Abstract
Oxidative damage and inflammation are related to the pathogenesis of age-related macular degeneration (AMD). Epidemiologic studies suggest that insufficient dietary lutein and zeaxanthin intake or lower serum zeaxanthin levels are associated with increased risk for AMD. The objective of this work is to test the protective effects of lutein and zeaxanthin against photo-oxidative damage to retinal pigment epithelial cells (RPE) and oxidation-induced changes in expression of inflammation-related genes. To mimic lipofuscin-mediated photo-oxidation in vivo, we used ARPE-19 cells that accumulated A2E, a lipofuscin fluorophore and photosensitizer, as a model system to investigate the effects of lutein and zeaxanthin supplementation. The data show that supplementation with lutein or zeaxanthin in the medium resulted in accumulation of lutein or zeaxanthin in the RPE cells. The concentrations of lutein and zeaxanthin in the cells were 2–14-fold of that detected in the medium, indicating that ARPE-19 cells actively take up lutein or zeaxanthin. As compared with untreated cells, exposure of A2E-containing RPE to blue light resulted in a 40–60% decrease in proteasome activity, a 50–80% decrease in expression of CFH and MCP-1, and an ~ 20-fold increase in expression of IL-8. The photo-oxidation-induced changes in expression of MCP-1, IL-8 and CFH were similar to those caused by chemical inhibition of the proteasome, suggesting that inactivation of the proteasome is involved in the photo-oxidation-induced alteration in expression of these inflammation-related genes. Incubation of the A2E-containing RPE with lutein or zeaxanthin prior to blue light exposure significantly attenuated the photo-oxidation-induced inactivation of the proteasome and photo-oxidation induced changes in expression of MCP-1, IL-8, and CFH. Together, these data indicate that lutein or zeaxanthin modulates inflammatory responses in cultured RPE in response to photo-oxidation. Protecting the proteasome from oxidative inactivation appears to be one of the mechanisms by which lutein and zeaxanthin modulate the inflammatory response. Similar mechanisms may explain salutary effects of lutein and zeaxanthin in reducing the risk for AMD.
Keywords: Lutein, zeaxanthin, RPE, photo-oxidation, proteasome, inflammation
Introduction
Age-related macular degeneration (AMD) is a multifactorial disease and a leading cause of blindness in industrialized countries. In addition to aging, genetic background and cigarette smoking, dietary factors also contribute to the onset and progression of AMD [1, 2]. A growing number of studies indicate that dietary lutein and zeaxanthin play significant protective roles against visual loss from AMD. Epidemiological evidence for these protective effects was first obtained from a large case-control study, which showed that individuals with high dietary intakes and high serum levels of lutein and zeaxanthin have a much lower rate of exudative AMD [3]. Results from many, but not all, subsequent epidemiologic and case-control studies support the conclusion that the risk for onset and progression of AMD is inversely related to lutein and zeaxanthin concentrations in the diet, plasma, and macular pigment [3–8]. Lutein is found in a broad spectrum of foods, such as corn, a variety of fruits, and green vegetables [9, 10]. In comparison, the dietary sources of zeaxanthin are limited. The major sources of zeaxanthin are egg yolks, corn, corn meal, Japanese persimmons, orange peppers and leafy greens [9, 10]. In typical American diets, the contents of lutein are ~ 5 times that of zeaxanthin. The ratio of lutein to zeaxanthin in human blood is similar to that found in typical American diets, indicating that lutein and zeaxanthin are equally absorbed by humans. However, it appears that zeaxanthin preferentially accumulates in the retina, particularly in the macular region [11]. The overall ratio of lutein to zeaxanthin in the whole retina is ~2:1, but the ratio of lutein to zeaxanthin in the central macula (0 to 0.25 mm eccentricity) is less than 1:2. The differential uptake and retentions of lutein and zeaxanthin in the retina may be related to the expression of their binding proteins in the retina [12, 13]. Roughly 50% of the zeaxanthin within the macula is the meso- isomer of zeaxanthin, a metabolite of lutein, indicating that dietary supplement of lutein could also increase meso-zeaxanthin concentrations in the macula [14]. Levels of lutein and zeaxanthin and their metabolites in the retina can be detected non-invasively by measuring the macular pigment optical density. An increase in dietary intake of lutein and zeaxanthin would increase the macular pigment optical density and provide better protection against photo-oxidation [15, 16].
Oxidative stress, particularly lipofuscin-mediated photo-oxidative damage, contributes to the onset and progress of AMD [17]. Thus, it is thought that lutein and zeaxanthin in the retina may protect against AMD by two different mechanisms: blocking harmful blue light and quenching reactive oxygen species. Both lutein and zeaxanthin absorb blue light, the most phototoxic visible light to which the retina is routinely exposed. Studies in quail provide direct evidence that long-term zeaxanthin supplementation results in an increase in retinal zeaxanthin concentrations and provides a protective effect against light-induced photoreceptor death [18, 19]. In rhesus monkeys, supplementation with lutein or zeaxanthin after long-term xanthophyll deficiency protected the fovea from blue light damage [20]. Lutein and zeaxanthin are also efficient quenchers of singlet oxygen and related reactive oxygen species [21, 22]. HPLC measurement of macular pigments in post-mortem retinas of normal and AMD patients demonstrates that elevated levels of lutein and zeaxanthin are associated with reduced odds ratios for AMD [23]. Protective effects of lutein and zeaxanthin against oxidative damage initiated by acute exposure to blue-light has also been demonstrated [20].
Recent studies indicate that innate immunity and inflammation are related to AMD pathogenesis [24, 25]. The evidence for the involvement of innate immunity and inflammation in AMD pathogenesis includes accumulation of immunoglobulin and complement components in drusen [26–28], the association between genetic variants of complement factor H, factor B, C2, C3, factor I and risk for AMD [29–37], and elevated serum CRP levels in AMD patients [38–40]. Emerging evidence indicates dietary lutein and zeaxanthin have anti-inflammation functions, including a reduction of serum levels of CRP and sICAM [41–44]. It is plausible that dietary lutein and zeaxanthin reduce the risk for AMD via modulating ocular or systemic inflammation. However, the mechanisms for such functions remain to be elucidated.
The ubiquitin-proteasome pathway (UPP) is the major non-lysosomal proteolytic pathway within cells [45–47]. Proteins destined for degradation are first conjugated with a polyubiquitin chain by the sequential action of three classes of enzymes: E1, E2 and E3. The ubiquitin-protein conjugates are then recognized and degraded by a large protease complex called the proteasome [46, 48]. The UPP has been involved in a myriad of cellular processes [47, 49], including regulation of immune response and inflammation [50, 51]. Dysfunction of the UPP has been implicated in the pathogenesis of many degenerative diseases such as Alzheimer’s disease [52], Parkinson’s disease [53], diabetic retinopathy [54] and cataract [55, 56]. A fully functional UPP is required for cells to cope with various stresses, including heavy metals [57], amino acid analogs and oxidation [58]. However, an extensive oxidative insult also impairs the function of critical components of the UPP [59–64]. Oxidative inactivation of the proteasome not only results in accumulation of damaged proteins [56], but also impairs cell signaling process [65].
Oxidative stress and inflammation are interrelated. Whereas oxidative stress triggers inflammatory responses [66, 67], inflammation also enhances the production of reactive oxygen species. Our recent work indicates that oxidative inactivation of the proteasome is a mechanistic link between oxidative stress and increased production of IL-8 in cultured RPE [68]. Since the RPE is a major ocular source of pro-inflammatory mediators and a primary target of photo-oxidative insult, oxidative impairment of the UPP in RPE may contribute to ocular inflammation and AMD-related lesions. To explore the mechanisms by which lutein and zeaxanthin may reduce the risk for AMD, we evaluated the effects of lutein and zeaxanthin supplementation on photo-oxidation induced impairment of the UPP in cultured RPE and the consequent inflammatory response. The data indicate that supplementation with lutein or zeaxanthin to cultured RPE ameliorates photo-oxidation-induced inactivation of the proteasome and partially reverses photo-oxidation-induced changes in expression of some inflammation-related genes.
