Abstract
As acetylcholinesterase (AChE) inhibitors are an important therapeutic strategy in Alzheimer’s disease, efforts are being made in search of new molecules with anti-AChE activity. The fact that naturally-occurring compounds from plants are considered to be a potential source of new inhibitors has led to the discovery of an important number of secondary metabolites and plant extracts with the ability of inhibiting the enzyme AChE, which, according to the cholinergic hypothesis, increases the levels of the neurotransmitter acetylcholine in the brain, thus improving cholinergic functions in patients with Alzheimer’s disease and alleviating the symptoms of this neurological disorder. This review summarizes a total of 128 studies which correspond to the most relevant research work published during 2006-2012 (1st semester) on plant-derived compounds, plant extracts and essential oils found to elicit AChE inhibition.
Keywords: Alzheimer’s Disease, acetylcholinesterase inhibitors, secondary metabolites, plant extracts, essential oils.
INTRODUCTION
Alzheimer’s disease (AD) is a progressive neuro-degenerative disorder associated with memory impairment and cognitive deficit. It is characterized by low levels of acetylcholine in the brain of AD patients. According to the cholinergic hypothesis, the inhibition of acetylcholinesterase (AChE), an enzyme that catalyzes acetylcholine hydrolysis, increases the levels of acetylcholine in the brain, thus improving cholinergic functions in AD patients. Further-more, although the general consensus concludes that AChE inhibitors (AChEi) can alleviate AD symptoms, they neither delay nor reverse the disease progress. Most of the drugs currently available for the treatment of AD are AChEi: tacrine (1), donezepil (2), rivastigmine (3) and galanthamine (4), all of which have limited effectiveness and some kind of side effect [1]. Tacrine (1) and donepezil (2), both from synthetic origin, were the first drugs approved for the treatment of cognitive loss in AD patients by US-FDA in 1993 and 1996, respectively. Rivastigmine (3) was approved in 2000 (US-FDA) and was designed from the lead compound physostigmine, a natural AChEi alkaloid. Galanthamine (4), a natural alkaloid first obtained from Galanthus spp. was approved by US-FDA in 2001. Huperzine A (5), an alkaloid found in Huperzia spp., is an AChEi commercialized as a dietary supplement for memory support and it is used to treat AD symptoms in China. This alkaloid has been thoroughly studied with promising results yielded particularly from the evaluation of cognitive performance of animals as well as from studies on its efficacy, tolerance and safety.
Taking into account that inhibitors 3, 4 and 5 are related to natural products and that AChEi are an important therapeutic strategy for the treatment of AD, many research groups have focused their studies on naturally-occurring compounds from plants as potential sources of either new or more effective AChEi. These studies led to the discovery of an important number of secondary metabolites as well as plant extracts, both of which are characterized by their ability to inhibit AChE. On the other hand, the fact that a significantly relevant number of research papers has been recorded in this field during the last decades can be clearly attributed to the development of colorimetric methods which allow a rapid and facile screening of a large number of samples. Ellman’s method is the most widely used for the detection of AChEi, even in complex mixtures, and for the quantification of anti-AChE inhibitory activity [2-6].
Several reviews on the newly discovered AChEi obtained from plants, fungus and marine organisms have also been published over the last years [7-10]. The majority of these AChEi belong to the alkaloid group, including indole, isoquinoline, quinolizidine, piperidine and steroidal alkaloids. On the other hand, several non-alkaloidal and potent AChEi have been obtained from natural sources, including terpenoids, flavonoids and other phenolic compounds. Interestingly, although literature demonstrates to be rich in the study on AChEi obtained from plants, this issue keeps on being the center of attention for research as confirmed by the increasing number of studies published every year. Therefore, the purpose of this review is to provide a comprehensive summary of the literature, particularly that published during 2006-2012 (1st semester) on plant-derived compounds, plant extracts and essential oils which have been reported to inhibit AChE. Readers interested not only in previous findings but also in synthetic/semisynthetic AChEi or natural AChEi of fungal, marine or microbial origin are recommended to see the above-mentioned reviews i.e. [7-10]. For the sake of brevity and in order to focus our attention on the most relevant findings, only those research papers reporting quantified results (IC50 and/or percentage of inhibition at a given concentration) were included. Extracts or essential oils with IC50 > 0.5 mg/ml were considered weakly active and were therefore not taken into account in the present review. With a few exceptions, only molecules with IC50 < 50 µM have been considered. Furthermore, unless otherwise stated, those results on AChE inhibition included in the present review refer to in vitro assays carried out with AChE from electric eel.
ALKALOIDS WITH AChE INHIBITORY ACTIVITY
The quinoline alkaloids 3-hydroxy-2,2,6-trimethyl-3,4,5,6-tetrahydro-2H-pyrano[3,2-c] quinoline-5-one (6), ribalinine (7) and methyl isoplatydesmine (8) isolated from the aerial parts of Skimmia laureola (Rutaceae) were found to be linear mixed inhibitors of AChE with Ki = 110.0, 30.0 and 30.0 µM, respectively [11]. These alkaloids were also observed to evidence butyrylcholinesterase (BChE) inhibition.
On the other hand, of the several alkaloids that were isolated from the active extracts of Esenbeckia leiocarpa (Rutaceae), leptomerine (9) and kokusaginine (10) with IC50 values of 2.5 and 46 µM, respectively, were observed to elicit AChE inhibitory activity [12]. The isolation of skimmianine (11), a furoquinoline alkaloid with very low AChE inhibitory activity, was also reported by the same authors. This alkaloid was observed in another Rutaceae, Zanthoxylum nitidum, exhibiting a moderate AChE inhibitory activity (IC50 = 8.6 µg/ml) [13].
Nelumbo nucifera is a well-known medicinal plant belonging to the Nelumbonaceae family which was studied due to its therapeutic potential [14]. N-methylasimilobine (12), an aporphine alkaloid with an IC50 = 1.5 µg/ml which was found to be a non-competitive inhibitor, was recently isolated from this plant [15]. In a random screening, two extracts of Beilschmiedia species were observed to exhibit AChE inhibition and a phytochemical study of B. alloiophylla and B. kunstleri revealed the presence of several alkaloids with IC50 values ranging between 2.0 and 10.0 µM [16]. The most potent AChEi were found to be 2-hydroxy-9-methoxyaporphine (13), laurotetanine (14), liriodenine (15) and oreobeiline (16) (IC50 = 2.0-5.0 µM), with anti-AChE activity comparable to huperzine A (IC50 = 1.8 µM). A significant AChE inhibitory activity was also observed in secoboldine (17), boldine (18), isoboldine (19), asimilobine (20) and 3-methoxynordomesticine (21) (IC50 = 8.4 - 10.0 µM).
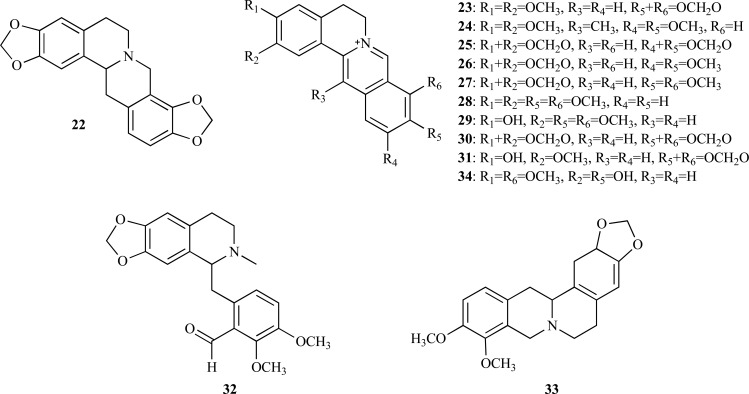
Research on plants from the genus Corydalis (Papaveraceae) which are used for the treatment of memory dysfunction in folk medicine reported the presence of benzylisoquinoline alkaloids with anti-AChE activity [7]. The ethanolic extract obtained from the tuber of C. turtschaninovii previously found to elicit AChE inhibition was selected to carry out a chemical study which led to the isolation of the isoquinoline alkaloids stylopine (22), epiberberine (23), pseudodehydrocorydaline (24), pseudocopsitine (25) and pseudoberberine (26). In the assay with mouse brain cortex as a source of AChE enzyme, the IC50 values obtained for each of these alkaloids were 15.8, 6.5, 8.4, 4.3 and 4.5 µM, respectively [17]. In addition, alkaloids 25 and 26, the two most active compounds, were found to elicit anti-amnesic activity [17, 18]. Alkaloids with benzylisoquinoline skeleton from Corydalis species having aromatic methylenedioxy groups and a quaternary atom of nitrogen were observed to show the strongest AChE inhibition [7, 17, 18]. In a more recent work, six protoberberine alkaloids 23, 27 - 31, were identified in rhizomes of Coptis chinensis which are traditionally used in Chinese medicine for the treatment of various diseases. Coptidis rhizomes and their alkaloids were reported to have cognitive-enhancing and neuroprotective effects and the analysis of the anti-AChE activity of these alkaloids showed that the IC50 values of berberine (27), palmatine (28), jateorrhizine (29), coptisine (30) and groenlandicine (31) ranged between 0.44 and 0.80 µM while that of epiberberine (23) was slightly higher (IC50 = 1.07 µM) [19]. Of these alkaloids, compounds 27, 30 and 31 were observed to have an aromatic methylenedioxy group. In this study groenlandicine (31) and berberine (27) were found to be the most active as BChE inhibitors and epiberberine (23) was observed to significantly inhibit β-secretase (BACE1) [19].
The alkaloids (+)-canadaline (32) and (+)-canadine (33), both isolated from Corydalis cava and with an IC50 = 20.1 and 12.4 µM, respectively, were observed to elicit a moderate inhibitory activity when tested with AChE from human blood [20].
On the other hand, Stephania venosa (Menispermaceae), a Thai medicinal plant, was found to show a high AChE inhibitory activity. The ethanolic extract of S. venosa was subjected to bioassay-guided fractionation to identify AChEi [21]. The following moderately active quaternary protoberberine alkaloids could be isolated: stepharanine (34), cyclanoline (35) and N-methyl stepholidine (36) with IC50 values of 14.10, 9.23 and 31.30 µM, respectively. A similar fractionation approach was followed to identify the compounds responsible for AChE inhibition in Chelidonium majus (Papaveraceae) [22]. Three active constituents were identified, namely 8-hydroxydihydrochelerythrine (37), 8-hydroxydihydrosanguinarine (38) and berberine (27). Compounds 37 and 38, with no previous record as AChEi, were found to elicit significant anti-AChE activity with an IC50 = 0.61 and 1.37 µM, respectively.
Taspine (39) was isolated from the alkaloid-enriched extract obtained from Magnolia x soulangiana (Magnoliaceae) [23]. This alkaloid was found not only to show a dose-dependent and long-lasting inhibitory effect on AChE (IC50 = 0.33 µM) but also to be more potent than galanthamine (IC50 = 3.2 µM) although its inhibitory activity is comparable to that of tacrine (IC50 = 0.22 µM). Similar observations were obtained when the in vitro assay was performed with human AChE (IC50 = 0.54 µM). Compound 39 resulted to be inactive against BChE, acting as a selective AChEi.
Catharanthus roseus (Apocynaceae) is a plant mainly known as a source of vincristine and vinblastine, two alkaloids found in its leaves and appreciated as anticancer compounds. Several other compounds with biological importance can be also found in C. roseus. For example, the alkaloid serpentine (40), isolated from the roots of this plant, was reported to be a potent in vitro AChEi (IC50 = 0.775 µM) compared with physostigmine (IC50 = 6.45 µM) [24].
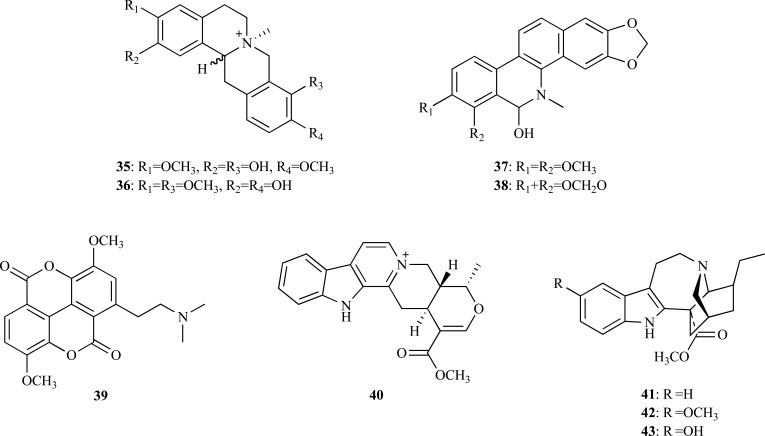
A bioassay-guided fractionation from the stems of Ervatamia hainanensis (Apocynaceae), a plant used in traditional Chinese medicine, allowed the isolation of several monoterpenoid indole alkaloids, some of them showing a potent AChE inhibitory activity [25]. For example, coronaridine (41) and voacangine (42), differing from each other only by the methoxy group attached to the aromatic ring, were observed to have an IC50 = 8.6 and 4.4 µM, respectively, these values being similar to that of galanthamine (3.2 µM). On the other hand, 10-hydro-xycoronaridine (43) was found to evidence a reduced AChE inhibition (IC50 = 29 µM), which was attributed to the introduction of a hydroxyl group to the aromatic ring. The indole alkaloids coronaridine (41) and voacangine (42), both detected in the stalks of Tabernaemontana australis (Apocynaceae), had been formerly identified as AChEi but no inhibition values were reported [26].
