Abstract
Pharmacological inhibitors of cyclin-dependent kinases (CDKs) have a wide therapeutic potential. Among the CDK inhibitors currently under clinical trials, the 2,6,9-trisubstituted purine (R)-roscovitine displays rather high selectivity, low toxicity, and promising antitumor activity. In an effort to improve this structure, we synthesized several bioisosteres of roscovitine. Surprisingly, one of them, pyrazolo[1,5-a]-1,3,5-triazine 7a (N-&-N1, GP0210), displayed significantly higher potency, compared to (R)-roscovitine and imidazo[2,1-f]-1,2,4-triazine 13 (N-&-N2, GP0212), at inhibiting various CDKs and at inducing cell death in a wide variety of human tumor cell lines. This approach may thus provide second generation analogues with enhanced biomedical potential.
Introduction
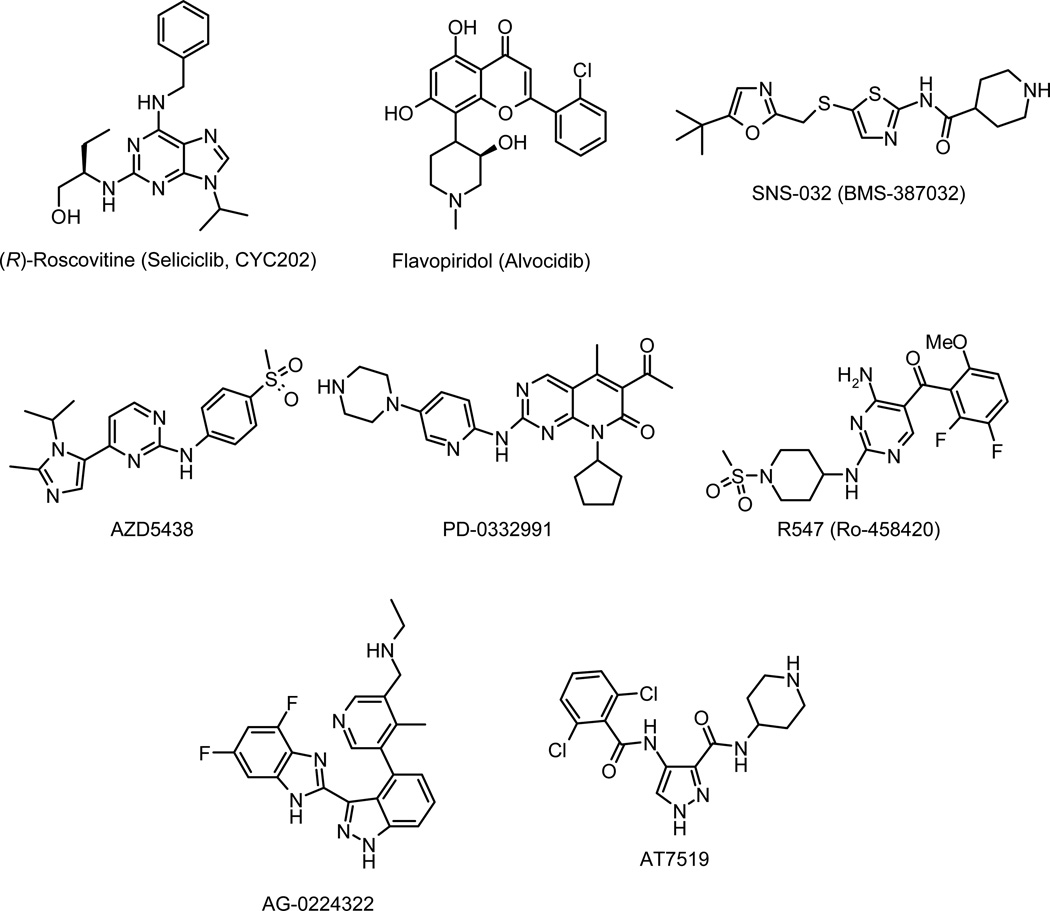
Pharmacological inhibition of cyclin-dependent kinases (CDKs) is anticipated to provide an effective approach to the control of multiple human diseases in the clinic including cancers, chronic neurodegenerative diseases (Alzheimer’s disease, Parkinson’s disease), “acute” neuronal disorders (stroke, traumatic brain injury, pain signaling), kidney diseases (glomerulonephritis, lupus nephritis, collapsing glomerulopathy, polycystic kidney disease, cisplatin-induced nephrotoxicity), pleural inflammation and arthritis, diabetes type 2, viral infections (HSV, HCMV, HPV, HIV), unicellular parasites (Plasmodium, Leishmania, etc…).1 Various CDK inhibitors have already been reported to exhibit antiproliferative activities and are in clinical trials against various cancers (Figure 1).2 Among them, flavopiridol and (R)-roscovitine were the first CDK inhibitors to enter in clinical phase. Second-generation CDK inhibitors such as SNS-032 (former BMS-387032), AG-24322, R547, AT7519, AT7119, AZD5438, and PD-0332991 are in advanced preclinical testing or in clinical trials.3,4 The discovery of the (R)-roscovitine has been reported in detail previously.5 This compound is an orally available 2,6,9-trisubstituted purine that specifically inhibits CDK1, CDK2, CDK3, CDK5, CDK7, and CDK9 in the submicromolar range. Preclinical studies demonstrate that (R)-roscovitine acts on multiple phases of the cell cycle and works by inducing cell cycle arrest and apoptosis.6
Figure 1.
CDK inhibitors in clinical trials: (R)-roscovitine, flavopiridol, SNS-032, AZD5438, PD-0332991, R547, AG-0224322 and AT7519.
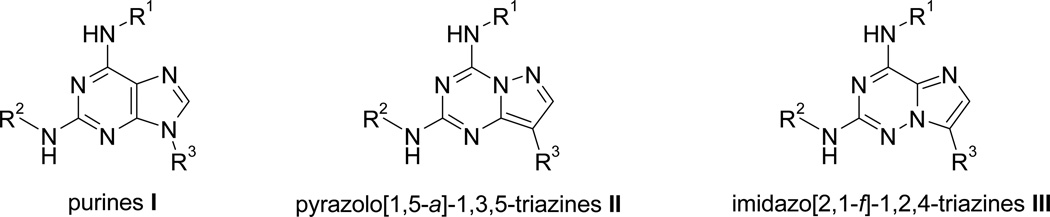
The 2,6,9-trisubstituted purine family I (Figure 2) has been the subject of intensive structure-activity relationship studies to identify more potent CDK inhibitory purine derivatives. Thus, the modification of the nature of the substituents on position 2, 6, and 9 led to the preparation of aminopurvalanol, purvalanol A and B, displaying higher inhibitory CDK activities in vitro.7 Among the classical approaches to drug design, the bioisosterism strategy8 is a powerful way to the rational design of new drugs. Several features are favourable to this approach such as (1) improvement of the metabolic stability of the drugs, (2) reduction of certain adverse affects, (3) optimization of pharmacokinetic and/or bioavailability, and (4) circumventing patent issues. In this article, we report a bioisosterism study leading to an original and promising new CDK inhibitor lead compound based on (R)-roscovitine.
Figure 2.
2,6,9-Trisubstituted purine CDK inhibitor scaffold I and related bioisosteres II and III.
The pyrazolo[1,5-a]-1,3,5-triazine moiety II or the imidazo[2,1-f]-1,2,4-triazine core III allows oneto consider the synthesis of a large number of "carbabioisosteres" of purine drugs (Figure 2). In the case of the pyrazolo[1,5-a]-1,3,5-triazine moiety, this scaffold perfectly mimicks the biophysicochemical properties of the purine ring9 while being more stable in vivo (no action of nucleosidases on C-8 position as encountered for the purine ring).10 By this approach, a large number of pyrazolo[1,5-a]-1,3,5-triazine derivatives have been designed for the last several decades as remarkable purine bioisosteres. Thus, this type of modification can lead to a large number of derivatives showing potent biological activities.11
These data prompted us to synthesize novel pyrazolo[1,5-a]-1,3,5-triazine and imidazo[2,1-f]-1,2,4-triazine roscovitine bioisosteres in order to compare their CDK inhibitory and antiproliferative activities to genuine (R)-roscovitine.
