Abstract
Context
Extreme obesity is associated with health and cardiovascular disease risks. Although gastric bypass surgery induces rapid weight loss and ameliorates many of these risks in the short term, long-term outcomes are uncertain.
Objective
To examine the association of Roux-en-Y gastric bypass (RYGB) with weight loss, diabetes mellitus, and other health risks 6 years after surgery.
Design, Setting, and Participants
A prospective Utah-based study conducted between July 2000 and June 2011 of 1156 severely obese (body mass index [BMI] ≥35) participants aged 18–72 years (82% women; mean BMI 45.9; 95% CI, 31.2–60.6) who sought and received RYGB surgery (n=418), sought but did not have surgery (n=417; control group 1), or were randomly selected from a population-based sample not seeking weight loss surgery (n=321; control group 2).
Main Outcome Measures
Weight loss, diabetes, hypertension, dyslipidemia, and health-related quality of life were compared between participants having RYGB surgery and control participants using propensity score adjustment.
Results
Six years after surgery, patients who received RYGB surgery (with 92.6% follow-up) lost 27.7% (95% CI, 26.6%–28.9%) of their initial body weight compared with 0.2% (95% CI, -1.1% to 1.4%) gain in control group 1 and 0% (95% CI, −1.2 to 1.2%) in control group 2. Weight loss maintenance was superior in patients who received RYGB surgery, with 94% (95% CI, 92%–96%) and 76% (95% CI, 72%–81%) of patients receiving RYGB surgery maintaining at least 20% weight loss 2 and 6 years after surgery, respectively. Diabetes remission rates 6 years after surgery were 62% (95% CI, 49%–75%) in the RYGB surgery group, 8% (95% CI, 0%–16%) in control group 1, and 6% (95% CI, 0%–13%) in control group 2, with remission odds ratios (ORs) of 16.5 (95% CI, 4.7–57.6; P<.001) vs control group 1 and 21.5 (95% CI, 5.4–85.6; P<.001) vs control group 2. The incidence of diabetes throughout the course of the study was reduced after RYGB surgery (2%; 95% CI, 0%–4%; versus 17%; 95% CI, 10%–24%; OR, 0.11; 95% CI, 0.04–0.34 compared with control group 1 and 15%; 95% CI, 9%–21%; OR, 0.21; 95% CI, 0.06–0.67 compared with control group 2; both P<.001). The numbers of participants with bariatric surgery-related hospitalizations were 33 (7.9%), 13 (3.9%), and 6 (2.0%) for RYGB surgery group and 2 control groups, respectively.
Conclusion
Among severely obese patients, compared with nonsurgical control patients, the use of RYGB surgery was associated with higher rates of diabetes remission and lower risk of cardiovascular and other health outcomes over 6 years.
INTRODUCTION
The prevalence of extreme obesity in the U.S. is increasing at a rate greater than moderate obesity.1,2 Unfortunately, lifestyle therapy is generally insufficient as a weight management intervention for patients who are extremely obese. To date, effective long-term weight loss through pharmacological therapy has been marginal, leaving bariatric surgery as the only reported medical intervention providing substantial, long-term weight loss for most patients who are severely obese.3 For this high-risk population, however, the number of studies reporting long-term weight loss following bariatric surgery are limited and generally have incomplete follow-up.4
This prospective study compared long-term weight loss and cardiometabolic end points in patients who were severely obese receiving Roux-en-Y gastric bypass (RYGB) surgery and in control patients who were severely obese who did not undergo surgery. This study tested the hypothesis that significant weight loss and cardiometabolic health benefits observed 2 years after surgery5 persists after 6 years.
METHODS
Study Design
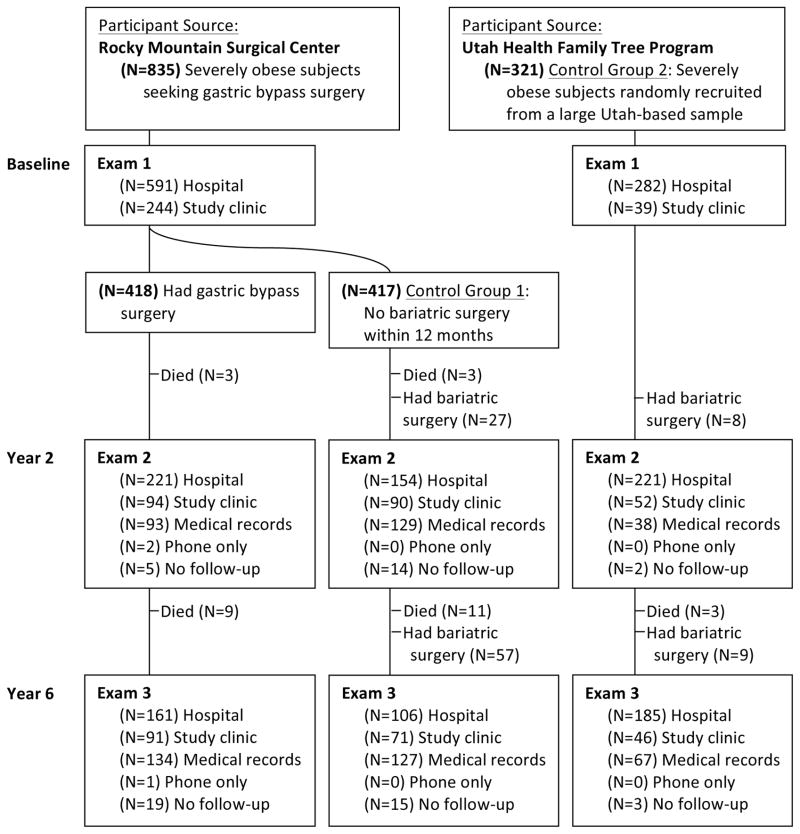
This Utah-based study, conducted between July 2000 and June 2011, included 1156 participants aged 18 to 72 years who were severely obese (body mass index [calculated as weight in kilograms divided by height in meters squared] ≥35), among whom patients surgically treated with RYGB surgery (n=418) were compared with 2 nonsurgical, nonintervened severely obese control groups (FIGURE 1). Control group 1 included participants seeking RYGB surgery at the same surgical center as the surgery group (Rocky Mountain Associated Physicians Inc, Salt Lake City, Utah) but who did not have surgery (n=417). Control group 2 was a population-based sample (n=321) of severely obese adults without prior history of bariatric surgery who were recruited at random from a large Utah database (Utah Health Family Tree Program, University of Utah School of Medicine, Salt Lake City, Utah).6,7 Group assignment and inclusion and exclusion criteria have been previously described,8 with additional details found in the eMethods (available at http://www.jama.com).
Figure 1. Utah Obesity Study Flow and Follow-Up Over 6 Years.