The genus Tabernaemontana is known for the wide variety of unusual bioactive indole alkaloids it produces. Among them, the bisindole alkaloids isolated from T. divaricata roots are an interesting example of new structures with potent AChE inhibitory activity. The crude alkaloid extract obtained from the root of T. divaricata was found to yield four bisindole alkaloids 44 - 47 [27]. The analysis of AChE inhibition revealed that 19,20-dihydrotabernamine (44) and 19,20-dihydroervahanine A (45) strongly inhibit AChE, with an IC50 = 0.227 and 0.071 µM, respectively, thus showing that they are significantly more active than galanthamine (IC50 = 0.594 µM). The fact that inhibition was found to be higher for compound 45 than for compound 44 suggests that the introduction of a carbomethoxy group at C16’ increases the enzymatic inhibition. In addition, taking into account that conodurine (46) and tabernaelegantine (47) were found to show no activity in AChE, it was suggested that the substitution at C11’ and C12’ is relevant for AChE inhibitory activity [27].
Uncaria rhynchophylla (Rubiaceae) is a Chinese medicine herb used to treat epilepsy. The alkaloid fraction from U. rhynchophylla is known for its antiepileptic and neuroprotective effects. Geissoschizine methyl ether (48), a strong AChEi, as well as six other weakly active alkaloids were recently isolated from this herb [28]. The active compound 48 was observed to inhibit AChE in a reversible and non-competitive way with an IC50 = 3.7 µg/ml.
The study of AChE inhibitory activity of Brazilian apocynacea Himatanthus lancifolius, commonly known as “agoniada”, led to the identification of active extracts in this plant and allowed the isolation of uleine (49), an active indole alkaloid, at a high concentration in the alkaloid fraction. The IC50 value observed for this alkaloid was 0.45 µM [29].
As to the Amaryllidaceae family, phytochemical research conducted in the last decades on this family revealed several alkaloids with moderate or potent inhibition of AChE [3, 7, 30]. In the search of new natural sources of galanthamine and other Amaryllidaceae alkaloids with anti-AChE activity, bulbs and leaves of Hippeastrum papilio collected in the South of Brazil were studied. Galanthamine (4), the already known alkaloids narwedine (50), haemanthamine (51), 11-hydroxyvittatine (52), 8-O-demethylmaritidine (53) and vittatine (54) as well as the new alkaloid 11(-hydro-xygalanthamine (55) were all isolated and of all of them galanthamine was obtained in significant amounts [31]. Compound 55 was observed to elicit AChE inhibition as other galanthamine-type alkaloids do, with an IC50 = 14.5 µM. Furthermore, because habranthine, epimer of 55, was observed to have an anti-AChE activity similar to that of galanthamine, it was concluded that β configuration at C11 is unfavorable for the interaction with AChE [3, 31]. Other potent AChEi, such as N-allylnorgalanthamine (56) and N-(14-methylallyl)norgalanthamine (57), were isolated from Leucojum aestivum, an amaryllidacea used for the industrial extraction of galanthamine [32]. N-alkylated galanthamine derivatives 56 and 57 were isolated together with galanthamine (4), epinorgalanthamine (58), narwedine (50) and lycorine (59), from the mother liquors obtained after the industrial production of galanthamine. Alkaloids 56 and 57, with IC50 values of 0.18 and 0.16 µM, respectively, resulted to be ten times more potent AChEi than galanthamine (IC50 = 1.82 µM).
The chemical investigation of Galanthus rizehensis, a wild-growing species from Turkey, allowed the isolation of two new Amaryllidaceae alkaloid N-oxides, incartine N-oxide (60) and lycorine N-oxide (61) and seven known alkaloids namely, 1-acetyl-β-carboline (62), incartine (63), N-trans feruloyltyramine (64), lycorine (59), O-methylnorbelladine (65), vittatine (54) and 11-hydroxyvittatine (52) [33]. The potential of these alkaloids as AChEi was analyzed but only incartine N-oxide (60) was observed to elicit a moderate inhibitory activity (IC50 = 34.50 µM), incartine (63) was observed to be weakly active (IC50 = 106.97 µM) and the other alkaloids were found to be inactive. In a bioassay-guided fractionation of an active extract obtained from bulbs of Nerine bowdenii, the Amaryllidaceae alkaloid undulatine (66) was identified as the most active component of the alkaloid fraction, with an IC50 = 37 µM [34].
Although benzylphenethylamine alkaloids were considered to belong exclusively to the Amaryllidaceae, some of them have been found to belong to other families [35]. A new example of this exception was found through the chemical investigation of Hosta plantaginea (Liliaceae) [36]. Seventeen benzylphenetylamine alkaloids, including five new alkaloids, 67-71, along with twelve known compounds [7-deoxy-trans-dihydronarciclasine, O-methyllycorenine, albomaculine, haemanthamine, O-demethylhaemanthamine, 8-O-demethylmaritadine, haemanthidine, yemenine C, lycorine, pseudolycorine, ungeremine (72) and norsanguinine (73)] were obtained. Some of these alkaloids were analyzed to determine whether they are AChEi or not.. Ungeremine (72) (IC50 = 3.85 µM), norsanguinine (73) (IC50 = 1.43 µM) and 8-demethoxy-10-O-methylhostasine (69) (IC50 = 2.32 µM) were all found to be potent AChE inhibitors.
After the isolation of the potent AChEi huperzine A (5) from Huperzia serrata (Lycopodiaceae), several plants belonging to the genus Lycopodium have been investigated in an attempt to find alkaloids with unusual skeletons that could have AChE inhibitory activity [7, 8, 37].Five new Lycopodium alkaloids, 11(-hydroxyfawcettidine (74), 2(,11(-dihydroxyfawcettidine (75), 8(,11(-dihydro-xyfawcettidine (76), 2β-hydroxylycothunine (77) and 8(-hydroxylycothunine (78), with the fawcettimine skeleton were isolated from L. serratum, along with three known alkaloids, lycothunine (79), serratine (80) and serratanidine (81) [38]. AChE inhibitory activity was analyzed for the alkaloid lycoposerramine-H (82) previously isolated from L. serratum [39] and for compounds 74, 75, 78. Alkaloids 75 and 82 were observed to inhibit AChE with an IC50 = 27.9 and 16.7 µM, respectively, while 74 and 78 were observed to show no anti-AChE activity. In another study, three new alkaloids (83 - 85) were isolated from L. carinatum, a species collected in Malasya [40]. Carinatumins A (83) and B (84) were observed to inhibit AChE from bovine erythrocytes with an IC50 = 4.6 and 7.0 µM, respectively, whereas carinatumin C (85) was observed to show no inhibition (IC50 > 100 µM). Alkaloids 83 and 84 were observed to exhibit an AChE inhibitory activity similar to that of huperzine A and huperzine B (IC50 = 0.8 and 8.0 µM). Alkaloids from L. casuarinoides were also isolated and three new compounds, lycoparins A-C (86 - 88), were characterized, of which lycoparin C (88) was found to show a moderate AChE inhibitory activity (from bovine erythrocytes) with an IC50 = 25 µM [41]. Lycoparin A (86) and lycoparin B (87), both having a carboxylic acid at C-15 and one or two N-methyl groups, were found to show no inhibitory activity.
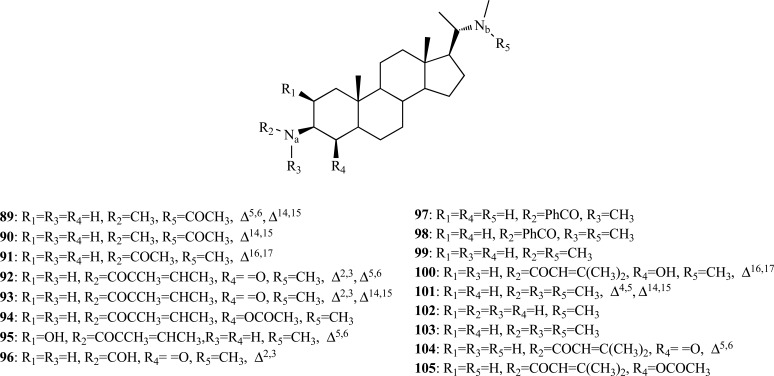
As to Sarcococca and Buxus species (Buxaceae), they are known to produce steroidal alkaloids, some of which were observed to evidence strong AChE inhibition [7, 42, 43]. New steroidal alkaloid AChEi from S. saligna and S. hookeriana were recently found. In the case of S. saligna, the study –which was a continuation of previous research [44, 45]– of the bioactive steroidal alkaloids of this species allowed the isolation of five new compounds (89-93) and two already known bases (94 and 95) [46]. The new alkaloids 5,14-dehydro-Na-demethylsaracodine (89), 14-dehydro-Na-demethylsaracodine (90), 16-dehydrosarcorine (91), 2,3-dehydrosarsalignone (92) and 14,15-dehydro-sarcovagine D (93), as well as the known compounds sarcovagine C (94) and salignarine C (95) were analyzed as anti-AChE agents. Only 91, 92 and 95 were observed to exhibit significant AChE inhibition (IC50 = 12.5, 7.0 and 19.7 µM, respectively). Compounds 89 - 92, 94 and 95 were also found to elicit strong and selective BChE inhibition [46]. The bioassay-guided chemical investigation of S. hookeriana allowed the isolation of two new pregnane-type steroidal alkaloids, hookerianamide H (96) and hookerianamide I (97) together with the known alkaloids Na-methylepipachysamine D (98), sarcovagine C (94) and dictyophlebine (99) [47]. Compounds 94, 96, 97, 98 and 99 were tested for their inhibitory properties towards AChE and all of them were observed to elicit significant inhibitory activity (IC50 2.9 – 34.1 µM) as well as a potent anti-BChE activity (IC50 0.3 – 3.6 µM). Further studies on S. hookeriana yielded two new 5α-pregnane-type steroidal alkaloids, hookerianamides J (100) and K (101) [48]. Furthermore, eight known steroidal alkaloids, hookerianamide H (96) and hookerianamide I (97), chonemorphine (102), N-methylpachysamine A (103), epipachysamine-E-5-en-4-one (104), vagenine A (105), 2,3-dehydrosarsalignone (92) and sarcovagine C (94), were isolated and characterized. Alkaloids 94, 100, 101, 102, 103 and 104 were analyzed as AChEi. Compounds 100, 101, 102 and 103 were observed to inhibit AChE moderately (IC50 22.1 – 48.5 µM) while 104 and 94 were found to be more active inhibitors (IC50 9.9 and 8.1 µM, respectively).
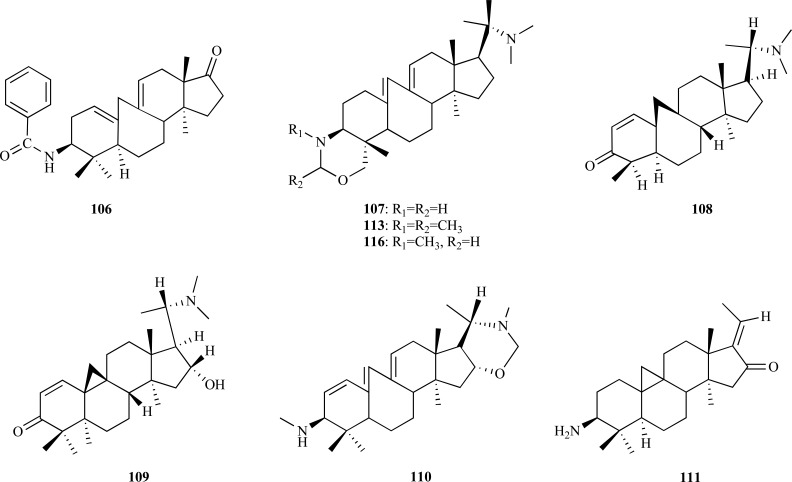
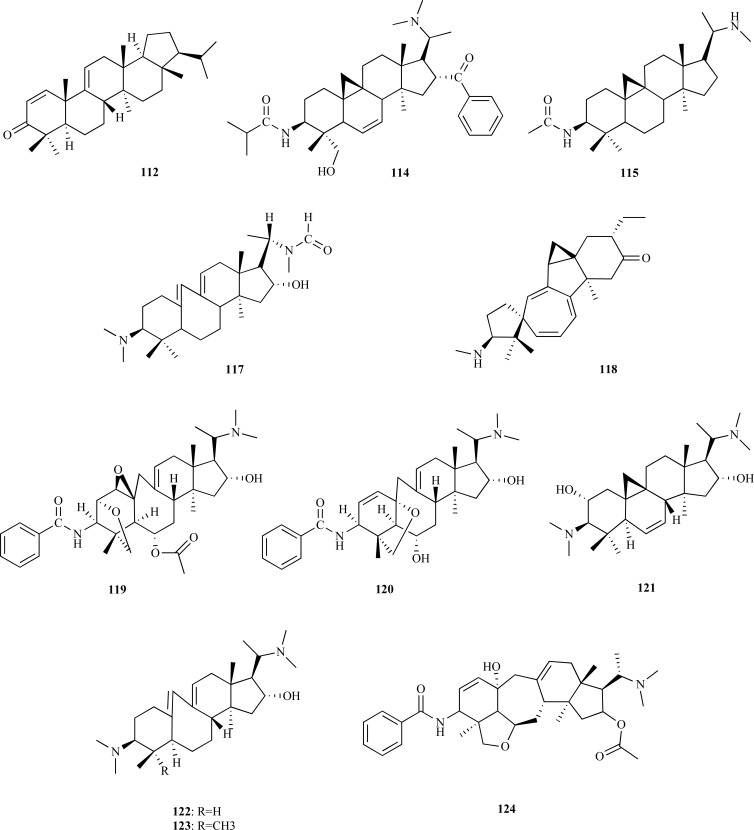
Phytochemical research on Buxus hyrcana allowed the identification of several Buxus alkaloids with cholinesterase inhibitory activity [43, 49]. Three new triterpenoidal alkaloids, namely 17-oxo-3-benzoylbuxadine (106), buxhyrcamine (107) and 31-demethylcyclobuxoviridine (108) along with sixteen known compounds, all tested as AChEi, were isolated and characterized in a recent study on B. hyrcana collected from Iran [50]. Weak AChE inhibitory activity was observed for Nb-dimethylcyclobuxoviricine (109), papillozine C (110), cyclobuxophylline O (111) and arbora-1,9(11)-dien-3-one (112) (IC50 = 35.4 - 47.9 µM). In the same in vitro assay, 17-oxo-3-benzoylbuxadine (106), buxhyrcamine (107), homomoenjodaramine (113), buxmicrophylline F (114), buxrugulosamine (115), moenjodaramine (116) and N20-formyl-buxaminol E (117) were observed to show moderate AChE inhibition (IC50 = 17.6 - 25.5 µM) while spirofornabuxine (118) was found to elicit a strong AChE inhibitory activity (IC50 = 6.3 µM).
