Abstract
Spinal motion palpation (SMP) is a standard component of a manual therapy examination despite questionable reliability. The present research is inconclusive as to the relevance of the findings from SMP, with respect to the patient’s pain complaints. Differences in the testing methods and interpretation of spinal mobility testing are problematic. If SMP is to be a meaningful component of a spinal examination, the methods for testing and interpretation must be carefully scrutinized. The intent of this narrative review is to facilitate a better understanding of how SMP should provide the examiner with relevant information for assessment and treatment of patients with spinal pain disorders. The concept of just noticeable difference is presented and applied to SMP as a suggestion for determining the neutral zone behavior of a spinal segment. In addition, the use of a lighter, or more passive receptive palpation technique, is considered as a means for increasing tactile discrimination of spinal movement behavior. Further understanding of the scientific basis of testing SMP may improve intra- and inter-examiner reliability. The significance of the findings from SMP should be considered in context of the patient’s functional problem. Methodological changes may be indicated for the performance of SMP techniques, such as central posterior-anterior (PA) pressure and passive intervertebral motion tests, in order to improve reliability. Instructors of manual therapy involved in teaching SMP should be knowledgeable of the neurophysiological processes of touch sensation so as to best advise students in the application of the various testing techniques.
Keywords: Spinal motion palpation, Tactile sensibility, Neurophysiology, Reliability
Introduction
Spinal motion palpation (SMP) is used by manual therapists to guide treatment interventions, yet reliable and valid testing methods have not been established.1–7 Presently, the Treatment Based Classification of patients with non-specific low back pain,8 and one Clinical Prediction Rule9 utilize segmental hypomobility as a criterion for identifying patients who would benefit from mobilization/manipulation. The potential for inappropriate or ineffective interventions may result from utilization of an examination tool with questionable reliability. Pain provocation tests have, to date, demonstrated greater reliability than SMP for detecting lumbar instability.10 Therefore, use of segmental motion tests as a criterion for recognizing patients with lumbar instability is likewise questionable.
The differences in interpretation of spinal motion behavior by palpation may relate to an examiner’s focus. For example, the examiner may concentrate on the amount of displacement of the spinal motion segment or its resistance to manual force or pressure. Examiners may also assess the velocity of vertebral displacement or the sequential order in which the vertebra are recruited during passive intervertebral motion testing. To more fully understand and interpret the SMP process, a review of touch sensation is appropriate. Understanding the mechanisms involved in touch sensation may help determine if modifications in motion palpation can improve reliability. Consequently, one objective of this review is to examine the various neurophysiological mechanisms involved in the processing of touch sensation.
Tactile Sensibility of the Human Hand
Understanding the tactile acuity of the hand and fingertips relative to spinal motion testing requires review of the different mechanoreceptors and end organs responsible for mediating the sense of touch.11 The relative densities and receptive fields of each type of mechanoreceptor determine the function of the mechanoreceptor in tactile perception. Four types of highly sensitive mechanoreceptor units in the glabrous (hairless) skin of the human hand have been identified — RA, PC, SA I, and SA II.12 The end organs of these mechanoreceptors are known, respectively, as Meissner corpuscles, Pacinian corpuscles, Merkel receptors, and Ruffini end organs.13
Mechanoreceptors mediating the sensation of touch are divided into two functional groups: slowly adapting and rapidly adapting.11 Slowly adapting mechanoreceptors, such as Merkel receptors in the skin and Ruffini corpuscles in the subcutaneous tissue layer beneath the glabrous skin of the hand, respond continuously to constant stimuli.
Rapidly adapting mechanoreceptors, such as the Meissner corpuscles in the glabrous skin of the hand and Pacinian corpuscles in the subcutaneous layer, respond to changes in velocity at the onset and/or end of a movement activity.11 The number of slow and rapid adapting mechanoreceptors in the glabrous skin area of the hand is about the same.13 The overall density of mechanoreceptors increases slightly from the palm to the proximal phalanx of the finger, but increases abruptly from the proximal phalanx of the finger to the tip of the finger. The RA and SA I mechanoreceptor units containing Meissner and Merkel’s corpuscles account for the greatest change in overall density from the palm to the finger tip (Fig. 1).
Figure 1.
