Abstract
Objective
To evaluate the effectiveness of a uniform single-center treatment protocol composed of high-dose methotrexate (MTX) and oral corticosteroids in a pediatric localized scleroderma (LS) cohort.
Methods
Thirty-six patients with LS were recruited. Patients with active disease, defined as erythematous lesions and/or new lesions, or expansion of existing lesions, were started on oral prednisone 2 mg/kg/day (maximum 60 mg/day) and subcutaneous (SC) MTX at 1 mg/kg/week (maximum 25 mg/week). Prednisone was tapered and kept at 0.25 mg/kg/day for 12 months. MTX SC was continued for 24 months, and then switched to oral administration to complete 36 months of therapy. Modified LS Skin Severity Index (mLoSSI) and the physician global assessment of disease activity (PGA-A) were used as outcome measures.
Results
Twenty-five patients with LS were female with a median age at onset of 7.86 years [interquartile range (IQR) 4.63–11.91]. Median disease duration from onset until start of this treatment regimen was 19.2 months (IQR 8.96–35.35). Median duration of followup was 36.40 months (IQR 29.39–45.36). All patients demonstrated significant improvement in mLoSSI at median 1.77 months (IQR 0.76–2.37, 95% CI 1.54, 2.01). PGA-A followed the same trend. No significant adverse reactions or flares were observed during therapy.
Conclusion
This single-center LS treatment protocol was effective and well tolerated. Clinical outcome in LS is affected by dose and route of administration of immunosuppressive regimens. Daily tapering dose of corticosteroids and parenteral MTX were effective in controlling LS activity without significant adverse reaction. This regimen should be considered as one of the therapies for LS clinical trials.
Key Indexing Terms: LOCALIZED SYSTEMIC SCLEROSIS, METHOTREXATE, PEDIATRIC RHEUMATIC DISEASE, CORTICOSTEROIDS
Localized scleroderma (LS) is the most common form of scleroderma affecting children1. The hallmark of LS is skin lesions with an inflammatory “active” phase demonstrated histologically by a dense dermal and subcutaneous lymphocytic infiltration, causing clinical signs of erythema and edema. This is followed by a “damage” phase characterized by closely packed homogenous dense collagen deposition leading to physical findings of fibrotic patches or linear bands of skin that are thick, hard, and discolored on the face, trunk, and extremities2,3. The fibrosis and resultant atrophy of the skin and underlying tissues including subcutaneous fat, muscle, tendons, and bone cause significant deformity and severe functional impairment in actively growing children4. It is during the early active phase of the disease that effective therapy is needed to minimize irreversible complications and permanent disability.
Despite its serious physical and mental consequences, optimal therapy for LS is unknown. Many topical and systemic regimens of therapy have been proposed, with varying results. Methotrexate (MTX) with or without corticosteroids (CS) in various regimens and modes of administration was reported to be an effective systemic therapy with varied success in pediatric and adult LS5,6,7,8,9,10. A recent survey indicates that most pediatric rheumatologists in North America treat their LS patients with MTX and CS combination as systemic therapy for those with moderate to severe disease, but the regimens varied broadly11. The more severe subtypes of LS require systemic therapy because they can be quite disfiguring, including linear scleroderma of the extremity and of face/scalp (en coup de sabre and Parry-Romberg syndrome), deep morphea, generalized morphea, and pansclerotic morphea11. In order to improve LS outcomes, a well designed clinical trial with objective outcome measures of disease activity is needed. We report our single-center experience in treating patients with LS using a uniform, effective regimen of MTX and CS combination therapy. We used our recently developed and validated LS outcome measure, the modified LS Skin Severity Index (mLoSSI)12. We are hopeful that our treatment regimen will be considered for the development of systemic therapy protocols for future clinical trials in LS.
MATERIALS AND METHODS
Patients
Patients with LS were recruited from the Scleroderma Clinic at the Children’s Hospital of Pittsburgh of the University of Pittsburgh Medical Center in a consecutive prospective manner from April 27, 2005, to December 5, 2009. Diagnosis and classification of LS were made according to the Peterson, et al recommendation13. All patients with LS were eligible if they had active disease, defined by the presence of erythematous or violaceous and/or new lesions. New lesions and expansion of lesions must have appeared within the past month. For new patients, this was dependent on both primary care physician and parent recall. In subsequent visits these were obtained objectively by the pediatric rheumatologist (KT or TA). Patients were recruited who had moderate to severe disease, such as the involvement of deep tissue, crossing a joint, covering a large surface area, in cosmetically concerning locations, and/or that had had topical therapy failure. Patients were excluded if they had major concomitant medical conditions, contraindications to the study medication (i.e., elevation of transaminases), or had been referred from distant pediatric rheumatology departments (and thus were unable to continue regular followup appointments). The University of Pittsburgh Institutional Review Board approved our study and informed consent was obtained from patients.
Treatment protocol
Prednisone was started at 2 mg/kg/day in 2 divided doses (maximum 60 mg/day) for 2 weeks. The dose was gradually tapered and kept at 1 mg/kg/day to complete 2 months, further tapered to 0.25 mg/kg/day for the majority of the remaining 10 months, and then discontinued. A sample of this protocol is provided for different patient weights in Table 1. The higher dosage of prednisone during the first 2 months is to aggressively treat with CS while awaiting MTX to take full effect, as described in the discussion. MTX was given at 1 mg/kg/week (maximum 25 mg/week) subcutaneously (SC) for 2 years, switched to an oral form at the same dose for 6 months, and then tapered to complete discontinuation over the following 6 months, for a total MTX course of 36 months. When MTX was switched to the oral form, it was administered in divided doses to maximize bioavailability, typically 12.5 mg on Saturday and 12.5 mg on Sunday. Oral folic acid was uniformly given at 1 mg/day daily throughout the week. Complete blood count, serum transaminases, blood urea nitrogen, and creatinine were collected to monitor for MTX side effects at each visit.