Results and Discussion
Chemistry
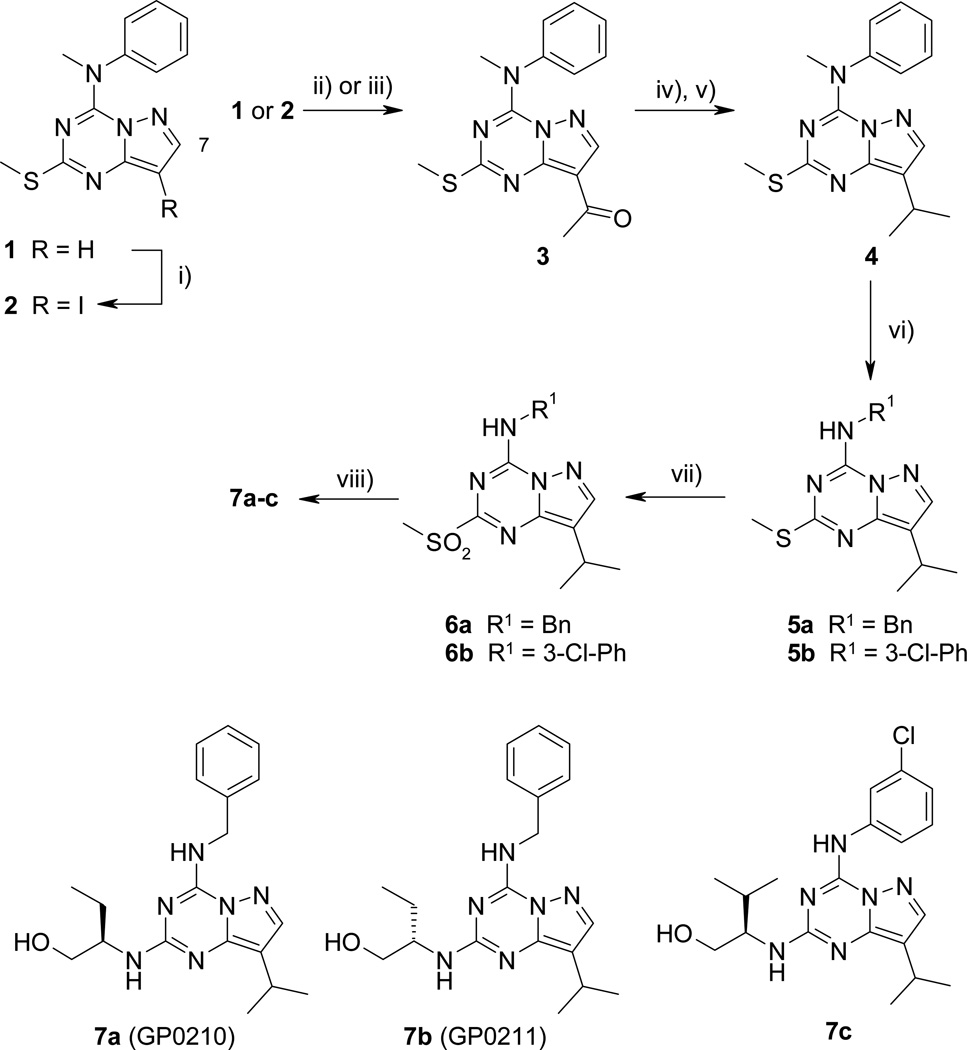
Pyrazolo[1,5-a]-1,3,5-triazine derivatives 7a–c were first prepared (Scheme 1). Regioselective iodination on C-8 position of 112 was carried out in the presence of N-iodosuccinimide to give 8-iodopyrazolo[1,5-a]-1,3,5-triazine 213 in 88% yield. Introduction of the acetyl group on C-8 position was first performed through a Stille palladium-catalyzed coupling reaction. Derivative 2 was treated with n-tributyl(1-ethoxyvinyl)tin in DMF, in the presence of a catalytic amount of freshly prepared tetrakis(triphenylphosphine)palladium (8 mol%) and LiCl to provide derivative 313 in good yield. Similarly, compound 3 was prepared from 1 by a Friedel-Crafts acylation.12 In a sealed tube, compound 1 was submitted to acylation reaction with acetyl chloride in the presence of 1 M SnCl4 in CH2Cl2 at 85 °C, affording compound 3 in 86% yield (versus 74% for two steps). The same reaction was carried out under various conditions (AcCl, AlCl3, CH2Cl2, room temperature to reflux or AcCl, SnCl4, CH2Cl2, reflux) leading to the total recovery of the starting material. Nucleophilic addition of 1 M MeMgBr in Et2O on 3 followed by hydrolysis by a freshly prepared NH4Cl solution gave tertiary alcohol, which was readily treated by sodium borohydride/trifluoroacetic acid in CH2Cl2 at room temperature to yield isopropyl derivative 4. Direct access to 4 from 1 via a Friedel-Crafts alkylation was investigated. Attempts were performed using 1, isopropyl chloride or iodide and Lewis acids (AlCl3, SnCl4, 1 M SnCl4 in CH2Cl2) under different temperature conditions (room temperature to 100 °C in a sealed tube). In each case, the starting material was recovered. As already mentioned in the literature,10 the N-methyl-N-phenylamino group on C-4 position is a good leaving group for nucleophilic addition-elimination reaction. Nucleophilic aromatic substitution on C-4 position of 4 was performed either in the presence of benzylamine or 3-chloroaniline, providing secondary amines 5a and 5b, respectively, in 84 and 40% yield. m-CPBA oxidation of methylsulfanyl group of 5 furnished sulfones 6a and 6b in fair yields. (R)-roscovitine bioisostere 7a (GP0210, N-&-N1)14 was obtained by nucleophilic aromatic substitution on C-2 position of 6a in the presence of (R)-(−)-2-aminobutanol. The enantiomer 7b was prepared from 6a and (S)-(+)-2-aminobutanol. Purvalanol A bioisostere 7c was obtained from 6b and (R)-(−)-2-amino-3-methylbutanol.
Scheme 1.
Reagents and conditions: i) NIS, CHCl3, reflux, 90 min, 88%; ii) AcCl, 1 M SnCl4, CH2Cl2, 85 °C, 17 h, sealed tube, 86%; iii) n-tributyl(1-ethoxyvinyl)stannane, Pd(PPh3)4, LiCl, DMF, 80–90 °C, 12 h, 84%; iv) a) 1 M MeMgBr, THF, 0 °C to rt, 20 min; b) NH4Cl; v) NaBH4, TFA, CH2Cl2, 0 °C to rt, 1 h, 89% (two step yield); vi) benzylamine, EtOH, 90 °C, 12 h, 5a = 84%; 3-chloroaniline, dioxane, 120°C, 12 h, 5a = 40%; vii) m-CPBA, CH2Cl2, 0 °C to rt, 3 h, 6a = 97%, 6b = 60%; viii) (R)-(−)-2-aminobutanol, dioxane, 140 °C, 12 h, 7a = 56%; (S)-(+)-2-aminobutanol, dioxane, 140 °C, 12 h, 7b = 67%, (R)-(−)-2-amino-3-methylbutanol, dioxane, 140 °C, 12 h 7c = 70%
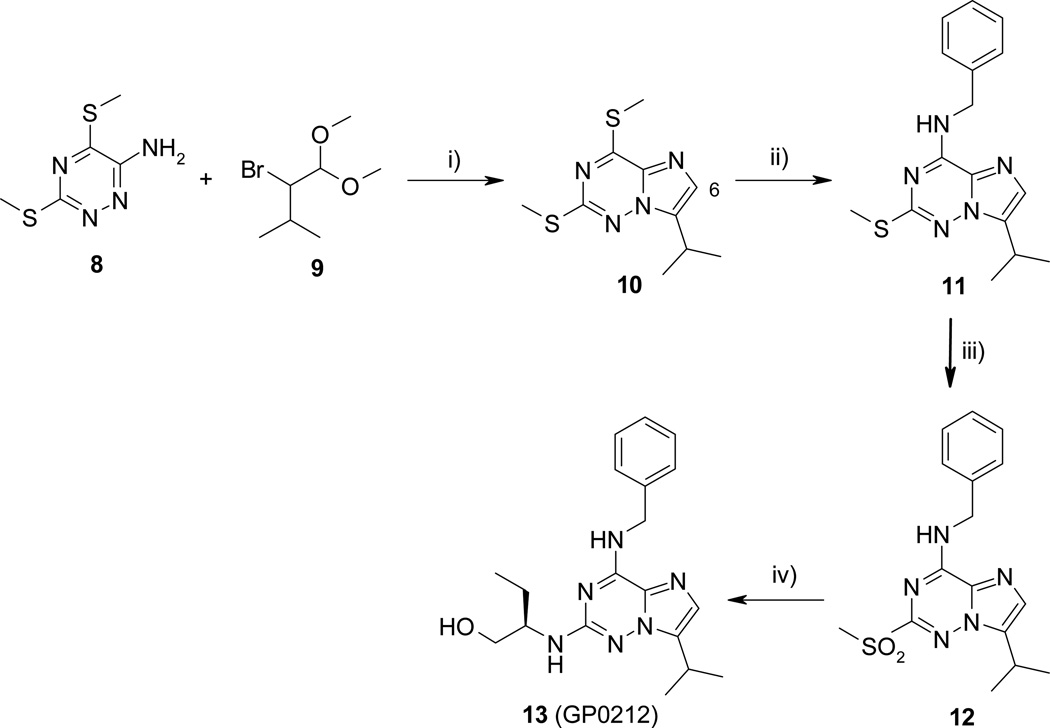
The synthesis of imidazo[2,1-f]-1,2,4-triazine bioisostere was carried out in four steps from the known 6-amino-3,5-bis(methylsulfanyl)-1,2,4-triazine 814 as depicted in scheme 2. Treatment of 1 with 2-bromo-3-methylbutyraldehyde dimethyl acetal 915 and molecular sieves 4Å in the presence of catalytic amount of camphorsulfonic acid in acetonitrile at reflux led to 10 in 60% yield. Nucleophilic displacement of the methylsulfonyl substitutent on C-4 position of 10 by benzylamine in excess gave compound 11 in good yield. It should be noted that the first experiments were carried out in ethanol at 50 °C, but even after a prolonged reaction time, the reactions were not complete. Optimal condition reaction was finally obtained when benzylamine was used as reagent and solvent. The 2-methylsulfanyl substituent was oxidized by m-CPBA to give the sulfone 12 in 81% yield. The ultimate nucleophilic aromatic substitution on C-2 position of 12 was carried out at 170 °C in the presence of (R)-(−)-2-aminobutanol in excess to afford (R)-roscovitine analogue 13 (N-&-N2, GP0212) in fair yield.
Scheme 2.