The crude methanolic extract of B. natalensis, a plant used to improve memory in the elderly by traditional healers in South Africa, was found to elicit AChE inhibition (IC50 = 28 µg/mL).The phytochemical study of this extract yielded seven compounds 119 - 125 which were found to show either moderate or strong AChE inhibition [51]. The alkaloids O2-natafuranamine (119), O10-natafuranamine (120), cyclonataminol (121) and 31-demethylbuxaminol (122) were isolated and characterized for the first time while buxaminol A (123) was isolated for the first time as a natural product. Buxafuranamide (124) and buxalongifolamidine (125) were already known compounds. Compounds 119, 120 and 124 were observed to exhibit a significantly higher AChE inhibitory activity compared to the rest, with IC50 values of 3.0, 8.5, and 14.0 µM, respectively. Compounds 121, 122, 123 and 125 were observed to be less effective as AChEi (IC50 = 22.9 – 30.2 µM).
The bulbs of Fritillaria species (Lilliaceae) which are known to be a traditional medicinal herb called “Beimu” in China are used as an antitussive, antiasthmatic and expectorant agent. In the past, in a chemical study carried out on alkaloids from F. imperialis bulbs new steroidal alkaloids with weak AChE inhibition and great selectivity towards BChE were identified [52]. Thus, taking into account this previous study, the bulbs from five Fritillaria species were studied and their alkaloids were identified and evaluated as cholinesterase inhibitors. Eighteen alkaloids were isolated and their effects on human whole blood cholinesterase were assayed. Results showed that N-demethyl-puqietinone (126) from F. puqiensis, hupeheninoside (127) from F. hupehensis, ebeiedinone (128) from F. ebeiensis var. purpurea, yibeinoside A (129) from F. pallidiflora and chuanbeinone (130) from F. delavayi showed good AChE inhibition, with IC50 values of 6.4, 16.9, 5.7, 6.5 and 7.7 µM, respectively. However, all of them were weaker AChEi than galanthamine (IC50 = 1.9 µM). Compounds 127, 128, 129 and 130 were found to be stronger inhibitors on plasma BChE than galanthamine, the positive control [53].
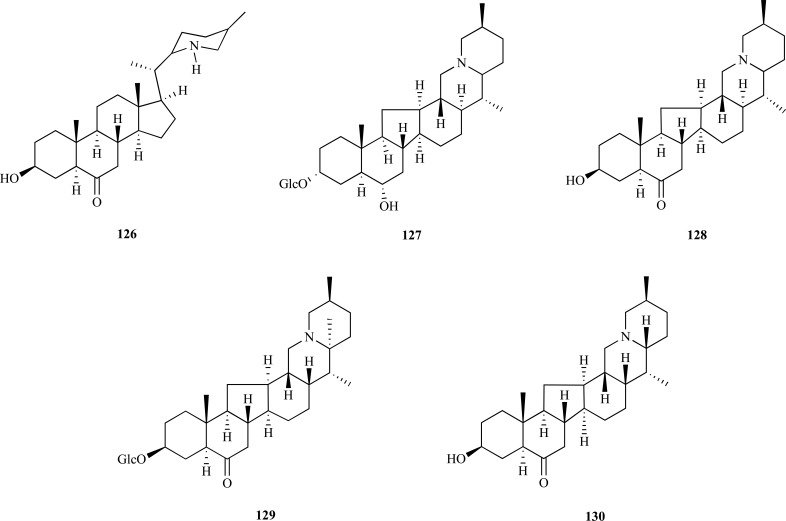
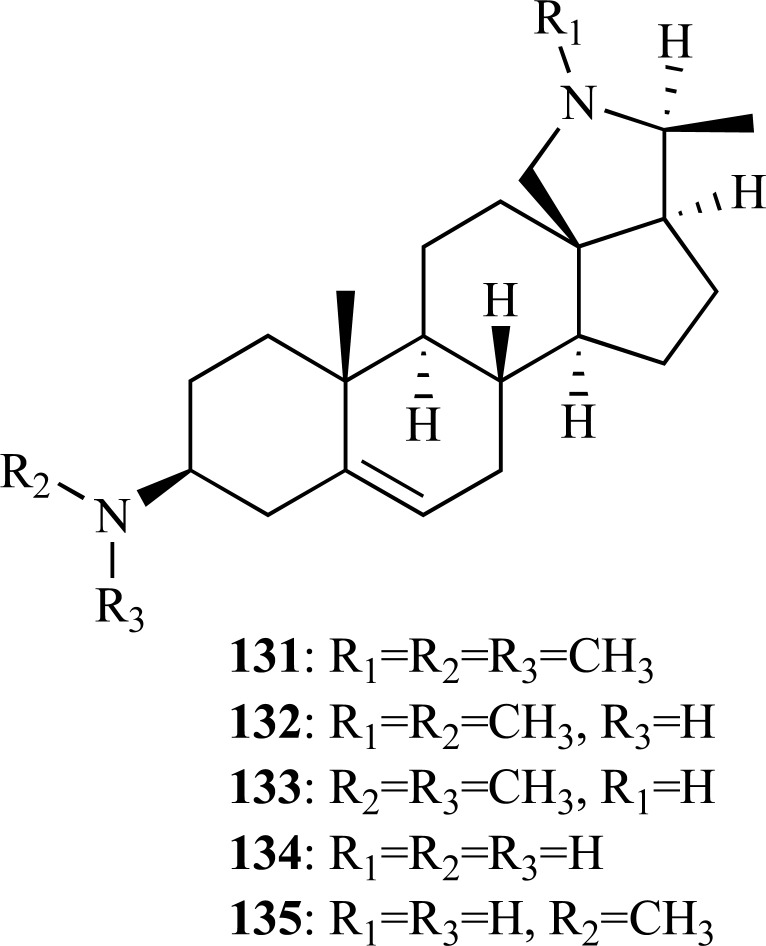
In addition, the following steroidal alkaloids: conessine (131), isoconessimine (132), conessimin (133), conarrhimin (134) and conimin (135) were isolated in a bioassay-guided fractionation from the seeds of Holarrhena antidysenterica (Apocynaceae), a common Tibetan drug [54]. Compounds 131, 133, 134 and 135 were identified as active constituents against AChE. Conessimin (133) was found to be the strongest AChE inhibitor with an IC50 = 4 µM whereas conessine (131), conarrhimin (134) and conimin (135) were found to be moderate AChE inhibitors (IC50 = 21 - 28 µM). These findings indicate that the elimination of the N-methyl group of pyrrolidine moiety induces a significant increase of activity while the cleavage of either one or two N-methyl groups at C-3 position reduces the inhibitory potency. Compound 133 was selected for a kinetic study through which it was demonstrated that its AChE inhibitory activity is both reversible and non-competitive. Molecular docking simulations of these compounds with AChE helped to understand their interactions with AChE and were consistent with the experimental results obtained [54].
NON-ALKALOIDAL COMPOUNDS WITH AChE INHIBITORY ACTIVITY
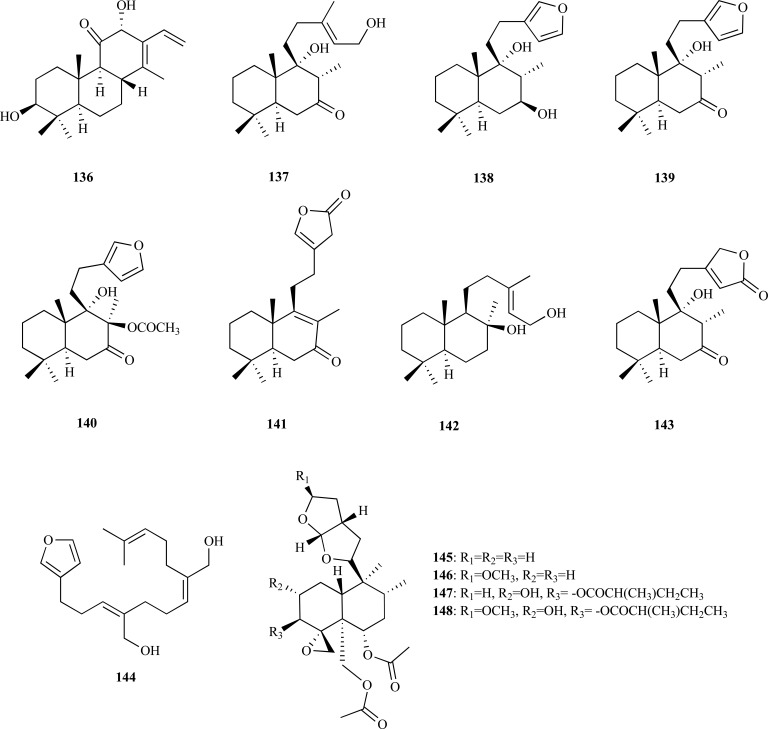
In spite of the fact that the majority of the most potent inhibitors known to date are alkaloids, several non-alkaloidal AChEi from the plant kingdom and with different structural characteristics (terpenoids, sterols, flavonoids and phenolic compounds, etc) have been recognized as promising lead compounds as anti-AD agents [7-10]. Until 2006 only a few diterpenoids demonstrated to inhibit AChE [7]. However, further recent research has reported a larger number of compounds belonging to this group with the ability to exert either moderate or strong AChE inhibitory activity. In addition, a new cassane diterpene named niloticane (136) was isolated from the ethyl acetate bark extract of Acacia nilotica subsp. kraussiana (Fabaceae), a plant used in African traditional medicine [55]. Niloticane (136) was found to show an AChE inhibitory activity similar to that of the positive control galanthamine (IC50 = 4 and 2 µM, respectively). In addition, one new (137) and six known (138 - 143) labdane-type diterpenoids were identified as AChE inhibitors present in an active extract obtained from Leonurus heterophyllus (Lamiaceae) by bioassay-guided fractionation [56]. Anti-AChE activity in 137 – 143 was analyzed in rat brain cortex as a source of AChE enzyme. Leoheteronin A (141) and leopersin G (143), both having a 15,16 epoxy group, were observed to be strong inhibitors with IC50 values of 11.6 and 12.9 µM, respectively. The new compounds leoheteronin F (137) and leoheteronin D (142) were found to show moderate inhibition with IC50 values of 16.1 and 18.4 µM, respectively. Leoheterin (138), hispanone (139) and galeopsin (140), all having a furan ring at the side chain, were found to be weakly active (IC50 = 38.5 - 42.7 µM).
Asparagus adscendens (Asparagaceae) is a medicinal plant traditionally used as a nerve tonic and remedy for memory impairments in Pakistan. Conypododiol (144), which was isolated from the chloroform fraction of the methanolic extract of A. adscendens, was found to elicit AChE and BChE inhibition with an IC50 = 2.17 and 11.21 µM, respectively [57]. This dual cholinesterase inhibitor was also observed to show potential as a bivalent ligand in molecular docking studies. Four non-competitive AChEi 145 – 148 were obtained in the chemical investigation of Ajuga bracteosa (Lamiaceae), another medicinal plant from Pakistan [58]. The diterpenoid dihydroajugapitin (148) was found to be the most active against AChE with an IC50 = 14.0 µM. Compared to compound 148, lupulin A (147), clerodinin A (146) and dihydroclerodin (145) were observed to be less efficient inhibitors (IC50 = 19.2, 26.5 and 35.2, respectively) and diterpenoids 145 - 148 were observed to elicit BChE inhibition. These findings indicate that the presence of a methoxy group at C-15 increases cholinesterase inhibitory potential.
