5.1902014. [DOI] [PubMed] [Google Scholar]