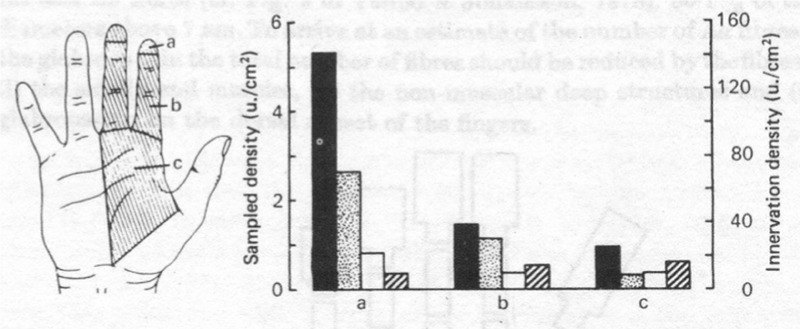
Overall density of mechanoreceptors at the finger tip, proximal finger, and palm demonstrating the finger tip to contain approximately five times more Meissner corpuscles than the palm. Filled dark column denotes Meissner corpuslces, stippled gray column denotes Merkel’s receptors, hollow white column denotes Pacinian corpuscles, and hatched column denotes Ruffini end organs (Johansson RS, Vallbo AB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J Physiol 1979; 286: 291).13
In addition to the proximal to distal mechanoreceptor density gradient from palm to finger tip, differences were also noted across finger tips. The highest concentration of mechanoreceptors is found on the index and middle fingers versus the thumb and radial half of the ring finger. Consequently, the higher density of mechanoreceptor units in the first and second finger tips may allow these fingers to be more suitable for SMP.
The receptive field of a mechanoreceptor corresponds to that region of the skin or subcutaneous tissue that is innervated by the terminals of the receptor and the surrounding tissue conducting the stimulus to the receptor. The size of the receptor field determines the ability of the receptors to discriminate spatial details.11,14 Smaller receptor fields are more discriminatory than larger receptor fields. Meissner’s corpuscles and Merkel’s cells located on the fingers have smaller receptor fields in contrast to Pacinian and Ruffini corpuscles. As a result, Meissner corpuscles and Merkel receptors on the fingers can resolve fine spatial differences. Pacinian and Ruffini corpuscles can only detect coarse spatial differences. Therefore, with respect to SMP, the smaller receptor fields of the Meissner corpuscles and Merkel receptors are more discriminatory for the recognition of the subtle spatial changes incurred by vertebral motion testing. In the brain, the cortical area representing the fingers is greater than the palm. Consequently, the size of the receptive field and its cortical representation are inversely related.15
Sensory discrimination, quantified by measuring two-point discrimination thresholds, varies throughout the body.11 Finger tip two-point discrimination is five times greater than on the palm. As a result, the greatest capacity for sensory discrimination resides in the fingertips where there is the highest density of mechanoreceptors, the smallest receptive fields, and the greatest cortical representation.11 Therefore, from a neurophysiological perspective, the finger tip assessment used in passive intervertebral motion testing has greater potential for motion discrimination than the palmar pressure used in producing posterior-anterior (PA) test force.
The rapidly adapting mechanoreceptors in the glabrous skin of the finger tips contain Meissner’s corpuscles, which are sensitive to low frequency sinusoidal mechanical stimuli and play a critical role in grip control.16 When assessing passive intervertebral motion, a steady skin indentation stimulus generated by sustained palpation pressure motion results in prompt inactivation of the rapidly adapting mechanoreceptors within several hundred milliseconds.11 The slowly adapting mechanoreceptors in the skin remain active to evaluate the spatial and temporal characteristics of the motion stimulus by providing ongoing information about the forces on the hand.16 Given the firing pattern behavior of the rapid adapting mechanoreceptors, the clinician involved in SMP should appreciate the significance of using light pressure to facilitate activation of mechanoreceptors as well as recognize the importance of evaluating the initial motion response.
Central Processing of Sensory Motion Stimuli
The detection of a movement stimulus is a primary function of higher cortical neuronal activity within areas 1, 2, and 3 of the somatosensory cortex.15 Understanding that the somatosensory cortex is the executer for detecting somatic sensation is fundamental to SMP. The role of our somatosensory cortex in the manual detection and coding of various movement activity demonstrates the importance of trusting our sensory perceptions of movement rather than critically analyzing the motion event by overutilization of the associative areas in the prefrontal cortex. An analogy perhaps applicable to the importance of recognizing the contribution of the somatosensory cortex in SMP detection relates to the intellect’s approach to learning how to dance by analyzing the steps or numbers instead of feeling or trusting their body movements. Touch perception should, therefore, involve a primary feeling process and not a primary thought process. Both educators of manual therapy and students should be aware that excessive prefrontal cortex critical analysis during SMP may actually interfere with accurate identification of the movement event given that central processing of the movement occurs within the somatosensory cortex.
Each of the four regions of the primary somatic sensory cortex receives input from all areas of the body surface. Area 1 receives input from the rapidly adapting cutaneous receptors and respective neurons whereas area 2 obtains input from the deep pressure receptors.15 The neural image in the sensory cortex of a non-painful sensory stimulus, however, does not always represent the exact sensation. In one study involving the fingers of awake monkeys, the pressure detecting Pacinian corpuscles in the subcutaneous region did not transmit an exact signal of a sensory stimulus.17 As a result, the cortical representation of the sensation in area 2 was different from the actual sensation. In contrast, the slow adapting Merkel and rapid adapting Meissner touch receptors in the skin transmitted a true neural image of the sensation in area 1 that was consistently reproduced in the somatosensory cortex.17 The clinical implication of touch sensors conducting a truer sensory signal than pressure receptors favors the use of finger tip palpation to detect spinal segmental motion versus manual pressure techniques such as spring tests.