RYGB indicates Roux-en-Y gastric bypass. Recruitment source and follow-up rates are depicted for the RYGB surgery group and comparative control groups. At year 2 examination (35 control participants) and year 5 examination (55 control participants), 101 total control participants had bariatric surgery subsequent to their baseline examination. Follow-up data were collected on all of the control participants who had postbaseline bariatric surgery, with the exception of 2 participants who were lost to follow-up at year 6 examination.
Study protocol was approved by the University of Utah and Intermountain Healthcare institutional review boards, and signed consent was obtained from all participants. No participants from this study were included in our previously-published mortality study.9
All participants underwent a baseline examination at the University of Utah Center for Clinical and Translational Science or at our center’s outpatient clinic as previously described (eMethods).8 Following this examination, patients in the surgical group underwent either an open or laparoscopic RYGB procedure by 1 of 3 surgeons.10,11 Control groups did not receive any weight loss intervention but were free to pursue weight loss therapies if desired.
Type 2 diabetes mellitus was defined as a fasting blood glucose level or at least 126 mg/dL (to convert to millimoles per liter, multiply by 0.055), hemoglobin A1c of at least 6.5%, or use of antidiabetic medication prescribed for diabetes. Hypertension was defined as a resting blood pressure of at least 140/90 mm Hg or if antihypertensive medications had been prescribed for blood pressure control. Dyslipidemia was considered present if fasting low-density lipoprotein cholesterol (LDL-C) was at least 160 mg/dL (to convert to millimoles per liter, multiply by 0.0259), fasting high-density lipoprotein cholesterol (HDL-C) was less than 40 mg/dL (to convert to millimoles per liter, multiply by 0.0259), or fasting triglycerides was at least 200 mg/dL (to convert to millimoles per liter, multiply by 0.0113), or if participants were using lipid-lowering medication. Remission of baseline prevalent disease was defined as normal levels of fasting glucose, hemoglobin A1c, lipids and resting blood pressure without reported medication use for the respective endpoint at each examination. Other variables included in this study are described in the eMethods.
Follow-up
All participants were invited to return for examinations at the University of Utah Center for Clinical and Translational Science or outpatient clinic at 2 and 6 years. For participants who could not be contacted or chose not to return for follow-up examinations, clinical and endpoint data were obtained through home visits, medical chart extraction or telephone contact (Figure 1). Statewide hospital surgical records (Utah Department of Health) were used to determine if any participants who could not be located had undergone bariatric surgery since baseline, and for all participants to identify hospitalizations associated with 138 common postbariatric surgery-related Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision (ICD-9) codes (eMethods). Vital status and cause of death were obtained from the National Death Index.12 Years between the baseline examination and subsequent hospitalization were calculated for each participant.
Statistical Analysis
For each examination, biochemical and blood pressure variables affected by medication were adjusted for medicated participants to their estimated premedication levels (eMethods). Propensity scores, or the probabilities of being in a specific study group at baseline, were created from a logistic regression model regressing baseline group membership on the baseline values of sex, age, body mass index, income, education level, and marital status, once for patients undergoing RYGB surgery vs control group 1 and again for patients undergoing RYGB surgery vs control group 2. Propensity scores adjust for baseline variable distribution differences among study groups. Changes in each outcome variable were compared between groups after adjusting for the baseline level of the outcome variable and the propensity score. Participants were excluded for missing variables on a variable-by-variable basis, and control participants who went on to have bariatric surgery were considered lost to follow-up. Sidek multiple comparison adjustments were made to P values and confidence intervals (18 multiple comparisons were assumed for continuous variables and 5 comparisons were assumed for dichotomous variables. All analyses used SAS version 9.2 (SAS Institute).
Logistic regression was used to analyze the group differences in incidence and remission of the disease end points (diabetes, dyslipidemia, and hypertension), because disease status was only ascertained at the time of each examination. Those participants with baseline prevalent disease were excluded from analyses of incidence, and only those with baseline prevalent disease were used for the remission analyses at examination 2 (year 2) and examination 3 (year 6).
Detailed sensitivity analyses were performed to assess model assumptions. Analyses of the medication-adjusted and propensity score-adjusted data were compared with (1) adjusted data using the covariates included in the propensity score, (2) a dataset in which all missing values were imputed using multiple imputation methodology, (3) a medication-adjusted dataset limited only to those participants who attended 1 of the 2 study clinics, and (4) a dataset in which the postsurgical measurements on control participants who had subsequent bariatric surgery were included in an intention-to-treat design; all participants with missing values had their missing values replaced by carrying the baseline observation forward to examination 3 (eMethods).
RESULTS
Participation Rates
At 6 years, 92.6% (387/418) of the surgical group, 72.9% (304/417) of control group 1 and 96.9% (311/321) of control group 2 had follow-up data (Figure 1). Before examination 3, 101 participants from the 2 control groups chose to have bariatric surgery and for 99 of these participants, follow-up contact and clinical data were obtained subsequent to their weight-loss surgery and used in the intention-to-treat analysis (eTable 5). After including these 99 examined control participants, overall follow-up rates were 92.6% for the surgical cohort, 92.6% for control group 1, and 98.1% for control group 2. Median (interquartile range) follow-up time was 2.2 (2.0–2.5) years for the year 2 examination and 5.8 (5.3–6.6) years for the year 6 examination.
Clinical Measures
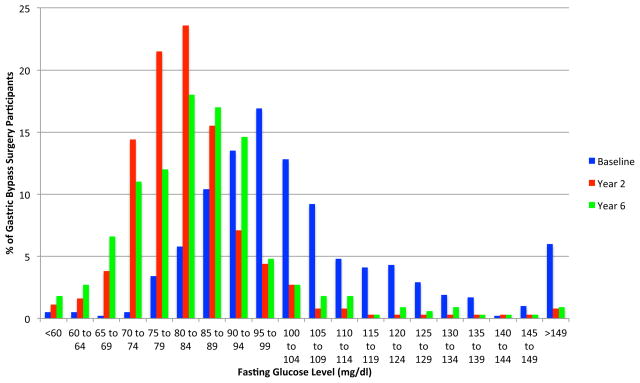
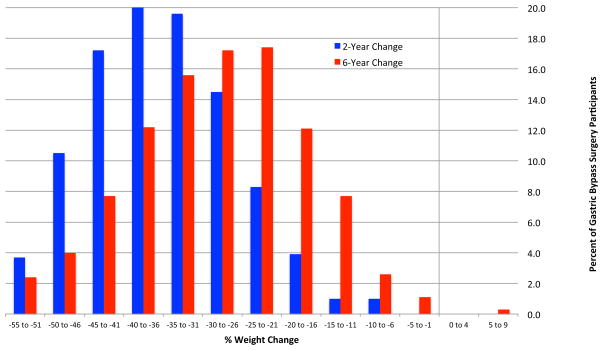
Participant ages ranged between 18 to 72 years (82% women) and 96% of the participants were non-Hispanic white; mean body mass index was 45.9 (95% CI, 31.2–60.6). Mean unadjusted weight loss in the surgical group was 34.9% (95% CI, 33.9%–35.8%) from baseline to year 2 and 27.7% (95% CI, 26.6%–28.9%) from baseline to year six, representing a 7.2% (95% CI, 4.6%–9.8%) regain in weight from years 2 to 6. Weight gain from baseline to year 6 was 0.2% (95% CI, −1.1%–1.4%) in control group 1 and 0% (95% CI, −1.2% to 1.2%) in control group 2. FIGURE 2 represents the frequency distribution of percentage unadjusted weight change from baseline to years 2 and 6 for the RYGB surgical group. At 2 years, 99% (95% CI, 98%–100%) of surgical patients had maintained more than 10% weight loss from baseline and 94% (95% CI, 92%–96%) had maintained more than 20% weight loss. At 6 years, 96% (95% CI, 94%–98%) of surgical patients had maintained more than 10% weight loss from baseline and 76% (95% CI, 72%–81%) had maintained more than 20% weight loss. Forty-nine percent of the RYGB group had baseline glucose levels of at least 100 mg/dL, whereas only 7% of this group had glucose concentrations of at least 100 mg/dL at 2 years, which slightly increased to 11% at 6 years (FIGURE 3).