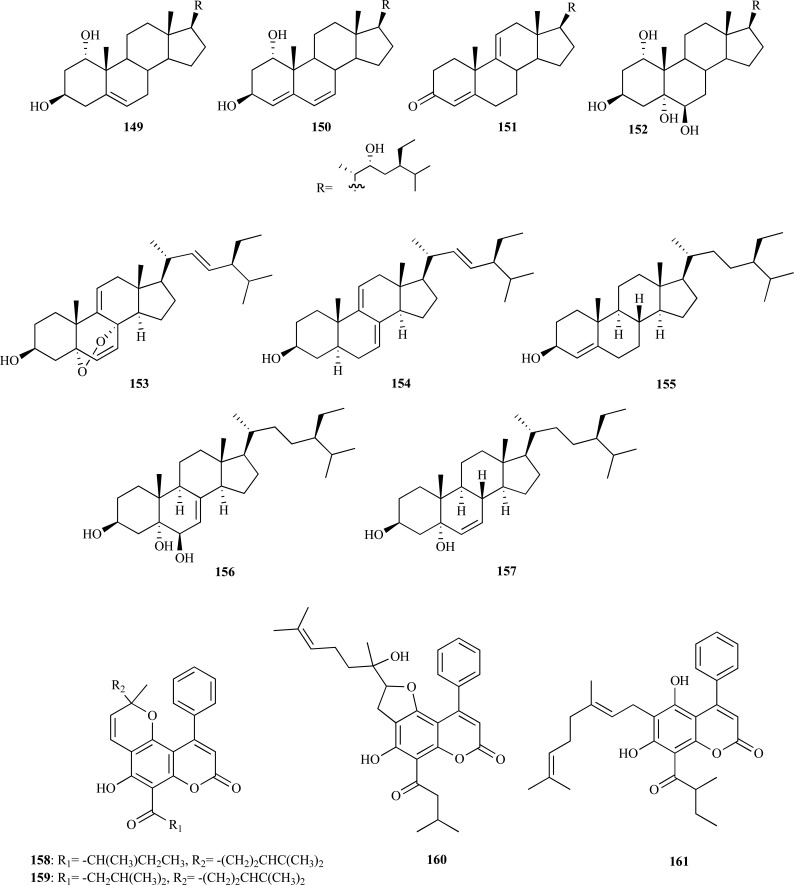
From the methanolic extract of Haloxylon recurvum (Chenopodiaceae), a plant used in Pakistan for the treatment of several neuronal disorders, four new C-24 alkylated sterols 149 – 152 and five known sterols 153 – 157 were isolated [59]. Compounds 149 – 157 were analyzed as AChEi and were found to inhibit AChE in a concentration-dependent manner acting as non-competitive inhibitors. Haloxysterol B (150) and haloxysterol C (151), whose IC50 values were 0.89 and 1.0 µM, respectively, were found to be the most active AChE inhibitors. Their inhibitory activity was observed to be similar to that of galanthamine (IC50 0.5 µM). Haloxysterol A (149) and 24-ethyl-cholest-6-ene-3,5-diol (157) were also observed to show potent AChE inhibition with IC50 values of 8.3 and 3.5 µM, respectively. Haloxysterol D (152), 5(,8(-epidioxy-(24S)-ethyl-cholest-6,9(11),22(E)-triene-3β-ol (153), (24S)-ethyl-cholest-7,9(11),22(E)-triene-3β-ol (154), lawsaritol (155) and 24-ethyl-cholest-7-ene-3,5,6-triol (156) were found to elicit a moderate anti-AChE activity (IC50 = 13.7 - 26.4 µM).
On the other hand, a bioassay-guided fractionation on the bark of Mesua elegans (Clusiaceae) allowed the isolation of the anti-AChE components responsible for the activity observed for the extract. Mesuagenin B (158) was the most potent inhibitor (IC50 = 0.7 µM) and mesuagenin A (159), mesuagenin D (160) and 5,7-dihydroxy-8-(3-methylbutanoyl)-6- (E)-3,7-dimethylocta-2,6-dienyl-4-phenyl-2H-chromen-2-one (161) were observed to elicit strong AChE inhibition with IC50 values of 1.06, 8.73 and 3.06 µM, respectively [60]. This bioassay-guided study is the first report of 4-phenylcoumarins as AChEi.
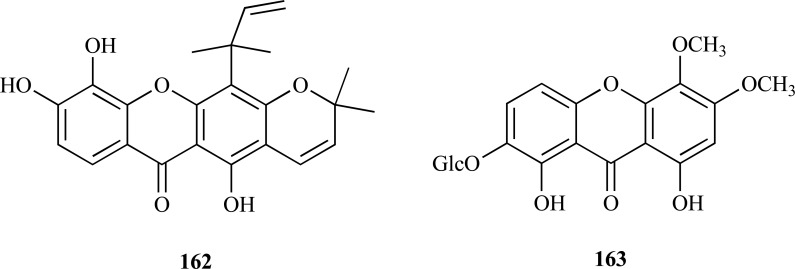
In the past, some examples of xanthones with moderate AChE inhibitory activity were reported [7]. Further recent research introduced two new xanthones, 162 and 163, to this group of AChEi also with moderate inhibitory activity. Macluraxanthone (162) which was obtained from the root of Maclura pomifera (Moraceae) was found to elicit non-competitive AChE inhibition (IC50 = 8.47 µM) [61]. Furthermore, docking studies yielded results supporting in vitro results. Triptexanthoside C (163) which was isolated from the methanolic extract of Gentianella amarella ssp. acuta (Gentianaceae) was observed to elicit AChE inhibition with an IC50 = 13.8 µM [62].
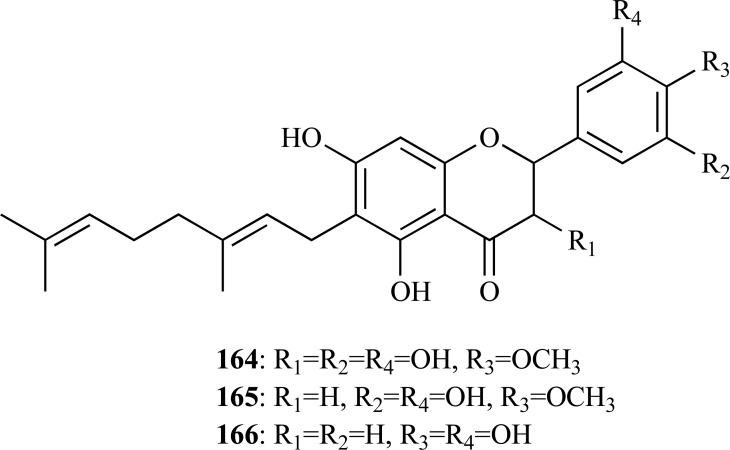
The methanolic extract of Paulownia tormentosa fruits, with a potent inhibitory activity against AChE, was subjected to bioactivity-guided fractionation which allowed the identification of some geranylated flavonoids, such as cholinesterase inhibitors, of which the most active resulted to be 6-geranyl-3,3’,5,5’,7-pentahydroxy-4’-methoxyflavane (164), 6-geranyl-3’,5,5’,7-tetrahydroxy-4’-methoxyflavanone (165) and diplacone (166), which were observed to show mixed-type inhibition of human AChE with IC50= 15.6, 22.9 and 7.2 µM, respectively [63]. In addition, the fact that these compounds were also observed to elicit significant BChE inhibition makes them interesting as potential dual inhibitors.
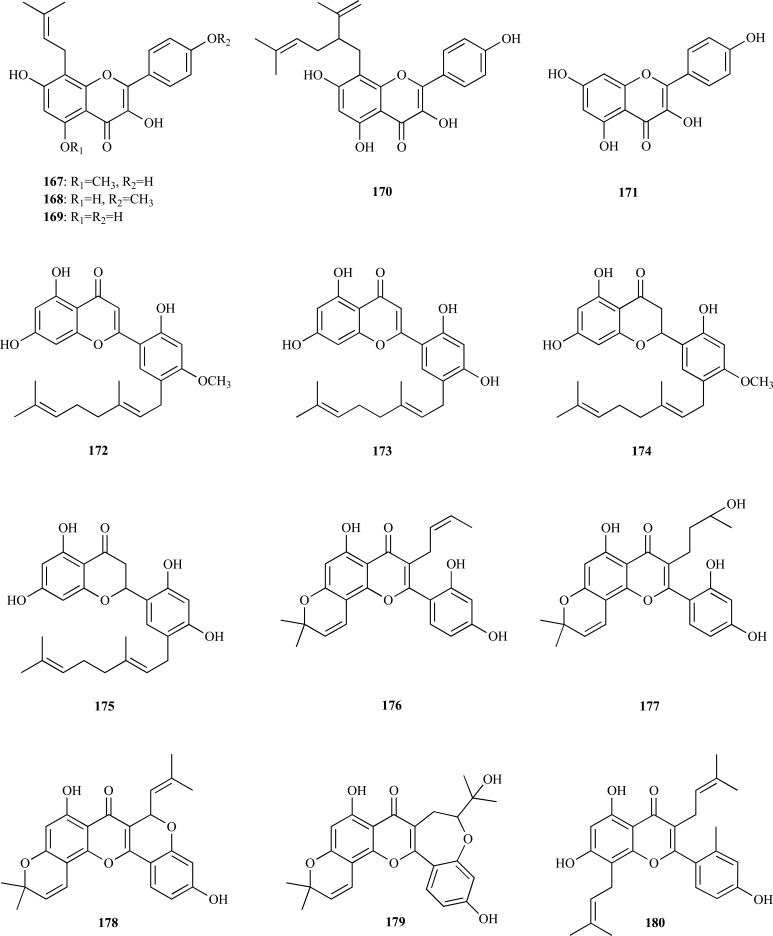
The flavonols present in Sophora flavescens (Fabaceae) were studied for several biological activities relevant for AD. Sophoflavescenol (167), icaritin (168), demethylanhydro-icaritin (169), 8-C-lavandurylkaempferol (170) and kaempferol (171) were all found to be good AChE inhibitors, with IC50 values of 8.37, 6.47, 6.67, 5.16 and 3.31 µM, respectively [64]. Compounds 167-171 were also found to elicit significant BChE and BACE1 inhibition.
The methanol extract from roots of Morus lhou (Moraceae), a polyphenol-rich plant, was found to yield nine flavonoids (172 - 180) of which eight showed AChE inhibition [65]. A new flavone, 5’-geranyl-4’-methoxy-5,7,2’-trihydroxyflavone (172), was identified as the most potent inhibitor (IC50 = 10.95 µM). 5’-geranyl-5,7,2’,4’-tetrahydroxyflavone (173), kuwanon U (174), kuwanon E (175), morusin (176), cyclomorusin (178), neocyclomorusin (179) and kuwanon C (180) were all observed to be moderate AChE inhibitors (IC50 = 16.21 - 36.4 µM) and morusinol (177) was observed to be weakly active (IC50 = 173.49 µM). C-3 prenylated flavones 176, 178, 179 and 180 were found to be noncompetitive inhibitors whereas those unsubstituted at C-3 172-175 were mixed inhibitors. Flavonoids 172 - 180 were also found to inhibit BChE [65].
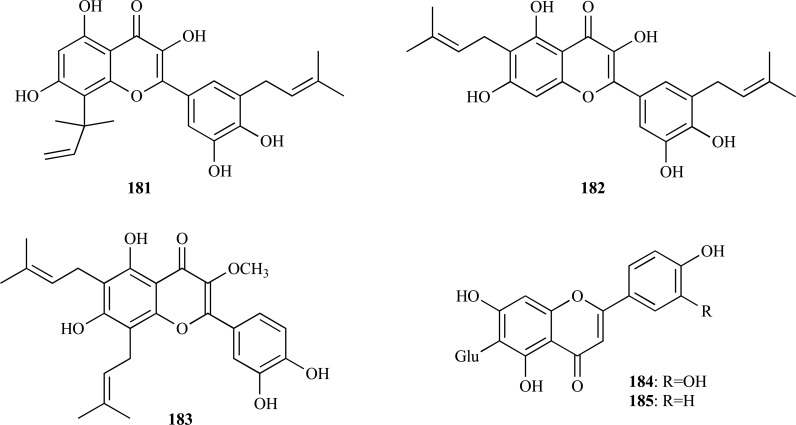
On the other hand, three potent AChEi were obtained from Broussonetia papyrifera, another plant belonging to the Moraceae family. From the ethanolic extract of the roots of B. papyrifera which was found to elicit cholinesterase inhibitory activity, prenylated flavonols 181 – 183 were isolated and characterized [66]. 8-(1,1-dimethylallyl)-5’-(3-methylbut-2-enyl)-3’,4’,5,7-tetrahydroxyflavonol (181), papyriflavonol (182) and broussoflavonol (183) were observed to inhibit human erythrocyte AChE with IC50 values of 0.82, 3.1 and 2.7 µM, respectively. Compound 181, the most potent, acted as a time-dependent, slow reversible inhibitor.
Isoorientin (184) and isovitexin (185) were identified as the compounds responsible for the AChE inhibition observed in the extracts from flowers and rhizomes of Iris pseudopumila (Iridaceae) from Italy [67]. Compound 184 was observed to be the highest inhibitor with an IC50 = 26.8 µM while 185, lacking the 3’-hydroxy group in ring B, was observed to show an IC50 value of 36.4 µM. Both compounds were also found to have the ability of significantly inhibiting BChE.
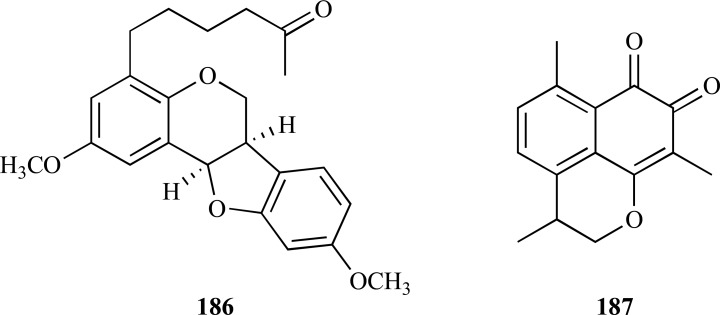
On the other hand, a pterocarpan with moderate AChE inhibition was isolated from the polar extract of Zygophyllum eurypterum (Zygophyllaceae) collected in Pakistan. Atricarpan D [(-)-2,9-dimethoxy-4-(5-oxohexyl)pterocarpan] (186) was observed to inhibit AChE with an IC50 = 20.5 µM [68]. Interestingly, three other pterocarpans with similar structure were obtained along with atricarpan D but they were found to be inactive against AChE. Nevertheless, the four pterocarpans were all found to be BChE inhibitors.