Understanding the relationship between the discharge of sensory neurons from touch receptors and the perceived intensity of the stimulus is essential for accurately detecting motion activity. The concept of just noticeable difference introduced by Weber18 and extended by Fechner19 refers to the smallest difference perceivable between a reference stimulus, such as a palpating finger, and a second stimulus, such as the initial motion induced at a spinal segment.19 When the motion stimulus exceeds the sensory threshold, the stimulus is detected by the palpation finger. The amount of motion stimulus necessary to create sensation is the sensation of the just noticeable difference.20,21
The sensitivity of a sensory system to differences in sensation is, therefore, a function of the strength of a stimulus. For example, the ability to feel a difference between 1 and 2 kg is easier than feeling a difference between 50 and 51 kg, despite the fact the difference between weights is the same. In accordance with the Fechner–Weber theory,19 the perception of motion or stiffness at a spinal segment is likely to be more discriminatory when the intensity of the force application is applied in a manner to detect the just (first) noticeable displacement. Clinical application of the Fechner–Webner19 theory to the delivery of PA forces onto spinous processes of a prone patient suggests that the examiner is less able to perceive motion displacement or stiffness when a force of 150 N is applied that results in 2.5° of segmental extension than if a force of 50 N is applied which results in 1° of segmental extension. A lighter test force, which detects the first noticeable displacement of spinal segmental movement, is likely to be a more accurate motion sensor than stronger test forces that produce more movement. Recognition of the neurophysiological significance for using lighter test forces coupled with the understanding that manual therapists often underestimate the amount of force generated in testing for spinal motion22 is an important concept for manual therapy instructors.
Most studies examining the encoding of information from peripheral tactile sensory systems have focused on the firing rates of individual neurons which are calculated by spike counts in a given period of time.16,23,24 A recent finding demonstrates that the firing rates assessed by the total number of spike counts provide information about the shape and force direction of an object contacted.25 In a comparative study, however, the information from tactile afferent neurons, with regard to surface curvature and force direction based on initial spike timing, was at least 2.2 and 1.6 times, respectively, larger than that of total spike counts during a 125-ms period of force increase.26 The practical application of this study regarding the importance of first spike timing in tactile perception of surface contour and force direction relates to the emphasis an examiner should place on the initial motion event induced by a passive motion test.
Age Factor in Affecting Tactile Perception
Tactile perception is influenced by many factors such as aging, finger tip conformation, usage or immobilization, vision, attention, and frequency usage. Aging results in structural modifications in both Meissner’s and Pacinian corpuscles as well as in a reduction in number and cross-sectional area.27–29 The vibrotactile sensitivity of pathways involving Pacinian corpuscles has been reported to be impaired with age.30 Older adults (mean ages of 55 and 65 years) also have a reduction in Meissner corpuscles in the index finger and an impaired touch threshold that is 2.5 times greater than younger control subjects (mean ages of 10, 20, and 35 years).31 In addition, older subjects (≥65 years of age) were found to have an average decline of 70% in two-point discrimination in the fingertips.32 The loss of spatial acuity with aging is not only related to the loss of skin receptors, but also to progressive thinning of the receptors.33 The atrophy of myelinated primary sensory neurons may also account for an age-related reduction in sensory nerve conduction velocity.34 Degradation of tactile senses as a result of aging may be a significant consideration with respect to reliability studies of SMP.