Figure 2. Frequency Distribution of Percentage Weight Change From Baseline to 2-Year and 6-Year Follow-up Examinations.
The percentages of participants in the gastric bypass surgery group are shown grouped by 5% of unadjusted baseline weight loss intervals at the 2-year and 6-year follow-up examinations.
Figure 3. Frequency Distribution of Fasting Glucose Measured at Baseline and 2-Year and 5-Year Follow-up Examinations.
The percentages of participants in the gastric bypass surgery group are shown grouped by unadjusted fasting glucose intervals of 5 mg/dL (to convert to mmol/L, multiply by 0.055) at baseline and 2-year and 6-year follow-up examinations.
TABLE 1 presents the comparisons of the unadjusted baseline means for each group and eTable 1 shows the baseline means after adjustment for propensity scores, indicating the degree that propensity score adjustment adequately adjusted for the baseline differences between groups. TABLE 2 shows the 6-year change differences between RYGB surgery and control group 1 and RYGB surgery and control group 2, adjusting for the baseline value of the outcome variable and control group-specific propensity scores. Six-year changes did not significantly differ between the 2 control groups for any variable (P values not shown in TABLE 2), despite significant baseline differences between control groups (TABLE 1). At 6 years, the patients in the RYGBsurgicy group showed sustained improvement vs control participants for all propensity score-adjusted and multiple comparison-adjusted variables (P<.05), with the exception of the 36-Item Short Form Health Survey (SF-36) mental component summary score (Table 2). At 6 years, the RYGB surgery group had a decrease in fasting glucose of 23.7 mg/dL (95% CI, 16.0–31.4 mg/dL) relative to control group 1 and a decrease of 19.5 mg/dL (95% CI, 12.5–26.5 mg/dL) relative to control group 2. In addition, the HDL-C level increased by 13.1 mg/dL (95% CI, 9.7–16.5 mg/dL) compared with either control group.
Table 1.
Unadjusted baseline results by study groupa
| Propensity Score Covariates | RYGB Surgery | Control Group 1 | Control Group 2 | |||
|---|---|---|---|---|---|---|
| No. of Patients | Mean (SD) | No. of Patients | Mean (SD) | No. of Patients | Mean (SD) | |
| Female sex, % | 418 | 84.4±0.4 | 417 | 84.4±0.4 | 321 | 76.0±0.4b |
| Age, y | 418 | 42.5±10.9 | 417 | 43.0±11.4 | 321 | 49.4±10.9c |
| BMI | 418 | 47.3±7.7 | 417 | 46.3±7.7 | 321 | 43.8±6.5c |
| Income category (scale 1–6) | 418 | 3.6±1.3 | 417 | 3.2±1.3c | 321 | 3.6±1.2 |
| Education, y | 418 | 14.1±2.1 | 417 | 13.9±2.3 | 321 | 13.8±2.1 |
| Married, % | 418 | 65.3 | 417 | 57.1d | 321 | 75.4b |
| Weight, kg | 418 | 133.9±26.9 | 417 | 129.8±24.9d | 321 | 124.0±23.1c |
| Waist circumference, cm | 418 | 136.0±17.9 | 417 | 134.6±17.2 | 321 | 130.9±15.8c |
| Body fat, % | 416 | 53.2±5.1 | 416 | 52.7±5.4 | 310 | 50.6±5.8c |
| SBP, mm Hg | 418 | 126.3±19.1 | 417 | 125.6±17.8 | 321 | 128.8±18.8 |
| DBP, mm Hg | 418 | 71.9±11.3 | 417 | 72.0±10.8 | 321 | 72.3±10.5 |
| Glucose, mg/dl | 415 | 101±30.9 | 417 | 107±39.1d | 321 | 107±33.7b |
| Insulin, μU/ml | 416 | 19.3±16.4 | 414 | 17.9±14.4 | 321 | 14.0±13.1c |
| HOMA IR | 415 | 4.9±4.7 | 414 | 4.8±4.3 | 321 | 3.7±3.9c |
| HbA1c, % | 416 | 5.8±1.1 | 412 | 6.0±1.2d | 319 | 6.0±1.1 |
| Total cholesterol, mg/dL | 417 | 188±34.0 | 417 | 185±37.7 | 321 | 189±37.8 |
| LDL-C, mg/dL | 417 | 109±27.3 | 416 | 107±27.5 | 321 | 109±27.6 |
| HDL-C, mg/dL | 417 | 46.6±11.5 | 416 | 44.8±11.0d | 321 | 47.0±10.9 |
| VLDL-C, mg/dL | 417 | 34.1±19.8 | 416 | 35.1±22.7 | 321 | 34.2±24.1 |
| Triglycerides, mg/dL | 417 | 186±96.9 | 416 | 193±122.0c | 321 | 186±184.6d |
| IWQOL-Lite total scoree | 411 | 31.4±16.5 | 407 | 34.9±18.4b | 317 | 54.5±19.5c |
| SF-36 physical component scoref | 401 | 31.4±9.3 | 400 | 33.3±9.7b | 314 | 39.3±10.2c |
| Sf-36 mental component scoreg | 401 | 41.4±11.7 | 400 | 40.4±12.0 | 314 | 47.8±11.4c |
Abbreviations: BM|, body mass index, calculated as weight in kilograms divided by height in meters squared; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; lWQOL impact of weight quality of life; LDL-C, low-density lipoprotein cholesterol; RYGB, Roux-en-Y gastric bypass; SBP, systolic blood pressure; SF-36, 36-Item Short Form Health Survey; VLDL-C very low-density lipoprotein cholesterol. SI conversions: To convert glucose to mmol/L, multiply by 0.055; total cholesterol, LDL-C, HDL-C, and VLDL-C to mmol/L, multiply by 0.0259; and triglycerides to mmol/L, multiply by 0.0113.