Materials and Methods
Materials
Lutein crystals were obtained from Kemin (Des Moines, IA USA). Zeaxanthin crystals were obtained from DSM (Basel, Switzerland) or purchased from Sigma Aldrich (St. Louis, MO, USA). Cell culture supplies were obtained from Invitrogen (Carlsbad, CA, USA). The DuoSet ELISA kits for human MCP-1 and human IL-8, were obtained from R&D Systems (Minneapolis, MN, USA). Mouse monoclonal antibody (capture antibody) to human CFH was purchased from Abcam (Cambridge MA, USA) and goat-polyclonal antibody (detecting antibody) to human CFH was purchased from EMD Chemicals (Gibbstown, NJ, USA). All other reagents were obtained from Sigma Aldrich (St. Louis, MO, USA).
Exposure to A2E and blue light
ARPE-19 cells were grown to confluence and then cultured in DMEM with 10% heat-inactivated fetal calf serum and 0.1 mM nonessential amino acid solution with or without 10 μM A2E for 10 days. The medium with fresh A2E was changed twice a week. To determine the protective effects of lutein and zeaxanthin, the cells that had accumulated A2E were incubated with or without 10 μM lutein of zeaxanthin for another 3 days in the absence of A2E. Stock solutions of lutein and zeaxanthin were prepared in DMSO in a concentration of 3.3 mM. The stock solutions were first diluted in fetal bovine serum (FBS) and then mixed with DMEM to a final concentration of 10 μM lutein or zeaxanthin and 10% FBS. All cells, including the controls, received same amount of DMSO (0.03%) and FBS (10%). After washing twice with complete medium with 10% FBS to remove unincorporated lutein or zeaxanthin, cell cultures were transferred to PBS with calcium, magnesium and glucose and were exposed to 430 nm light delivered from a tungsten halogen source (430 nm ± 20; 10 minutes; 2.62 mW/cm2). The cells were then incubated for an additional 6 hours in DMEM with 1% FBS. After collection of the media, cells were washed twice with cold PBS and then the dishes were placed on ice and the cells were harvested with a cell scraper. Cells that had neither accumulated A2E nor been exposed to blue light were used as controls. The control cells were treated in the same manner as the cells that were exposed to A2E and blue light. Levels of MCP-1, IL-8 and CFH in the medium were determined by ELISA. The latter were performed according to the manufacturer’s instructions. Total RNA was also isolated from the cells for the quantitation of mRNA levels of MCP-1, IL-8 and CFH.
Lutein and zeaxanthin determination
To determine the intake of lutein and zeaxanthin by ARPE-19, confluent monolayers of ARPE-19 cells were incubated with 1 and 10 μM lutein or zeaxanthin in the medium containing 10% FBS for 3 days. After washing three times with DMEM containing 10% FBS, the cells were then collected and levels of lutein or zeaxanthin in the cells were determined by HPLC method as described previously [69]. The presence of FBS, which contains lipoproteins, in the washing solution helps to carry these carotenoids and reduce non-specific adherence of these carotenoids to the cell membrane. However, we cannot rule out the possibility that some of the carotenoids detected the cells was due to non-specific adherence to the cell membrane.
Proteasome activity assay
ARPE-19 cells were lysed in 25 mM Tris-HCl buffer, pH 7.6. The chymotrypsin-like activity of the proteasome was determined using the fluorogenic peptide succinyl-Leu-Leu-Val-Tyr-amidomethylcoumarin (LLVY-AMC) as a substrate [70]. The mixture, containing 20 μl of cell supernatant in 25 mM Tris-HCl, pH 7.6, was incubated at 25 °C with the peptide substrate (LLVY-AMC at 25 μM) in a buffer containing 50 mM Tris-HCl, pH 8.0, 100 mM NaCl, 5 mM EDTA, 1 mM EGTA, 3 mM NaN3 and 0.04% 3-[(3-Cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS). The final volume of the assay was 200 μl. Rates of reactions were measured in a temperature-controlled microplate fluorometric reader. Excitation/emission wavelengths were 380/440 nm. Proteasome activity was defined as the portion of peptidase activity in the cell extracts that was inhibited by 20 μM MG132, a potent proteasome inhibitor. Ubiquitin conjugates in the cell lysate were determined by Western Blotting with antibodies specific to ubiquitin and levels of β-actin were used as a protein loading control.
Results
Cultured ARPE-19 cells actively uptake lutein and zeaxanthin
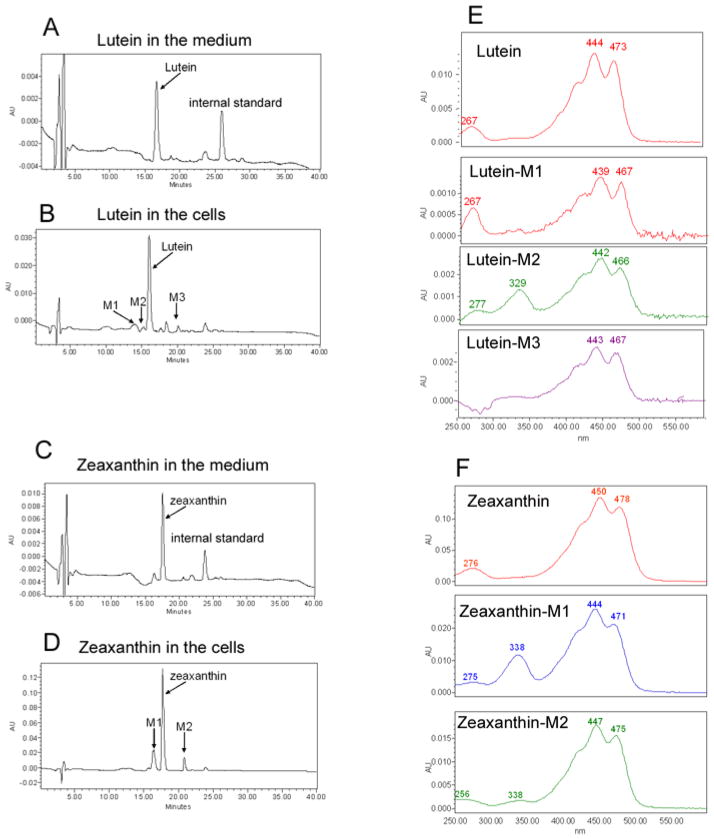
To determine the potential mechanisms by which lutein and zeaxanthin protect the RPE, we investigated uptake of lutein and zeaxanthin by cultured RPE. ARPE-19 cells cultured with normal DMEM contained no detectable lutein or zeaxanthin. When cells were cultured in the presence of 1 or 10 μM lutein for 3 days, concentrations of lutein in the cells increased to 805 or 2233 ng/mg protein (~14 and ~39 μM), respectively. When the cells were cultured in the presence of 1 or 10 μM zeaxanthin for 3 days, the concentrations of zeaxanthin in the cells increased to 296 or 1389 ng/mg protein (~5 and ~24 μM), respectively. The concentrations of lutein and zeaxanthin in the cells were at least 2–14 fold higher than the concentrations in the medium, suggesting that the RPE cells either actively take up or trap lutein and zeaxanthin. Furthermore, a small amount of putative metabolites of lutein or zeaxanthin were detected in RPE cells, but not in the medium (Fig. 1, Compare panels B to A and D to C). These metabolites of lutein or zeaxanthin in RPE had similar, but not identical, absorbency spectra of lutein or zeaxanthin (Fig. 1, panels E and F), suggesting these putative metabolites are isomers of lutein or zeaxanthin.
Fig. 1. Cultured RPE cells actively take up lutein and zeaxanthin.
Confluent ARPE-19 cells were cultured in media containing 1 or 10 μM of lutein or zeaxanthin for 3 days. The medium was removed and the cells were washed three times with medium containing 10% bovine serum. Then the cells were collected. Levels of lutein and zeaxanthin in the medium and in the cells were determined by reversed phase HPLC with a C30 column. This figure showed the chromatograms (monitored at 450 nm) and spectra of lutein and zeaxanthin in the medium and cells upon supplementation with 10 μM lutein or zeaxanthin for 3 days. Panel A: chromatogram of lutein in the medium; panel B: chromatogram of lutein and its putative metabolites in cells; panel C: chromatogram of zeaxanthin in the medium; panel D: chromatogram of zeaxanthin and its metabolites in cells; panel E: spectra of lutein and its metabolites in cells; panel F: spectra of zeaxanthin and its putative metabolites in cells.