Skin Conformation and Tactile Acuity
The ability of the finger tip skin to conform to the spatial details of a surface or object is also a factor in tactile spatial acuity. Skin conformance has been found to account for 50% of the variance in tactile spatial acuity in young subjects.35 Subjects with more compliant finger tip skin had greater conformation to the skin surface palpated than subjects with less finger tip compliant skin.35 In another study on finger tip skin conformance, a 30 g force produced 1.5 mm of skin indentation while an increase of force to 100 g only caused an additional 0.6 mm of indentation.36 Therefore, forces greater than 30 or 40 g result in minimal additional finger tip skin conformation. Subjects with the most compliant skin on the finger tips produced large indentation (compliance) with light force and were found to have substantially lower tactile perception thresholds. Therefore, the manner in which the local skin tissue is distorted determines the accuracy of tactile spatial acuity rather than the intensity of pressure delivered. Since skin conformance was found to be virtually identical in young and old subjects, the loss of spatial acuity with aging is most likely due to changes in the neural mechanisms.35
Visual Tactile Enhancement of Tactile Perception
Tactile perception is reportedly improved by visual stimulus.37–39 Viewing the hand while palpating versus viewing a neutral object (a flat piece of wood) appearing in the same spatial location as the hand enhances the sense of touch. Study participants had faster tactile reaction times,37 improved two point discrimination,38 and better grating discrimination thresholds (ability to discern alternate grooves and ridges with the fingertips) while viewing the hand.39 The concept of visual-tactile enhancement by viewing the hand allows a preset or an anticipatory tuning of neural circuits in the primary somatosensory cortex that facilitates the processing of a subsequent tactile stimulus.40
The importance of the visual cortex in tactile perception has been further demonstrated by increased activation of the visual cortex near the parieto-occipital fissure on positron emission tomography during tactile discrimination of ridges and grooves that were either wide apart or narrow (grating orientation). This is an example of a cross-modal interaction that characterizes normal perception whereby visual imagery is used to enhance tactile sensing.41 Conversely, blocking visual cortical processing disrupts tactile discrimination of orientation.42 Increased activity in the visual cortical areas during tactile shape discrimination has been noted on functional MRIs.43 Given the enhancement of tactile perception secondary to the activation of the visual cortex, the use of visual imagery in motion palpation may help to improve accuracy and consistency in detecting spinal segmental movement.
Attentive State and Sensory Processing
The attention an examiner devotes to spinal motion palpation may also determine the outcome interpretation. Attention has been defined as the ‘state of mind wherein an individual expects particular information and prepares to perceive and act on that information’.44 Being attentive is, therefore, an active process whereby one attends to a specific stimulus when there are various competing stimuli. Manipulating the attentive state has been shown to modify the processing of sensory information and alter the perception of vibrotactile stimuli and texture changes.45,46 Psychophysiological studies have demonstrated the detection of tactile stimuli is best when attention is directed toward the tactile modality and poorest when directed away from the tactile modality and towards a visual modality.47,48 Manipulating the examiner’s attention by means of different colored LED instructional cues significantly influenced the ability of subjects to detect and discriminate small changes in texture on the palmar surface of the distal phalanx of the middle finger of the dominant hand using passive touch.49 Both accuracy and reaction time significantly improved when the subject’s attention was selectively directed toward four different textured surfaces, as compared to when attention was misdirected towards a visual stimulus. In regard to the teaching and learning of SMP, the examiner’s attention should be directed towards the patient’s body.
Sensory Perception Enhancement through Movement Stimulus
Although the perception of coarse textures, such as embossed dots on a page or the array of teeth on a comb, can be discriminated by spatial cues (size, shape, density, and arrangement of the structure),50 the perception of fine texture is difficult using spatial cues alone.51 Perception of fine textures (particle sizes of 9–15 μm) is possible because of the ability to detect and discriminate information-rich vibrations that are produced when the skin (finger tip) moves across a surface (spinal segments) or when a surface moves on the skin. Eliminating movement interferes with the perception of fine surfaces because, without movement, there is little cutaneous vibration.52 Thus, a temporal code (vibrotaction) provides an essential contribution to the perception of fine textured surfaces while a spatial code predominates for the detection and discrimination of coarse textures. The Pacinian channels play a more important role than the RA (Meissner and Merkel mechanoreceptors) channels in discriminating fine textured surfaces. Both channels demonstrate greater discrimination with low-frequency vibrations (10 and 30 Hz) than with higher frequency vibrations (100 and 300 Hz).48
The speed at which a fine textured surface moves over the skin of the finger tip, therefore, affects the ability to discriminate a sensory stimulus. This study suggests that a slow induction of spinal movement during SMP testing may enhance tactile discrimination.
Significance of Frequency of Use on Tactile Perception
Psychophysiological studies have demonstrated that tactile spatial resolution is greater in blind subjects than in age-matched sighted subjects.53–55 Blind subjects show no age-related decline in tactile acuity in comparison to age-matched sighted subjects who demonstrate a decrease in tactile acuity of nearly 1% per year.56 The tactile acuity of blind subjects did not correlate with Braille reading speed, the amount of Braille reading per day, the age in which Braille was learned, or the years of blindness.56 The use of active touch (tactile scanning with the fingers to explore objects)57 in activities of daily living by the blind appears to be the most important factor in the preservation of tactile acuity across the life span rather than Braille reading.56 The difference observed may relate to the reliance on vision by sighted people to distinguish between keys, coins, or clothing fabric texture, whereas blind people rely completely on tactile shape or texture discrimination. Sighted individuals handle and touch the same objects as blind people, but, in contrast to the blind, are not required to focus all of their attention on the geometry and texture of the objects being discriminated.