Reagents and conditions: i) CSA, Molecular Sieves 4Å, MeCN, 70 °C, 8 h, 60%; ii) BnNH2, 50 °C, 16 h, 95%; iii) m-CPBA, CH2Cl2, rt, 16 h, 81%; iv) (R)-(−)-2-aminobutanol, 170 °C, 4 h, 60%
Biological Evaluation
Inhibition of Cyclin-Dependent Kinases
We first tested the synthesized compounds on a selection of CDKs (CDK1, CDK2, CDK5, CDK7, and CDK9), known to be inhibited by (R)-roscovitine.6 Results show that all compounds were active on these kinases in the submicromolar range (Table 1). Compound 13 was essentially identical to (R)-roscovitine in terms of its effects on the 5 CDKs. In contrast, the roscovitine bioisostere 7a was significantly more potent, by a factor of 3–5, than either 13 or (R)-roscovitine. The purvalanol A analogue 7c was also more potent but, surprisingly, totally inactive on CDK7/cyclin H and CDK9/cyclin T. The enzymatic effects and selectivity of both 7a and 13 are described in more detail elsewhere.14
Table 1.
Effects of (R)-Roscovitine, Purvalanol A and Its Four Analogues on the Catalytic Activity of Various CDKsa
| IC50 (µM)b | |||||
|---|---|---|---|---|---|
| Compound | CDK1/ cyclin B |
CDK2/ cyclin A |
CDK5/ p25 |
CDK7/ cyclin H |
CDK9/ cyclin T |
| (R)-Roscovitine | 0.33 | 0.22 | 0.27 | 0.80 | 0.23 |
| Purvalanol A7a | 0.004 | 0.070 | 0.075 | NDc | ND |
| 7a | 0.073 | 0.04 | 0.07 | 0.50 | 0.043 |
| 7b | ND | ND | 0.13 | ND | ND |
| 7c | 0.08 | 0.06 | 0.06 | > 100 | > 100 |
| 13 | 0.40 | 0.22 | 0.32 | 0.60 | 0.20 |
(R)-Roscovitine and its analogues were tested at various concentrations for their effects on 5 CDKs.
IC50 values were calculated from the dose-response curves and are reported in µM.
ND, not determined.
Inhibition of Tumor Cell Proliferation
We next tested the synthesized compounds for their effects on cell survival using the human neuroblastoma SH-SY5Y and human embryonic kidney HEK293 cell lines (Table 2). Results show that while compound 13 and (R)-roscovitine share a similar efficacy, 7a and its (S)-isomer 7b are significantly more active (5–6 fold). Interestingly, 7c was completely inactive, possibly accounting for its lack of effects on CDK7 and CDK9. Compound 7a was next evaluated against the full panel NCI of 60 tumor cell lines (mean graphs data for 7a and (R)-roscovitine, see Supporting Information, Figures S1 and S2).17 The biological data were compared with those available for (R)-roscovitine (Table 3). Results show that compound 7a is on average more potent at inhibiting cell proliferation than (R)-roscovitine (14-fold in terms of GI50, 9-fold in terms of TGI), but only modestly more potent at inducing net cell death (LC50). Nevertheless, the cytotoxic effect of 7a is preferably observed on melanoma cancer cell lines (see Table 3). Furthermore, like for (R)-roscovitine, no specific tumor type displayed any particular sensitivity to compound 7a. Finally, we performed a cell proliferation assay on a small panel of 6 human cell lines derived from lung (A549), prostate (PC3), cervix (Hela), kidney (293T) cancers, B-lymphoma (Raji), and myeloma (U937) (see Supporting Information, Figure S3). 7a inhibited the proliferation of all cell lines in a dose-dependent fashion. Total growth inhibition was nearly achieved at 5 µM for the other cell lines. In this assay as well, 7a proved significantly more potent than (R)-Roscovitine (Supporting Information, Figure S3), which only partially inhibited tumor cell proliferation at 10 µM. All these data confirm that 7a is >5-fold more potent than its parent molecule, (R)-roscovitine. A more detailed investigation on the intracellular mechanism of action of 7a and 13, in comparison with (R)-roscovitine, as well as the co-crystal structures of 7a and (R)-roscovitine with CDK2/cyclin A, is presented elsewhere.14
Table 2.
Effects of (R)-Roscovitine, Purvalanol A and Its Four Analogues on the Survival of Two Cell Linesa
| IC50 (µM)b | ||
|---|---|---|
| Compound | SH-SY5Y | HEK293 |
| (R)-Roscovitine | 16.1 | 48.9 |
| 7a | 2.7 | 7.6 |
| 7b | 2.0 | 7.2 |
| 7c | > 100 | > 100 |
| 13 | 15.4 | 26.8 |
Compounds were tested at various concentrations for their effects on the survival of the human neuroblastoma SH-SY5Y cells and human embryonic kidney cells (HEK293).
Cell survival was estimated 48 h after the addition of each compound using the MTS reduction assay. IC50 values were calculated from the dose-response curves and are reported in µM.
Table 3.
Effects of (R)-Roscovitine and 7a on Cell Proliferation and Cell Death as Assayed in the NCI 60 Cell Line Screening Panela
| GI50 (µM)b | TGI (µM)c | LC50 (µM)d | ||||
|---|---|---|---|---|---|---|
| Cell line | (R)-Roscovitine | 7a | (R)-Roscovitine | 7a | (R)-Roscovitine | 7a |
| Leukemia | ||||||
| CCRF-CEM | > 100 | 0.65 | > 100 | 9.26 | > 100 | > 100 |
| HL-60(TB) | 19.9 | 0.41 | > 100 | 2.68 | > 100 | > 100 |
| K-562 | 50.1 | 2.44 | > 100 | 7.38 | > 100 | > 100 |
| MOLT-4 | 25.1 | 2.18 | > 100 | 9.45 | > 100 | > 100 |
| RPMI-8226 | 25.1 | 2.23 | > 100 | 6.93 | > 100 | > 100 |
| SR | 5.01 | 0.25 | > 100 | 1.59 | > 100 | > 100 |
| NSCL cancer | ||||||
| A549/ATCC | 12.6 | 1.58 | > 100 | 41.4 | > 100 | > 100 |
| EKVX | 19.9 | 2.41 | 50.1 | 9.13 | > 100 | > 100 |
| HOP-62 | 25.1 | 2.10 | > 100 | 8.72 | > 100 | > 100 |
| HOP-92 | 50.1 | 1.67 | > 100 | 7.10 | > 100 | > 100 |
| NCI-H226 | 19.9 | 1.84 | > 100 | 10.1 | > 100 | > 100 |
| NCI-H23 | 12.6 | 1.71 | 50.1 | 7.79 | > 100 | > 100 |
| NCI-H322M | 31.6 | 1.86 | > 100 | 8.92 | > 100 | > 100 |
| NCI-H460 | 25.1 | 1.05 | > 100 | 10.9 | > 100 | > 100 |
| NCI-H522 | 15.8 | 1.46 | 63.1 | 8.98 | > 100 | > 100 |
| Colon cancer | ||||||
| COLO 205 | 12.6 | 1.12 | 31.6 | 3.15 | > 100 | 8.86 |
| HCC-2998 | 31.6 | 1.20 | > 100 | 3.27 | > 100 | 8.96 |
| HCT-116 | 5.01 | 0.51 | > 100 | 15.0 | > 100 | > 100 |
| HCT-15 | 19.9 | 0.87 | > 100 | 10.5 | > 100 | > 100 |
| HT29 | 19.9 | 2.00 | > 100 | 15.5 | > 100 | > 100 |
| KM12 | 5.01 | 0.71 | 63.1 | 2.79 | > 100 | 9.30 |
| SW-620 | 79.4 | 1.38 | > 100 | 5.56 | > 100 | > 100 |
| CNS cancer | ||||||
| SF-268 | 19.9 | 1.82 | > 100 | 7.20 | > 100 | > 100 |
| SF-295 | 25.1 | 0.81 | > 100 | 4.74 | > 100 | > 100 |
| SF-539 | 15.8 | 1.22 | 79.4 | 3.62 | > 100 | 11.9 |
| SNB-19f | NDe | 1.44 | ND | 12.3 | ND | > 100 |
| SNB-75 | 12.6 | 1.31 | 31.6 | 3.33 | > 100 | 8.48 |
| U251f | 50.1 | 1.40 | > 100 | 10.5 | > 100 | > 100 |
| Melanoma | ||||||
| LOX IMVI | 12.6 | 1.07 | > 100 | 10.3 | > 100 | 34.4 |
| MALME-3M | 19.9 | 2.60 | 50.1 | 6.99 | > 100 | 43.5 |
| M14 | 25.1 | 2.00 | > 100 | 7.93 | > 100 | 34.3 |
| SK-MEL-2 | 15.8 | 1.47 | 39.8 | 6.00 | > 100 | > 100 |
| SK-MEL-28 | ND | 1.16 | ND | 3.82 | ND | 16.9 |
| SK-MEL-5 | 10 | 1.78 | 31.6 | 4.96 | 79.4 | 17.4 |
| UACC-257 | ND | 3.56 | ND | 15.0 | ND | > 100 |
| UACC-62 | 7.9 | 1.37 | 31.6 | 5.50 | > 100 | 31.1 |
| Ovarian cancer | ||||||
| IGROV1 | 6.3 | 0.78 | > 100 | > 100 | > 100 | > 100 |
| OVCAR-3 | 12.6 | 1.15 | > 100 | 4.60 | > 100 | 42.4 |
| OVCAR-4 | 31.6 | 2.57 | > 100 | 37.0 | > 100 | > 100 |
| OVCAR-5 | 39.8 | 1.85 | > 100 | 15.0 | > 100 | > 100 |
| OVCAR-8 | ND | 2.15 | ND | 54.8 | ND | > 100 |
| SK-OV-3 | 79.4 | 5.55 | > 100 | 40.2 | > 100 | > 100 |
| Renal cancer | ||||||
| 786-0 | 25.1 | 1.98 | > 100 | 10.7 | > 100 | > 100 |
| A498 | 50.1 | 1.25 | > 100 | 3.44 | > 100 | 9.47 |
| ACHN | 12.6 | 0.81 | 79.4 | 10.7 | > 100 | 49.1 |
| CAKI-1 | 6.3 | 1.02 | > 100 | 8.35 | > 100 | > 100 |
| RXF 393 | 5.0 | 0.25 | > 100 | 0.79 | > 100 | 4.25 |
| SN12C | ND | 2.41 | ND | 14.4 | ND | 58.1 |
| TK-10 | 63.1 | 2.00 | > 100 | 10.9 | > 100 | > 100 |
| UO-31 | 12.6 | 1.59 | > 100 | > 100 | > 100 | > 100 |
| Prostate cancer | ||||||
| PC-3 | 19.9 | 0.93 | 63.1 | 12.3 | > 100 | > 100 |
| DU-145 | 15.8 | 1.30 | > 100 | 9.52 | > 100 | > 100 |
| Breast cancer | ||||||
| MCF7 | 12.6 | 0.59 | > 100 | 7.99 | > 100 | > 100 |
| NCI/ADR-RESg | 6.3 | 2.08 | 63.1 | 15.7 | > 100 | > 100 |
| MDA-MB-231-ATCC | 39.8 | 2.37 | > 100 | 9.47 | > 100 | > 100 |
| HS 578T | ? | 1.59 | 12.6 | 6.16 | 79.4 | > 100 |
| MDA-MB-435h | 12.6 | 2.06 | 50.1 | 10.1 | > 100 | 41.4 |
| BT-549 | ND | 1.56 | ND | 5.29 | ND | 36.1 |
| T-47D | 16.8 | 2.48 | 79.4 | 13.3 | > 100 | > 100 |
| MDA-MB-468 | ND | 1.88 | ND | 5.19 | ND | 63.5 |
| MG_MIDi | 19.3 | 1.41 | 78.3 | 8.71 | 99.4 | 61.6 |
Data obtained from NCI’s in vitro-oriented tumor cells screen ((R)-Roscovitine = NSC 701554; 7a = NSC 743927).