Based on isomer spectra and retention times, lutein M1 could be assigned as oxo-lutein, lutein M2 as 13-cis isomer of lutein and lutein M3 as 9-cis isomer of lutein. The oxo-product was not observed for zeaxanthin and the 13-cis and 9-cis isomers of zeaxanthin corresponded to M1 and M2, respectively.
Supplementation with lutein and zeaxanthin prevents photo-oxidative inactivation of the proteasome
The UPP is the primary proteolytic system in RPE, with important roles in many cellular functions. Although a fully functional UPP is required for cells to recover from oxidative stress, the UPP itself is also a target of severe oxidative stress. We previously demonstrated that the proteasome is one of the components of the UPP that are vulnerable to oxidative inactivation [64]. Impairment of the UPP in the RPE alters the expression of AMD-related genes, such as VEGF, MCP-1 and IL-8 [68, 71, 72]. To begin to decipher how carotenoids may protect the proteasome from photo-oxidative inactivation, we tested the effects of lutein or zeaxanthin on photo-oxidative inactivation of the proteasome in cultured RPE. As shown previously [64], exposure of A2E-containing RPE cells to blue light resulted in ~60% decrease in proteasome activity (Fig. 2A). However, if A2E-containing RPE cells were incubated with 10 μM lutein or zeaxanthin prior to blue light exposure, the inactivation of proteasome was substantially attenuated (Fig. 2A). Whereas incubation with 10 μM lutein almost completely blocked the photo-oxidative inactivation of the proteasome, the same concentration of zeaxanthin only provided 50% of the protection (Fig. 2A). The greater protective effect of lutein, as compared to zeaxanthin, may be related to its higher cellular accumulation. As presented in the above paragraph, when incubated with the same level of lutein or zeaxanthin for the same period, the RPE cells accumulated nearly twice the amount of lutein as that of zeaxanthin. For example, after incubation with 10 μM lutein or zeaxanthin for three days, concentrations of lutein in RPE cells reached ~39 μM, whereas concentrations of zeaxanthin in the cells were only ~24 μM. Consistent with the role of the proteasome in the degradation of ubiquitinated proteins, photo-oxidation induced inactivation of the proteasome was associated with an increase in levels of ubiquitin conjugates (Fig. 2B, compare lanes 2 with 1). Supplementation of the A2E-containing cells with lutein or zeaxanthin prior to blue light exposure also attenuated the photo-oxidation-induced accumulation of ubiquitin-proteins conjugates in the cells (Fig 2B, compare lanes 3 and 4 with 2). The limited accumulation of ubiquitin conjugates in the presence of lutein or zeaxanthin may be due to less oxidative stress or greater proteasome activity. Together, these data indicate that accumulation of lutein and/or zeaxanthin in RPE provides a protection against photo-oxidation, including protecting the proteasome from photo-oxidative inactivation.
Fig. 2. Accumulation of lutein and zeaxanthin in RPE cells prevents photo-oxidative inactivation of the proteasome in ARPE-19 cells.
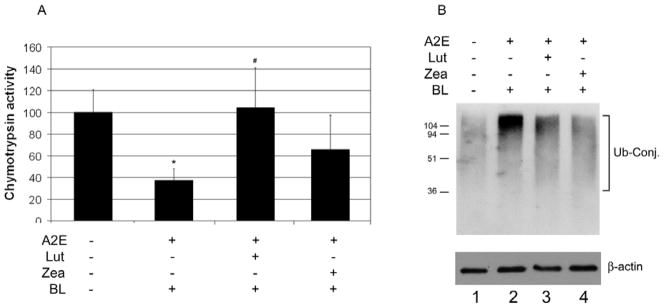
Confluent cultured ARPE-19 cells were loaded with A2E alone or loaded with A2E and then supplemented with 10 μM lutein or zeaxanthin for 3 days. The cells were then exposed to blue light for 10 min and harvested. The chymotrypsin-like activity of the proteasome in the cells was determined using a fluorogenic peptide as a substrate (panel A) and levels of ubiquitin conjugates were determined by Western blotting analysis using levels of β-actin as a loading control (panel B). The experiments were repeated twice with triplicates each time. The data in panel A are mean ± SD of the results from 6 samples in each group. The proteasome activity in control cells (neither treated with A2E nor exposed to blue light) was arbitrarily designated as 100 and the rest were expressed as relative activities. * indicates p<0.05 as compared the control cells. # indicates p<0.05 as compared to cells that were loaded with A2E and exposed to blue light.
Supplementation with lutein and zeaxanthin prevents photo-oxidation-induced alteration in expression of inflammation-related genes
Since the proteasome in RPE plays an important role in regulating the expression of inflammation-related genes [68, 72], we hypothesized that photo-oxidative inactivation of the proteasome would alter the expression of inflammation related genes and the inflammatory response. Similar to the results of chemical inhibition of the proteasome, exposure of A2E-containing RPE to blue light decreased the expression and secretion of MCP-1 (Fig. 3) and increased the expression and secretion of IL-8 (Fig. 4). To investigate the roles of lutein and zeaxanthin in modulating the inflammatory response to photo-oxidation, we supplemented A2E-containing RPE cells with lutein or zeaxanthin prior to blue light exposure and determined their effects on photo-oxidation-induced expression of these inflammation-related genes. The data showed that supplementation with lutein or zeaxanthin blocked the A2E and blue light-induced decrease in MCP-1 expression (Fig. 3A) and secretion (Fig. 3B). The extents of the protection of lutein and zeaxanthin against A2E and blue light-induced decline in expression and secretion of MCP-1 were comparable to their ability to protect the proteasome from inactivation. Supplementation with lutein or zeaxanthin also suppressed the photo-oxidation induced increase in expression and secretion of IL-8 (Fig. 4). It appears that zeaxanthin was more effective than lutein in suppressing photo-oxidation induced expression and secretion of IL-8, although it was less effective in protecting the proteasome from inactivation. The data indicate that lutein and zeaxanthin may regulate the expression of IL-8 by other mechanisms in addition to protecting the proteasome from photo-oxidative inactivation.
Fig. 3. Accumulation of lutein or zeaxanthin in A2E- containing RPE cells attenuates blue light induced down regulation of MCP-1.
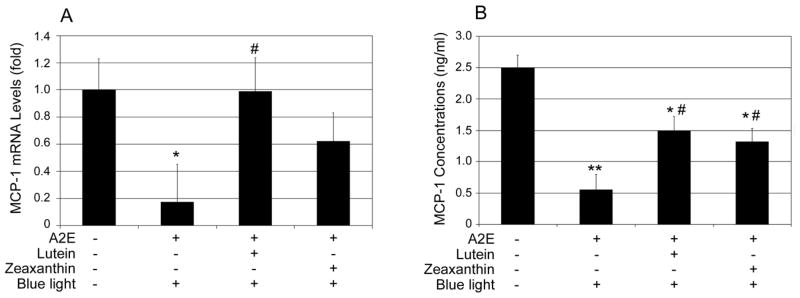
Confluent ARPE-19 cells that were loaded with A2E alone or loaded with A2E and then incubated with 10 μM lutein or zeaxanthin were exposed to blue light for 10 min. The cells were then cultured in medium containing 1% FBS for another 6 h and the medium was collected and cells were harvested. Cells that were neither loaded with A2E nor exposed to blue light were used controls. Levels of mRNA for MCP-1 in the cells were determined by real-time quantitative RT-PCR using levels of mRNA for GAPDH as a reference (panel A) and protein levels of MCP-1 in the medium were determined by ELISA (panel B). The relative levels of mRNA for MCP-1 in control cells were arbitrarily designated as 1 and relative levels of mRNA for MCP-1 in treated cells were expressed as fold of that in the control cells. The data are mean ± SD of the results from 6 samples in each group. * indicates p<0.05 and ** indicates p<0.01 as compared the control cells that were neither treated with A2E nor blue light. # indicates p<0.05 as compared to cells that were loaded with A2E and exposed to blue light.