Further support for the contention that increased use, reliance, and focus improve tactile perception has been demonstrated in a study that immobilized hands and arms of 31 subjects for an average of 5.8 weeks.58 Following the immobilization period, the spatial two-point discrimination thresholds on the tip of the index finger were significantly higher for the immobilized index finger when compared to the non-immobilized finger. Discrimination thresholds of the immobilized hand returned to control levels on the non-immobilized side in 2–3 weeks. Immobilization also appears to have an effect on cortical activation. In contrast to the enlargement of cortical maps from increased use, 2 weeks of immobilization of the index finger resulted in a significant decrease in blood oxygen levels, as measured by functional MRI, within the related S1 area of the somatosensory cortex. The reduced vascular activation in the somatosensory cortex secondary to enforced immobilization was parallel to the impairment in tactile perception.58
Increased frequency of use of the hands may also account for the significantly higher spatial acuity in professional pianists as compared to nonmusicians.59 Tai Chi practitioners, likewise, have been shown to have tactile spatial acuity greater than age-matched gender controls.60 The results of this study are not due to direct touch tactile training, but perhaps from the Tai Chi practitioner’s mental attention on the body (particularly the hands and fingers) as slow movement activity is performed. Both the pianist and the practitioner of Tai Chi have intensive practice routines potentially enhancing neuronal efficiency and cortical organization of tactile acuity leading to lower spatial discrimination thresholds.
Summary
Many variables affect tactile perception: finger tip utilized, intensity of the motion stimulus (or degree of manual pressure utilized), speed of the motion induced, visual focus to the tactile task, attentiveness to the motion exam, finger tip conformation to the spatial details of the surface, texture of the surface, age of the examiner, and frequency of use of the finger tips (Table 1). Evidence exists for using either the first or second fingers for spinal motion palpation because of the higher number of mechanoreceptors located in these finger tips. Finger contact that optimizes skin conformation to the body surface is recommended in order to lower thresholds of tactile perception in favor of the amount of finger pressure. A passive receptive approach to spinal motion palpation involving palpation contact with minimal palpation pressure enhances the activation of touch mechanoreceptors such as Meissner’s corpuscles and Merkel’s endings and conducts a true signal of the motion event to the somatosensory cortex.
Table 1. SMP clinical considerations to improve accuracy and reliability.
| Variables affecting tactile perception | Possible ramifications for SMP |
| Finger tip palpation versus palmar palpation | Utilize one of the first two finger tips to assess passive intervertebral motion versus palmer pressure to analyze vertebral spring |
| Trust of the somatosensory impression of the movement stimulus analyzed by palpation | Excessive over-analysis of a movement stimulus assessed by palpation may lead to false interpretation of the motion event. SMP is centrally processed in the somatosensory cortex not the pre-frontal cortex |
| Use of touch sensors or pressure sensors | Touch sensors conduct a truer signal of the motion event to the somatosensory cortex than pressure sensors |
| Palpatory contact or force used | Use of light contact or force is more accurate than greater force for motion palpation |
| Analysis of the motion event | Focus on palpating the initial motion elicited instead of concentrating solely on the amount of motion |
| Age | Degradation of tactile receptors with age may affect SMP discrimination. Take care of your hands and fingers |
| Finger tip skin conformation to the spatial details of the body surface being palpated | Skin conformation of the hand and finger tip to the body part palpated enhances tactile perception more than the amount of pressure |
| Actual viewing of the body | Actual viewing of the body facilitates the processing of tactile stimuli by presetting neural circuits in the somatosensory cortex |
| Visual imagery | Visual imagery enhances tactile sensory discrimination |
| Attentiveness to the motion task | Attentional direction to SMP may significantly influences tactile detection and discrimination |
| Test movement induction | Slow test movement induction enhances tactile perception of fine textured surfaces (i.e. skin) |
| Frequency of use | Frequent practice of motion palpation may improve tactile acuity |
Recognition of the just noticeable difference between the motion stimulus induced by the test and the motion event may also increase tactile perception of the movement. Spinal segmental motion analysis of the first noticeable movement induced by a gentle test movement stimulus places emphasis on the evaluation of the neutral zone behavior in the spinal motion segment. For example, during passive physiological intervertebral motion testing of lumbar segmental forward bending, the manual therapist palpates the ease in which the spinous processes separate upon the first arrival of motion at the segment instead of concentrating on the degree to which the spinous processes separate. If examining the initial resistance-free movement (described as the neutral zone of the spinal motion segment) enhances tactile perception, intertherapist reliability may improve. Examination of spinal segmental motion behavior using the just noticeable difference concept may also contribute to the clinical understanding of spinal segmental instability and provide support for motor control management strategies.