Two-sided P values are unadjusted for multiple comparisons. P values are control groups 1 and 2 vs RYGB surgery group. Income categories were grouped according to 1 (<$10000); 2 ($10000–$29999); 3 ($30000–$49999); 4 ($50000–$69999); 5 ($70000–$99999); and 6 (>$100000).
P< .01.
P< .001.
P< .05.
Range of scores (0–1 00, with 1 00 being best and normative mean of 94.7); a meaningful individual change is considered 7.7 to 12 points depending on baseline severity.13
Range of scores (1 2–69, with 69 being best); meaningful change for either scale is 5 points with a normative mean of 50.14
Range of scores (8–73, with 73 being best); meaningful change for either scale is 5 points with a normative mean of 50.14
Table 2.
Propensity Score-Adjusted 6-Year Change by Study Group and Group Differencesa
| Study Variables | RYGB Surgery Mean (95% CI) | Control Group 1 Mean (95% CI) | RYGB Surgery vs Control Group 1 Difference | RYGB Surgery Mean (95% CI) | Control Group 2 Mean (95% CI) | RYGB Surgery vs Control Group 2 Difference |
|---|---|---|---|---|---|---|
| Weight, kg | −36.8 (−39.2,−34.4) (379) | −0.4 (−3.1,2.3) (299) | −36.4b (−40.1,−32.7) | −37.0 (−39.4,−34.5) (379) | −0.2 (−3.0,2.6) (296) | −36.7b (−40.6,−32.8) |
| Waist circumference, cm | −28.7 (−31.5,−25.9) (249) | 0.6 (−2.8,4.0) (172) | −29.3b (−33.8,−24.8) | −28.5 (−31.3,−25.7) (249) | 0.3 (−2.7,3.3) (225) | −28.8b (−33.1,−24.6) |
| Body fat, % | −5.6 (−6.4,−4.8) (244) | −0.3 (−1.2,0.7) (171) | −5.3b (−6.5,−4.0) | −5.5 (−6.3,−4.6) (244) | 0.0 (−0.9,1.0) (209) | −5.5b (−6.8,−4.2) |
| SBP, mm Hg | −5.8 (−8.8,−2.8) (358) | 3.6 (0.2,6.9) (288) | −9.3b (−13.9,−4.8) | −6.0 (−9.0,−3.1) (358) | 0.9 (−2.4,4.0) (293) | −6.9b (−11.5,−2.3) |
| DBP, mm Hg | −1.0 (−3.3,1.2) (358) | 4.5 (2.0,7.1) (288) | −5.6b (−9.0,−2.2) | −1.3 (−3.5,1.0) (358) | 3.1 (0.6,5.6) (293) | −4.4c (−7.9,−0.9) |
| Glucose, mg/dL | −14.6 (−19.6,−9.6) (336) | 9.1 (3.4,14.9) (262) | −23.7b (−31.4,−16.0) | −15.0 (−19.5,−10.5) (336) | 4.5 (−0.4,9.5) (281) | −19.5b (−26.5,−12.5) |
| Insulin, μU/mL | −11.8 (−13.5,−10.1) (256) | −2.7 (−4.6,−0.8) (201) | −9.1b (−11.6,−6.6) | −10.0 (−11.7,−8.4) (256) | −2.1 (−3.8,−0.4) (248) | −7.9b (−10.4,−5.4) |
| HOMA-IR | −3.1 (−3.7,−2.6) (253) | −0.2 (−0.8,0.4) (201) | −2.9b (−3.8,−2.1) | −2.8 (−3.3,−2.2) (253) | −0.2 (−0.7,0.4) (248) | −2.6b (−3.4,−1.8) |
| HbA1c, % | −0.4 (−0.5,−0.2) (250) | 0.2 (0.0,0.3) (202) | −0.5b (−0.7,−0.3) | −0.3 (−0.5,−0.2) (250) | 0.1 (0.0,0.3) (245) | −0.5b (−0.7,−0.2) |
| Total cholesterol, mg/dL | −13.7 (−20.4,−6.9) (295) | 16.8 (9.6,24.1) (255) | −30.5b (−40.6,−20.4) | −13.2 (−19.9,−6.6) (295) | 13.8 (6.9,20.8) (271) | −27.1b (−37.1,−17.0) |
| LDL-C, mg/dL | −9.3 (−15.2,−3.3) (291) | 19.4 (13.0,25.8) (251) | −28.7b (−37.6,−19.8) | −8.8 (−14.5,−3.1) (291) | 19.4 (13.4,25.4) (270) | −28.2b (−36.8,−19.5) |
| HDL-C, mg/dL | 11.0 (8.7,13.2) (291) | −2.1 (−4.6,0.3) (251) | 13.1b (9.7,16.5) | 10.4 (8.2,12.7) (291) | −2.7 (−5.0,−0.3) (270) | 13.1b (9.7,16.5) |
| VLDL-C, mg/dL | −17.1 (−20.3,−13.9) (284) | −2.9 (−6.4,0.5) (239) | −14.2b (−18.9,−9.4) | −16.1 (−19.4,−12.8) (284) | −4.5 (−7.9,−1.1) (262) | −11.6b (−16.6,−6.7) |
| Triglycerides, mg/dL | −66.8 (−80.6,−52.9) (290) | 0.3 (−14.7,15.2) (251) | −67.0b (−87.7,−46.4) | −63.6 (−78.5,−48.7) (290) | −0.7 (−16.2,−14.8) (270) | −62.9b (−85.3,−40.4) |
| IWQOL-Lite total scored | 45.1 (41.6,48.6) (241) | 13.2 (9.0,17.4) (168) | 31.9b (26.4,37.5) | 42.8 (39.3,46.2) (241) | 8.6 (5.0,12.2) (226) | 34.2b (28.7,39.7) |
| SF-36 physical component scored | 12.5 (10.5,14.5) (230) | 2.2 (−0.1,4.6) (167) | 10.2b (7.1,13.4) | 11.6 (9.6,13.6) (230) | 0.4 (−1.7,2.4) (219) | 11.2b (8.2,14.3) |
| Sf-36 mental component scored | 4.2 (2.0,6.4) (230) | 4.7 (2.1,7.2) (167) | −0.5 (−3.8,2.9) | 3.4 (1.2,5.6) (230) | 2.7 (0.5,5.0) (219) | 0.6 (−2.6,3.9) |
Abbreviations: DBP, diastolic blood pressure; HBA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment ofinsulin resistance; IWQOL, impact of weight quality of life; LDL-C, low-density lipoprotein cholesterol; RYGB; Roux-en-Y gastric bypass; SBP, systolic blood pressure; SF-36, 36-Item Short Form Health Survey; VLDL-C very low-density lipoprotein cholesterol.