Table 1.
Oral corticosteroid taper sample for patients with localized scleroderma. The oral corticosteroid was prednisone or prednisolone. Significant stages of corticosteroid tapering are in bold type.
| Day | Wk | Mo | 10-kg Child | 20-kg Child | ≥ 30-kg Child | Maximum Dose* |
|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 10 mg BID | 20 mg BID | 30 mg BID | 2 MKD or 60 mg |
| 14 | 2 | 20 mg QD | 40 mg QD | 60 mg QD | ||
| 28 | 4 | 1 | 15 mg QD | 30 mg QD | 50 mg QD | |
| 42 | 6 | 12.5 mg QD | 25 mg QD | 40 mg QD | ||
| 56 | 8 | 2 | 10 mg QD | 20 mg QD | 30 mg QD | 1 MKD or 30 mg |
| 70 | 10 | 9 mg QD | 17.5 mg QD | 25 mg QD | ||
| 84 | 12 | 3 | 8 mg QD | 15 mg QD | 20 mg QD | |
| 98 | 14 | 7 mg QD | 12.5 mg QD | 17.5 mg QD | ||
| 112 | 16 | 4 | 6 mg QD | 10 mg QD | 15 mg QD | 0.5 MKD or 15 mg |
| 126 | 18 | 5 mg QD | 10 mg QD | 12.5 mg QD | ||
| 140 | 20 | 5 | 4 mg QD | 7.5 mg QD | 10 mg QD | |
| 154 | 22 | 3 mg QD | 7.5 mg QD | 10 mg QD | ||
| 168 | 24 | 6 | 2 mg QD | 5 mg QD | 7.5 mg QD | 0.25 MKD or 7.5 mg |
Maximum dose is whichever daily dose is the lower of the 2 options listed. BID: twice daily; MKD: milligram per kilogram per day; QD: once daily.
Outcome measures
The modified LS Skin Severity Index (mLoSSI)12 and physician global assessment of disease activity (PGA-A) were used as outcome measures. Briefly, mLoSSI is a semiquantitative clinical skin examination measuring the degree of erythema and dermal thickness, and the presence of new lesion/s or enlargement of the existing lesions in 18 cutaneous anatomic sites (face/scalp, neck, chest, abdomen, upper and lower back, right and left arms, forearms, hands, thighs, legs, and feet)12. The 100-mm visual analog scale was used to quantify the PGA-A of LS disease activity12. These outcome measures were obtained every 2–4 weeks until lesions became inactive, and then were recorded every 8 weeks during therapy and followup period. Response to therapy was defined by absence of erythema and/or decrease in skin thickness and no new lesion or enlargement of existing lesions. Lesion diameter and width were measured with a ruler and photographs were taken during study visits. Expansion of a lesion would be considered as enlargement of the lesion within the past month in comparison to previous ruler measurements or gross changes in photographs. If a patient was new to the practice, parent report of the lesion noticeably “growing” over the past month was acceptable.
The authors were responsible for examining and scoring the patient’s mLoSSI and PGA-A during their SSc clinic visits. The interrater reliability coefficients of the mLoSSI and PGA-A between the 2 physicians were 0.84 (95% CI 0.72, 0.93) and 0.90 (95% CI 0.80, 0.96), respectively.
Statistical analysis
All statistical analyses were performed using SPSS v. 18 (SPSS Inc., Chicago, IL, USA). Mean (SD) or median interquartile range (IQR) was used to described data where appropriate. Kaplan-Meier analysis was used to calculate the median time to respond with a 95% CI. The changes of mLoSSI and PGA-A were assessed by Wilcoxon signed-rank test. All statistical tests were 2-sided, and a p value < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
Thirty-six patients with a median age at LS onset of 7.86 years (IQR 4.63–11.91) were included in our study (Table 2). One additional patient was initially enrolled but did not start medication and was lost to followup after the first appointment, and therefore was not included in the study analysis. The 36 patients were compliant with medications and followup appointments. The majority of patients (32/36) completed the full 36-month protocol at the time of data analysis. The only exception to the described treatment regimen was the clinician’s choice to initiate 30 mg of MTX SC instead of using 25 mg as the maximum weekly dose in a single patient due to extensive involvement of disease, including linear scleroderma of the arm and leg with length and circumference discrepancy, generalized plaque morphea throughout the trunk and neck, and Parry-Romberg features of the face.
Table 2.
Demographic and baseline characteristics of patients with localized scleroderma (LS).
| Characteristics | |
|---|---|
| Total | 36 |
| Female, n (%) | 25 (70) |
| White, n (%) | 34 (94.4) |
| LS subtype, n (%) | |
| Linear extremity | 12 (33.3) |
| Linear face/scalp | 6(16.7) |
| Generalized morphea | 5(13.9) |
| Mixed (linear and GM) | 7(19.4) |
| Subcutaneous morphea | 3 (8.3) |
| Plaque morphea | 3 (8.3) |
| Age at onset, yrs, median (IQR) | 7.86(4.63–11.91) |
| Age at last followup, yrs, median (IQR) | 13.15(9.89–16.61) |
| Disease duration before therapy started, mo, median (IQR) | 19.15(8.96–35.35) |
| Duration of followup, mo, median (IQR) | 36.40 (29.39–45.36) |
GM: generalized morphea; IQR: interquartile range.