Fig. 4. Accumulation of lutein or zeaxanthin in A2E- containing RPE cells attenuates blue light induced up-regulation of IL-8.
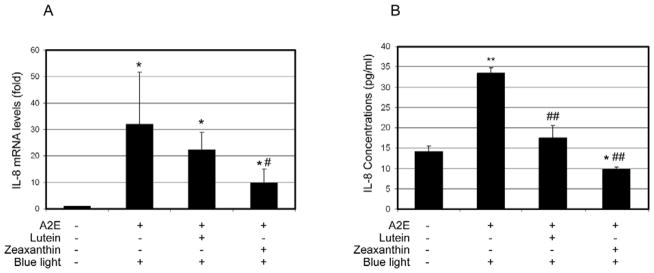
Confluent ARPE-19 cells that were loaded with A2E alone or loaded with A2E and then incubated with 10 μM lutein or zeaxanthin were exposed to blue light for 10 min. The cells were then cultured in medium containing 1% FBS for another 6 h and the media were collected and cells were harvested. Cells that were neither loaded with A2E nor exposed to blue light were used controls. Levels of mRNA for IL-8 in the cells were determined by real-time quantitative RT-PCR using levels of mRNA for GAPDH as a reference (panel A) and protein levels of Il-8 in the medium were determined by ELISA (panel B). The relative levels of mRNA for IL-8 in control cells were arbitrarily designated as 1 and relative levels of mRNA for Il-8 in treated cells were expressed as fold of that in the control cells. The data are mean ± SD of the results from 6 samples in each group. * indicates p<0.05 and ** indicates p<0.01 as compared the control cells. # indicates p<0.05 and ## indicates p< 0.01 as compared to cells that were loaded with A2E and exposed to blue light.
Supplementation with lutein and zeaxanthin prevents photo-oxidation-induced down-regulation of CFH
Recent studies indicate that over-activation of the complement system is related to the pathogenesis of AMD [73]. It is known that oxidative stress, including A2E-mediated photo-oxidative stress, triggers complement activation and renders RPE vulnerable to complement attack [66, 74]. CFH is a negative regulator of the alternative pathway of complement activation; it binds to activated C3 (C3b) and promotes its proteolytic inactivation by complement factor I [75, 76]. Oxidative activation of the complement may be related to decreased levels of activity of CFH. It has been reported that oxidative stress suppresses the secretion of CFH by RPE cells [77]. Here we determined the effects of lutein and zeaxanthin on photo-oxidation-induced down-regulation of CFH. Exposure of A2E-containing cells to blue light resulted in a 60–70% reduction in mRNA levels of CFH (Fig 5A.) and >80% decrease in levels of secreted CFH (Fig 5B). Supplementation of lutein or zeaxanthin to A2E-loaded cells prior to blue light exposure partially prevented the decrease in the expression of CFH (Fig 5A). However, supplementation of lutein or zeaxanthin did not prevent the photo-oxidation-induced decline in the secretion of CFH (Fig. 5B). It is possible that different regulatory steps of CFH production, such as transcription, translation, modification or secretion could be damaged by photo-oxidation, and each of these steps may have different vulnerability to photo-oxidative stress. Since lutein and zeaxanthin cannot completely blocks photo-oxidation under these experimental conditions, supplementation with lutein or zeaxanthin may alleviate the effects of photo-oxidation-induced damage to some, but not all, of these steps, and have different effects on levels of CFH mRNA and secreted protein.
Fig. 5. Accumulation of lutein and zeaxanthin in A2E- containing RPE cells attenuated blue light induced down-regulation of CFH.
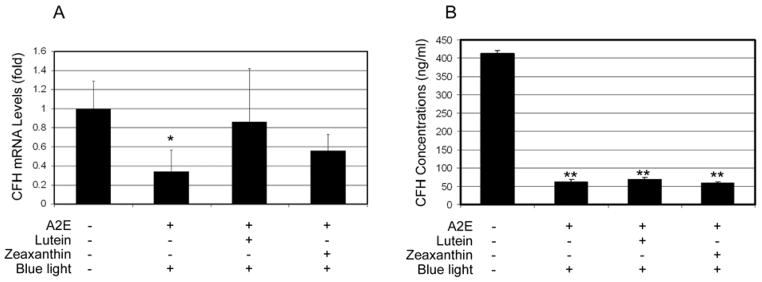
Confluent ARPE-19 cells that were loaded with A2E alone or loaded with A2E and then incubated with 10 μM lutein or zeaxanthin were exposed to blue light for 10 min. The cells were then cultured in medium containing 1% FBS for another 6 h and the media were collected and cells were harvested. Cells that were neither loaded with A2E nor exposed to blue light were used controls. Levels of mRNA for CFH in the cells were determined by real-time quantitative RT-PCR using levels of mRNA for GAPDH as a reference (panel A) and protein levels of CFH in the medium were determined by ELISA (panel B). The relative levels of mRNA for CFH in control cells were arbitrarily designated as 1 and relative levels of mRNA for CFH in treated cells were expressed as fold of that in the control cells. The data are mean ± SD of the results from 6 samples in each group. * indicates p<0.05 and ** indicates p<0.01 as compared to the control cells that were neither treated with A2E nor blue light.
Proteasome inhibition decreases the expression and secretion of CFH
Previous studies indicate that proteasome inactivation is involved in the oxidation-induced changes in expression and secretion of MCP-1 and IL-8 [68, 71, 72]. To investigate whether the oxidation-induced down-regulation of CFH is also related to proteasome inactivation, we determined the effects of proteasome inhibition on expression and secretion of CFH. As shown in Figure 6, incubating ARPE-19 cells with MG132 or epoxomicin, two cell permeable proteasome inhibitors, resulted a significant decrease in mRNA levels (Fig 6A) and secreted protein levels (Fig 6B) of CFH. These data indicate that the proteasome is also involved in regulating the expression of CFH and that oxidative inactivation of the proteasome appears to be one of the mechanisms by which photo-oxidation- reduces the expression and secretion of CFH.
Fig. 6. Inhibition of the proteasome in RPE cells down-regulates CFH expression.
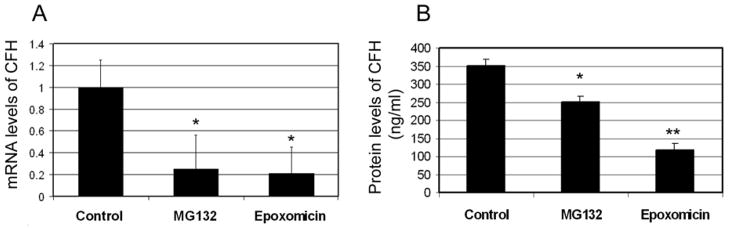
Confluent cultured ARPE-19 cells were incubated in fresh medium in the absence or presence of 10 μM MG132 or 5 μM epoxomicin for 8 h. Levels of mRNA for CFH in the cells were determined by real-time quantitative RT-PCR using levels of mRNA for GAPDH as a reference (panel A) and protein levels of CFH in the medium were determined by ELISA (panel B). The relative levels of mRNA for CFH in control cells were arbitrarily designated as 1 and relative levels of mRNA for CFH in MG132- or epoxomicin-treated cells were expressed as fold of that in the control cells. The data are mean ± SD of the results from 4 samples in each group. * indicates p<0.05 and ** indicates p<0.001 as compared to the controls.
Discussion
Oxidative stress and inflammation are interrelated biological events [78] and both are implicated in the pathogenesis of AMD [67, 79, 80]. There is a vicious cycle in which oxidative stress triggers inflammatory responses, and inflammation also enhances the production of reactive oxygen species, all of them causing oxidative damage [81, 82]. Due to its high metabolic rate and age-related accumulation of lipofuscin, the RPE is a primary target of photo-oxidative damage in the eye [17]. The RPE is also a major source of cytokines that regulate inflammatory response in the retina [68, 83, 84]. The vicious interaction between oxidative stress and inflammatory responses in RPE may contribute to the onset and progression of AMD. Any approach that breaks the vicious cycle between oxidative stress and inflammation in RPE could be a potential strategy for prevention or treatment of AMD.