A study conducted on AChE and BChE inhibitory activity of coumarins and naphtoquinones obtained from Mansonia gagei (Sterculiaceae) proposed a novel class of cholinesterase inhibitor, mansonones or 1,2-naphtoquinones [69]. The level of cholinesterase inhibition observed in this study seemed to correlate to the presence of a fused pyran ring and a substituent at C-6 being present in the molecule. Mansonone E (187) was observed to be the most active AChE (IC50 = 23.5 µM) and BChE inhibitor.
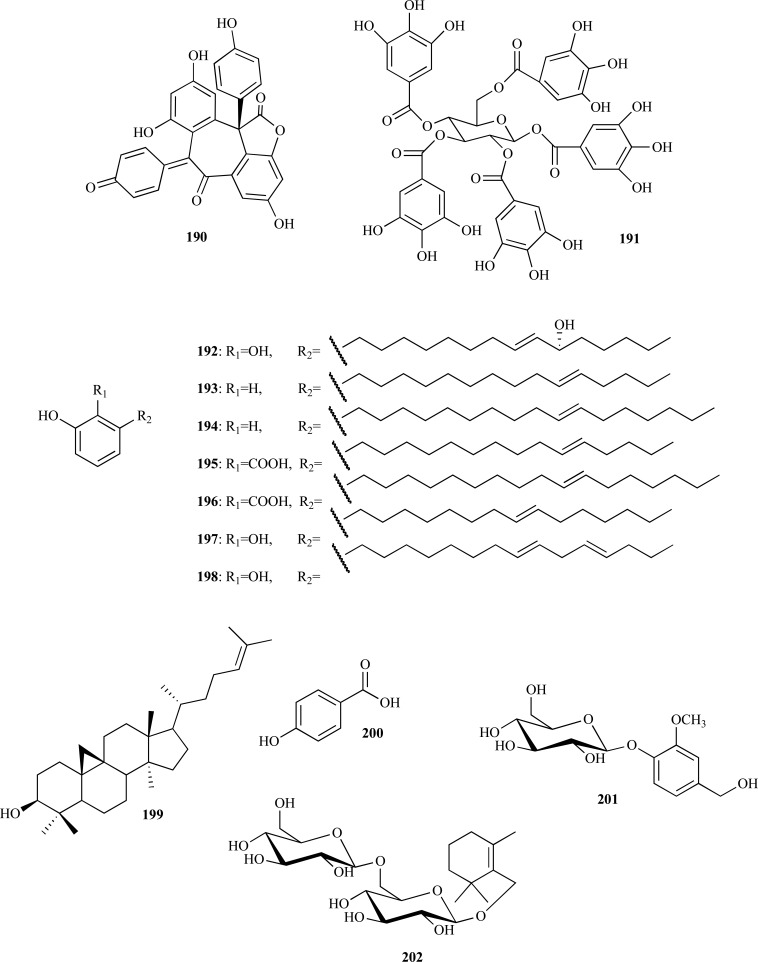
In several studies published during the period covered in the present review various phenolic compounds with different structural characteristics were reported as AChEi. Some of them are structurally simple such as gallic acid (188, IC50 = 5.85 µM) and ellagic acid (189, IC50 = 45.63 µM) [70]. Hopeahainol A (190), which was identified as a new compound isolated from Hopea hainensis, was observed to elicit a notable AChE inhibition (IC50 = 4.33 µM) with respect to huperzine A (IC50 = 1.6 µM), as a reversible mixed-type inhibitor [71].
The bioassay-guided fractionation of the extract from Terminalia chebula (Combretaceae) fruits allowed the isolation of 1,2,3,4,6-penta-O-galloyl-β-D-glucose (191) which demonstrated to be a significant AChE inhibitor (IC50 = 29.9 µM) [72]. This gallotanin which has been also isolated from other different sources and which is known by its diverse biological activities, was observed to exert good BChE inhibition and potent antioxidant activity (FRAP assay) in this study.
The bioassay-guided extraction of the stem bark of Knema laurina (Myristicaceae) yielded two active fractions (dichloromethane and hexane) which were subjected to chromatographic separation. That latter yielded five alkenyl phenol and salicylic acid derivatives 192 - 196, of which 192 and 193 were new compounds [73]. Compounds 192, 195 and 196, all having salicylic acid moiety, were observed to strongly inhibit AChE with an IC50 = 3.182, 2.172 and 0.573 µM, respectively. Compounds 193 and 194, with no carboxyl moiety, were observed to be good AChE inhibitors (IC50 = 17.224 and 13.114 µM, respectively). These findings suggest that the acidic group is key to good AChE inhibition. It was also observed that anti-AChE activity dramatically decreased when the acidic and the phenolic hydroxy group were methylated. Two catechol alkenyls were isolated from the fruits of Semecarpus anacardium (Anacardiaceae), a species used in Ayurvedic medicine for retarding and treatment of memory loss [74]. Compounds 197 and 198 were identified as active components of the dichloromethane extract through a fractionation guided by the detection of AChE inhibition. Microplate assay revealed that these catechol alkenyls are moderate and weak selective AChEi. Compound 197, with a double bond in the aliphatic chain, was identified as a stronger inhibitor (IC50 = 39.7 µM) with respect to compound 198, with two double bonds in the aliphatic chain (IC50 = 108 µM).
On the other hand, four structurally diverse AChEi were isolated from the polar extract of Nelumbo nucifera (Nelumbonaceae) stamens [75]. Cycloartenol (199), p-hydroxybenzoic acid (200), vanilloloside (201) and nuciferoside (202) were found to elicit good and noncompetitive inhibition against AChE with an IC50 = 11.89, 20.07, 4.55 and 3.2 µM, respectively. In the same study, compounds 199, 200 and 202 were observed to exert moderate BChE inhibition and compounds 199 - 202 were found to show no inhibition against BACE1.
PLANT EXTRACTS, FRACTIONS AND ESSENTIAL OILS WITH AChE INHIBITORY ACTIVITY
Table 1 summarizes the studies published from 2006 to 2012 on plant extracts, fractions and essential oils that have been found to be good AChE inhibitors (IC50 < 500 µg/mL). Those plants included in other recent reviews were omitted [76, 77]. Extracts and fractions under further phytochemical studies that led to the discovery of AChE inhibitors were also omitted. Whenever possible, reference is made to the part of the plant used in each study reported. AChE inhibitory activity is reported in the same way as it was reported by authors and IC50 values were chosen instead of inhibition percentages when both were available.
Table 1.
Plant Extracts, Fractions and Essential Oils with AChE Inhibitory Activity
| Family and Botanical Name | Type of Extract (Solvent) | Plant's Parts | AChE Inhibition (%) | IC50 | Refs. |
|---|---|---|---|---|---|
| Acanthaceae | |||||
| Andrographis paniculata | H2O:EtOH | Aerial | 222.41 µg/ml | [78] | |
| Amaranthaceae | |||||
| Salsola oppositifolia | Alkaloids | Aerial | 70.0 µg/ml | [79] | |
| Salsola soda | Alkaloids | Aerial | 64.1 µg/ml | [79] | |
| Salsola tragus | Alkaloids | Aerial | 30.2 µg/ml | [79] | |
| Amaryllidaceae | |||||
| Crinum jagus | MeOH | Leaf | 74.25 (42 µg/ml) | [80] | |
| Crinum moorei | 50% MeOH PE DCM EtOH | Bulb | 21.5 µg/ml 18.9 µg/ml 2.9 µg/ml 22.5 µg/ml | [81] | |
| Nerine undulata | Alkaloids | Bulb | 14.3 µg/ml a | [82] | |
| Scadoxus multiflorus | Alkaloids | Bulb | 313.5 µg/mla | [82] | |
| Sprekelia formosissima | Alkaloids | Bulb | 209.7 µg/mla | [82] | |
| Zephyranthes grandiflora | Alkaloids | Bulb | 39.2 µg/mla | [83] | |
| Anacardiaceae | |||||
| Harpephyllum caffrum | DCM MeOH | Leaf Stem bark Leaf | 0.17 mg/ml 0.02 mg/ml 0.12 mg/ml | [84] | |
| Pistacia atlantica | H2O | Leaf | 0.87 µg/ml | [85] | |
| Pistacia lentiscos | H2O | Leaf | 13.67 µg/ml | [85] | |
| Sclerocarya birrea | DCM MeOH | Young stem Leaf Operculum Young stem | 0.15 mg/ml 0.10 mg/ml 0.35 mg/ml 0.47 mg/ml | [84] | |
| Spondias mombin | MeOH | Root bark | 64.77 (42 µg/ml) | [80] | |
| Apiaceae | |||||
| Centella asiatica | H2O:EtOH | Whole plant | 106.55 µg/ml | [78] | |
| Apocynaceae | |||||
| Geissospermum vellosii | Alkaloids | Stem bark | 2.9 µg/ml | [86] | |
| Araceae | |||||
| Colocasia antiquorum | 50% MeOH PE DCM | Tuber | 7.9 µg/ml 6.4 µg/ml 168.1 µg/ml | [81] | |
| Pinellia ternata | Alkaloids | Tuber | 56.2 µg/ml | [87] | |
| Arecaceae | |||||
| Phoenix dactylifera | Hexane | Seed | 52.96 (300 µg/ml) | [88] | |
| Asparagaceae | |||||
| Leopoldia comosa | Hexane | Bulb | 104.9 µg/ml | [89] | |
| Asphodelaceae | |||||
| Aloe ferox | 50% MeOH PE DCM | Leaf | 84.0 µg/ml 37.7 µg/ml 62.6 µg/ml | [81] | |
| Asteraceae | |||||
| Achyrocline tomentosa | Organic | Aerial | 0.4847 mg/ml | [90] | |
| Arnica chamissonis ssp. foliosa | MeOH Hexane | Flower | 43 µg/ml 29 µg/ml | [91] | |
| Chromolaena tequendamensis | MeOH | Whole plant | 359.36 mg/l | [92] | |
| Eupatorium viscidum | Organic | Aerial | 0.4792 mg/ml | [90] | |
| Pulicaria stephanocarpa | CHCl3 | Leaf | 61.43 (0.2 mg/ml) | [93] | |
| Schistocarpha sinforosi | MeOH | Whole plant | 145.31 mg/l | [92] | |
| Trichocline reptans | Organic | Aerial | 0.1118 mg/ml | [90] | |
| Berberidaceae | |||||
| Berberis darwinii | MeOH | Stem bark | 1.23 µg/ml | [94] | |
| Boraginaceae | |||||
| Onosma bracteata | MeOH | Leaf | 59.73 (250 µg/ml) | [95] | |
| Buddlejaceae | |||||
| Buddleja salviifolia | DCM:MeOH (1:1) | Whole plant | 0.05 mg/ml | [96] | |
| Burseraceae | |||||
| Boswellia socotranao | CHCl3 | Resin | 71.21 (0.2 mg/ml) | [93] | |
| Cistaceae | |||||
| Cistus laurifolius | EtOH | Leaf | 80.07 (200 µg/ml) | [97] | |
| Combretaceae | |||||
| Terminalia bellirica | MeOH | Fruit | 14.37 µg/ml | [70] | |
| Convolvulaceae | |||||
| Evolvulus alsinoides | H2O:EtOH | Whole plant | 141.76 µg/ml | [78] | |
| Ipomoea asarifolia | MeOH | Leaf | 0.12 µg/ml | [98] | |
| Crassulaceae | |||||
| Kalanchoe brasiliensis | EtOAc | Leaf | 0.16 mg/ml | [98] | |
| Cucurbitaceae | |||||
| Eureiandra balfourii | MeOH | Tuber | 58.61 (0.2 mg/ml) | [93] | |
| Cupressaceae | |||||
| Juniperus phoenicea | EtOH | Leaf | 53.44 (400 µg/ml) | [99] | |
| Juniperus turbinata | Phenolic | Leaf | 83.84 (400 µg/ml) | [99] | |
| Ericaceae | |||||
| Rhododendron yedoense var. poukhanense | 80% MeOH | Bark | 169.01 µg/ml | [100] | |
| Eucommiaceae | |||||
| Eucommia ulmoides | H2O | Bark | 172 µg/ml | [101] | |
| Euphorbiaceae | |||||
| Alchornia laxiflora | MeOH | Stem bark | 41.12 (42 µg/ml) | [80] | |
| Cephalocroton socotranus | CHCl3 | Bark | 51.1 (0.2 mg/ml) | [93] | |
| Jatropha curcas | MeOH | Leaf | 0.25 mg/ml | [98] | |
| Jatropha gossypiifolia | MeOH | Leaf | 0.05 mg/ml | [98] | |
| Fabaceae | |||||
| Acacia nilotica | H2O | Root | 0.079 mg/mlb | [102] | |
| Acacia raddiana | H2O | Bark | 33.91 µg/ml | [85] | |
| Cassia obtusifolia | EtOH | Seed | 81.6 µg/ml c | [103] | |
| Chamaecrista mimosoides | DCM:MeOH (1:1) H2O | Root | 0.03 mg/ml 0.35 mg/ml | [96] | |
| Genista tenera | EtOAc | Aerial | 77.0 (70 µg/ml) | [105] | |
| Peltophorum pterocarpum | MeOH | Leaf Stem bark | 49.5 (42 µg/ml) 68.85 (42 µg/ml) | [80] | |
| Schotia brachypetala | DCM:MeOH (1:1) H2O | Bark | 0.27 mg/ml 0.49 mg/ml | [96] | |
| Senna alata | EtOAc | Leaf | 0.08 mg/ml | [98] | |
| Spatholobus suberectus | H2O EtOH | Whole plant | 85 µg/ml 9 µg/ml | [104] | |
| Trigonella foenum-graecum | EtOAc Alkaloids | Seed | 53.00 µg/ml 9.23 µg/ml | [106] | |
| Gobulariaceae | |||||
| Globularia alypum | H2O | Root | 16.67 µg/ml | [85] | |
| Guttiferaceae | |||||
| Callophyllum inophyllurn | MeOH | Root bark | 56.52 (42 µg/ml) | [80] | |
| Hypericaceae | |||||
| Hypericum perforatum | MeOH | Whole plant | 178 µg/ml | [91] | |
| Illiciaceae | |||||
| Illicium verum | H2O:EtOH Butanol EtOAc CHCl3 Oil | Fruit | 58.67 µg/ml 44.94 µg/ml 83.75 µg/ml 103.03 µg/ml 39.89 µg/ml | [107] | |
| Lamiaceae | |||||
| Cyclotrichium niveum | EtOAc DCM | Whole plant | 83.11 (250 µg/ml) 70.82 (250 µg/ml) | [108] | |
| Hyssopus officinials | Hexane | Whole plant | 55.0 (400 µg/ml) | [91] | |
| Lavandula viridis | MeOH | Aerial | 244.55 µg/ml | [109] | |
| Marrubium vulgare | Acetone | Aerial | 62.70 (25 µg/ml) | [110] | |
| Origanum ehrenbergii | Essential oil | Aerial | 0.3 µg/ml | [111] | |
| Origanum majorana | Essential oil | Leaf | 36.40 µg/ml | [112] | |
| Origanum syriacum | Essential oil | Aerial | 1.7 µg/ml | [111] | |