The majority of patients were female (70.0%), white (94.4%), and had moderate to severe disease, with 91.7% being non-plaque morphea (Table 2). Three patients with plaque morphea were included because all had actively expanding lesions despite topical therapy, with 2 of the patient’s lesions covering a large surface area of the trunk and the third having a lesion on her neck (cosmetically sensitive area). The median number of lesions was 3 (IQR 2–5). Table 3 illustrates the baseline mLoSSI scores, with a median of 8 (IQR 4–15), and baseline PGA-A scores, with a median of 61 (IQR 36–82).
Table 3.
Modified Localized Scleroderma Skin Severity Index (mLoSSI) and physician global assessment-activity (PGA-A) scores for baseline and followup visits.
| Visit | ||||||||
|---|---|---|---|---|---|---|---|---|
| V1 | V2 | V3 | V4 | V5 | V6 | V7 | V8 | |
| Months from initial visit | ||||||||
| Mean (SD) | 0 | 1.3 (1.2) | 2.6(1.8) | 4.1 (2.3) | 6.1(2.9) | 8.5 (3.5) | 11.5(4.7) | 13.8 (4.3) |
| mLoSSI | ||||||||
| Median | 8 | 3 | 2 | 1 | 1 | 0 | 0 | 0 |
| IQR | 4–15 | 1–5 | 0–4 | 0–2 | 0–2 | 0–1 | 0–1 | 0–1 |
| Range | 2–44 | 0–12 | 0–13 | 0–11 | 0–12 | 0–11 | 0–8 | 0–9 |
| New lesion | ||||||||
| Median | 0 | — | — | — | — | — | — | — |
| IQR | 0–3 | — | — | — | — | — | — | — |
| Range | 0–15 | — | — | — | — | — | — | — |
| Enlarged | ||||||||
| Median | 0 | — | — | — | — | — | — | — |
| IQR | 0–3 | — | — | — | — | — | — | — |
| Range | 0–9 | — | — | — | — | — | — | — |
| Erythema | ||||||||
| Median | 3 | 1 | 0 | 0 | — | — | — | — |
| IQR | 1–5 | 0–1 | 0–0 | 0–0 | — | — | — | — |
| Range | 0–6 | 0–6 | 0–3 | 0–1 | — | — | — | — |
| Skin thickness | ||||||||
| Median | 2 | 2 | 1 | 1 | 1 | 0 | 0 | 0 |
| IQR | 0–5 | 0–4 | 0–3 | 0–2 | 0–1 | 0–1 | 0–1 | 0–1 |
| Range | 0–12 | 0–12 | 0–12 | 0–11 | 0–11 | 0–11 | 0–9 | 0–8 |
| PGA-A* | ||||||||
| Median | 61 | 22 | 8 | 2 | 1 | 0 | 0 | 0 |
| IQR | 36–82 | 13–60 | 1–32 | 0–24 | 0–20 | 0–6 | 0–4 | 0–0 |
| Range | 7–97 | 0–84 | 0–78 | 0–68 | 0–37 | 0–36 | 0–38 | 0–9 |
n = 27 for complete PGA-A. The change in median mLoSSI, its components (new lesion, enlargement of lesion, erythema, and skin thickness), and PGA-A activity are described at each visit since the initiation of systemic therapy regimen. New lesion and enlarged lesion components are scored as either 0 (none) or 3 (present), while erythema and skin thickness components are scored 0–3 (mild-moderate-severe) per anatomic site. IQR: interquartile range.
Prior to initiation of the treatment protocol, patients had a median disease duration after onset of 19.15 months (IQR 8.96–35.35). Eight patients (22.2%) had no prior therapy and 25 patients (69.4%) were using various topical therapies including topical CS, vitamin D analogs, and tacrolimus. Three patients (8.4%) had previous systemic therapy (1 MTX and 2 CS). The patient treated with MTX had polyarticular juvenile idiopathic arthritis in the previous several years, which was in remission prior to the onset of LS. One of the patients treated with CS was on prior intravenous (IV) pulse methylprednisolone for treatment of LS and the other patient had only a 3-week history of oral prednisone therapy for LS; both patients had partial response to these therapies, had not been taking the therapies > 6 months, and had active disease upon enrollment.
Nine of the 36 patients (25%) had extracutaneous manifestations (joint contractures being the most common), and 2 patients had > 1 manifestation. Eight patients had LS lesions transversing a joint and causing contracture; one of those patients had concurrent arthritis (synovitis documented by magnetic resonance imaging). One patient had arthritis independent of LS, with onset and remission of arthritis several years prior to LS onset; arthritis did not recur. The degree of joint contracture improved in all 8 patients; however, mild to moderate contractures remained in 4 patients at the last followup visit. Two patients had eye findings, both with linear LS of the extremity not affecting the face or scalp. One had Sjögren’s keratoconjunctivitis sicca, occurring at the onset of LS, and the other had scleritis that developed 1 year into therapy while the patient took 25 mg of MTX SC weekly. These patients were treated topically for their eye conditions, which improved. Of interest was the dramatic improvement of vitiligo in a patient with this concurrent skin condition, affecting both the upper and lower extremities bilaterally; it became barely noticeable 1 year into systemic therapy.