Results from this study show that photo-oxidative stress inactivates the proteasome in RPE and subsequently alters the expression of inflammation-related genes, such as down-regulation of MCP-1 and CFH and up-regulation of IL-8. These data are consistent with our previous work which indicates that inactivation of the proteasome is a mechanistic link between oxidative stress and altered inflammatory responses [68, 71, 72]. Furthermore, the data indicate that supplementation with lutein or zeaxanthin can partially break the vicious cycle between oxidative stress and inflammatory response in RPE cells via protecting the proteasome from inactivation. The effects of lutein and zeaxanthin in protecting RPE from photo-oxidative damage and their roles in modulating inflammation-related genes may be one of the mechanisms by which dietary lutein and zeaxanthin reduce the risk for AMD.
Photo-oxidative inactivation of the proteasome was consistent with previous reports that the proteasome is a target of oxidative stress [64, 65, 68, 70, 85]. Since the proteasome is involved in regulating the activation of NF-κB, a master regulator of inflammation processes [86], inactivation of the proteasome alters the expression of inflammation-related genes [68, 72, 87, 88]. The effects of oxidative stress on MCP-1 and IL-8 expression appear to depend on the extent of oxidative stress. Mild oxidative stress may promote the expression of MCP-1 and IL-8 by stimulating NF-κB activation [89, 90]. However, extensive oxidative stress may inactivate the proteasome and subsequently inhibit NF-κB activation and result in down-regulation of MCP-1 (Fig. 3 ). Chemical inhibition of the proteasome also reduced the expression of MCP-1 in RPE [72, 87, 91]. Extensive oxidative stress may inactivate the proteasome and subsequently activate the p38 MAP kinase, and thus promoting the expression of IL-8 [68, 71]. It was also reported that oxidative stress suppresses the expression of CFH in RPE [77, 92]. However, the underlying mechanism for the oxidative regulation of CFH expression in RPE remains to be elucidated. We speculate that inactivation of the proteasome by extensive oxidative stress may be involved in this process, as proteasome activity is required for activation of many transcription factors [93, 94].
It has long been suspected that accumulation of lutein and zeaxanthin in the retina may protect the retina by filtering high-energy blue light and/or by quenching reactive oxygen species [95]. The highest concentration of lutein and zeaxanthin was detected in the Henle fiber layer in the foveal region, where the concentrations of these carotenoids can be as high as 1 mM. The anatomical localization and deep yellow color of carotenoids may reduce the exposure of photoreceptor and RPE to blue light and subsequently reduce the photo-mediated production of reactive oxygen species, such as singlet oxygen. Lutein and zeaxanthin are also excellent quenchers of singlet oxygen and triplet states of photoactive molecules [96, 97]. The capacities of lutein and zeaxanthin in quenching singlet oxygen are superior to that of α-tocopherol in a cell free system [97]. The antioxidant function of lutein and zeaxanthin for protecting RPE from photo-oxidation requires their local presentation. Indeed, lutein and zeaxanthin were also detected in human RPE, although at relatively lower levels as compared to neuronal retina [98, 99]. Results from these experiments show that cellular concentrations of lutein and zeaxanthin were 2–14 fold higher than that in the medium, indicating that cultured human RPE actively takes up and accumulate lutein and zeaxanthin upon supplementation (Fig. 1). If RPE cells in vivo also actively take up lutein and zeaxanthin, choroidal blood supplies would be a source of these carotenoids. The other sources of lutein or zeaxanthin for RPE may be phagocytosed photoreceptor outer segments that are enriched with these carotenoids [100]. Thus, we hypothesize that high dietary intake of lutein and zeaxanthin may not only increase macular pigment density, but also the concentrations of lutein and zeaxanthin in RPE. However, the concentrations of lutein and zeaxanthin used in this study were substantially higher than the levels that were detected in human RPE/choroids. Although the data obtained from these experiments indicate that accumulation of lutein and zeaxanthin in RPE has beneficial effects against photo-oxidation, the protective effects of lutein and zeaxanthin using this acute cell cultural photo-oxidation model may not actually reflect the processes within the RPE in vivo. Future experiments using chronic photo-oxidation model and physiologically relevant concentrations of lutein and zeaxanthin are warranted.
An increasing body of evidence indicated dysregulation of inflammatory response, including improper complement activation is involved in the pathogenesis of AMD [24]. It is also known that dietary lutein and zeaxanthin play a role in modulating inflammatory responses [42–44, 101–103]. However, it remained unclear how dietary lutein and zeaxanthin modulates inflammatory response. Results from this work suggest that protection of the proteasome from oxidative inactivation appears to be one of mechanisms by which lutein and zeaxanthin modulate the inflammatory response to photo-oxidative stress. The proteasome is involved in many aspects of cellular functions. In addition to selective degradation of damaged or obsolete proteins, the proteasome is involved in regulation of signal transduction and expression via controlling the levels of regulatory proteins and transcription factors. For example, proteasome-mediated degradation of inhibitors of NF-κB is required for activation of the NF-κB pathway [104–106]. We found that inhibition of the proteasome in RPE suppressed the expression and secretion of MCP-1 and the suppression is related to down regulation of NF-κB signaling pathway [72]. The down-regulation of MCP-1 may have physiological consequences since MCP-1 knockout mice developed AMD-like phenotypes [107]. We also found the inactivation of the proteasome enhances the expression and secretion of IL-8 by activation of the p38 MAPK signaling pathway [68, 71]. Elevated levels of IL-8 may not only promote inflammation, but also trigger neovascularization, because IL-8 is a neutrophil attractant and a strong pro-angiogenesis factor [108–111]. This study showed that inactivation of the proteasome also contributed to the down-regulation of CFH upon photo-oxidative stress (Fig 6). Although it is unknown at present how proteasome inhibition suppresses the expression of CFH, it is likely that the proteasome is involved in regulating levels of transcription factors and signaling molecules that control the expression of CFH. The down-regulation of CFH may play a role in complement activation [66] and complement attack of the RPE [74] in response to oxidative stress.
Together, these results suggest that inactivation the proteasome is a mechanistic link between oxidative stress and altered expression of inflammation-related genes. Supplementation of lutein or zeaxanthin in RPE protected the proteasome from inactivation and attenuated the changes in expressions of these inflammation-related genes. This may be one of the mechanisms by which dietary lutein and zeaxanthin modulate ocular and systemic inflammation and reduce the risk for AMD.
Photo-oxidation inactivates the proteasome in retina pigment epithelial cells (RPE)
Inactivation of the proteasome alters the expression of inflammation-related genes.
RPE cells accumulate lutein and zeaxanthin upon supplementation.
Lutein or zeaxanthin attenuates photo-oxidation–induced proteasome inactivation.
Lutein or zeaxanthin attenuates the expression of inflammation-related genes.