| Pycnostachys reticulata | 50% MeOH EtOH | Leaf | 28.8 µg/ml 8.8 µg/ml | [81] | |
| Salvia chionantha | Essential oil | Aerial | 56.7 (500 µg/ml) | [113] | |
| Salvia fruticosa | DCM | Whole plant | 51.07 (100 µg/ml) | [114] | |
| Salvia leriifolia | Essential oil | Aerial | 0.32 µl/ml | [115] | |
| Salvia miltiorrhiza | H2O EtOH | Root | 50 µg/ml 5 µg/ml | [104] | |
| Teucrium royleanum | MeOH | Whole plant | 52.4 (40 µg/0.2ml) | [116] | |
| Menispermaceae | |||||
| Stephania pierrei | EtOH | Tuber | 5.68 µg/ml | [117] | |
| Tinospora cordifolia | MeOH | Stem | 38.36 µg/ml | [118] | |
| Moraceae | |||||
| Dorstenia gigas | CHCl3 | Leaf | 65.12 (0.2 mg/ml) | [93] | |
| Ficus religiosa | MeOH | Stem bark | 73.69 µg/ml | [118] | |
| Myristicaceae | |||||
| Myristica fragrans | H2O:EtOH | Seed | 133.28 µg/ml | [78] | |
| Embelia ribes | MeOH | Root | 23.04 µg/ml | [118] | |
| Orchidaceae | |||||
| Orchis mascula | MeOH | Root | 56.99 (250 µg/ml) | [119] | |
| Paeoniaceae | |||||
| Paeonia lactiflora | H2O EtOH | Root | 20 µg/ml 8 µg/ml | [104] | |
| Paeonia veitchii | H2O EtOH | Root | 14 µg/ml 45 µg/ml | [104] | |
| Papaveraceae | |||||
| Corydalis intermedia | MeOH H2O | Whole plant Tuber Whole plant Tuber | 84 (100 µg/ml) 97 (100 µg/ml) 57 (100 µg/ml) 78 (100 µg/ml) | [120] | |
| Papaveraceae | |||||
| Corydalis solida ssp. laxa | MeOH H2O | Whole plant Tuber Whole plant Tuber | 89 (100 µg/ml) 96 (100 µg/ml) 78 (100 µg/ml) 85 (100 µg/ml) | [120] | |
| Corydalis solida ssp. slivenensis | MeOH H2O | Whole plant Tuber Whole plant Tuber | 82 (100 µg/ml) 97 (100 µg/ml) 48 (100 µg/ml) 87 (100 µg/ml) | [120] | |
| Phyllantaceae | |||||
| Emblica officinalis | MeOH | Fruit | 29.26 µg/ml | [70] | |
| Pinaceae | |||||
| Pinus halepensis | EtOH Essential oil | Needle Twig | 60.15 (200 µg/ml) 83.91 (200 µg/ml) | [121] | |
| Pinus heldreichii subsp. leucodermis | Essential oil | Needle | 51.1 µg/ml | [122] | |
| Pinus nigra subsp. nigra | Essential oil | Needle | 94.4 µg/ml | [122] | |
| Pinus nigra subsp. calabrica | Essential oil | Needle | 101.5 µg/ml | [122] | |
| Pinus pinaster | Pycnogenol | Bark | 63.33 (200 µg/ml) | [121] | |
| Piperaceae | |||||
| Piper nigrum | EtOH | Fruit | 30.67 µg/ml | [117] | |
| Poaceae | |||||
| Cymbopogon jawarancusa | MeOH | Whole plant | 72.36 (250 µg/ml) | [95] | |
| Cymbopogon schoenanthus | Essential oil | Fresh leaf (mountain reg./ desert reg.) Dried leaf (mountain reg./ desert reg.) Dried root (mountain reg./ desert reg.) | 0.26 / 0.67 mg/ml 0.44 / 0.52 mg/ml 0.27 / 0.32 mg/ml | [123] | |
| Cymbopogon schoenanthus | Hexane DCM EtOAc MeOH H2O | Shoot (mountain reg./ desert reg.) | 0.50 / 0.54 mg/ml 0.57 / 0.30 mg/ml 0.23 / 0.30 mg/ml 0.23 / 0.25 mg/ml 0.46 / 0.04 mg/ml | [124] | |
| Polygonaceae | |||||
| Fallopia multiflora | H2O EtOH | Root | 13 µg/ml 65 µg/ml | [104] | |
| Rheum palmatum | H2O EtOH | Root and Rizhome | 32 µg/ml 18 µg/ml | [104] | |
| Ruprechtia apetala | EtOH | Aerial | 0.0779 mg/ml | [90] | |
| Portulacaceae | |||||
| Portulaca oleracea | Alkaloids | Upper part | 29.4 µg/ml | [87] | |
| Rhamnaceae | |||||
| Rhamnus prinoides | H2O | Root | 0.201 mg/mlb | [102] | |
| Rosaceae | |||||
| Leucosidea sericea | PE DCM MeOH PE | Leaf Stem | 0.16 mg/ml 0.14 mg/ml 0.24 mg/ml 0.26 mg/ml | [125] | |
| Rubiaceae | |||||
| Galium odoratum | Hexane | Whole plant | 53.1 (400 µg/ml) | [91] | |
| Morinda citrifolia | EtOH CHCl3 | Fruit | 138.4 µg/ml 78.11 µg/ml | [126] | |
| Morinda lucida | MeOH | Leaf | 40.15 (42 µg/ml) | [80] | |
| Rutaceae | |||||
| Citrus aurantifolia | Essential oil | Leaf | 139 µg/ml | [127] | |
| Citrus medica | Essential oil | Peel | 171.3 µg/ml | [128] | |
| Ruta graveolens | MeOH Hexane | Whole plant | 59.1 (100 µg/ml) | 34 µg/ml | [91] |
| Zanthoxylum coco | Organic | Aerial | 0.1579 mg/ml | [90] | |
| Solanaceae | |||||
| Solanum leucocarpum | MeOH | Whole plant | 204.59 mg/l | [92] | |
| Withania somnifera | MeOH | Root | 33.38 µg/ml | [118] | |
| Witheringia coccoloboides | MeOH | Whole plant | 220.68 mg/l | [92] | |
| Valerianaceae | |||||
| Nardostachys jatamansi | H2O:EtOH MeOH | Rhizome | 130.11 µg/ml 47.21 µg/ml | [78] [118] | |
| Zingiberaceae | |||||
| Kaempfera parviflora | EtOH | Rhizome | 20.64 µg/ml | [117] | |
DCM: dichloromethane; MeOH: methanol; EtOH: ethanol; PE: petroleum ether; EtOAc: ethyl acetate
Human blood AChE.
Bovine erythrocyte AChE.
Mouse brain homogenized.
Fig. (1).
Fig. (2).
Fig. (3).
Fig. (4).
Fig. (5).
Fig. (6).
Fig. (7).
Fig. (8).
Fig. (9).
Fig. (10).
Fig. (11).
Fig. (12).
Fig. (13).
Fig. (14).
Fig. (15).
Fig. (16).
Fig. (17).
Fig. (18).
Fig. (19).
Fig. (20).
Fig. (21).
Fig. (22).
Fig. (23).
Fig. (24).
Fig. (25).
ACKNOWLEDGEMENTS
CONICET, CIC, UNS and ANPCYT.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Chopra K, Misra S, Kuhad A. Current perspectives on pharmacotherapy of Alzheimer’s. Expert. Opin. Pharmacother. . 2011;12:335–350. doi: 10.1517/14656566.2011.520702. [DOI] [PubMed] [Google Scholar]
- 2.Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 3.Lopez S, Bastida J, Viladomat F, Codina C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. . 2002;71: 2521–2529. doi: 10.1016/s0024-3205(02)02034-9. [DOI] [PubMed] [Google Scholar]
- 4.Rhee IK, van de Meent M, Ingkaninan K, Verpoorte R. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combinatioin with bioactivity staining. J. Chromatogr. A . 2001;915: 217–223. doi: 10.1016/s0021-9673(01)00624-0. [DOI] [PubMed] [Google Scholar]
- 5.Marston A, Kissling J, Hostettmann K. A rapid TLC bioautographic method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem. Anal. . 2002;13:51–54. doi: 10.1002/pca.623. [DOI] [PubMed] [Google Scholar]
- 6.Di Giovanni S, Borloz A, Urbain A, Marston A, Hostettmann K, Carrupt PA, Reist M. In vitro screening assays to identify natural or synthetic acetylcholinesterase inhibitors: thin layer chromatography versus microplate methods. Eur. J. Pharm. Sci. . 2008;33:109–119. doi: 10.1016/j.ejps.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Houghton PJ, Ren Y, Howes MJ. Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. . 2006; 23:181–199. doi: 10.1039/b508966m. [DOI] [PubMed] [Google Scholar]
- 8.Williams P, Sorribas A, Howes MJ. Natural Products as a source of Alzheimer’s drugs leads. Nat. Prod. Rep. . 2011; 28: 48–77. doi: 10.1039/c0np00027b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukherjee PK, Kumar V, Mal M, Houghton PJ. Acetylcholinesterase inhibitors from plants. Phytomedicine . 2007;14: 289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Orhan G, Orhan I, Subutay-Oztekin N, Ak F, Sener B. Contemporary anticholinesterase pharmaceuticals of natural origin and their synthetic analogues for the treatment of Alzheimer's disease. Recent. Pat. CNS Drug. Discov. . 2009; 4: 43–51. doi: 10.2174/157488909787002582. [DOI] [PubMed] [Google Scholar]
- 11.Rahman AU, Khalid A, Sultana N, Ghayur MN, Mesaik MA, Khan MR, Gilani AH, Choudhary MI. New natural cholinesterase inhibiting and calcium channel blocking quinoline alkaloids. J. Enzyme Inhib. Med. Chem. . 2006; 21:703–710. doi: 10.1080/14756360600889708. [DOI] [PubMed] [Google Scholar]
- 12.Cardoso-Lopes EM, Maier A, da Silva MR, Regasini LO, Simote SY, Lopes NP, Pirani JR, da Silva Bolzani V, Marx Young MC. Alkaloids from stems of Esenbeckia leiocarpa Engl.(Rutaceae) as potential treatment for Alzheimer Disease. Molecules . 2010;15:9205–9213. doi: 10.3390/molecules15129205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Z, Zhang D, Ren J, Yang M. Skimmianine, a furoquinoline alkaloid from Zanthoxylum nitidum as a potential acetylcholinesterase inhibitor. Med. Chem. Res. . 2012; 21:722–725. [Google Scholar]
- 14.Mukherjee PK, Mukherjee D, Maji AK, Rai S, Heinrich M. The sacred lotus (Nelumbo nucifera) - phytochemical and therapeutic profile. J. Pharm. Pharmacol. . 2009;61: 407–422. doi: 10.1211/jpp/61.04.0001. [DOI] [PubMed] [Google Scholar]
- 15.Yang Z, Zhang X, Du J, Ma ZJ, Guo F, Li S, Yao XJ. An aporphine alkaloid from Nelumbo nucifera as an acetylcholinesterase inhibitor and the primary investigation for structure-activity correlations. Nat. Prod. Res. . 2012; 26:387–392. doi: 10.1080/14786419.2010.487188. [DOI] [PubMed] [Google Scholar]
- 16.Mollataghi A, Coudiere E, Hadi AH, Mukhtar MR, Awang K, Litaudon M, Ata A. Anti-acetylcholinesterase, anti-a-glucosidase, anti-leishmanial and anti-fungal activities of chemical constituents of Beilschmiedia species. Fitoterapia . 2012;83: 298–302. doi: 10.1016/j.fitote.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Hung TM, Na M, Dat NT, Ngoc TM, Youn U, Kim HJ, Min BS, Lee J, Bae K. Cholinesterase inhibitory and anti-amnesic activity of alkaloids from Corydalis turtschaninovii. J. Ethnopharmacol. . 2008;119:74–80. doi: 10.1016/j.jep.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 18.Hung TM, Ngoc TM, Youn UJ, Min BS, Na M, Thuong PT, Bae K. Anti-amnesic activity of pseudocoptisine from Corydalis tuber. Biol. Pharm. Bull. . 2008;31:159–162. doi: 10.1248/bpb.31.159. [DOI] [PubMed] [Google Scholar]
- 19.Jung HA, Min BS, Yokozawa T, Lee JH, Kim YS, Choi JS. Anti-Alzheimer and antioxidant activities of Coptidis Rhizoma alkaloids. Biol. Pharm. Bull. . 2009;32:1433–1438. doi: 10.1248/bpb.32.1433. [DOI] [PubMed] [Google Scholar]
- 20.Chlebek J, Macáková K, Cahlíkovi L, Kurfürst M, Kunes J, Opletal L. Acetylcholinesterase and butyrylcholinesterase inhibitory compounds from Corydalis cava (Fumariaceae) Nat. Prod. Commun. . 2011;6: 607–610. [PubMed] [Google Scholar]
- 21.Ingkaninan K, Phengpa P, Yuenyongsawad S, Khorana N. Acetylcholinesterase inhibitors from Stephania venosa tuber. J. Pharm. Pharmacol. . 2006;58: 695–700. doi: 10.1211/jpp.58.5.0015. [DOI] [PubMed] [Google Scholar]
- 22.Cho KM, Yoo ID, Kim WG. 8-Hydroxydihydrochelerythrine and 8-Hydroxydihydrosanguinarine with a potent acetylcholinesterase inhibitory activity from Chelidonium majus L. Biol. Pharm. Bull. . 2006; 29: 2317–2320. doi: 10.1248/bpb.29.2317. [DOI] [PubMed] [Google Scholar]