Changes in outcome measures
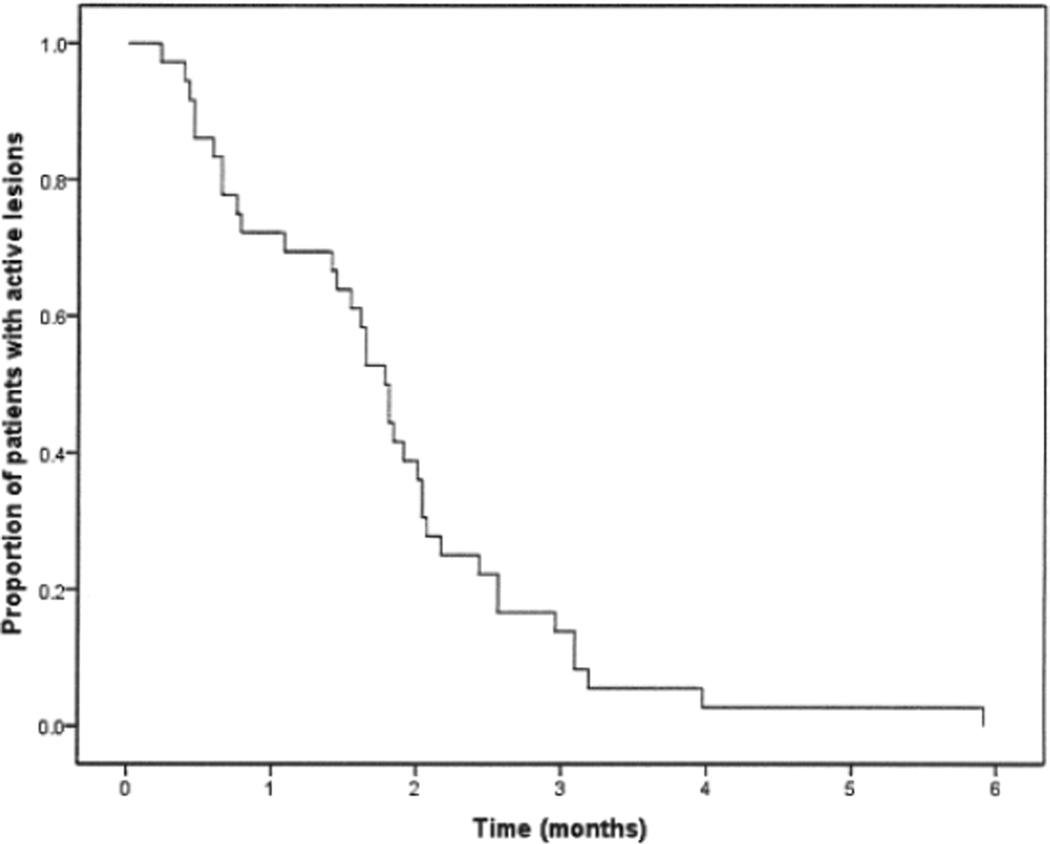
With a median duration of followup of 36.4 months (IQR 29.39–45.36), significant drops in mLoSSI and its components from baseline were observed in all patients shortly after initiation of therapy, as shown in Table 3 and Figure 1. Median time for improvement (decrease in erythema and/or skin thickness) was 1.08 months (IQR 0.49–1.88; 95% CI 0.60, 1.57). Median time to reach inactive disease (no erythema or decrease in skin thickness) was 1.77 months (IQR 0.76–2.37; 95% CI 1.54, 2.01). Figures 2 and 3 depict these changes. Improvement of disease damage measures, such as subcutaneous atrophy, dermal atrophy, and dyspigmentation, are also depicted. A formal analysis of the improvement of disease damage measures is under way at our institution. None of the patient’s mLoSSI scores increased while medication was slowly tapered over time. At the last followup, all patients’ mLoSSI scores decreased significantly from baseline (p < 0.001) and were maintained. Only 4 patients had mLoSSI > 0, which was attributed to dermal thickness. The PGA-A followed the same trends in the 27 LS patients who had complete PGA-A data (p = 0.003), as exhibited in Table 3. No patients developed LS flares while on our 36-month treatment protocol, as exemplified by no new lesions, extension of lesions, or increase in erythema and/or skin thickness during sequential visits, as shown in Table 3.
Figure 1.
Kaplan-Meier survival plot demonstrating response to therapy. The proportion of localized scleroderma patients with active lesions decreased over time (months) after therapy.
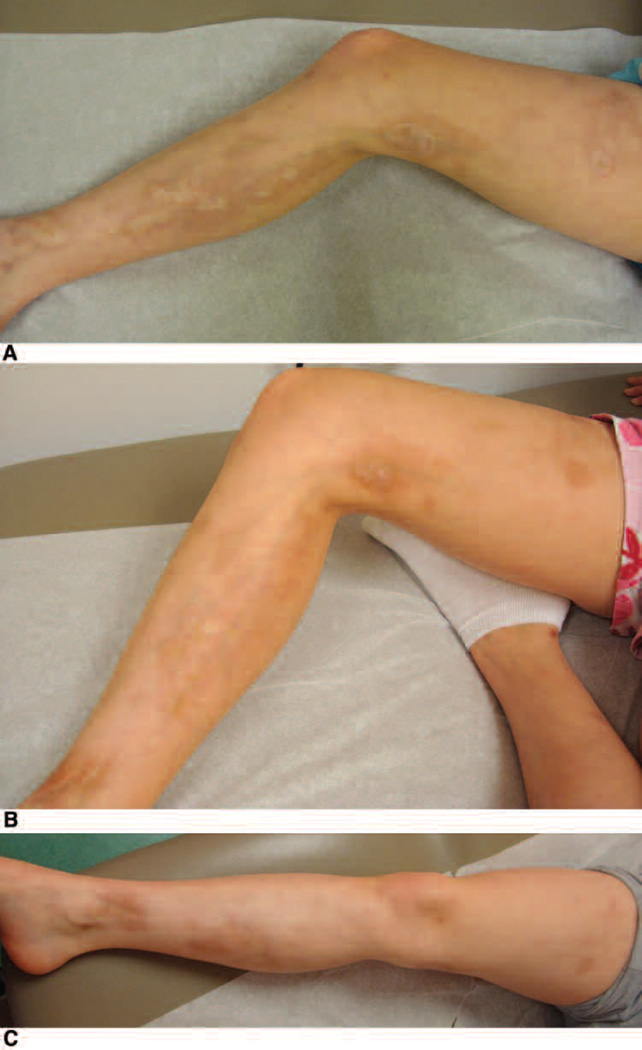
Figure 2.