Acknowledgments
This work is supported by USDA AFRI Award 2009-35200-05014, NIH grant EY 011717, USDA contract 1950-510000-060-01A, and Dennis L. Gierhart Charitable Gift Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chiu CJ, Taylor A. Nutritional antioxidants and age-related cataract and maculopathy. Exp Eye Res. 2007;84:229–245. doi: 10.1016/j.exer.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Chiu CJ, Taylor A, editors. Nutritional antioxidants, dietary carbohydrates, and age-related maculopathy and cataract. Humana Press; 2010. [Google Scholar]
- 3.Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. Jama. 1994;272:1413–1420. [PubMed] [Google Scholar]
- 4.Bone RA, Landrum JT, Dixon Z, Chen Y, Llerena CM. Lutein and zeaxanthin in the eyes, serum and diet of human subjects. Exp Eye Res. 2000;71:239–245. doi: 10.1006/exer.2000.0870. [DOI] [PubMed] [Google Scholar]
- 5.Mares-Perlman JA, Fisher AI, Klein R, Palta M, Block G, Millen AE, Wright JD. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. Am J Epidemiol. 2001;153:424–432. doi: 10.1093/aje/153.5.424. [DOI] [PubMed] [Google Scholar]
- 6.Snellen EL, Verbeek AL, Van Den Hoogen GW, Cruysberg JR, Hoyng CB. Neovascular age-related macular degeneration and its relationship to antioxidant intake. Acta Ophthalmol Scand. 2002;80:368–371. doi: 10.1034/j.1600-0420.2002.800404.x. [DOI] [PubMed] [Google Scholar]
- 7.Robman L, Vu H, Hodge A, Tikellis G, Dimitrov P, McCarty C, Guymer R. Dietary lutein, zeaxanthin, and fats and the progression of age-related macular degeneration. Can J Ophthalmol. 2007;42:720–726. doi: 10.3129/i07-116. [DOI] [PubMed] [Google Scholar]
- 8.Obana A, Hiramitsu T, Gohto Y, Ohira A, Mizuno S, Hirano T, Bernstein PS, Fujii H, Iseki K, Tanito M, Hotta Y. Macular carotenoid levels of normal subjects and age-related maculopathy patients in a Japanese population. Ophthalmology. 2008;115:147–157. doi: 10.1016/j.ophtha.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 9.Holden JM, Eldridge AL, Beecher GR, Buzzard IM, Bhagwat AS, Davis CS, Douglass LW, Gebhardt ES, Haytowitz D, Schakel S. Carotenoid Content of US. Foods: An Update of the Database. Journal of Food Ccomposion and Analysis. 1999;12 [Google Scholar]
- 10.Sommerburg O, Keunen JE, Bird AC, van Kuijk FJ. Fruits and vegetables that are sources for lutein and zeaxanthin: the macular pigment in human eyes. Br J Ophthalmol. 1998;82:907–910. doi: 10.1136/bjo.82.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr. 1995;62:1448S–1461S. doi: 10.1093/ajcn/62.6.1448S. [DOI] [PubMed] [Google Scholar]
- 12.Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J Biol Chem. 2004;279:49447–49454. doi: 10.1074/jbc.M405334200. [DOI] [PubMed] [Google Scholar]
- 13.Bhosale P, Li B, Sharifzadeh M, Gellermann W, Frederick JM, Tsuchida K, Bernstein PS. Purification and partial characterization of a lutein-binding protein from human retina. Biochemistry. 2009;48:4798–4807. doi: 10.1021/bi9004478. [DOI] [PubMed] [Google Scholar]
- 14.Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas, III: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2005;46:692–702. doi: 10.1167/iovs.02-1192. [DOI] [PubMed] [Google Scholar]
- 15.Johnson EJ, Chung HY, Caldarella SM, Snodderly DM. The influence of supplemental lutein and docosahexaenoic acid on serum, lipoproteins, and macular pigmentation. Am J Clin Nutr. 2008;87:1521–1529. doi: 10.1093/ajcn/87.5.1521. [DOI] [PubMed] [Google Scholar]
- 16.Connolly EE, Beatty S, Loughman J, Howard AN, Louw MS, Nolan JM. Supplementation with all three macular carotenoids: response, stability, and safety. Invest Ophthalmol Vis Sci. 2011;52:9207–9217. doi: 10.1167/iovs.11-8025. [DOI] [PubMed] [Google Scholar]
- 17.Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80:595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Thomson LR, Toyoda Y, Langner A, Delori FC, Garnett KM, Craft N, Nichols CR, Cheng KM, Dorey CK. Elevated retinal zeaxanthin and prevention of light-induced photoreceptor cell death in quail. Invest Ophthalmol Vis Sci. 2002;43:3538–3549. [PubMed] [Google Scholar]
- 19.Thomson LR, Toyoda Y, Delori FC, Garnett KM, Wong ZY, Nichols CR, Cheng KM, Craft NE, Dorey CK. Long term dietary supplementation with zeaxanthin reduces photoreceptor death in light-damaged Japanese quail. Exp Eye Res. 2002;75:529–542. doi: 10.1006/exer.2002.2050. [DOI] [PubMed] [Google Scholar]
- 20.Barker FM, 2nd, Snodderly DM, Johnson EJ, Schalch W, Koepcke W, Gerss J, Neuringer M. Nutritional Manipulation of Primate Retinas. V: Effects of Lutein, Zeaxanthin and n--3 Fatty Acids on Retinal Sensitivity to Blue Light Damage. Invest Ophthalmol Vis Sci. 2011 doi: 10.1167/iovs.10-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeum KJ, Aldini G, Chung HY, Krinsky NI, Russell RM. The activities of antioxidant nutrients in human plasma depend on the localization of attacking radical species. J Nutr. 2003;133:2688–2691. doi: 10.1093/jn/133.8.2688. [DOI] [PubMed] [Google Scholar]
- 22.Li B, Ahmed F, Bernstein PS. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Arch Biochem Biophys. 2010;504:56–60. doi: 10.1016/j.abb.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE. Macular pigment in donor eyes with and without AMD: a case-control study. Invest Ophthalmol Vis Sci. 2001;42:235–240. [PubMed] [Google Scholar]
- 24.Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Rickman CB, Bok D, Hageman GS, Johnson LV. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel M, Chan CC. Immunopathological aspects of age-related macular degeneration. Semin Immunopathol. 2008;30:97–110. doi: 10.1007/s00281-008-0112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umeda S, Suzuki MT, Okamoto H, Ono F, Mizota A, Terao K, Yoshikawa Y, Tanaka Y, Iwata T. Molecular composition of drusen and possible involvement of anti-retinal autoimmunity in two different forms of macular degeneration in cynomolgus monkey (Macaca fascicularis) Faseb J. 2005;19:1683–1685. doi: 10.1096/fj.04-3525fje. [DOI] [PubMed] [Google Scholar]
- 27.Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006;51:137–152. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Souied EH, Leveziel N, Richard F, Dragon-Durey MA, Coscas G, Soubrane G, Benlian P, Fremeaux-Bacchi V. Y402H complement factor H polymorphism associated with exudative age-related macular degeneration in the French population. Mol Vis. 2005;11:1135–1140. [PubMed] [Google Scholar]
- 30.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 32.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 33.Postel EA, Agarwal A, Caldwell J, Gallins P, Toth C, Schmidt S, Scott WK, Hauser MA, Haines JL, Pericak-Vance MA. Complement factor H increases risk for atrophic age-related macular degeneration. Ophthalmology. 2006;113:1504–1507. doi: 10.1016/j.ophtha.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 34.Despriet DD, Klaver CC, Witteman JC, Bergen AA, Kardys I, de Maat MP, Boekhoorn SS, Vingerling JR, Hofman A, Oostra BA, Uitterlinden AG, Stijnen T, van Duijn CM, de Jong PT. Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. Jama. 2006;296:301–309. doi: 10.1001/jama.296.3.301. [DOI] [PubMed] [Google Scholar]
- 35.Simonelli F, Frisso G, Testa F, di Fiore R, Vitale DF, Manitto MP, Brancato R, Rinaldi E, Sacchetti L. Polymorphism p.402Y>H in the complement factor H protein is a risk factor for age related macular degeneration in an Italian population. Br J Ophthalmol. 2006;90:1142–1145. doi: 10.1136/bjo.2006.096487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau LI, Chen SJ, Cheng CY, Yen MY, Lee FL, Lin MW, Hsu WM, Wei YH. Association of the Y402H polymorphism in complement factor H gene and neovascular age-related macular degeneration in Chinese patients. Invest Ophthalmol Vis Sci. 2006;47:3242–3246. doi: 10.1167/iovs.05-1532. [DOI] [PubMed] [Google Scholar]