- 23.Rollinger JM, Schuster D, Baier E, Ellmerer EP, Langer T, Stuppner H. Taspine bioactivity-guided isolation and molecular ligand-target insight of a potent acetylcholinesterase inhibitor from Magnolia x soulangiana. J. Nat. Prod. . 2006; 69: 1341–1346. doi: 10.1021/np060268p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira DM, Ferreres F, Oliveira JM, Gaspar L, Faria J, Valentão P, Sottomayor M, Andrade PB. Pharmacological effects of Catharanthus roseus root alkaloids in acetylcholinesterase inhibition and cholinergic neurotransmission. Phytomedicine . 2010; 17: 646–652. doi: 10.1016/j.phymed.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Zhan ZJ, Yu Q, Wang ZL, Shan WG. Indole alkaloids from Ervatamia hainanensis with potent acetylcholinesterase inhibition activities. Bioorg. Med. Chem. Lett. . 2010; 20: 6185–6187. doi: 10.1016/j.bmcl.2010.08.123. [DOI] [PubMed] [Google Scholar]
- 26.Andrade MT, Lima JA, Pinto AC, Rezende CM, Carvalho MP, Epifanio RA. Indole alkaloids from Tabernaemontana australis (Muell.Arg) Miers that inhibit acetylcholinesterase enzyme. Bioorg. Med. Chem. . 2005; 13: 4092–4095. doi: 10.1016/j.bmc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 27.Ingkaninan K, Changwijit K, Suwanborirux K. Vobasinyl-iboga bisindole alkaloids, potent acetylcholinesterase inhibitors from Tabernaemontana divaricata root. J. Pharm. Pharmacol. . 2006;58:847–852. doi: 10.1211/jpp.58.6.0015. [DOI] [PubMed] [Google Scholar]
- 28.Yang Z, Duan DZ, Du J, Yang MJ, Li S, Yao XJ. Geissoschizine methyl ether, a corynanthean-type indole alkaloid from Uncaria rhynchophylla as a potential acetylcholinesterase inhibitor. Nat. Prod. Res. . 2012; 26: 22–28. doi: 10.1080/14786419.2010.529811. [DOI] [PubMed] [Google Scholar]
- 29.Seidl C, Correia BL, Stinghen AE, Santos CA. Acetylcholinesterase inhibitory activity of uleine from Himatanthus lancifolius. Z. Naturforsch. C. . 2010; 65: 440–444. doi: 10.1515/znc-2010-7-804. [DOI] [PubMed] [Google Scholar]
- 30.Jin Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. . 2007; 24:886–905. doi: 10.1039/b502163b. [DOI] [PubMed] [Google Scholar]
- 31.de Andrade JP, Berkov S, Viladomat F, Codina C, Zuanazzi JA, Bastida J. Alkaloids from Hippeastrum papillo. Molecules . 2011; 16:7097–7104. doi: 10.3390/molecules16087097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berkov S, Codina C, Viladomat F, Bastida J. N-Alkylated galanthamine derivatives: Potent acetylcholinesterase inhibitors from Leucojum aestivum. Bioorg. Med. Chem. Lett. . 2008; 18: 2263–2266. doi: 10.1016/j.bmcl.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Sarikaya BB, Kaya GI, Onur MA, Viladomat F, Codina C, Bastida J, Somer NU. Alkaloids from Galanthus rizehensis. Phytochem. Lett. . 2012;5:367–370. [Google Scholar]
- 34.Rijn RM, Rhee IK, Verpoorte R. Isolation of acetylcholinesterase inhibitory alkaloids from Nerine bowdenii. Nat. Prod. Res. . 2010; 24: 222–225. doi: 10.1080/14786410802263758. [DOI] [PubMed] [Google Scholar]
- 35.Mulholland DA, Crouch N, Decker B, Smith M T. The isolation of the Amaryllidaceae alkaloid crinamine from Dioscorea dregeana. Biochem. Syst. Ecol. . 2002;30: 183–185. [Google Scholar]
- 36.Wang YH, Zhang ZK, Yang FM, Sun QY, He HP, Di YT, Mu SZ, Lu Y, Chang Y, Zheng QT, Ding M, Dong JH, Hao XJ. Benzylphenethylamine Alkaloids from Hosta plantaginea with Inhibitory Activity against Tobacco Mosaic Virus and Acetylcholinesterase. J. Nat. Prod. . 2007;70: 1458–1461. doi: 10.1021/np0702077. [DOI] [PubMed] [Google Scholar]
- 37.Ma X, Gang DR. The Lycopodium alkaloids. Nat. Prod. Rep. . 2004; 21:752–772. doi: 10.1039/b409720n. [DOI] [PubMed] [Google Scholar]
- 38.Katakawa K, Nozoe A, Kogure N, Kitajima M, Hosokawa M, Takayama H. Fawcettimine-related alkaloids from Lycopodium serratum. J. Nat. Prod. . 2007;70: 1024–1028. doi: 10.1021/np0700568. [DOI] [PubMed] [Google Scholar]
- 39.Takayama H, Katakawa K, Kitajima M, Yamaguchi K, Aimi N. Ten new Lycopodium alkaloids having the lycopodane skeleton isolated from Lycopodium serratum Thunb. Chem. Pharm. Bull. (Tokyo) . 2003;51: 1163–1169. doi: 10.1248/cpb.51.1163. [DOI] [PubMed] [Google Scholar]
- 40.Choo CY, Hirasawa Y, Karimata C, Koyama K, Sekiguchi M, Kobayashi J, Morita H. Carinatumins A–C, new alkaloids from Lycopodium carinatum inhibiting acetylcholinesterase. Bioorg. Med. Chem. . 2007; 15: 1703–1707. doi: 10.1016/j.bmc.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Hirasawa Y, Kato E, Kobayashi J, Kawahara N, Goda Y, Shiro M, Morita H. Lycoparins A-C, new alkaloids from Licopodium casuarinoides inhibiting acetylcholinesterase. Bioorg. Med. Chem. . 2008; 16: 6167–6171. doi: 10.1016/j.bmc.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 42.Howes M-JR, Houghton PJ. Acetylcholinesterase inhibitors of natural origin. Int. J. Biomed. Pharm. Sci. . 2009;3: 67–86. [Google Scholar]
- 43.Devkota KP, Lenta BN, Fokou PA, Sewald N. Terpenoid alkaloids of the Buxaceae family with potential biological importance. Nat. Prod. Rep. . 2008; 25: 612–630. doi: 10.1039/b704958g. [DOI] [PubMed] [Google Scholar]
- 44.Rahman AU, Ul-Haq Z, Khalid A, Anjum S, Khan MR, Choudhary MI. Pregnane-Type steroidal alkaloids of Sarcococca saligna: a new class of cholinesterase inhibitors. Helv. Chim. Acta . 2002;85: 678–688. [Google Scholar]
- 45.Rahman AU, Feroz F, Ul-Haq Z, Nawaz SA, Khan MR, Choudhary MI. New steroidal alkaloids from Sarcococca saligna. Nat. Prod. Res. . 2003; 17: 235–241. doi: 10.1080/1057563021000051086. [DOI] [PubMed] [Google Scholar]
- 46.Rahman AU, Feroz F, Naeem I, Ul-Haq Z, Nawaz SA, Khan N, Khan MR, Choudhary MI. New pregnane-type steroidal alkaloids from Sarcococca saligna and their cholinesterase inhibitory activity. Steroids Source. 2004; 69:735–741. doi: 10.1016/j.steroids.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 47.Devkota KP, Lenta BN, Choudhary MI, Naz Q, Fekam FB, Rosenthal PJ, Sewald N. Cholinesterase inhibiting and antiplasmodial steroidal alkaloids from Sarcococca hookeriana. Chem. Pharm. Bull. (Tokyo) . 2007;55: 1397–1401. doi: 10.1248/cpb.55.1397. [DOI] [PubMed] [Google Scholar]
- 48.Devkota KP, Lenta BN, Wansi J, Choudhary MI, Kisangau DP, Naz Q, SamreenSewald N. Bioactive 5?-Pregnane-type steroidal alkaloids from Sarcococca hookeriana. J. Nat. Prod. . 2008;71: 1481–1484. doi: 10.1021/np800305b. [DOI] [PubMed] [Google Scholar]
- 49.Ata A. In Studies in Natural Products Chemistry 1st Edition, Vol.38 Rahman A. :225–245. [Google Scholar]
- 50.Ata A, Iverson CD, Kalhari KS, Akhter S, Betteridge J, Meshkatalsadat MH, Orhan I, Sener B. Triterpenoidal alkaloids from Buxus hyrcana and their enzyme inhibitory, anti-fungal and anti-leishmanial activities. Phytochemistry . 2010;71: 1780–1786. doi: 10.1016/j.phytochem.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 51.Matochko WL, James A, Lam CW, Kozera D, Ata A, Gengan R. Triterpenoidal alkaloids from Buxus natalensis and their acetylcholinesterase inhibitory activity. J. Nat. Prod. . 2010;73: 1858–1862. doi: 10.1021/np100494u. [DOI] [PubMed] [Google Scholar]
- 52.Rahman AU, Akhtar MN, Choudhary MI, Tsuda Y, Sener B, Khalid A, Parvez M. New steroidal alkaloids from Fritillaria imperialis and their cholinesterase inhibiting activities. Chem. Pharm. Bull. . 2002;50: 1013–1016. doi: 10.1248/cpb.50.1013. [DOI] [PubMed] [Google Scholar]
- 53.Lin BQ, Ji H, Li P, Fang W, Jiang Y. Inhibitors of acetylcholine esterase in vitro - Screening of steroidal alkaloids from Fritillaria species. Planta Med. . 2006;72:814–818. doi: 10.1055/s-2006-947168. [DOI] [PubMed] [Google Scholar]
- 54.Yang Z, Duan DZ, Xue WW, Yao XJ, Li S. Steroidal alkaloids from Holarrhena antidysenterica as acetylcholinesterase inhibitors and the investigation for structure–activity relationships. Life Sci. . 2012;90:929–933. doi: 10.1016/j.lfs.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 55.Eldeen IM, VanHeerden FR, VanStaden J. In vitro biological activities of niloticane, a new bioactive cassane diterpene from the bark of Acacia nilotica subsp. kraussiana. J. Ethnopharmacol. . 2010; 128:555–560. doi: 10.1016/j.jep.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 56.Hung TM, Luan TC, Vinh BT, Cuong TD, Min BS. Labdane-type diterpenoids from Leonurus heterophyllus and their cholinesterase inhibitory activity. Phytother. Res. . 2011; 25: 611–614. doi: 10.1002/ptr.3307. [DOI] [PubMed] [Google Scholar]
- 57.Khan I, Nisar M, Khan N, Saeed M, Nadeem S, Fazal-ur-Rehman; Ali F, Karim N, Kaleem WA, Qayum M, Ahmad H, Khan IA. Structural insights to investigate Conypododiol as a dual cholinesterase inhibitor from Asparagus adscendens. Fitoterapia . 2010;81: 1020–1025. doi: 10.1016/j.fitote.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 58.Riaz N, Nawaz SA, Mukhtar N, Malik A, Afza N, Ali S, Ullah S, Muhammad P, Choudhary MI. Isolation and enzyme-inhibition studies of the chemical constituents from Ajuga bracteosa. Chem. Biodivers. . 2007; 4:72–83. doi: 10.1002/cbdv.200790008. [DOI] [PubMed] [Google Scholar]
- 59.Ahmed E, Nawaz SA, Malik A, Choudhary MI. Isolation and cholinesterase-inhibition studies of sterols from Haloxylon recurvum. Bioorg. Med. Chem. Lett. . 2006; 16:573–580. doi: 10.1016/j.bmcl.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 60.Awang K, Chan G, Litaudon M, Ismail NH, Martin MT, Gueritte F. 4-Phenylcoumarins from Mesua elegans with acetylcholinesterase inhibitory activity. Bioorg. Med. Chem. . 2010; 18:7873–7877. doi: 10.1016/j.bmc.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 61.Khan MT, Orhan I, Senol FS, Kartal M, Sener B, Dvorská M, Smejkal K, Slapetová T. Cholinesterase inhibitory activities of some flavonoid derivatives and chosen xanthone and their molecular docking studies. Chem. Biol. Interact. . 2009; 181:383–389. doi: 10.1016/j.cbi.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 62.Urbain A, Marston A, Sintro Grilo L, Bravo J, Purev O, Purevsuren B, Batsuren D, Reist M, Carrupt P-A, Hostettmann K. Xanthones from Gentianella amarella ssp.acuta with acetylcholinesterase and monoamine oxidase inhibitory activities. J. Nat. Prod. . 2008;71:895–897. doi: 10.1021/np070690l. [DOI] [PubMed] [Google Scholar]