Linear scleroderma affecting the right thigh and leg. Improvement in erythema, dyspigmentation, and skin thickness demonstrated over a 6-month period. A. First visit before systemic therapy initiated. B. Two months after systemic therapy initiated. C. Six months after systemic therapy initiated.
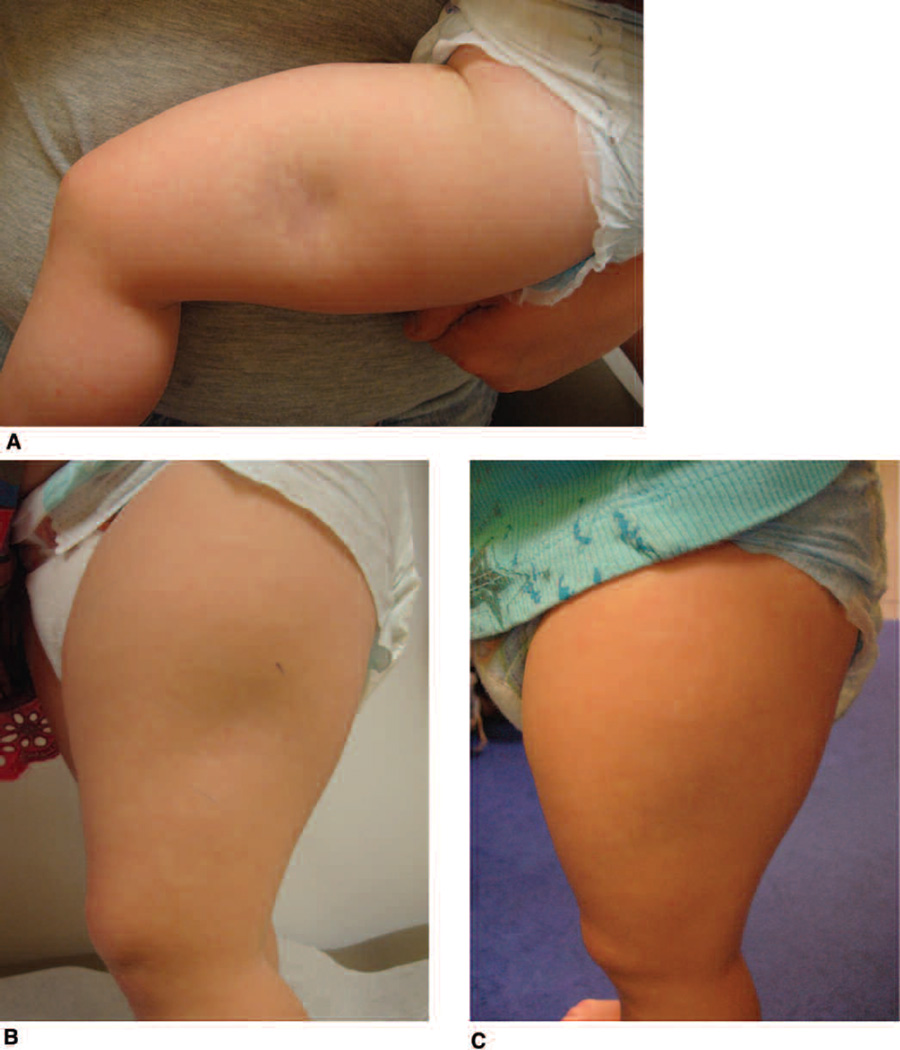
Figure 3.
Deep morphea affecting the left thigh. Improvement in erythema/violaceous color and subcutaneous atrophy demonstrated over a 6-month period. A. First visit before initiation of systemic therapy. B. Two months after initiation of systemic therapy. C. Six months after initiation of systemic therapy.
Safety profiles
There were no serious adverse drug reactions (ADR). The most common ADR was Cushingoid facies, affecting 23 patients (63.88%), and this dissipated completely once tapered from prednisone. Seven patients (19.44%) developed anticipatory emesis before MTX injection. One patient each developed oral candidiasis and transient liver transaminase elevation. Further, light striae occurred in 2 patients (5.56%). None of these ADR required discontinuation of therapy. Height was recorded at each visit and a stunted growth pattern was not documented in any of the subjects.
DISCUSSION
We report a well tolerated and effective MTX and CS combination therapy regimen for patients with LS. The protocol used in our LS cohort comprised a high dose of MTX SC (1 mg/kg/week, maximum 25 mg/week) for a full 24 months, followed by tapering to oral form at the same total dose for 6 months. Given continued disease remission, oral MTX was tapered to achieve a full discontinuation in another 6 months, completing a full 36 months of MTX therapy. MTX was combined with an uninterrupted oral daily dose of prednisone initially at 2 mg/kg/day, then further tapered to 0.25 mg/kg/day to complete a full 12 months of CS therapy. There was no recurrence of active LS lesions during the course of therapy.