- 37.Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, Smith RT, Hageman GS, Dean M, Allikmets R. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaumberg DA, Christen WG, Buring JE, Glynn RJ, Rifai N, Ridker PM. High-sensitivity C-reactive protein, other markers of inflammation, and the incidence of macular degeneration in women. Arch Ophthalmol. 2007;125:300–305. doi: 10.1001/archopht.125.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N. Association between C-reactive protein and age-related macular degeneration. Jama. 2004;291:704–710. doi: 10.1001/jama.291.6.704. [DOI] [PubMed] [Google Scholar]
- 40.Seddon JM, George S, Rosner B, Rifai N. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol. 2005;123:774–782. doi: 10.1001/archopht.123.6.774. [DOI] [PubMed] [Google Scholar]
- 41.Walston J, Xue Q, Semba RD, Ferrucci L, Cappola AR, Ricks M, Guralnik J, Fried LP. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol. 2006;163:18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- 42.Koutsos EA, Garcia Lopez JC, Klasing KC. Carotenoids from in ovo or dietary sources blunt systemic indices of the inflammatory response in growing chicks (Gallus gallus domesticus) J Nutr. 2006;136:1027–1031. doi: 10.1093/jn/136.4.1027. [DOI] [PubMed] [Google Scholar]
- 43.Hozawa A, Jacobs DR, Jr, Steffes MW, Gross MD, Steffen LM, Lee DH. Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: the Coronary Artery Risk Development in Young Adults (CARDIA)/Young Adult Longitudinal Trends in Antioxidants (YALTA) study. Clin Chem. 2007;53:447–455. doi: 10.1373/clinchem.2006.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seddon JM, Gensler G, Klein ML, Milton RC. C-reactive protein and homocysteine are associated with dietary and behavioral risk factors for age-related macular degeneration. Nutrition. 2006;22:441–443. doi: 10.1016/j.nut.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Ciechanover A. The ubiquitin proteolytic system and pathogenesis of human diseases: a novel platform for mechanism-based drug targeting. Biochem Soc Trans. 2003;31:474–481. doi: 10.1042/bst0310474. [DOI] [PubMed] [Google Scholar]
- 46.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 47.Shang F, Taylor A. Function of the ubiquitin proteolytic pathway in the eye. Exp Eye Res. 2004;78:1–14. doi: 10.1016/j.exer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 49.Welchman RL, Gordon C, Mayer RJ. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat Rev Mol Cell Biol. 2005;6:599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- 50.Qureshi N, Vogel SN, Van Way C, 3rd, Papasian CJ, Qureshi AA, Morrison DC. The proteasome: a central regulator of inflammation and macrophage function. Immunol Res. 2005;31:243–260. doi: 10.1385/IR:31:3:243. [DOI] [PubMed] [Google Scholar]
- 51.Kloetzel PM. Generation of major histocompatibility complex class I antigens: functional interplay between proteasomes and TPPII. Nat Immunol. 2004;5:661–669. doi: 10.1038/ni1090. [DOI] [PubMed] [Google Scholar]
- 52.Hope AD, de Silva R, Fischer DF, Hol EM, van Leeuwen FW, Lees AJ. Alzheimer’s associated variant ubiquitin causes inhibition of the 26S proteasome and chaperone expression. J Neurochem. 2003;86:394–404. doi: 10.1046/j.1471-4159.2003.01844.x. [DOI] [PubMed] [Google Scholar]
- 53.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 54.Fernandes R, Ramalho J, Pereira P. Oxidative stress upregulates ubiquitin proteasome pathway in retinal endothelial cells. Mol Vis. 2006;12:1526–1535. [PubMed] [Google Scholar]
- 55.Dudek EJ, Shang F, Valverde P, Liu Q, Hobbs M, Taylor A. Selectivity of the ubiquitin pathway for oxidatively modified proteins: relevance to protein precipitation diseases. Faseb J. 2005;19:1707–1709. doi: 10.1096/fj.05-4049fje. [DOI] [PubMed] [Google Scholar]
- 56.Shang F, Nowell TR, Jr, Taylor A. Removal of oxidatively damaged proteins from lens cells by the ubiquitin-proteasome pathway. Exp Eye Res. 2001;73:229–238. doi: 10.1006/exer.2001.1029. [DOI] [PubMed] [Google Scholar]
- 57.Tsirigotis M, Zhang M, Chiu RK, Wouters BG, Gray DA. Sensitivity of mammalian cells expressing mutant ubiquitin to protein damaging agents. J Biol Chem. 2001;11:11. doi: 10.1074/jbc.M109023200. [DOI] [PubMed] [Google Scholar]
- 58.Shang F, Deng G, Liu Q, Guo W, Haas AL, Crosas B, Finley D, Taylor A. Lys6-modified ubiquitin inhibits ubiquitin-dependent protein degradation. J Biol Chem. 2005;280:20365–20374. doi: 10.1074/jbc.M414356200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jahngen-Hodge J, Obin M, Gong X, Shang F, Nowell T, Gong J, Abasi H, Blumberg J, Taylor A. Regulation of ubiquitin conjugating enzymes by glutathione following oxidative stress. J Biol Chem. 1997;272:28218–28226. doi: 10.1074/jbc.272.45.28218. [DOI] [PubMed] [Google Scholar]
- 60.Obin M, Shang F, Gong X, Handelman G, Blumberg J, Taylor A. Redox regulation of ubiquitin-conjugating enzymes: mechanistic insights using the thiol-specific oxidant diamide. FASEB Journal. 1998;12:561–569. doi: 10.1096/fasebj.12.7.561. [DOI] [PubMed] [Google Scholar]
- 61.Yao D, Gu Z, Nakamura T, Shi ZQ, Ma Y, Gaston B, Palmer LA, Rockenstein EM, Zhang Z, Masliah E, Uehara T, Lipton SA. Nitrosative stress linked to sporadic Parkinson’s disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci U S A. 2004;101:10810–10814. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishii T, Sakurai T, Usami H, Uchida K. Oxidative modification of proteasome: identification of an oxidation-sensitive subunit in 26 s proteasome. Biochemistry. 2005;44:13893–13901. doi: 10.1021/bi051336u. [DOI] [PubMed] [Google Scholar]
- 63.Caballero M, Liton PB, Epstein DL, Gonzalez P. Proteasome inhibition by chronic oxidative stress in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003;308:346–352. doi: 10.1016/s0006-291x(03)01385-8. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Zhou J, Fernandes AF, Sparrow JR, Pereira P, Taylor A, Shang F. The proteasome: a target of oxidative damage in cultured human retina pigment epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:3622–3630. doi: 10.1167/iovs.07-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu M, Bian Q, Liu Y, Fernandes AF, Taylor A, Pereira P, Shang F. Sustained oxidative stress inhibits NF-kappaB activation partially via inactivating the proteasome. Free Radic Biol Med. 2009;46:62–69. doi: 10.1016/j.freeradbiomed.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou J, Jang YP, Kim SR, Sparrow JR. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2006;103:16182–16187. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernandes AF, Zhou J, Zhang X, Bian Q, Sparrow J, Taylor A, Pereira P, Shang F. Oxidative inactivation of the proteasome in retinal pigment epithelial cells. A potential link between oxidative stress and up-regulation of interleukin-8. J Biol Chem. 2008;283:20745–20753. doi: 10.1074/jbc.M800268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson EJ, Hammond BR, Yeum KJ, Qin J, Wang XD, Castaneda C, Snodderly DM, Russell RM. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am J Clin Nutr. 2000;71:1555–1562. doi: 10.1093/ajcn/71.6.1555. [DOI] [PubMed] [Google Scholar]
- 70.Bulteau AL, Lundberg KC, Humphries KM, Sadek HA, Szweda PA, Friguet B, Szweda LI. Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J Biol Chem. 2001;276:30057–30063. doi: 10.1074/jbc.M100142200. [DOI] [PubMed] [Google Scholar]