SI conversions: To convert glucose to mmol/L, multiply by 0.055; total cholesterol, LDL-C, HDL-C, and VLDL-C to mmol/L, multiply by 0.0259; and triglycerides to mmol/L, multiply by 0.0113.
The sample size of RYGB surgery group and 2 control groups excludes deaths, lost-to-follow-up participants, and control participants who had subsequent bariatric surgery. Because propensity scores were derived separately for each control group vs RYGB surgery group, 2 propensity score-adjusted means are provided for the RYGB surgery group. Group differences are RYGB surgery minus either control group of 6-year changes. Two-sided P values and 95% CIs are adjusted for multiple comparisons. P values are RYGB surgery group vs the respective control group comparison of 6-year change means.
P<.001.
P<.01.
Sensitivity analyses showed that the propensity score-adjusted results (Table 2) were similar to the covariate-adjusted results (eTable 2). Also, all significant variables in Table 2 remained significantly different when analysis was restricted to participants who were examined at both baseline and 6-year visits in either of our 2 standardized clinics (eTable 3) and when imputed values for missing measurements were analyzed (eTable 4). Even the most conservative intention-to-treat analysis with baseline observations carried forward for missing values showed significant improvements in patients in the RYGB surgery group the compared with the control groups (eTable 5).
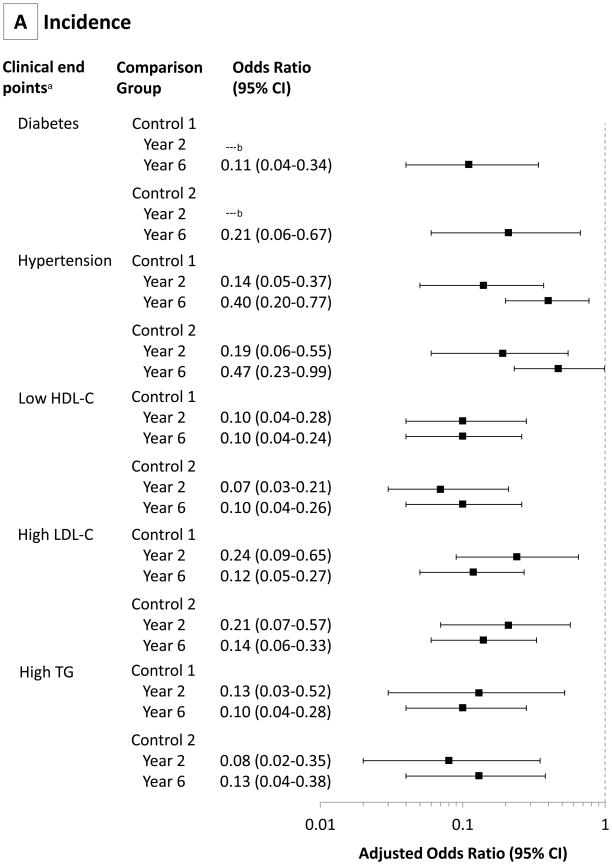
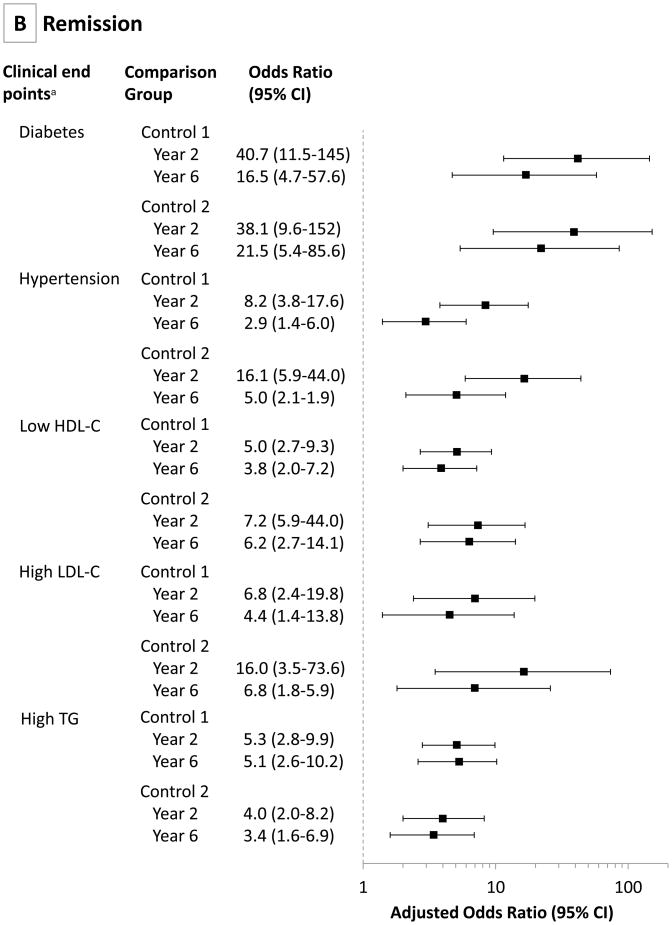
Table 3 shows the incidence and remission of diabetes, hypertension, high LDL-C, low HDL-C, and high triglycerides (prevalence also shown in eTable 6), and FIGURE 4 shows the propensity score-adjusted odds ratios (ORs) for these five variables. Remission of diabetes for the RYGB surgery group was 75% (95% CI, 63%–87%) at year 2, decreasing to 62% (95% CI, 49%–75%) at year 6. The 6-year RYGB surgery group remission rates were significantly higher than the 2 control groups (62%; 95% CI, 49%–75% for the RYGB surgery group; vs 8%; 95% CI, 0%–16%[OR, 16.5; 95% CI, 4.7–57.6; P<.001] for control group 1; and 6%; 95% CI, 0%–13% [OR, 21.5; 95% CI, 5.4–85.6; P,.001] for control group 2) (Table 3 and Figure 4). At the same time, diabetes incidence following RYGB surgery was significantly lower than the 2 control groups (2%; 95% CI, 0%–4%; vs 17%; 95% CI, 10%–24% [OR, 0.11; 95% CI, 0.04–0.34; P<.001]; and 15%; 95% CI, 9%–21% [OR, 0.21; 95% CI, 0.06–0.67; P<.001]; respectively). Remission rates of hypertension at year 6 remained significantly improved in the RYGB surgical group compared with 2 control groups (42%; 95% CI, 32%–52%; vs 18%; 95% CI, 9%–27% [OR, 2.9; 95% CI, 1.4–6.0]; and 9%; 95% CI, 3%–15% [OR, 5.0; 95% CI, 2.1–11.9]; respectively). Low HDL-C remission rates were also significantly improved at year 6 in the RYGB surgery group compared with 2 control groups (67%; 95% CI, 57%–77%; vs 34%; 95% CI, 23%–45% [OR, 3.8; 95% CI, 2.0–7.2]; and 18%; 95% CI, 8%–28% [OR, 6.2; 95% CI, 2.7–14.1]; respectively), with similar remission rates for high LDL-C and triglycerides.