Although this was not a randomized placebo-controlled clinical trial (RCT), compared to a recent study by Zulian, et al10 using oral MTX with oral CS (3 months) versus placebo and oral CS, our results are encouraging because none of our patients had a flare within the 36 months of therapy on combination MTX SC and high-dose oral CS. In the RCT, despite an initial favorable response in all subjects, at the end of 12 months, 32% (15 patients) in the MTX and oral CS group relapsed with a flare10. The general definitions for disease activity and flare were similar between these 2 studies; however, the outcome measures used in Zulian’s cohort were a combination of thermography, computerized skin size assessment, and skin thickness, which differed from our outcome measures. In addition, their criteria for defining improvement may be more stringent, as a 10% decrease in lesion temperature was required. Their findings certainly supported the use of MTX compared to placebo. Our findings suggest that maximizing MTX with SC administration and providing higher and longer maintenance oral CS may be even more effective for LS. However, this will only be accurately appraised by head-to-head comparison of different MTX and CS therapy regimens using the same outcome measures in an RCT fashion.
It is a well accepted practice for both rheumatologists and dermatologists to use systemic medications to treat severe and disabling forms of LS, which include lesions that are resistant to topical therapy or in a cosmetically unacceptable location (face and scalp, transverse a joint), deep subcutaneous involvement, or lesions larger than a single plaque lesion. Traditionally, MTX and/or CS are the treatments of choice. Earlier open-label studies of monotherapy in adults reported 67% efficacy with oral MTX7 and 82% efficacy of oral CS as monotherapy for LS14. However, studies over the past 10 years in children have reported benefit with about 90% efficacy from combined MTX and CS, and have greatly influenced the manner in which pediatric rheumatologists treat LS5,8,9. Although comparing previous studies involving either monotherapy or combination of CS and MTX is not easy because most studies were performed retrospectively with different methods of medication administration and dosages, outcome measures, and patient populations (pediatric vs adult LS), most clinicians recognize the benefit of combination therapy demonstrated in the more recent studies. A recent survey shows that over 80% of North American pediatric rheumatologists treat moderate-severe LS with MTX and CS in combination11.
Systemic CS are a cornerstone in the treatment of active LS to reduce the early inflammatory components of the disease, and are the most likely reason for the dramatic decline in mLoSSI within 2 months observed in both our cohort and others using combination therapy5,6,8,9,15. The oral CS with placebo arm of a recent RCT also supports the role of CS as an effective induction agent in the early inflammatory stage10. When choosing CS administration, it has been our experience that intermittent CS, either oral alternate-day or IV-pulse regimens, do not sustain LS disease remission as compared to continuous therapy (Medsger TA, personal communication). Additional benefit is sparing the child and family hospital visits and IV catheterization. Because the MTX effect may not be reached in the first 8 weeks, a higher CS dose (2 mg/kg/day) was necessary to induce LS remission. CS were rapidly tapered when disease activity was under control. Although CS are very effective induction agents, without a concomitant disease-modifying antirheumatic drug (DMARD) serving as maintenance therapy, LS tends to flare once CS are tapered and discontinued. A high re-flare rate was observed by Zulian, et al10, with 17/24 (70%) flaring in the oral CS and placebo arms. Joly, et al observed 6/17 (35%) flaring and requiring retreatment with CS14
MTX is the DMARD most commonly used in LS treatment, supported by over 90% of 158 North American pediatric rheumatologists surveyed11. MTX may act more on the chronic inflammatory and fibrotic components of the disease and serve as maintenance therapy in LS, allowing tapering and discontinuation of CS8. Supporting a possible antifibrotic role of MTX was a “skin softening” effect demonstrated by Seyger, et al in an adult LS series7 and van den Hoogen, et al in an adult systemic sclerosis trial16. MTX is a dihydrofolate reductase inhibitor, affecting both DNA and RNA synthesis. However, its exact mechanism in modifying LS disease is unclear, perhaps by interfering with cytokine expression. In both juvenile and adult rheumatoid arthritis, MTX has been shown to decrease levels of soluble interleukin 2 (IL-2) receptors and serum levels of IL-2, IL-6, and IL-8, supporting a possible mechanistic role in LS because these cytokines have been found to be elevated in LS17,18,19,20,21,22. Route of MTX administration determines its bioavailability, especially in higher doses (> 10 mg/m2/week), thus we uniformly use SC administration in our protocol to maximize MTX immunomodulatory effects needed to maintain LS remission23. The higher MTX dose (1 mg/kg/week) used as lower nonparenteral MTX treatment may be less effective5,6,8,9,15. The duration of MTX treatment was arbitrarily chosen based on our own institutional experience of encountering patients having a flare months after tapering therapy after a treatment duration of 2 years. Other studies have also indicated that disease recurrence may be encountered with a shorter duration (< 2 years) of MTX therapy. Weibel, et al found that out of 16 patients who had stopped MTX therapy (after inactive disease for 20 ± 12 months), 7 (44%) relapsed between 5 and 32 months (mean 16 months)9. In Uziel, et al, 1/10 patients had a flare after 1 year of therapy (3 months after discontinuing MTX)8. In Cox, et al, 3/10 patients had a flare after a mean 23-month treatment duration, with 6 months mean relapse time after MTX discontinuation15. Indeed, the appropriate duration of MTX therapy remains to be determined. We require a longer followup period to accurately define our therapy protocol treatment duration. This process is under way in our institution.