GI50 is the molar concentration causing 50% growth inhibition of tumor cells.
TGI is the molar concentration giving total growth inhibition.
LC50 is the molar concentration leading to 50% net cell death.
ND = not determined.
CNS cell lines SNB-19 and U251 are derived from the same individual.
NCI/ADR-RES is an ovarian tumor cell line, not a breast tumor line.
MDA-MB-435 is a melanoma cell line, not a breast cancer cell line cell line.
MG_MID (mean graph midpoint) is the arithmetical mean value for all tested cancer lines.
In Vivo Antitumor Activity of Compound 7a and (R)-Roscovitine
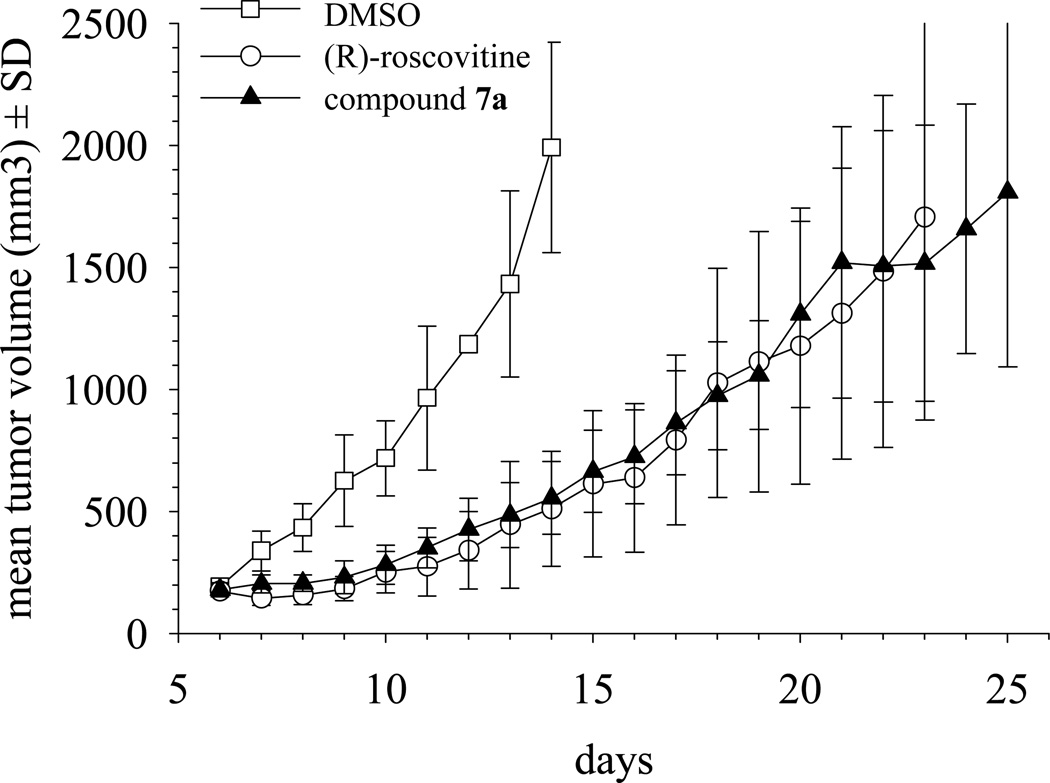
An Ewing’s sarcoma xenograft mouse model system established as described in the Experimental Section was used to compare the antitumor activity of 7a and (R)-roscovitine in vivo. This model was selected on the basis of its clear-cut sensitivity to roscovitine.18 Compound 7a was evaluated at 25 mg/kg, and (R)-roscovitine was tested at 50 mg/kg. Because compound 7a was roughly 2 times more potent in vitro than (R)-roscovitine (average on 5 kinases, Table 1), the dosing for compound 7a was chosen as half of that of roscovitine, so that both compounds could be compared on the basis of similar activity. Results showed those 8 days after treatment initiation, and both compounds had slowed tumor growth considerably (Figure 3). At 25 mg/kg, compound 7a was as active as (R)-roscovitine used at a concentration 2-fold higher (50 mg/kg). When tumors in control animals reached an average volume about 2000 mm3, and animals had to be sacrificed to comply with institutional animal care and use guidelines, tumors in drug- treated animals had reached and average volume of only ~550 mm3 (a near 73% inhibition of tumor growth). The inhibitory effect of both drugs on tumor growth was also manifested by the fact that tumors in treated animals took an additional period of about 15 days to reach the maximum volume allowed by institutional tumor burden standards (Figure 3).
Figure 3.
In vivo antitumor activity of 7a. Compound 7a treatment of Ewing’s sarcoma xenografts established in mice resulted in tumor growth delay similar to that produced by (R)-Roscovitine. Xenografts established by sc inoculation of A4573 cells (4 × 106 per animal) in nude mice were grown to a mean volume of about 195 mm3. Animals carrying tumors were randomized into three groups. One group (n = 6) was treated with Compound 7a in DMSO, administered as single daily ip injections, at a dose of 25 mg/kg, for 5 days. Another group (n = 6) received (R)-Roscovitine under the same conditions, except that the dose was 50 mg/kg. The control group (n = 6) received ip injections of DMSO following identical schedules. Results shown correspond to one of the experimental replicas. Tumor growths in compound 7a and (R)-roscovitine -treated animals were significantly slower than the
Conclusion
A rather straightforward bioisostere design approach, applied to the clinical drug (R)-roscovitine, has allowed us to generate one analogue that displays unexpected enhanced inhibitory activity towards the CDK targets. This translates into enhanced cell death inducing activity, a favorable property, which was confirmed on more than 60 cell lines, and an improved in vitro biological activity, which was confirmed in a first animal tumor model. Altogether this work shows that minor modification can lead to better second generation analogues of (R)-roscovitine. These findings support further clinical investigation to determine the therapeutic potential of 7a.
Experimental Section
Chemistry
General Methods
Commercial reagents (Fluka, Aldrich) were used without purification. Solvents were distillated prior to use. (R)-Roscovitine was synthesized as described previously6 and kindly provided by Dr. Hervé Galons and Nassima Oumata (Université René Descartes, Paris 5). Melting points were determined using a Büchi capillary instrument and are uncorrected. IR spectra were recorded on a Perkin-Elmer Spectrum One. 1H spectra were recorded on a Bruker Advance 300 MHz spectrometer. Chemical shifts are reported in ppm (δ) relative to tetramethylsilane as an internal standard. Mass spectra were recorded with a Perkin-Elmer SCIEX API spectrometer. Elemental analyses were performed on a Thermoquest Flash 1112 series EA analyzer. Thin Layer Chromatography (TLC) analyses were conducted on aluminium sheets silica gel Merck 60F254. The spots were visualized using an ultraviolet light. Flash chromatography was carried out on silica gel 60 (40–63 µm, Merck) using the indicated solvents. The light petroleum ether (PE) refers to the fraction boiling at 40–60°C.