The concept of just noticeable difference may also apply to the central PA test commonly used to assess vertebral motion stiffness at the segmental level. Induction of gentle PA force would less likely influence adjacent vertebral segments and thus be more specific to the test segment, but may also allow for greater sensory discrimination of neutral zone behavior than a stronger force used to assess total amount of displacement. Perhaps previous studies on spinal motion palpation have placed too much emphasis on segmental range of motion and not enough focus on motion quality such as ease or resistance to movement.
To improve tactile perception, the manual therapist is also encouraged to use visual focus and/or visual imagery to the spinal region to be examined when possible. Full attention to the motion test has also been found to facilitate tactile detection and awareness. Since motion awareness and perception accuracy is enhanced through repetition, the manual therapist is encouraged to use SMP as a routine part of every spinal pain patient examination. With respect to passive intervertebral motion testing, the examiner should also consider inducing the segmental motion slowly, since the ability to discriminate the sensory motion stimulus is enhanced at slower speeds.
Manual therapists applying spinal motion tests to help determine the nature of a patient’s motion impairment and guide treatment decisions should be aware of the various factors that may influence the interpretation of a spinal segmental motion event.
Researchers examining reliability of spinal motion examination techniques used by manual therapists need to account for the variables that affect tactile perception in the design of studies assessing spinal segmental movement. Additional consideration of the variables that affect tactile perception is necessary to obtain meaningful information from SMP tests that help decide an appropriate intervention. The decision to utilize manipulation for the purpose of improving spinal mobility, or a motor control exercise approach to provide spinal stabilization, may be determined at least in part from an accurate interpretation of spinal motion by palpation. The accuracy in interpreting spinal segmental motion by palpation is, therefore, likely to affect treatment outcome.
References
- 1.Binkley J, Stratford PW, Gill C. Interrater reliability of lumbar accessory motion mobility testing. Phys Ther. 1995;75:786–92; discussion 793–5 [DOI] [PubMed] [Google Scholar]
- 2.Maher C, Adams R. Reliability of pain and stiffness assessments in clinical manual lumbar spine examination. Phys Ther. 1994;74:801–11 [DOI] [PubMed] [Google Scholar]
- 3.Schneider M, Erhard R, Brach J, Tellin W, Imbarlina F, Delitto A. Spinal palpation for lumbar segmental mobility and pain provocation: an interexaminer reliability study. J Manipulative Physiol Ther. 2008;31:465–73 [DOI] [PubMed] [Google Scholar]
- 4.Seffinger MA, Najm WI, Mishra SI, Adams A. Reliability of spinal palpation for diagnosis of back and neck pain: a systematic review of the literature. Spine. 2004;29(19):E413–25 [DOI] [PubMed] [Google Scholar]
- 5.Huijbregts P. Spinal motion palpation: a review of reliability studies. J Man Manip Ther. 2002;10:24–9 [Google Scholar]
- 6.Maher CG, Latimer J, Adams R. An investigation of the reliability and validity of posteroanterior spinal stiffness judgments made using a reference-based protocol. Phys Ther. 1998:78:829–37 [DOI] [PubMed] [Google Scholar]
- 7.Landel R, Kulig K, Fredericson M, Li B, Powers CM. Intertester reliability and validity of motion assessments during lumbar spine accessory motion testing. Phys Ther. 2008;88:43–9 [DOI] [PubMed] [Google Scholar]
- 8.Delitto A, Erhardt R, Bowling R. A treatment-based classification approach to low back syndrome: identifying and staging patients for conservative treatment. Phys Ther. 1995;75:470–85 [DOI] [PubMed] [Google Scholar]
- 9.Flynn T, Fritz J, Whitman J, Wainner R, Magel J, Rendeiro D, et al. A clinical prediction rule for classifying patients with low back pain who demonstrate short-term improvement with spinal manipulation. Spine. 2002;27:2835–43 [DOI] [PubMed] [Google Scholar]
- 10.Hicks GE, Fritz JM, DeLitto A, Mishock J. Interrater reliability of clinical examination measures for identification of lumbar segmental instability. Arch Phys Med Rehabil. 2003;84:1858–64 [DOI] [PubMed] [Google Scholar]