Table 3.
Incidence and Remission Rates for Each Study Groupa
| (No./Total) | Roux-en-Y Gastric Bypass Surgery | Control Group 1 | Control Group 2 | |||
|---|---|---|---|---|---|---|
|
|
||||||
| Incidence, % [95% CI] | ||||||
| (No./Total) | Year 2 | Year 6 | Year 2 | Year 6 | Year 2 | Year 6 |
| Diabetes | 0 [0–0%] (0/299) | 2 [0–4%] (7/290) | 5 [1–9%] (12/255) | 17 [10–24%] (36/207) | 7 [2–12%] (14/213) | 15 [9–21%] (31/207) |
| Hypertension | 4 [1–7%] (9/234) | 16 [10–22%] (34/220) | 22 [15–29%] (49/219) | 31 [22–40%] (53/169) | 23 [14–32%] (34/147) | 33 [23–43%] (46/141) |
| Low HDL-C | 3 [0–6%] (8/246) | 5 [1–9%] (11/242) | 26 [18–34%] (57/217) | 32 [23–41%] (58/183) | 32 [24–40%] (65/201) | 38 [29–47%] (73/191) |
| High LDL-C | 3 [1–5%] (9/334) | 4 [1–7%] (13/328) | 12 [7–17%] (38/321) | 25 [18–32%] (66/265) | 18 [12–24%] (44/248) | 30 [22–38%] (71/237) |
| High Triglycerides | 2 [0–4%] (4/237) | 3 [0–6%] (8/234) | 14 [8–20%] (32/232) | 25 [17–33%] (48/194) | 22 [14–30%] (40/186) | 28 [19–37%] (50/177) |
| Remission, % [95% CI] | ||||||
| (No./Total) | Year 2 | Year 6 | Year 2 | Year 6 | Year 2 | Year 6 |
| Diabetes | 75 [63–87%] (66/88) | 62 [49–75%] (54/87) | 7 [0–14%] (6/93) | 8 [0–16%] (6/72) | 6 [0–13%] (5/88) | 6 [0–13%] (5/83) |
| Hypertension | 53 [43–63%] (90/169) | 42 [32–52%] (68/164) | 12 [5–19%] (18/152) | 18 [9–27%] (23/128) | 6 [1–11%] (9/157) | 9 [3–15%] (14/153) |
| Low HDL-C | 66 [57–75%] (108/165) | 67 [57–77%] (107/161) | 27 [18–36%] (46/170) | 34 [23–45%] (43/126) | 15 [6–24%] (17/111) | 18 [8–28%] (19/108) |
| High LDL-C | 57 [42–72%] (43/76) | 53 [38–68%] (40/75) | 17 [5–29%] (11/66) | 22 [6–38%] (10/46) | 6 [0–14%] (4/64) | 10 [0–20%] (6/61) |
| High Triglycerides | 69 [60–78%] (119/173) | 71 [62–80%] (120/168) | 29 [20–38%] (45/155) | 33 [22–44%] (38/117) | 29 [19–39%] (36/126) | 34 [23–45%] (40/119) |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotien cholesterol.
95% CIs were adjusted for multiple comparisons.
Figure 4. Propensity Score-Adjusted Odds Ratios Comparing Incidence and Remission Rates of Diabetes, Hypertension, and Dyslipidemia Determined at Years 2 and 6 in RYGB Surgery and Control Groups 1 and 2.
RYGB indicates Roux-en-Y gastric bypass. Odds ratios are adjusted for a propensity score composed of age, sex, baseline body mass index, income, education level, and marital status (95% Cls are adjusted for multiple comparisons). Clinical end points for both incidence and remission rates were defined as type 2 diabetes (a fasting concentration of blood glucose ≥126 mg/dL, hemoglobin A1c ≥6.5, or use of antidiabetic medication); hypertension (resting blood pressure ≥140/90 mm Hg or use of antihypertensive medications); and dyslipidemia (a fasting concentration of measured low-density lipoprotein cholesterol [LDL-C] ≥160 mg/dL, high-density lipoprotein cholesterol [HDL-C] <40 mg/dL, or triglycerides ≥200 mg/dL, or use of lipid-lowering medication). No estimate was available for year 2 diabetes incidence (there was no incident diabetes in the RYGB surgery group at 2 years).
There were 29 deaths in study participants at the end of the 6-year follow-up (12 in participants in the RYGB surgery group [3%], 14 in control group 1 [3%], and 3 in control group 2 [1%]) (eTable 7). None of the deaths in the RYGB surgery group occurred within 30 days following surgery. All 4 suicides and 2 of the 3 poisonings of undetermined intention occurred in the surgical group. Because of the small numbers of events, Fisher exact test was used to analyze the cumulative incidence of suicide, which was significantly higher in the surgical group compared with the combined control groups (Mantel-Haenszel logit OR, 18; 95% CI, 1–385; Fisher exact test, P = 0.02). Suicide incidence between the surgical group and either of the control groups alone was not significantly different. The 30-day RYGB surgery perioperative complication rate was 3%. The numbers of hospitalizations with bariatric surgery-related ICD-9 and CPT codes were 38 (9.1%) for the RYGB surgery group, 15 (4.5%) for control group 1, and 8 (2.6%) for control group 2 (eTable 8). When using numbers of participants rather than number of hospitalizations, the numbers and percents were 33 (7.9%), 13 (3.9%), and 6 (2.0%), respectively. The majority (61%) of the patients receiving RYGB surgery had their hospitalization occur during the first 2 years after surgery.
DISCUSSION
Our study reports significant weight loss and 6-year improvements in major cardiovascular and metabolic risk factors in patients receiving RYGB surgery compared with severely obese control participants, including frequent remission and lower incidence of diabetes, dyslipidemia and hypertension. In contrast, cardiovascular and metabolic status of severely obese control participants generally worsened during the 6-year period.