8-Iodo-4-(N-methyl-N-phenylamino)-2-methylsulfanylpyrazolo[1,5-a]-1,3,5-triazine (2)
A solution of 4-(N-methyl-N-phenylamino)-2-methylsulfanylpyrazolo[1,5-a]-1,3,5-triazine 112,13 (940 mg, 3.46 mmol) and NIS (1.09 g, 4.85 mmol) in CHCl3 (35 mL) was stirred at reflux for 30 min. The solvent was evaporated in vacuo. The residue was dissolved in CH2Cl2 (50 mL) and washed with a saturated aqueous Na2S2O3 solution (3 × 20 mL). The organic layer was dried over MgSO4 and concentrated in vacuo. The crude product was purified by flash chromatography (EP/Et2O 9:1) to afford 2 (1.21 g, 88%) as a pale red solid. Mp 162–163 °C (Et2O/PE). IR (KBr): ν 3085, 2915, 1605, 1585, 745, 695 cm−1. 1H NMR (300 MHz, CDCl3): δ 7.61 (s, 1H, H7), 7.39-7.36 (m, 3H, Harom), 7.16 (broad d, 2H, J = 7.1 Hz, Harom), 3.71 (s, 3H, CH3), 2.59 (s, 3H, CH3). MS (ESI): m/z 398 (M + H+). Anal. (C13H12IN5S) C, H, N.
8-Acetyl-4-(N-methyl-N-phenylamino)-2-methylsulfanylpyrazolo[1,5-a]-1,3,5-triazine (3)
Method A : A mixture of 1 (871 mg, 3.21 mmol), acetyl chloride (458 mL, 6.42 mmol) and 1 M SnCl4 in CH2Cl2 (16.1 mL) was stirred in a sealed tube for 17 h at 85 °C. The reaction mixture was then poured into crushed ice, and extracted with EtOAc (3 × 15 mL). The combined organic extracts were dried over MgSO4 and concentrated in vacuo. The crude residue was purified by flash chromatography (CH2Cl2/EP/EtOAc 5:6:1) to provide 3 (868 mg, 86%) as a solid. Method B : A mixture of 2 (513 mg, 1.3 mmol), freshly prepared Pd(PPh3)4 (223 mg, 0.19 mmol), and LiCl (137 mg, 3.23 mmol) in DMF (10 mL) was added to n-tributyl(1-ethoxyvinyl)stannane (627 µL, 1.94 mmol). The solution was stirred for 12 h at 90 °C. The solvent was evaporated in vacuo. The crude residue was purified by flash chromatography (CH2Cl2/EP/EtOAc 5:6:1) to give 3 (341 mg, 84%) as a solid. Mp 223-225 °C (MeOH). IR (KBr): ν 3005, 2935, 1670, 1560, 755, 700 cm−1. 1H NMR (300 MHz, CDCl3): δ 8.08 (s, 1H, H7), 7.45-7.38 (m, 3H, Harom), 7.19-7.16 (m, 2H, Harom), 3.75 (s, 3H, CH3), 2.70 (s, 3H, CH3), 2.59 (s, 3H, CH3). MS (ESI): m/z 314 (M + H+). Anal. (C15H15N5OS) C, H, N.
4-(N-Methyl-N-phenylamino)-8-(1-methylethyl)-2-methylsulfanylpyrazolo[1,5-a]-1,3,5-triazine (4)
At 0 °C, a solution of 3 M MeMgBr in Et2O (297 µL, 2.58 mmol) was added dropwise to a solution of 3 (269 mg, 0.86 mmole) in THF (7 mL). After completion of the addition, the solution was stirred at room temperature for an additional 20 min. The reaction mixture was neutralized by a freshly prepared aqueous NH4Cl solution (5 mL). The aqueous layer was extracted with CH2Cl2 (5 mL) and EtOAc (2 × 5 mL). The combined organic extracts were dried over MgSO4 and concentrated. The oily residue was used without further purification. A solution of the intermediate in CH2Cl2 (9 mL) was added at 0 °C under nitrogen atmosphere to a solution of NaBH4 (113 mg, 3.00 mmol) in trifluoroacetic acid (9 mL). The reaction mixture was stirred for 1 h at room temperature and then neutralized by a 1 M NaOH solution (15 mL). The aqueous layer was extracted with CH2Cl2 (3 × 10 mL). The combined organic extracts were dried over MgSO4 and concentrated in vacuo. The residue was purified by flash chromatography (PE/Et2O 9:1) to afford 4 as a solid (241 mg, 89%). Mp 85–87 °C (EtOAc/PE); IR (KBr): ν 3055, 2960, 2865, 1615, 1535, 1460, 750, 695 cm−1. 1H NMR (300 MHz, CDCl3): δ 7.54 (s, 1H, H7), 7.41-7.31 (m, 3H, Harom), 7.19-7.15 (m, 2H, Harom), 3.70 (s, 3H, CH3), 3.14 (hept, 1H, J = 6.8 Hz, CH), 2.55 (s, 3H, CH3), 1.27 (d, 6H, J = 6.8 Hz, 2 CH3). MS (ESI): m/z 314 (M + H+). Anal. (C16H19N5S) C, H, N.
4-(N-Benzylamino)-8-(1-methylethyl)-2-methylsulfanylpyrazolo[1,5-a]-1,3,5-triazine (5a)
In a sealed tube, a solution of 4 (120 mg, 0.38 mmol) and benzylamine (209 µL, 1.91 mmol) in ethanol (1 mL) was heated for 12 h at 90 °C. Concentration of the reaction mixture provided the crude product that was purified by flash chromatography (PE/Et2O 4:1) to afford 5a as a solid (101 mg, 84%). Mp 139–141 °C (MeOH). IR (KBr): ν 3280, 2960, 1630, 1605 cm−1. 1H NMR (300 MHz, CDCl3): δ 7.69 (s, 1H, H7), 7.33-7.28 (m, 5H, Harom), 6.93 (t, 1H, J = 6.0 Hz, NH), 4.78 (d, 2H, J = 6.0 Hz, CH2), 3.15 (hept, 1H, J = 7.0 Hz, CH), 2.57 (s, 3H, CH3), 1.32 (d, 6H, J = 7.0 Hz, 2 CH3). MS (ESI): m/z 314 (M + H+). Anal. (C16H19N5S) C, H, N.
4-(3-Chloroanilino)-8-(1-methylethyl)-2-methylsulfanylpyrazolo[1,5-a]-1,3,5-triazine (5b)
In a sealed tube, a solution of 4 (120 mg, 0.38 mmol) and 3-chloroaniline (204 µL, 1.91 mmol) in dioxane (1 mL) was heated for 12 h at 120 °C. After concentration in vacuo, the residue was purified by flash chromatography (PE/Et2O 20:1 then 9:1) to afford 5a as a solid (51 mg, 40%). Mp 85–87 °C (EtOAc/PE). IR (KBr): ν 3335, 2955, 1615, 1570 cm−1. 1H NMR (300 MHz, CDCl3): δ 8,.47 (s, 1H, NH), 7.93 (s, 1H, Harom), 7.82 (s, 1H, H7), 7.60 (d, 1H, J = 7.5 Hz, Harom), 7.32 (t, 1H, J = 8.1 Hz, Harom), 7.16 (d, 1H, J = 7.4 Hz, Harom), 3.19 (hept, 1H, J = 7.0 Hz, CH), 2.61 (s, 3H, CH3), 1.35 (d, 6H, J = 7.0 Hz, 2 CH3). MS (ESI): m/z 334 (M + H+). Anal. (C15H16ClN5S) C, H, N.
4-(N-Benzylamino)-8-(1-methylethyl)-2-methylsulfonylpyrazolo[1,5-a]-1,3,5-triazine (6a)
Under an inert atmosphere, 50% m-CPBA (249 mg, 0.72 mmole) was added to a solution of 5a (74 mg, 0.24 mmol) in CH2Cl2 (5 mL) at 0 °C. The reaction mixture was stirred for 1 h at 0 °C and was allowed to warm up to room temperature for 3 h. The reaction mixture was diluted with CH2Cl2 (10 mL), washed with a saturated aqueous NaHCO3 solution (20 mL), then H2O (20 mL). The organic phase was dried over MgSO4 and concentrated in vacuo. The crude product was purified by flash chromatography (PE/EtOAc 4:1 then 2:1) to afford 6a as a solid (81 mg, 97%). Mp 123–125 °C (EtOAc/PE). IR (KBr): ν 3395, 2955, 2870, 1645, 1595, 1465, 1045, 750, 700 cm−1. 1H NMR (300 MHz, CDCl3): δ 7.83 (s, 1H, H7), 7.43 (t, 1H, J = 5.9 Hz, NH), 7.30-7.26 (m, 5H, Harom), 4.82 (d, 2H, J = 5.9 Hz, CH2), 3.29 (s, 3H, CH3), 3.19 (hept, 1H, J = 7.0 Hz, CH), 1.28 (d, 6H, J = 7.0 Hz, 2 CH3). MS (ESI): m/z 346 (M + H+). Anal. (C16H19N5O2S) C, H, N.