- 71.Fernandes AF, Bian Q, Jiang JK, Thomas CJ, Taylor A, Pereira P, Shang F. Proteasome inactivation promotes p38 mitogen-activated protein kinase-dependent phosphatidylinositol 3-kinase activation and increases interleukin-8 production in retinal pigment epithelial cells. Mol Biol Cell. 2009;20:3690–3699. doi: 10.1091/mbc.E08-10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fernandes AF, Guo W, Zhang X, Gallagher M, Ivan M, Taylor A, Pereira P, Shang F. Proteasome-dependent regulation of signal transduction in retinal pigment epithelial cells. Exp Eye Res. 2006 doi: 10.1016/j.exer.2006.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Charbel Issa P, Chong NV, Scholl HP. The significance of the complement system for the pathogenesis of age-related macular degeneration - current evidence and translation into clinical application. Graefes Arch Clin Exp Ophthalmol. 2011;249:163–174. doi: 10.1007/s00417-010-1568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thurman JM, Renner B, Kunchithapautham K, Ferreira VP, Pangburn MK, Ablonczy Z, Tomlinson S, Holers VM, Rohrer B. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J Biol Chem. 2009;284:16939–16947. doi: 10.1074/jbc.M808166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodriguez de Cordoba S, Esparza-Gordillo J, Goicoechea de Jorge E, Lopez-Trascasa M, Sanchez-Corral P. The human complement factor H: functional roles, genetic variations and disease associations. Mol Immunol. 2004;41:355–367. doi: 10.1016/j.molimm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 76.Lutz HU, Jelezarova E. Complement amplification revisited. Mol Immunol. 2006;43:2–12. doi: 10.1016/j.molimm.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 77.Wu Z, Lauer TW, Sick A, Hackett SF, Campochiaro PA. Oxidative stress modulates complement factor H expression in retinal pigmented epithelial cells by acetylation of FOXO3. J Biol Chem. 2007;282:22414–22425. doi: 10.1074/jbc.M702321200. [DOI] [PubMed] [Google Scholar]
- 78.Nicholls SJ. The complex intersection of inflammation and oxidation: implications for atheroprotection. J Am Coll Cardiol. 2008;52:1379–1380. doi: 10.1016/j.jacc.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 79.Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- 80.Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci. 2006;7:860–872. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- 81.Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic Biol Med. 2010;48:1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khaper N, Bryan S, Dhingra S, Singal R, Bajaj A, Pathak CM, Singal PK. Targeting the vicious inflammation-oxidative stress cycle for the management of heart failure. Antioxid Redox Signal. 2010;13:1033–1049. doi: 10.1089/ars.2009.2930. [DOI] [PubMed] [Google Scholar]
- 83.Larrayoz IM, Huang JD, Lee JW, Pascual I, Rodriguez IR. 7-ketocholesterol-induced inflammation: involvement of multiple kinase signaling pathways via NFkappaB but independently of reactive oxygen species formation. Invest Ophthalmol Vis Sci. 2010;51:4942–4955. doi: 10.1167/iovs.09-4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 85.Conconi M, Petropoulos I, Emod I, Turlin E, Biville F, Friguet B. Protection from oxidative inactivation of the 20S proteasome by heat-shock protein 90. Biochem J. 1998;333(Pt 2):407–415. doi: 10.1042/bj3330407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uetama T, Ohno-Matsui K, Nakahama K, Morita I, Mochizuki M. Phenotypic change regulates monocyte chemoattractant protein-1 (MCP-1) gene expression in human retinal pigment epithelial cells. J Cell Physiol. 2003;197:77–85. doi: 10.1002/jcp.10342. [DOI] [PubMed] [Google Scholar]
- 88.Elner VM, Burnstine MA, Strieter RM, Kunkel SL, Elner SG. Cell-associated human retinal pigment epithelium interleukin-8 and monocyte chemotactic protein-1: immunochemical and in-situ hybridization analyses. Exp Eye Res. 1997;65:781–789. doi: 10.1006/exer.1997.0380. [DOI] [PubMed] [Google Scholar]
- 89.Higgins GT, Wang JH, Dockery P, Cleary PE, Redmond HP. Induction of angiogenic cytokine expression in cultured RPE by ingestion of oxidized photoreceptor outer segments. Invest Ophthalmol Vis Sci. 2003;44:1775–1782. doi: 10.1167/iovs.02-0742. [DOI] [PubMed] [Google Scholar]
- 90.Suzuki M, Tsujikawa M, Itabe H, Du ZJ, Xie P, Matsumura N, Fu X, Zhang R, Sonoda KH, Egashira K, Hazen SL, Kamei M. Chronic photo-oxidative stress and subsequent MCP-1 activation as causative factors for age-related macular degeneration. J Cell Sci. 2012 doi: 10.1242/jcs.097683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parry GC, Martin T, Felts KA, Cobb RR. IL-1beta-induced monocyte chemoattractant protein-1 gene expression in endothelial cells is blocked by proteasome inhibitors. Arterioscler Thromb Vasc Biol. 1998;18:934–940. doi: 10.1161/01.atv.18.6.934. [DOI] [PubMed] [Google Scholar]
- 92.Lau LI, Chiou SH, Liu CJ, Yen MY, Wei YH. The effect of photo-oxidative stress and inflammatory cytokine on complement factor H expression in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2011;52:6832–6841. doi: 10.1167/iovs.11-7815. [DOI] [PubMed] [Google Scholar]
- 93.Muratani M, Tansey WP. How the ubiquitin-proteasome system controls transcription. Nat Rev Mol Cell Biol. 2003;4:192–201. doi: 10.1038/nrm1049. [DOI] [PubMed] [Google Scholar]
- 94.Collins GA, Tansey WP. The proteasome: a utility tool for transcription? Curr Opin Genet Dev. 2006;16:197–202. doi: 10.1016/j.gde.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 95.Bernstein PS. Nutritional Interventions against Age-Related Macular Degeneration. Acta Hortic. 2009;841:103–112. doi: 10.17660/actahortic.2009.841.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cantrell A, McGarvey DJ, Truscott TG, Rancan F, Bohm F. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch Biochem Biophys. 2003;412:47–54. doi: 10.1016/s0003-9861(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 97.Kim SR, Nakanishi K, Itagaki Y, Sparrow JR. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp Eye Res. 2006;82:828–839. doi: 10.1016/j.exer.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 98.Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res. 2001;72:215–223. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- 99.Sommerburg OG, Siems WG, Hurst JS, Lewis JW, Kliger DS, van Kuijk FJ. Lutein and zeaxanthin are associated with photoreceptors in the human retina. Curr Eye Res. 1999;19:491–495. doi: 10.1076/ceyr.19.6.491.5276. [DOI] [PubMed] [Google Scholar]
- 100.Subczynski WK, Wisniewska A, Widomska J. Location of macular xanthophylls in the most vulnerable regions of photoreceptor outer-segment membranes. Arch Biochem Biophys. 2010;504:61–66. doi: 10.1016/j.abb.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Herpen-Broekmans WM, Klopping-Ketelaars IA, Bots ML, Kluft C, Princen H, Hendriks HF, Tijburg LB, van Poppel G, Kardinaal AF. Serum carotenoids and vitamins in relation to markers of endothelial function and inflammation. Eur J Epidemiol. 2004;19:915–921. doi: 10.1007/s10654-004-5760-z. [DOI] [PubMed] [Google Scholar]
- 102.Gonzalez S, Astner S, An W, Goukassian D, Pathak MA. Dietary lutein/zeaxanthin decreases ultraviolet B-induced epidermal hyperproliferation and acute inflammation in hairless mice. J Invest Dermatol. 2003;121:399–405. doi: 10.1046/j.1523-1747.2003.12355.x. [DOI] [PubMed] [Google Scholar]
- 103.Sasaki M, Ozawa Y, Kurihara T, Noda K, Imamura Y, Kobayashi S, Ishida S, Tsubota K. Neuroprotective effect of an antioxidant, lutein, during retinal inflammation. Invest Ophthalmol Vis Sci. 2009;50:1433–1439. doi: 10.1167/iovs.08-2493. [DOI] [PubMed] [Google Scholar]
- 104.Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing of NF-κB procursor protein and the activation of NF-κB. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 105.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 106.Yaron A, Gonen H, Alkalay I, Hatzubai A, Jung S, Beyth S, Mercurio F, Manning AM, Ciechanover A, Ben-Neriah Y. Inhibition of NF-kappa-B cellular function via specific targeting of the I-kappa-B-ubiquitin ligase. Embo J. 1997;16:6486–6494. doi: 10.1093/emboj/16.21.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 108.Simonini A, Moscucci M, Muller DW, Bates ER, Pagani FD, Burdick MD, Strieter RM. IL-8 is an angiogenic factor in human coronary atherectomy tissue. Circulation. 2000;101:1519–1526. doi: 10.1161/01.cir.101.13.1519. [DOI] [PubMed] [Google Scholar]
- 109.Yoshida S, Ono M, Shono T, Izumi H, Ishibashi T, Suzuki H, Kuwano M. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol Cell Biol. 1997;17:4015–4023. doi: 10.1128/mcb.17.7.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 111.Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, Binion DG. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–8515. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]