At 2 years, the surgical group lost 34.9% of their initial weight and at 6 years, their mean weight loss was 27.7%, representing a weight regain of approximately 7%. Two randomized clinical trials involving intensive lifestyle weight loss therapy, The Diabetes Prevention Program Outcome study had a 7.5% weight loss at 1 year, with 2.1% weight loss at 4 years of follow-up, and the Action for Health in Diabetes study had an 8.6% weight loss at 1 year, with 6.2% weight loss at 4 years of follow-up, both randomized clinical trials involving intensive lifestyle weight-loss therapies.15–19 A recently reported randomized clinical trial comparing bariatric surgery and intensive medical therapy demonstrated a mean weight loss of 5.2% for the medical therapy group measured at 1 year.20 Considering the 5% to 9% weight loss at 1 year with only 2% to 6% weight loss after 4 years of intensive lifestyle-based and medication-based therapy, the weight loss maintenance of 28% from baseline measured at 6 years in our Utah study is quite significant. These findings are similar to the results of the prospective, controlled Swedish Obese Subjects study that also reported a 7% mean weight regain among patients after gastric bypass surgery from 2 years (32% weight loss from baseline) to 10 years (25% weight loss).21 The amount of weight loss sustained long term may affect the durability of cardiovascular disease risk factor improvements and explain differential results across bariatric surgical procedures.21,22
Although some recurrences of diabetes among patients undergoing RYGB surgery occurred, 62% remission of diabetes was maintained at year 6. Similar findings have been reported by DiGiorgi and colleagues.23 Although maintenance of diabetes remission at 6 years is somewhat less than the 75% to 80% remission rates reported in studies with shorter follow-up periods,24–29 the continued protective association of RYGB surgery was underscored by a 5- to 9-fold reduction in the risk of new diabetes in surgical patients compared with nonsurgical control participants. In addition, the dramatic improvement seen in fasting glucose concentrations at year 2 remained at year 6, with only 11% of the RYGB surgery group having a fasting glucose concentration of at least 100 mg/dL. To our knowledge, 3 randomized controlled trials20,29,30 comparing patients with diabetes with bariatric surgical procedures or intensive medical therapy have been reported. Dixon et al30 reported that 2 years after gastric banding type 2 diabetes remission was 73% compared with 13% after conventional-therapy. Using the remission of diabetes definition proposed by Buse et al,31 Mingrone et al29 found 75% diabetes remission at 2 years for gastric bypass, 95% for biliopancreatic diversion, and no remission for the conventional medical therapy group. In addition, Schauer et al20 reported that 42% of gastric bypass, 37% of sleeve-gastrectomy, and 12% of medical therapy groups achieved the primary endpoint of a glycated hemoglobin level of 6% or less after 1 year. The promising results for diabetes management from these 3 short-term studies are supported by our longer-term follow-up of diabetes remission after bariatric surgery.
Consideration should also be given to the possibility that despite a worsening of diabetes remission rates over time, the years of improved glycemic control following bariatric surgery may have the end result of reduced microvascular disease.32 Obesity is associated with premature and accelerated coronary atherosclerosis,33,34 and improvements in coronary risk factors after bariatric surgery have been predicted to lower the 10-year risk of ischemic heart disease events by approximately 50%.35 Our study demonstrated a sustained improvement in cardiovascular risk factors measured at 6 years. Our prior study showed a significant 2-year increase in HDL-C,5 and despite a 7% weight regain from year 2 to 6, HDL-C did not decrease in the RYGB surgery group in our current study.
Reasons for the small but significantly increased incidence of suicides in the surgical compared with combined control groups (P=0.02) are not known, but these results are consistent with our previously reported mortality data.9 The absence of improvement in the SF-36 mental component score in the surgical group during this period was in contrast to the marked improvements in the SF-36 physical component score and the overall quality of life score. Bocchieri et al36 noted that numerous life changes occur after bariatric surgery that may generate tension and pose special social, psychological, and lifestyle challenges. Preoperative and postoperative psychological assessment of social and emotional status related to postbariatric surgical expectations and the potential risk of self-destructive behavior might be warranted.
A weakness of many bariatric surgery studies has been poor rates of participant retention, introducing a potential bias (ie, patients who regain weight may not return for subsequent screening).37 Strengths of our study were the high combined 6-year participation and follow-up rate, and thorough sensitivity analysis to confirm that data obtained outside of our primary research centers did not influence study conclusions.
Inclusion of 2 severely obese control groups allowed broad inferences to be made regarding the benefits of gastric bypass surgery. The first control group provided an opportunity to follow severely obese patients who, similar to enrolled surgical cases, sought gastric bypass surgery and were more clinically comparable to study participants who subsequently had gastric bypass surgery.5 The second control group was older, less severely obese, and reported a higher health-related quality of life. Despite these baseline differences, the 6-year changes were similar between control groups, resulting in the same conclusions when comparing either control group with patients in the RYGB surgery group. Propensity score adjustment for baseline group differences further confirmed this conclusion. The large outcome variable effect sizes after RYGB surgery and associated extremely low P values (eTable 4 and eTable 5) suggest that remaining biases would need to be very large to explain the observed results and that baseline differences between groups, sampling errors, or statistical issues did not falsely inflate the beneficial association of surgically-induced weight loss.
In conclusion, significant weight loss was sustained for an average of 6 years in the majority of patients having RYGB surgery. Diabetes remission was also sustained and the incidence of diabetes was much lower during the 6-year follow-up period in patients in the RYGB surgery compared with the severely obese control participants. Similarly, metabolic and cardiovascular risk profiles during the 6 years of follow-up remained significantly improved after RYGB surgery. These findings are important considering the rapid increase in total numbers of bariatric surgical operations performed in the United States and worldwide,38,39 and may have significant ramifications for the projected 31 million US individuals meeting criteria for bariatric surgery.40
Supplementary Material
Acknowledgments
Funding/Support: This research is supported by NIH (NIDDK) Grant DK-55006, and Public Health Service research grant No. M01-RR00064 from the National Center for Research Resources.
Role of the Sponsor: The National Institutes of Health had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Ronette Kolotkin receives royalties for use of the IWQOL-Lite and is a consultant for Vivus, Orexigen, and GlaxoSmithKline.
Online-Only Material: eMethods, eTables 1 through 8, and Author Video Interview are available at http://www.jama.com.
Author Contributions: Drs. Steven Hunt and Ted Adams had full access to all of the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Adams, LaMonte, Hopkins, Gress, Walker, Cloward, Litwin, Hunt.
Acquisition of data: Adams, Davidson, Litwin, Kolotkin, Pendleton, Strong, Vinik, Wanner, Walker, Greenwood.
Analysis and interpretation of data: Adams, Davidson, Litwin, Kolotkin, Hopkins, Gress, Crosby, Hunt.
Drafting of the manuscript: Adams, Davidson, Litwin, Hunt.
Critical revision of the manuscript for important intellectual content: Adams, Davidson, Litwin, Kolotkin, LaMonte, Hopkins, Gress, Walker, Hunt.
Statistical analysis: Davidson, Gress, Hunt.
Obtained funding: Adams, Litwin, Hunt.
Administrative, technical, clinical or material support: Adams, Davidson, Litwin, Pendleton, Strong, Hammoud, McKinlay, Simper, Smith, Hunt.
Study supervision: Adams, Davidson, Hunt.
Additional Contributions: We thank RC Halverson, MD, Charles B. Edwards, MD, Gerald N. Goodman, MD and the late David K. Miller, MD, bariatric surgeons with Rocky Mountain Associated Physicians, Inc., SLC, UT USA. Acknowledgments are also expressed for clinical and technical support from the staff of the Cardiovascular Genetics Division who assisted with recruitment and clinical testing (including Sara Frogley, Loni Gardner, and Sawsan Ibrahim), the Huntsman Center for Clinical and Transitional Science and their very capable staff, and the Cardiology Division, University of Utah School of Medicine. No financial compensation was given the surgeons but salary support was received by the above named staff members.