4-(3-Chloroanilino)-8-(1-methylethyl)-2-methylsulfonylpyrazolo[1,5-a]-1,3,5-triazine (6b)
Under an inert atmosphere, 50% m-CPBA (159 mg, 0.93 mmole) was added to a solution of 5a (51 mg, 0.15 mmol) in CH2Cl2 (3.5 mL) at 0 °C. The reaction mixture was stirred for 1 h at 0 °C and was allowed to warm up to room temperature for 3 h. The reaction mixture was diluted with CH2Cl2 (10 mL), washed with a saturated aqueous NaHCO3 solution (10 mL), then H2O (10 mL). The organic phase was dried over MgSO4 and concentrated in vacuo. The crude product was purified by flash chromatography (PE/EtOAc 9:1 then 2:1) to afford 6a as a solid (34 mg, 60%). Mp 172–174 °C (EtOAc/PE). IR (KBr): ν 3295, 2960, 2860, 1625, 1570, 1030, 755, 675 cm−1. 1H NMR (300 MHz, CDCl3): δ 8.71 (s, 1H, NH), 8.02 (s, 1H, H7), 7.78-7.76 (m, 2H, Harom), 7.35 (t, 1H, J = 8.3 Hz, Harom), 7.18 (d, 1H, J = 8.3 Hz, Harom), 3.38 (s, 3H, CH3), 3.29 (hept, 1H, J = 7.0 Hz, CH), 1.37 (d, 6H, J = 7.0 Hz, 2 CH3). MS (ESI): m/z 366 (M + H+). Anal. (C15H16ClN5O2S) C, H, N.
2-((R)-1-Amino-2-butanol)-4-(N-benzylamino)-8-(1-methylethyl)pyrazolo[1,5-a]-1,3,5-triazine (7a)
In a sealed tube, a solution of 6a (104 mg, 0.30 mmol) and (R)-(−)-2-aminobutanol (142 µL, 1.50 mmol) in dry dioxane (1 mL) was heated for 12 h at 140 °C. After evaporation of the solvent, the residue was purified by flash chromatography (PE/EtOAc 2:1) to afford 7a as a solid (60 mg, 56%). Mp 37–39 °C. (c = 0.50, CH2Cl2). IR (film): ν 3275, 2960, 2875, 1650, 1600, 1560, 1435, 765, 695 cm−1. 1H NMR (300 MHz, CDCl3): δ 7.56 (s, 1H, H7), 7.33-7.24 (m, 5H, Harom), 7.10 (s, 1H, NH), 5.23 (s, 1H, OH), 4.73-4.65 (m, 2H, CH2), 4.00-3.88 (m, 1H, CH), 3.83 (d, 1H, J = 10.8 Hz, CH2O), 3.66 (dd, 1H, J = 7.3, 10.8 Hz, CH2O), 3.02 (hept, 1H, J = 6.6 Hz, CH), 1.70-1.52 (m, 2H, CH2), 1.28 (d, 6H, J = 6.6 Hz, 2 CH3), 1.03 (t, 3H, J = 7.4 Hz, CH3). MS (EI+VE): m/z 354 (M+). Anal. (C19H26N6O) C, H, N.
2-((S)-1-Amino-2-butanol)-4-(N-benzylamino)-8-(1-methylethyl)pyrazolo[1,5-a]-1,3,5-triazine 7b
In a sealed tube, a solution of 6a (84 mg, 0.24 mmole) and (S)-(−)-2-aminobutanol (115 µL, 1.22 mmol) in dry dioxane (1 mL) was heated for 12 h at 140 °C. After evaporation of the solvent, the residue was purified by flash chromatography (PE/EtOAc 1:2) to afford 7b as a solid (58 mg, 67%). Mp 37–39 °C. (c = 0.50, CH2Cl2). Anal. (C19H26N6O) C, H, N.
2-((1R)-(1-Methylethyl)-2-hydroxyethylamino)-4-(3-chloroanilino)-8-(1-methylethyl) pyrazolo[1,5-a]-1,3,5-triazine (7c)
In a sealed tube, a solution of 6b (34 mg, 0.09 mmole) and (R)-(−)-2-amino-3-methylbutanol (47.5 mg, 0.46 mmol) in dry dioxane (1 mL) was heated for 12 h at 140 °C. After evaporation of the solvent, the residue was purified by flash chromatography (PE/EtOAc 4:1) to afford 7c as a solid (25 mg, 70%). Mp 161–163 °C (EtOAc). (c = 0.27, CH2Cl2). IR (KBr): ν 3360, 3310, 2955, 1635, 1615, 1575, 1420, 1070, 760, 680 cm−1. 1H NMR (300 MHz, DMSO-d6 + D2O à 75°C): δ 8.05 (s, 1H, Harom), 7.88 (d, 1H, J = 8.0 Hz, Harom), 7.77 (s, 1H, H7), 7.38 (t, 1H, J = 8.0 Hz, Harom), 7.16 (d, 1H, J = 8.0 Hz, Harom), 3.83 (q, 1H, J = 5.9 Hz, CH), 3.60-3.50 (m, 2H, CH2O), 2.96 (hept, 1H, J = 7.0 Hz, CH), 1.92-2.02 (m, 1H, CH), 1.27 (d, 6H, J = 7.0 Hz, 2 CH3), 0.94 (d, 3H, J = 6.8 Hz, CH3), 0.93 (d, 3H, J = 6.8 Hz, 3 CH3). MS (ESI): m/z 389 (M + H+). Anal. (C19H25ClN6O) C, H, N.
7-(1-Methylethyl)-2,4-bis(methylsulfanyl)imidazo[2,1-f]-1,2,4-triazine (10)
A solution of triazine 814 (558 mg, 2.96 mmol), camphorsulphonic acid (50 mg, 0.21 mmol), 2-bromo-3-methylbutyraldehyde dimethyl acetal 915 (0.7 mL), and MS 4Å (50 mg) in dry MeCN (30 mL) were heated at reflux for 48 h. The reaction was completed by addition of 9 (0.5 mL) to the medium at 24 h. The solids were removed by filtration and washed with CH2Cl2. After evaporation in vacuo, the residue was taken up in CH2Cl2 (40 mL) and washed with saturated aqueous NaHCO3 solution (10 mL), then brine (10 mL). The organic phase was dried over MgSO4 and concentrated in vacuo. The crude product was purified by flash chromatography (PE/EtOAc 95:5) to give 10 (452 mg, 60%) as a solid. Mp 85–86 °C. IR (KBr): ν 2960, 1570, 1510, 1435, 1355, 1150 cm−1. 1H NMR (300 MHz, CDCl3): δ 7.40 (s, 1H, H6), 3.40 (hept, 1H, J = 7.0 Hz, CH), 2.66 (s, 3H, CH3), 2.60 (s, 3H, CH3), 1.39 (d, 6H, J = 7.0 Hz, 2 CH3). MS (ESI): m/z 255 (M + H+). Anal. (C10H14N4S2) C, H, N.
4-Benzylamino-7-(1-methylethyl)-2-(methylsulfanyl)imidazo[2,1-f]-1,2,4-triazine (11)
A mixture of 10 (228 mg, 0.90 mmol) and benzylamine (1 mL) was stirred at 50 °C for 4 h. Amine in excess was removed at 10−2mm Hg using Kugelrohr apparatus. The solid residue was recrystallized from EtOH to yield 11 (232 mg, 83%). Mother liquors were concentrated and the crude residue was purified by flash chromatography (PE/EtOAc 7:3) gel to give an additional quantity (35 mg) of 11 (overall yield: 95%). Mp 161–162 °C (EtOH). IR (KBr): ν 3245, 2960, 1620, 1340, 1270, 1195 cm−1. 1H NMR (300 MHz, CDCl3): δ 7.30-7.34 (m, 5H, Harom), 7.13 (s, 1H, H6), 6.94 (broad s, 1H, NH), 4.79 (d, 2H, J = 5.6 Hz, CH2), 3.35 (hept, 1H, J = 7.0 Hz, CH), 2.55 (s, 3H, CH3), 1.37 (d, 6H, J = 7.0 Hz, 2 CH3). MS (ESI): m/z 314 (M + H+). Anal. (C16H19N5S) C, H, N.
4-Benzylamino-7-(1-methylethyl)-2-(methylsulfonyl)imidazo[2,1-f]-1,2,4-triazine (12)
To an ice cold solution of 11 (331 mg, 1.06 mmol) in CH2Cl2 (10 mL) was added 70% m-CPBA (781 mg, 3.17 mmol) in small portions. The reaction was stirred for 30 min at 0 °C then 3.5 h at room temperature. The mixture was diluted in CH2Cl2 (10 mL), washed with saturated aqueous NaHCO3 solution (10 mL), then brine (10 mL). The organic phase was dried over MgSO4 and concentrated in vacuo. The crude residue was purified by flash chromatography (PE/EtOAc 85:15 to 1:1) to provide 12 (295 mg, 81%) as a solid. Mp 132–133 °C (EtOH). IR (KBr): ν 3250, 2970, 1620, 1380, 1330, 1145, 970, 755 cm−1. 1H NMR (300 MHz, CDCl3): δ 7.40 (broad s, 1H, NH), 7.31-7.39 (m, 6H, Harom + H6), 4.88 (d, 2H, J = 5.8 Hz, CH2), 3.43 (hept, 1H, J = 7.0 Hz, CH), 3.32 (s, 3H, CH3), 1.39 (d, 6H, J = 7.0 Hz, 2 CH3). MS (ESI): m/z 346 (M + H+). Anal. (C16H19N5O2S2) C, H, N.