Although our treatment protocol may be more aggressive than others, it was well tolerated, with the type and frequency of side effects similar to those previously reported8,9. We also tapered the CS dosage quickly in the beginning from 2 mg/kg/day to 1 mg/kg/day within the first 2 months of therapy. The most common ADR was Cushingoid facies (64%), which was completely reversible and well accepted by patients in our cohort because we educated them about this potential ADR in advance. Seven patients (19%) developed anticipatory nausea and vomiting (ANV). ANV is a learned response to parenteral chemotherapy that develops in up to 25% of patients by the fourth treatment cycle24. Ameliorating ANV remains a challenge, and pharmacotherapy may not be successful24. In our cohort, ANV tended to develop in older patients and was mild in nature. None of these ADR merited MTX discontinuation. There was no serious infection observed during the therapy.
We chose to use mLoSSI as the primary and PGA-A as the secondary outcome measure of LS disease activity to determine the response to therapy. These reliable and validated semiquantitative measures are readily available in routine clinical settings without requiring sophisticated equipment. However, training may be required in order to minimize inter-and intrarater variation12,25. The mLoSSI identifies total cutaneous LS disease activity in regard to its severity (degree of erythema and induration, new lesion development, and enlargement of existing lesion) and the extent of disease by assessing LS lesions in 18 cutaneous surface anatomic sites12. The PGA-A not only measures LS cutaneous activity, but also assesses the extracutaneous disease activity12. The tools are user friendly and require short assessment time, especially in experienced hands. A potential limitation to our study was the presence of 2 raters for these tools; however, the interrater variability was quite low and repeatability was excellent. Another potential issue for the mLoSSI at baseline was recall bias of parent/patient when asked about an expanding lesion within the past month during the initial visit. In subsequent visits, the lesion expansion was measured objectively by the physician.
Our study is unique in that we used a uniform treatment protocol for all our referral patients with LS. The majority of our LS subtypes were non-plaque morphea with moderate-severe disease, which required systemic therapy, making the results of our study generalizable to the rheumatologist’s daily practice referral. The outcome measures, mLoSSI and PGA-A, are user friendly and feasible for routine care of patients with LS.
To our knowledge this was the first time the mLoSSI has been observed longitudinally in a prospective treatment study. As demonstrated here and in our previous reports, mLoSSI is sensitive to change and correlates well with PGA-A12. We did not have a predefined percentage decrease in the mLoSSI to determine effectiveness, but used significant rank-sum difference of scores between visits to support effectiveness. The limitations in our study included the small number of patients recruited, lack of control or concurrent comparison treatment group, and relatively short followup duration after discontinuation of the therapy. However, the positive response and lack of disease flare during 36 months of therapy were dramatic.
Effective and optimal therapy for LS is needed to improve overall LS outcomes. This can only be achieved by a careful clinical trial design. We report our MTX and CS combination regimen to be effective and well tolerated and thus it should be considered as one of the protocols for future LS clinical trials. Longer duration of followup is needed to define the proper duration of this treatment protocol to minimize recurrence rates.
ACKNOWLEDGMENT
Supported by Divisional Funds.
We thank Sally Pino, MD, Soamarat Vilaiyuk, MD, and Katherine Kurzinski, BS, for their assistance with organizing and maintaining the Pittsburgh Localized Scleroderma Database. We also thank Thomas Medsger Jr, MD, for his continued mentoring and support.
REFERENCES
- 1.Peterson LS, Nelson AM, Su WP, Mason T, O’Fallon WM, Gabriel SE. The epidemiology of morphea (localized scleroderma) in Olmsted County 1960–1993. J Rheumatol. 1997;24:73–80. [PubMed] [Google Scholar]
- 2.Sommer A, Gambichler T, Bacharach-Buhles M, von Rothenburg T, Altmeyer P, Kreuter A. Clinical and serological characteristics of progressive facial hemiatrophy: A case series of 12 patients. J Am Acad Dermatol. 2006;54:227–233. doi: 10.1016/j.jaad.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Zulian F, Athreya BH, Laxer R, Nelson AM, Feitosa de Oliveira SK, Punaro MG, et al. Juvenile localized scleroderma: Clinical and epidemiological features in 750 children. An international study. Rheumatology. 2006;45:614–620. doi: 10.1093/rheumatology/kei251. [DOI] [PubMed] [Google Scholar]
- 4.Falanga V, Medsger TA, Jr, Reichlin M, Rodnan GR. Linear scleroderma. Clinical spectrum, prognosis, and laboratory abnormalities. Ann Intern Med. 1986;104:849–857. doi: 10.7326/0003-4819-104-6-849. [DOI] [PubMed] [Google Scholar]
- 5.Fitch PG, Rettig P, Burnham JM, Finkel TH, Yan AC, Akin E, et al. Treatment of pediatric localized scleroderma with methotrexate. J Rheumatol. 2006;33:609–614. [PubMed] [Google Scholar]