- 11.Martin JH, Jessel TM. Modality coding in the somatic sensory system. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science. 3rd ed New York: Elsevier; 1991. p. 344–6 [Google Scholar]
- 12.Knipestol M, Vallbo AB. Single unit analysis of mechanoreceptor activity from the human glabrous skin. Acta Physiol Scand. 1970;80:178–195 [DOI] [PubMed] [Google Scholar]
- 13.Johansson RS, Vallbo AB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptor units in glabrous skin. J Physiol. 1979;286:283–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson RS, Vallbo AB. Tactile sensory coding in the glabrous skin of the human hand. Trends Neurosci. 1983;6:27–32 [Google Scholar]
- 15.Kandel ER, Jessel TM. Touch. In: Kandel ER, Schwartz JH, Jessel TM, editors. Principles of neural science. 3rd ed New York: Elsevier; 1991. p. 374–80 [Google Scholar]
- 16.Johnson KO, Yoshioka T, Vega-Bermudez F. Tactile functions of mechanoreceptive afferents innervating the hand. J Clin Neurophysiol. 2000;6:539–55 [DOI] [PubMed] [Google Scholar]
- 17.Phillips JR, Johnson KO, Hsiaso SS. Spatial pattern representation and transformation in monkey somatosensory cortex. Proc Natl Acad Sci USA. 1988;85:1317–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber EH. Der Tastsinn und das Gemeingefuhl. In: Wagner R, editor. Hanworterbuch der Physiologie. Vol. III, Abt. 2 Brunschweig: Vieweg; 1846. p. 481–588 [Google Scholar]
- 19.Fechner G. Elements of psychophysics. New York: Holt, Rinehart and Winston; 1966 [Google Scholar]
- 20.Stevens SS. Psychophysics: introduction to its perceptual, neural, and social prospects. New York: Wiley; 1975 [Google Scholar]
- 21.Herman IP. Physics of the human body. New York: Springer; 2007. p. 25–6 [Google Scholar]
- 22.Simmonds MJ, Kumar S, Lechelt E. Use of a spinal model to quantify the forces and motion that occur during therapists’ tests of spinal motion. Phys Ther. 1995;75(3):212–22 [DOI] [PubMed] [Google Scholar]
- 23.Craig JC, Rollman GB. Somesthesis. Annu Rev Psychol. 1999;50:305–31 [DOI] [PubMed] [Google Scholar]
- 24.Goodwin AW, Wheat HE. Sensory signals in neural populations underlying tactile perception and manipulation. Annu Rev Neurosci. 2004;27:53–77 [DOI] [PubMed] [Google Scholar]
- 25.Johansson RS, Birznieks I. First spikes in ensembles of human tactile afferents code complex spatial fingertip events. Nat Neurosci. 2004;7:1170–77 [DOI] [PubMed] [Google Scholar]
- 26.Saal HP, Vijayakumar S, Johansson RS. Information about complex fingertip parameters individual human tactile afferent neurons. J Neurosci. 2009;29(25):8022–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens SS. On the brightness of lights and the loudness of sounds. Science. 1953;118:576 [Google Scholar]
- 28.Cauna N, Mannan G. The structure of human digital pacinian corpuscles (corpus cula lamellose) and its functional significance. J Anat. 1958;92:1–20 [PMC free article] [PubMed] [Google Scholar]
- 29.Bolton CF, Winkelman RK, Dyck PJ. A quantitative study of meissner’s corpuscles in man. Neurology. 1966;16:1–9 [DOI] [PubMed] [Google Scholar]
- 30.Verrillo RT. Change in vibrotactile threshold as a function of age. Sens Processes. 1979;3:49–59 [PubMed] [Google Scholar]
- 31.Bruce MF. The relation of tactile thresholds to histology in the fingers of elderly people. J Neurol Neurosurg Psychiatry. 1980;43:730–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens JC, Alvarez-Reeves M, Dipietro L, Mack GW, Green BG. Decline of tactile acuity in aging: a study of body site, blood flow, and lifetime habits of smoking and physical activity. Somatosens Mot Res. 2003:20:271–9 [DOI] [PubMed] [Google Scholar]
- 33.Stevens JC, Patterson MQ. Dimensions of spatial acuity in the touch sense: changes over the life span. Somatosens Mot Res. 1995;12:29–47 [DOI] [PubMed] [Google Scholar]
- 34.Bergman E, Ulfhake B. Evidence for loss of myelinated input to the spinal cord in senescent rats. Neurobiol Aging. 2002;23:271–86 [DOI] [PubMed] [Google Scholar]
- 35.Vega-Bermudez F, Johnson KO. Fingertip skin conformance accounts, in part, for differences in tactile acuity in young subjects, but not for the decline in spatial acuity with aging. Percept Psychophys. 2004;66:60–7 [DOI] [PubMed] [Google Scholar]
- 36.Vega-Bermudez F, Johnson KO. SA I and RA receptive fields, response variability, and population responses mapped with a probe array. J Neurophysiol. 1999;81:2701–10 [DOI] [PubMed] [Google Scholar]