2-((R)-1-Amino-2-butanol)-4-(N-benzylamino)-7-(1-methylethyl)imidazolo[2,1-f]-1,2,4-triazine (13)
A mixture of 12 (253 mg, 0.73 mmol) and (R)-(−)-2-aminobutanol (0.35 mL, 3.7 mmol) was heated for 4 h at 170 °C. Excess of amine was removed by Kugelrohr distillation at 10−2 mmHg and the crude residue was purified by flash chromatography (PE/EtOAc 6:4 to 1:1) to give 13 (155 mg, 60%) as a solid. Mp 105–106 °C (PE/EtOAc 8:2). (c = 0.50, CHCl3). IR (KBr): ν 3330, 3220, 3100, 1615, 1570, 1540, 1450, 1370, 1055, 755, 720 cm−1. 1H NMR (300 MHz, CDCl3): δ 7.34-7.27 (m, 5H, Harom), 7.08 (s, 1H, H6), 6.89 (broad s, 1H, NH), 4.73 (d, 2H, J = 5.5 Hz, CH2), 4.57 (d, 1H, J = 6.4 Hz, OH), 3.89-3.78 (m, 2H, CH2O + CH), 3.70-3.60 (m, 1H, CH2O), 3.37 (broad s, 1H, NH), 3.24 (hept, 1H, J = 7.0 Hz, CH), 1.73-1.51 (m, 2H, CH2), 1.35 (d, 3H, J = 7.0 Hz, CH3), 1.34 (d, 3H, J = 7.0 Hz, CH3), 1.02 (t, 1H, J = 7.4 Hz, CH3). MS (EI + VE): m/z 354 (M+). Anal. (C19H26N6O) C, H, N.
Biology
Protein Kinase Assays
CDK1/cyclin B (native affinity purified from starfish oocytes), CDK2/cyclin A, CDK7/cyclin H and CDK9/cyclin T (recombinant, expressed in baculovirus-infected insect cells) and CDK5/p25 (recombinant, expressed in E. coli) were assayed in the presence of 15 µM ATP as previously described.16 IC50 values were determined from dose-response curves and are expressed in µM.
Cell Cultures and Reagents
A549, PC3, Hela and 293T cells were cultured in complete medium (DMEM without phenol red, supplemented with 10% heat-inactivated fetal calf serum, 2 mM Glutamax-I, 100 IU/mL penicillin, 100 µg/mL streptomycin). Raji and U937 cells were cultured in complete medium (RPMI 1640 without phenol red supplemented with 10% heat-inactivated fetal calf serum, 2 mM Glutamax-I, 100 IU/mL penicillin, 100 µg/mL streptomycin). All cells were kept at 37 °C in a humidified atmosphere (5% CO2 in air). Exponentially growing cells were used for all experiments. Cell culture reagents were purchased from Invitrogen. SH-SY5Y human neuroblastoma cell line was grown in DMEM supplemented with 2mM L-glutamine (Invitrogen, Cergy Pontoise, France), plus antibiotics and a 10% volume of FCS (Invitrogen). Cell viability was determined by means of the MTS (3- (4,5-dimethylthiazol-2-yl)-5- (3-carboxymethoxyphenyl)-2- (4-sulfophenyl)-2H -tetrazolium) method as previously described in detail.18 Roscovitine was purchased from Sigma-Aldrich and dissolved in DMSO to make a 10 mM stock, which was then diluted as desired into complete medium. The DMSO concentration was kept equal to 0.1% in all cell culture wells.
Cell Proliferation Assays
Five thousand cells of each cell line were seeded in 90 µL of culture medium / well in 96 well-plates (Greiner, #655098) and we incubated the plates for 6 h at 37 °C. We then added different concentrations of (R)-roscovitine or compound 7a (GP0210) in triplicate and plates were incubated for 72 h. To quantify viable cells at the beginning of the experiment, a Vialight assay (Cambrex, #LT07-121) was used in a control plate where cells were treated with 0.1% DMSO. Briefly, 50 µL of cell lysis buffer were added, followed by a 10 min incubation at room temperature. Then 100 µL of Vialight assay buffer were added and after a 2 min incubation luminescence was recorded in each well with an Envision plate reader (Perkin Elmer). The average luminescence value was calculated on 6 wells for each cell line. After 72 h, a Vialight assay was performed on all test plates and on the control plate.
In Vivo Antitumor Activity
Experiments to evaluate the antitumor activity of compound 7a were carried out essentially as described14,19 under protocols approved by the Georgetown University Animal Care and Use Committee, using immunodeficient, male (5–6 weeks old) athymic nude (BALB/c nu/nu) mice purchased from the National Cancer Institute.20 Mice were injected sc into the right posterior flank with 4 × 106 A4573 Ewing’s sarcoma cells in 100 µl of Matrigel basement membrane matrix (BD Biosciences, San Jose, California). Once tumors reached a mean volume of about 195 mm3, mice were randomized into three groups (six animals per group) and treatment was initiated. Experimental groups were treated with compound 7a or (R)-roscovitine, dissolved in DMSO, and administered as a single daily ip injection, at a dose of 25 or 50 mg/kg, respectively, for 5 days. The control group received ip injections of DMSO following an identical schedule. Tumor growth was followed for up to 4 weeks after the first injection. Tumor volumes were calculated by the formula V=(½)a × b2, where a is the longest tumor axis, and b is the shortest tumor axis. Whenever tumors reached the maximum volume allowed by institutional tumor burden guidelines, animals were sacrificed by asphyxiation with CO2. Tumors were immediately excised from euthanized animals and measured. Data are given as mean values ± SD. Statistical analysis of differences between groups was performed by a one-way ANOVA, followed by an unpaired Student’s t-test
Supplementary Material
Acknowledgment
This research was supported by grants from the EEC (FP6-2002-Life Sciences & Health, PRO-KINASE Research Project) (L.M.), the “Cancéropole Grand-Ouest” grant (L.M.), from the “Institut National du Cancer” (INCa), Cancer Détection d’innovations 2006 (L.M.), from the “Ligue Nationale contre le Cancer” (Comité du Finistère) (L.M.), and by U.S. NIH grant PO1-CA74175 (V.N.). K.B. was supported by a fellowship from the “Ministère de la Recherche” and from the “Association pour la Recherche sur le Cancer”. We are grateful to J. Boix for the SH-SY5Y cell line.
References
- 1.(a) Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J. Clin. Oncol. 2006;24:1770–1783. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]; (b) Du J, Widlund HR, Horstmann MA, Ramaswamy S, Ross K, Huber WE, Nishimura EK, Golub TR, Fisher DE. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell. 2004;6:565–576. doi: 10.1016/j.ccr.2004.10.014. [DOI] [PubMed] [Google Scholar]; (c) Alvi AJ, Austen B, Weston VJ, Fegan C, MacCallum D, Gianella-Borradori A, Lane DP, Hubank M, Powell JE, Wei W, Taylor AMR, Moss PAH, Stankovic T. A novel CDK inhibitor, CYC202 (R-roscovitine), overcomes the defect in p53-dependent apoptosis in B-CLL by down-regulation of genes involved in transcription regulation and survival. Blood. 2005;105:4484–4491. doi: 10.1182/blood-2004-07-2713. [DOI] [PubMed] [Google Scholar]; (d) Cruz JC, Tsai LH. Cdk5 deregulation in the pathogenesis of Alzheimer‘s disease. Trends Mol. Med. 2004;10:452–458. doi: 10.1016/j.molmed.2004.07.001. [DOI] [PubMed] [Google Scholar]; (e) Smith PD, O’Hare MJ, Park DS. CDKs: taking on a role as mediators of dopaminergic loss in Parkinson’s disease. Trends Mol. Med. 2004;10:445–451. doi: 10.1016/j.molmed.2004.07.003. [DOI] [PubMed] [Google Scholar]; (f) Zhang M, Li J, Chakrabarty P, Bu B, Vincent I. Cyclin-dependent kinase inhibitors attenuate protein hyper-phosphorylation, cytoskeletal lesion formation, and motor defects in Niemann-Pick Type C mice. Am. J. Pathol. 2004;165:843–853. doi: 10.1016/S0002-9440(10)63347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Wang J, Liu S, Fu Y, Wang JH, Lu Y. Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nat. Neurosci. 2003;6:1039–1047. doi: 10.1038/nn1119. [DOI] [PubMed] [Google Scholar]; (h) Di Giovanni S, Movsesyan V, Ahmed F, Cernak I, Schinelli S, Stoica B, Faden AI. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc. Natl. Acad. Sci. U.S.A. 2005;102:8333–8338. doi: 10.1073/pnas.0500989102. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Pareek TK, Keller J, Kesavapany S, Pant HC, Iadarola MJ, Brady RO, Kulkani AB. Cyclin-dependent kinase 5 activity regulates pain signaling. Proc. Natl. Acad. Sci. U.S.A. 2006;103:791–796. doi: 10.1073/pnas.0510405103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Pareek TK, Keller J, Kesavapany S, Agarwal N, Kuner R, Pant HC, Iadarola MJ, Brady RO, Kulkarni AB. Cyclin-dependent kinase 5 modulates nociceptive signaling through direct phosphorylation of transient receptor potential vanilloid 1. Proc. Natl. Acad. Sci. U.S.A. 2007;104:660–665. doi: 10.1073/pnas.0609916104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Gherardi D, D’Agati V, Chu T-HT, Barnett A, Gianella-Borradori A, Gelman IH, Nelson PJ. Reversal of collapsing glomerulopathy in mice with the cyclin-dependent kinase inhibitor CYC202. J. Am. Soc. Nephrol. 