- 6.Kreuter A, Gambichler T, Breuckmann F, Rotterdam S, Freitag M, Stuecker M, et al. Pulsed high-dose corticosteroids combined with low-dose methotrexate in severe localized scleroderma. Arch Dermatol. 2005;141:847–852. doi: 10.1001/archderm.141.7.847. [DOI] [PubMed] [Google Scholar]
- 7.Seyger MM, van den Hoogen FH, de Boo T, de Jong EM. Low-dose methotrexate in the treatment of widespread morphea. J Am Acad Dermatol. 1998;39(2 Pt 1):220–225. doi: 10.1016/s0190-9622(98)70079-9. [DOI] [PubMed] [Google Scholar]
- 8.Uziel Y, Feldman BM, Krafchik BR, Yeung RS, Laxer RM. Methotrexate and corticosteroid therapy for pediatric localized scleroderma. J Pediatr. 2000;136:91–95. doi: 10.1016/s0022-3476(00)90056-8. [DOI] [PubMed] [Google Scholar]
- 9.Weibel L, Sampaio MC, Visentin MT, Howell KJ, Woo P, Harper JI. Evaluation of methotrexate and corticosteroids for the treatment of localized scleroderma (morphoea) in children. Br J Dermatol. 2006;155:1013–1020. doi: 10.1111/j.1365-2133.2006.07497.x. [DOI] [PubMed] [Google Scholar]
- 10.Zulian F, Martini G, Vallongo C, Vittadello F, Falcini F, Patrizi A, et al. Methotrexate in juvenile localized scleroderma: A randomised, double-blind, placebo-controlled trial. Arthritis Rheum. 2011;63:1998–2006. doi: 10.1002/art.30264. [DOI] [PubMed] [Google Scholar]
- 11.Li SC, Feldman BM, Higgins GC, Haines KA, Punaro MG, O’Neil KM. Treatment of pediatric localized scleroderma: Results of a survey of North American pediatric rheumatologists. J Rheumatol. 2010;37:175–181. doi: 10.3899/jrheum.090708. [DOI] [PubMed] [Google Scholar]
- 12.Arkachaisri T, Vilaiyuk S, Li S, O’Neil KM, Pope E, Higgins GC, et al. The localized scleroderma skin severity index and physician global assessment of disease activity: A work in progress toward development of localized scleroderma outcome measures. J Rheumatol. 2009;36:2819–2829. doi: 10.3899/jrheum.081284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson LS, Nelson AM, Su WR. Classification of morphea (localized scleroderma) Mayo Clin Proc. 1995;70:1068–1076. doi: 10.4065/70.11.1068. [DOI] [PubMed] [Google Scholar]
- 14.Joly P, Bamberger N, Crickx B, Belaich S. Treatment of severe forms of localized scleroderma with oral corticosteroids: follow-up study on 17 patients. Arch Dermatol. 1994;130:663–664. [PubMed] [Google Scholar]
- 15.Cox D, O’Regan G, Collins S, Byrne A, Irvine A, Watson R. Juvenile localised scleroderma: A retrospective review of response to systemic treatment. Ir J Med Sci. 2008;177:343–346. doi: 10.1007/s11845-008-0217-0. [DOI] [PubMed] [Google Scholar]
- 16.van den Hoogen FH, Boerbooms AM, Swaak AJ, Rasker JJ, van Lier HJ, van de Putte LB. Comparison of methotrexate with placebo in the treatment of systemic sclerosis: A 24 week randomized double-blind trial, followed by a 24 week observational trial. Br J Rheumatol. 1996;35:364–372. doi: 10.1093/rheumatology/35.4.364. [DOI] [PubMed] [Google Scholar]
- 17.Barrera P, Haagsma CJ, Boerbooms AM, van Riel PL, Borm GF, van de Putte LB, et al. Effect of methotrexate alone or in combination with sulphasalazine on the production and circulating concentrations of cytokines and their antagonists. Longitudinal evaluation in patients with rheumatoid arthritis. Br J Rheumatol. 1995;34:747–755. doi: 10.1093/rheumatology/34.8.747. [DOI] [PubMed] [Google Scholar]
- 18.Rose CD, Fawcett PT, Gibney K, Doughty RA, Singsen BH. Serial measurements of soluble interleukin 2 receptor levels (sIL2-R) in children with juvenile rheumatoid arthritis treated with oral methotrexate. Ann Rheum Dis. 1994;53:471–474. doi: 10.1136/ard.53.7.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seitz M, Loetscher P, Dewald B, Towbin H, Rordorf C, Gallati H, et al. Methotrexate action in rheumatoid arthritis: Stimulation of cytokine inhibitor and inhibition of chemokine production by peripheral blood mononuclear cells. Br J Rheumatol. 1995;34:602–609. doi: 10.1093/rheumatology/34.7.602. [DOI] [PubMed] [Google Scholar]
- 20.Ihn H, Sato S, Fujimoto M, Kikuchi K, Takehara K. Demonstration of interleukin 8 in serum samples of patients with localized scleroderma. Arch Dermatol. 1994;130:1327–1328. [PubMed] [Google Scholar]
- 21.Ihn H, Sato S, Fujimoto M, Kikuchi K, Takehara K. Demonstration of interleukin-2, interleukin-4 and interleukin-6 in sera from patients with localized scleroderma. Arch Dermatol Res. 1995;287:193–197. doi: 10.1007/BF01262331. [DOI] [PubMed] [Google Scholar]
- 22.Uziel Y, Krafchik BR, Feldman B, Silverman ED, Rubin LA, Laxer RM. Serum levels of soluble interleukin-2 receptor. A marker of disease activity in localized scleroderma. Arthritis Rheum. 1994;37:898–901. doi: 10.1002/art.1780370618. [DOI] [PubMed] [Google Scholar]
- 23.Wallace CA. The use of methotrexate in childhood rheumatic diseases. Arthritis Rheum. 1998;41:381–391. doi: 10.1002/1529-0131(199803)41:3<381::AID-ART2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Morrow GR, Rosenthal SN. Models, mechanisms and management of anticipatory nausea and emesis. Oncology. 1996;53(Suppl 1):4–7. doi: 10.1159/000227633. [DOI] [PubMed] [Google Scholar]
- 25.Arkachaisri T, Pino S. Localized scleroderma severity index and global assessments: A pilot study of outcome instruments. J Rheumatol. 2008;35:650–657. [PubMed] [Google Scholar]