References
- 1.Freedman DS, Khan LK, Serdula MK, Galuska DA, Dietz WH. Trends and correlates of class 3 obesity in the United States from 1990 through 2000. JAMA. 2002;288(14):1758–1761. doi: 10.1001/jama.288.14.1758. [DOI] [PubMed] [Google Scholar]
- 2.Sturm R. Increases in clinically severe obesity in the United States, 1986–2000. Arch Intern Med. 2003;163(18):2146–2148. doi: 10.1001/archinte.163.18.2146. [DOI] [PubMed] [Google Scholar]
- 3.Flum DR, Belle SH, King WC, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5):445–454. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kushner RF, Noble CA. Long-term outcome of bariatric surgery: an interim analysis. Mayo Clin Proc. 2006;81(10 Suppl):S46–51. doi: 10.1016/s0025-6196(11)61180-4. [DOI] [PubMed] [Google Scholar]
- 5.Adams TD, Pendleton RC, Strong MB, et al. Health outcomes of gastric bypass patients compared to nonsurgical, nonintervened severely obese. Obesity (Silver Spring) 2010;18(1):121–130. doi: 10.1038/oby.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt SC, Williams RR, Barlow GK. A comparison of positive family history definitions for defining risk of future disease. J Chron Dis. 1986;39:809–821. doi: 10.1016/0021-9681(86)90083-4. [DOI] [PubMed] [Google Scholar]
- 7.Williams RR, Hunt SC, Barlow GK, et al. Health family trees: A tool for finding and helping young members of coronary and cancer prone pedigrees in Texas and Utah. Am J Public Health. 1988;78:1283–1286. doi: 10.2105/ajph.78.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams TD, Avelar E, Cloward T, et al. Design and rationale of the Utah obesity study. A study to assess morbidity following gastric bypass surgery. Contemp Clin Trials. 2005;26(5):534–551. doi: 10.1016/j.cct.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 10.Smith SC, Edwards CB, Goodman GN, Halversen RC, Simper SC. Open vs laparoscopic Roux-en-Y gastric bypass: comparison of operative morbidity and mortality. Obes Surg. 2004;14(1):73–76. doi: 10.1381/096089204772787329. [DOI] [PubMed] [Google Scholar]
- 11.Smith SC, Goodman GN, Edwards CB. Roux-en-Y Gastric Bypass: A 7-year Retrospective Review of 3,855 Patients. Obes Surg. 1995;5(3):314–318. doi: 10.1381/096089295765557700. [DOI] [PubMed] [Google Scholar]
- 12.Horm J. Assignment of probabilistic scores to National Death Index record matches. In: Bildgrad R, editor. National Death Index Plus: Coded Causes of Death. Hyattsville: Division of Vital Statistics, National Center for Health Statistics, CDC; 1996. pp. A5–A12. [Google Scholar]
- 13.Crosby RD, Kolotkin RL, Williams GR. An integrated method to determine meaningful changes in health-related quality of life. J Clin Epidemiol. 2004;57(11):1153–1160. doi: 10.1016/j.jclinepi.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 15.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wadden TA, West DS, Neiberg RH, et al. One-year Weight Losses in the Look AHEAD Study: Factors Associated With Success. Obesity (Silver Spring) 2009;17(4):713–722. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170(17):1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 22.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 23.DiGiorgi M, Rosen DJ, Choi JJ, et al. Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis. 2010;6(3):249–253. doi: 10.1016/j.soard.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Thaler JP, Cummings DE. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150(6):2518–2525. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
- 25.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Annals of surgery. 2003;238(4):467–484. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wittgrove AC, Clark GW. Laparoscopic gastric bypass, Roux-en-Y- 500 patients: technique and results, with 3–60 month follow-up. Obesity surgery. 2000;10(3):233–239. doi: 10.1381/096089200321643511. [DOI] [PubMed] [Google Scholar]
- 27.Torquati A, Lutfi R, Abumrad N, Richards WO. Is Roux-en-Y gastric bypass surgery the most effective treatment for type 2 diabetes mellitus in morbidly obese patients? J Gastrointest Surg. 2005;9(8):1112–1116. doi: 10.1016/j.gassur.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–256. e245. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 29.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 30.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299(3):316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 31.Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32(11):2133–2135. doi: 10.2337/dc09-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmet P, Alberti KG. Surgery or medical therapy for obese patients with type 2 diabetes? N Engl J Med. 2012;366(17):1635–1636. doi: 10.1056/NEJMe1202443. [DOI] [PubMed] [Google Scholar]
- 33.Madala MC, Franklin BA, Chen AY, et al. Obesity and age of first non-ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2008;52(12):979–985. doi: 10.1016/j.jacc.2008.04.067. [DOI] [PubMed] [Google Scholar]
- 34.Wee CC, Girotra S, Weinstein AR, Mittleman MA, Mukamal KJ. The relationship between obesity and atherosclerotic progression and prognosis among patients with coronary artery bypass grafts the effect of aggressive statin therapy. J Am Coll Cardiol. 2008;52(8):620–625. doi: 10.1016/j.jacc.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Benraouane F, Litwin SE. Reductions in cardiovascular risk after bariatric surgery. Current opinion in cardiology. 2011;26(6):555–561. doi: 10.1097/HCO.0b013e32834b7fc4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams TD, Pendleton RC, Strong MB, et al. Health outcomes of gastric bypass patients compared to nonsurgical, nonintervened severely obese. Obesity (Silver Spring) 2010;18(1):121–130. doi: 10.1038/oby.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bocchieri LE, Meana M, Fisher BL. Perceived psychosocial outcomes of gastric bypass surgery: a qualitative study. Obes Surg. 2002;12(6):781–788. doi: 10.1381/096089202320995556. [DOI] [PubMed] [Google Scholar]
- 38.Dixon JB, Murphy DK, Segel JE, Finkelstein EA. Impact of laparoscopic adjustable gastric banding on type 2 diabetes. Obes Rev. 2012;13(1):57–67. doi: 10.1111/j.1467-789X.2011.00928.x. [DOI] [PubMed] [Google Scholar]
- 39.Robinson MK. Surgical treatment of obesity--weighing the facts. N Engl J Med. 2009;361(5):520–521. doi: 10.1056/NEJMe0904837. [DOI] [PubMed] [Google Scholar]
- 40.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294(15):1909–1917. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 41.Poirier P, Cornier MA, Mazzone T, et al. Bariatric surgery and cardiovascular risk factors: a scientific statement from the American Heart Association. Circulation. 2011;123(15):1683–1701. doi: 10.1161/CIR.0b013e3182149099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.