- 37.Phillips JR, Johnson KO. Tactile spatial resolution: III. A continuum mechanics model of skin predicting mechanoreceptor responses to bars, edges, and gratings. J Neurophysiol. 1981;46:1204–25 [DOI] [PubMed] [Google Scholar]
- 38.Tipper SP, Lloyd D, Shorland B, Dancer C, Howard LA, McGlone F. Vision influences tactile perception without propioception orienting. Neuroreport. 1998;9:1741–4 [DOI] [PubMed] [Google Scholar]
- 39.Kennett S, Taylor-Clarke M, Haggard P. Noninformative vision improves the spatial resolution of touch in humans. Curr Biol. 2001;11:1188–91 [DOI] [PubMed] [Google Scholar]
- 40.Taylor-Clarke M, Kennett S, Haggard P. Persistence of visual-tactile enhancement in humans. Neurosci Lett. 2004;354:22–5 [DOI] [PubMed] [Google Scholar]
- 41.Fiorio M, Haggard P. Viewing the body prepares the brain for touch: effects of TMS over somatosensory cortex. Eur J Neurosci. 2005;22:773–7 [DOI] [PubMed] [Google Scholar]
- 42.Sathian K, Zangaladze A. Feeling with the mind’s eye: contribution of the visual cortex to tactile perception. Behav Brain Res. 2002;135:127–32 [DOI] [PubMed] [Google Scholar]
- 43.Deibert E, Kraut M, Kremen S, Hart J. Neural pathways in tactile object recognition. Neurology. 1999;52:1413–7 [DOI] [PubMed] [Google Scholar]
- 44.Picton TW, Stuss DT, Marshall KC. Attention and the brain. In: Friedman SL, Klivington KA, Peterson RW, editors. The brain, cognition and education New York: Academic Press; 1986. p. 19–79 [Google Scholar]
- 45.Whang KC, Burton H, Shulman GL. Selective attention in vibrotactile tasks: detecting the presence and absence of amplitude change. Percept Psychophys. 1991;50:157–65 [DOI] [PubMed] [Google Scholar]
- 46.Sathian K, Burton H. The role of spatially selective attention on the tactile perception of texture. Percept Psychophys. 1991;50:237–48 [DOI] [PubMed] [Google Scholar]
- 47.Boulter LR. Attention and reaction times to signals of uncertain modality. J Exp Psychol (Hum Percept Perf). 1977;3:379–88 [DOI] [PubMed] [Google Scholar]
- 48.Post LJ, Chapman CE. The effects of cross-modal manipulations of attention on the detection of vibrotactile stimuli in humans. Somatosens Mot Res. 1991;8:149–57 [DOI] [PubMed] [Google Scholar]
- 49.Zompa IC, Chapman CE. Effects of cross-modal manipulations of attention on the ability of human subjects to discriminate changes in texture. Somatosens Mot Res. 1995;12:87–102 [DOI] [PubMed] [Google Scholar]
- 50.Blake DT, Hsiao SS, Johnson KO. Neural Coding mechanisms in tactile pattern recognition: the relative contributions of slowly and rapidly adapting mechanoreceptors to perceived roughness. J Neurosci. 1997;17:7480–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hollins M, Risner SR. Evidence for the duplex theory of tactile texture perception. Percept Psychophys. 2000;62:695–705 [DOI] [PubMed] [Google Scholar]
- 52.Hollins M, Bensmaia SJ, Roy EA. Vibrotaction and texture perception. Behav Brain Res. 2002;135:51–6 [DOI] [PubMed] [Google Scholar]
- 53.Goldreich D, Kanics IM. Tactile acuity is enhanced in blindness. J Neurosci. 2003;23:3439–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldreich D, Kanics IM. Performance of blind and sighted humans on a tactile grating detection task. Percept Psychophys. 2006;68:1363–71 [DOI] [PubMed] [Google Scholar]
- 55.van Boven RW, Hamilton RW, Kauffman RH, Keenan JP, Pascual-Leone A. Tactile spatial resolution in blind raille readers. Neurology. 2000;54:2230–6 [DOI] [PubMed] [Google Scholar]
- 56.Legge GE, Madison C, Vaughn BN, Cheong AM, Miller JC. Retention of high tactile acuity throughout the life span in blindness. Percept Psychophys. 2008;70:1471–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gibson JJ. Observations of active touch. Psychol Rev. 1962;69:477–91 [DOI] [PubMed] [Google Scholar]
- 58.Lissek S, Wilimzig C, Stude P, Pleger B, Kalisch T, Maier C, et al. Immobilization impairs tactile perception and shrinks somatosensory cortical maps. Curr Biol. 2009;19:837–42 [DOI] [PubMed] [Google Scholar]
- 59.Ragert P, Schmidt A, Altenmuller E, Dinse HR. Superior tactile performance and learning in professional pianists: evidence of meta-plasticity in musicians. Eur J Neurosci. 2004;19:473–8 [DOI] [PubMed] [Google Scholar]
- 60.Kerr CE, Shaw JR, Wasserman RH, Chen VW, Kanojia A, Bayer T, et al. Tactile acuity in experienced Tai Chi practitioners: evidence for use dependent plasticity as an effect of sensory-attentional training. Exp Brain Res. 2008;188:317–22 [DOI] [PMC free article] [PubMed] [Google Scholar]