2004;15:1212–1222. doi: 10.1097/01.asn.0000124672.41036.f4. [DOI] [PubMed] [Google Scholar]; (l) Griffin SV, Krofft RD, Pippin JW, Shankland SJ. Limitation of podocyte proliferation improves renal function in experimental crescentic glomerulonephritis. Kidney Int. 2005;67:977–986. doi: 10.1111/j.1523-1755.2005.00161.x. [DOI] [PubMed] [Google Scholar]; (m) Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor Roscovitine. Nature. 2006;444:949–952. doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]; (n) Price PM, Yu F, Kaldis P, Aleem E, Nowak G, Safirstein RL, Megyesi J. Dependence of cisplatin-induced cell death in vitro and in vivo on cyclin-dependent kinase 2. J. Am. Soc. Nephrol. 2006;17:2434–2442. doi: 10.1681/ASN.2006020162. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, Caldicott A, Martinez-Losa M, Walker TR, Duffin R, Gray M, Crescenzi E, Martin MC, Brady HJ, Savill JS, Dransfield I, Haslett C. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat. Med. 2006;12:1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]; (p) Wei F-Y, Nagashima K, Ohshima T, Saheki Y, Lu Y-F, Matsushita M, Yamada Y, Mikoshiba K, Seino Y, Matsui H, Tomizawa K. Cdk5-dependent regulation of glucose-stimulated insulin secretion. Nat. Med. 2005;11:1104–1108. doi: 10.1038/nm1299. [DOI] [PubMed] [Google Scholar]; (q) Kitani K, Oguma S, Nishiki TI, Ohmori I, Galons H, Matsui H, Meijer L, Tomizawa K. A Cdk5 inhibitor enhances the induction of insulin secretion by exendin-4 both in vitro and in vivo. J. Physiol. Sci. 2007;57:235–239. doi: 10.2170/physiolsci.RP006607. [DOI] [PubMed] [Google Scholar]; (r) Pumfery A, De La Fuente C, Berro R, Nekhai S, Kashanchi F, Chao S-H. Potential use of pharmacological cyclin-dependent kinase inhibitors as anti-HIV therapeutics. Curr. Pharm. Des. 2006;12:1949–1961. doi: 10.2174/138161206777442083. [DOI] [PubMed] [Google Scholar]; (s) Doerig C, Billker O, Pratt D, Endicott J. Protein kinases as targets for antimalarial intervention: Kinomics, structure-based design, transmission-blockade, and targeting host cell enzymes. Biochim. Biophys. Acta. 2005;1754:132–150. doi: 10.1016/j.bbapap.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 2.Knockaert M, Greengard P, Meijer L. Pharmacological inhibitors of cyclin-dependent kinases. Trends Pharmacol. Sci. 2002;123:417–425. doi: 10.1016/s0165-6147(02)02071-0. [DOI] [PubMed] [Google Scholar]
- 3.Sharma Sapra P, Sharma R, Tyagi R. Inhibitors of cyclin dependent kinases: useful targets for cancer treatment. Curr. Cancer Drug Targets. 2008;8:53–75. doi: 10.2174/156800908783497131. [DOI] [PubMed] [Google Scholar]
- 4.Malumbres M, Pevarello P, Barbacid M, Bischoff JR. CDK inhibitors in cancer therapy: what is next? Trends Pharmacol. Sci. 2008;29:16–21. doi: 10.1016/j.tips.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Meijer L, Raymond E. Roscovitine and other purines as kinase inhibitors. From starfish oocytes to clinical trials. Acc. Chem. Res. 2003;36:417–425. doi: 10.1021/ar0201198. [DOI] [PubMed] [Google Scholar]
- 6.Meijer L, Bettayeb K, Galons H. (R)-Roscovitine (CYC202, Seliciclib) In: Smith PJ, Yue EW, editors. Inhibitors of Cyclin-Dependent Kinases as Anti-tumor Agents; Enzyme Inhibitors Series, Volume 3. Chapter 9. Boca Raton, FL: CRC Press; 2006. pp. 187–226. [Google Scholar]
- 7.(a) Gray NS, Wodicka L, Thunnissen A-MWH, Norman TC, Kwon S, Espinoza FH, Morgan DO, Barnes G, LeClerc S, Meijer L, Kim S-H, Lockhart DJ, Schultz PG. Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science. 1998;281:533–538. doi: 10.1126/science.281.5376.533. [DOI] [PubMed] [Google Scholar]; (b) Chang Y-T, Gray NS, Rosania GR, Sutherlin DP, Kwon S, Norman TC, Sarohia R, Leost M, Meijer L, Schultz PG. Synthesis and application of functionally diverse 2,6,9-trisubstituted purine libraries as CDK inhibitors. Chem. Biol. 1999;6:361–375. doi: 10.1016/S1074-5521(99)80048-9. [DOI] [PubMed] [Google Scholar]
- 8.(a) Patani GA, LaVoie EJ. Bioisosterism: a rational approach in drug design. Chem. ReV. 1996;96:3147–3176. doi: 10.1021/cr950066q. [DOI] [PubMed] [Google Scholar]; (b) Moreira Lima L, Barreiro EJ. Bioisosterism: a useful strategy for molecular modification and drug design. Curr. Med. Chem. 2005;12:23–49. doi: 10.2174/0929867053363540. [DOI] [PubMed] [Google Scholar]
- 9.Raboisson P, Schultz D, Muller C, Reimund J-M, Pinna G, Mathieu R, Bernard P, Do Q-T, DesJarlais RL, Justiano H, Lugnier C, Bourguignon J-J. Cyclic nucleotide phosphodiesterase type 4 inhibitors: evaluation of pyrazolo[1,5-a]-1,3,5-triazine ring system as an adenine bioisostere. Eur. J. Med. Chem. 2008;43:816–829. doi: 10.1016/j.ejmech.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Raboisson P, Baurand A, Cazenave J-P, Gachet C, Schultz D, Spiess B, Bourguignon J-J. General approach toward the synthesis of C-nucleoside pyrazolo[1,5-a]-1,3,5-triazines and their 3′,5′-bisphosphate C-nucleotide analogues as the first reported in vivo stable P2Y1-receptor antagonists. J. Org. Chem. 2002;67:8063–8071. doi: 10.1021/jo026268l. [DOI] [PubMed] [Google Scholar]
- 11.Dolzhenko AV, Dolzhenko AV, Chui W-K. Pyrazolo[1,5-a][1,3,5]triazines (5-aza-9-deazapurines): synthesis and biological activity. Heterocycles. 2008;75:1575–1622. [Google Scholar]
- 12.Raboisson P, Schultz D, Lugnier C, Bourguignon JJ. Efficient synthesis of 8-substituted pyrazolo[1,5-a]-1,3,5-triazines by regioselective acylation. Tetrahedron Lett. 2002;43:9501–9503. [Google Scholar]
- 13.Popowycz F, Bernard P, Raboisson P, Joseph B. Synthesis of 8-substituted pyrazolo[1,5-a]-1,3,5-triazine derivatives via palladiumcatalyzed cross-coupling reactions. Synthesis. 2007:367–374. [Google Scholar]
- 14.Bettayeb K, Sallam H, Ferandin Y, Popowycz F, Fournet G, Hassan M, Echalier A, Bernard P, Endicott J, Joseph B, Meijer L. N-&-N, a new class of cell death-inducing, kinase inhibitors derived from the purine roscovitine. Mol. Cancer Ther. 2008;7:2713–2724. doi: 10.1158/1535-7163.MCT-08-0080. [DOI] [PubMed] [Google Scholar]
- 15.(a) Dudfield PJ, Le V-D, Lindell SD, Rees CW. Synthesis of C-ribosyl imidazo[2,1-f][1,2,4]triazines as inhibitors of adenosine and AMP deaminases. J. Chem. Soc. Perkin Trans. 1999;20:2929–2936. [Google Scholar]; (b) Tzeng CC, Motola NC, Panzica RP. Synthesis of certain 5,6-diamino-as-triazines: precursors for fused heterocyclic systems. J. Org. Chem. 1983;48:1271–1275. [Google Scholar]
- 16.Rasmussen PB, Boewadt S. Ketene chemistry. 2. A general procedure for the synthesis of 2-alkoxycyclopropanecarboxylic esters and acids starting from aldehydes and ketene. Synthesis. 1989:114–117. [Google Scholar]
- 17.(a) Paull KD, Hamel E, Malspeis L. Prediction of biochemical mechanism of action from the in vitro antitumor screen of the National Cancer Institute. In: Foye WO, editor. Cancer Chemotherapeutic Agents. Chapter 2. Washington, DC: American Chemical Society; 1995. pp. 9–45. Also available online at http://dtp.nci.nih.gov/docs/compare/compare.html. [Google Scholar]; (b) Holbeck SL. Update on NCI in vitro drug screen utilities. Eur. J. Cancer. 2004;40:785–793. doi: 10.1016/j.ejca.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Tirado OM, Mateo-Lozano S, Notario V. Roscovitine is an effective inducer of apoptosis of Ewing’s sarcoma family tumor cells in vitro and in vivo. Cancer Res. 2005;65:9320–9327. doi: 10.1158/0008-5472.CAN-05-1276. [DOI] [PubMed] [Google Scholar]
- 19.Ribas J, Boix J. Cell differentiation, caspase inhibition, and macromolecular synthesis blockage, but not BCL-2 or BCL-XL proteins, protect SH-SY5Y cells from apoptosis triggered by two CDK inhibitory drugs. Exp. Cell Res. 2004;295:9–24. doi: 10.1016/j.yexcr.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Developmental Therapeutics Program NCI/NIH. [Accessed October 2007]; http://dtp.cancer.gov/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.