- 63.Cho JK, Ryu YB, Curtis-Long MJ, Ryu HW, Yuk HJ, Kim DW, Kim HJ, Lee WS, Park KH. Cholinesterase inhibitory effects of geranylated flavonoids from Paulownia tormentosa fruits. Bioorg. Med. Chem. . 2012:2595–2602. doi: 10.1016/j.bmc.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 64.Jung HA, Jin SE, Park JS, Choi JS. Antidiabetic complications and anti-alzheimer activities of sophoflavescenol, a prenylated flavonol from Sophora flavescens, and its structure-activity relationship. Phytother. Res. . 2011; 25:709–715. doi: 10.1002/ptr.3326. [DOI] [PubMed] [Google Scholar]
- 65.Kim JY, Lee WS, Kim YS, Curtis-Long MJ, Lee BW, Ryu YB, Park KH. Isolation of cholinesterase-inhibiting flavonoids from Morus lhou. J. Agric. Food Chem. . 2011;59: 4589–4596. doi: 10.1021/jf200423g. [DOI] [PubMed] [Google Scholar]
- 66.Ryu HW, Curtis-Long MJ, Jung S, Jeong IY, Kim DS, Kang KY, Park KH. Anticholinesterase potential of flavonols from paper mulberry (Broussonetia papyrifera) and their kinetic studies. Food Chem. . 2012; 132: 1244–1250. doi: 10.1016/j.foodchem.2011.11.093. [DOI] [PubMed] [Google Scholar]
- 67.Conforti F, Rigano D, Menichini F, Loizzo MR, Senatore F. Protection against neurodegenerative diseases of Iris pseudopumila extracts and their constituents. Fitoterapia . 2009;80: 62–67. doi: 10.1016/j.fitote.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 68.Ahmad VU, Iqbal S, Nawaz SA, Choudhary MI, Farooq U, Ali ST, Ahmad A, Bader S, Kousar F, Arshad S, Tareen RB. Isolation of four new pterocarpans from Zygophyllum eurypterum (syn.Z. atriplicoides) with enzyme-inhibition properties. Chem. Biodivers. . 2006;3:996–1003. doi: 10.1002/cbdv.200690109. [DOI] [PubMed] [Google Scholar]
- 69.Changwong N, Sabphon C, Ingkaninan K, Sawasdee P. Acetyl- and butyryl-cholinesterase inhibitory activities of Mansorins and Mansonones. Phytother. Res. . 2012; 26:392–396. doi: 10.1002/ptr.3576. [DOI] [PubMed] [Google Scholar]
- 70.Nag G, De B. Acetylcholinesterase inhibitory activity of Terminalia chebula, Terminalia bellerica and Emblica officinalis and some phenolic compounds. Int. J. Pharm. Pharm. Sci. . 2011;3: 121–124. [Google Scholar]
- 71.Ge HM, Zhu CH, Shi DH, Zhang LD, Xie DQ, Yang J, Ng SW, Tan RX. Hopeahainol A.An acetylcholinesterase inhibitor from Hopea hainanensis. Chemistry . 2008; 14:376–381. doi: 10.1002/chem.200700960. [DOI] [PubMed] [Google Scholar]
- 72.Sancheti S, Um BH, Seo SH. 1,2,3,4,6-penta-O-galloyl-ß-D-glucose A cholinesterase inhibitor from Terminalia chebula. S. Afr. J. Bot. . 2010;76: 285–288. [Google Scholar]
- 73.Akhtar MN, Lam KW, Abas F, Maulidiani H, Ahmad S, Shah SA, Rahman AU, Choudhary MI, Lajis NH. New class of acetylcholinesterase inhibitors from the stem bark of Knema laurina and their structural insights. Bioorg. Med. Chem. Lett. . 2011; 21: 4097–4103. doi: 10.1016/j.bmcl.2011.04.065. [DOI] [PubMed] [Google Scholar]
- 74.Adhami H R, Linder T, Kaehlig H, Schuster D, Zehl M, Krenn L. Cathechol alkenyls from Semecarpus anacardium.Acetylcholinesterase inhibition and binding mode predictions. . J. Ethnopharmacol. 2012; 139: 142–148. doi: 10.1016/j.jep.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 75.Jung HA, Jung YJ, Hyun SK, Min BS, Kim DW, Jung JH, Choi JS. Selective cholinesterase inhibitory activities of a new monoterpene diglycoside and other constituents from Nelumbo nucifera stamens. Biol. Pharm. Bull. . 2010;33: 267–272. doi: 10.1248/bpb.33.267. [DOI] [PubMed] [Google Scholar]
- 76.Adewusi EA, Moodley N, Steenkamp V. Medicinal plants with cholinesterase inhibitory activity a review. Afr. J. Biotechnol. . 2010;9:8257–8276. [Google Scholar]
- 77.BarbosaFilho JM, Medeiros KCP, Diniz MF, Batista LM, Athayde-Filho PF, Silva MS, da-Cunha EVL, Silva Almeida JRG, Quintans-Júnior LJ. Natural products inhibitors of the enzyme acetylcholinesterase. Braz. J. Pharmacogn. . 2006; 16: 258–285. [Google Scholar]
- 78.Mukherjee PK, Kumar V, Houghton PJ. Screening of Indian medicinal plants for acetylcholinesterase inhibitory activity. Phytother. Res. . 2007; 21: 1142–1145. doi: 10.1002/ptr.2224. [DOI] [PubMed] [Google Scholar]
- 79.Tundis R, Menichini F, Conforti F, Loizzo MR, Bonesi M, Statti G, Menichini F. A potential role of alkaloid extracts from Salsola species (Chenopodiaceae) in the treatment of Alzheimer's disease. J. Enzyme Inhib. Med. Chem. . 2009; 24:818–824. doi: 10.1080/14756360802399662. [DOI] [PubMed] [Google Scholar]
- 80.Elufioye TO, Obuotor EM, Sennuga AT, Agbedahunsi JM, Adesanya SA. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some selected Nigerian medicinal plants. Rev. Bras. Farmacogn. . DOI: 10.1590/S0102-695X2010000400002. 2010 [Google Scholar]
- 81.Fawole OA, Amoo SO, Ndhlala AR, Light ME, Finnie JF, Van Staden J. Anti-inflammatory, anticholinesterase, antioxidant and phytochemical properties of medicinal plants used for pain-related ailments in South Africa. J. Ethnopharmacol. . 2010 ;127: 235–241. doi: 10.1016/j.jep.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 82.Cahlíková L, Benešová N, Macáková K, Urbanová K, Opletal L. GC/MS analysis of three Amaryllidaceae species and their cholinesterase activity. Nat. Prod. Commun. . 2011; 6: 1255–1258. [PubMed] [Google Scholar]
- 83.Cahlíková L, Valterová I, Macáková K, Opletal L. Analysis of Amaryllidaceae alkaloids from Zephyranthes grandiflora by GC/MS and their cholinesterase activity. Rev. Bras. Farmacogn. . DOI: 10.1590/S0102-695X2011005000089. 2011 [PubMed] [Google Scholar]
- 84.Moyo M, Ndhlala AR, Finnie JF, VanStaden J. Phenolic composition, antioxidant and acetylcholinesterase inhibitory activities of Sclerocarya birrea and Harpephyllum caffrum (Anacardiaceae) extracts. Food Chem. . 2010; 123: 69–76. [Google Scholar]
- 85.Benamar H, Rached W, Derdour A, Marouf A. Screening of Algerian medicinal plants for acetylcholinesterase inhibitory activity. J. Biol. Sci. . 2010; 10: 1–9. [Google Scholar]
- 86.Lima JA, Costa RS, Epifânio RA, Castro NG, Rocha MS, Pinto AC. Geissospermum vellosii stembark.Anticholinesterase activity and improvement of scopolamine-induced memory deficits. Pharmacol. Biochem. Be. . 2009;92:508–513. doi: 10.1016/j.pbb.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 87.Yang Z, Zhang D, Ren J, Yang M, Li S. Acetylcholinesterase inhibitory activity of the total alkaloid from traditional Chinese herbal medicine for treating Alzheimer's disease. Med. Chem. Res. . 2012; 21:734–738. [Google Scholar]
- 88.Sekeroglu N, Senol FS, Orhan IE, Gulpinar AR, Kartal M, Sener B. In vitro prospective effects of various traditional herbal coffees consumed in Anatolia linked to neurodegeneration. Food Res. Int. . 2012; 45: 197–203. [Google Scholar]
- 89.Loizzo MR. Radical scavenging activity and cholinesterase inhibitory activity of Leopoldia comosa (L.) bulbs. Progr. Nutr. 2011,; 13:300–303. [Google Scholar]
- 90.Carpinella MC, Andrione DG, Ruiz G, Palacios SM. Screening for acetylcholinesterase inhibitory activity in plant extracts from Argentina. Phytother. Res. . 2010; 24: 259–263. doi: 10.1002/ptr.2923. [DOI] [PubMed] [Google Scholar]
- 91.Wszelaki N, Kuciun A, Kiss AK. Screening of traditional European herbal medicines for acetylcholinesterase and butyrylcholinesterase inhibitory activity. Acta Pharm. . 2010; 60: 119–128. doi: 10.2478/v10007-010-0006-y. [DOI] [PubMed] [Google Scholar]
- 92.Niño J, Hernández JA, Correa YM, Mosquera OM. In vitro inhibition of acetylcholinesterase by crude plant extracts from Colombian flora. Mem. Inst. Oswaldo Cruz. . 2006; 101:783–785. doi: 10.1590/s0074-02762006000700013. [DOI] [PubMed] [Google Scholar]
- 93.Bakthir H, Ali NAA, Arnold N, Teichert A, Wessjohann L. Anticholinesterase activity of endemic plant extracts from Soqotra. Afr. J. Tradit. Complement. Altern. Med. . 2011;8: 296–299. doi: 10.4314/ajtcam.v8i3.65292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Habtemariam S. The therapeutic potential of Berberis darwinii stem-bark: Quantification of berberine and in vitro evidence for Alzheimer's disease therapy. Nat. Prod. Commun. . 2011; 6: 1089–1090. [PubMed] [Google Scholar]
- 95.Ashraf M, Ahmad K, Ahmad I, Ahmad S, Arshad S, Shah SM, Nasim F. Acetylcholinesterase and NADH oxidase inhibitory activity of some medicinal plants. J. Med. Plant. Res. . 2011;5: 2086–2089. [Google Scholar]
- 96.Adewusi EA, Moodley N, Steenkamp V. Antioxidant and acetylcholinesterase inhibitory activity of selected southern African medicinal plants. S. Afr. J. Bot. . 2011;77: 638–644. [Google Scholar]
- 97.Akkol EK, Orhan IE, Yesilada E. Anticholinesterase and antioxidant effects of the ethanol extract, ethanol fractions and isolated flavonoids from Cistus laurifolius L. leaves. Food Chem. . 2012; 131: 626–631. [Google Scholar]
- 98.Feitosa CM, Freitas RM, Luz NNN, Bezerra MZB, Trevisan MTS. Acetylcholinesterase inhibition by some promising Brazilian medicinal plants. Braz. J. Biol. . 2011;71:783–789. doi: 10.1590/s1519-69842011000400025. [DOI] [PubMed] [Google Scholar]
- 99.Tavares L, McDougall GJ, Fortalezas S, Stewart D, Ferreira RB, Santos CN. The neuroprotective potential of phenolic-enriched fractions from four Juniperus species found in Portugal. Food Chem. . 2012; 135:562–570. doi: 10.1016/j.foodchem.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 100.Lee S-H, Sancheti SA, Bafna MR, Sancheti SS, Seo S-Y. Acetylcholineterase inhibitory and antioxidant properties of Rhododendron yedoense var.poukhanense bark. J. Med. Plants Res. . 2011;5: 248–254. [Google Scholar]
- 101.Kwon S-H, Lee H-K, Kim JA, Hong SI, Kim S-Y, Jo T-H, Park Y-I, Lee C-K, Kim Y-B, Lee S-Y, Jang C-G. Neuroprotective effects of Eucommia ulmoides Oliv.bark on amyloid beta25 35-induced learning and memory impairments in mice. Neurosci. Lett. . 2011; 487: 123–127. doi: 10.1016/j.neulet.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 102.Crowch CM, Okello EJ. Kinetics of acetylcholinesterase inhibitory activities by aqueous extracts of Acacia nilotica (L. and Rhamnus prinoides (L H r.) Afr. J. Pharm. Pharmacol. . 2009;3: 469–475. [Google Scholar]
- 103.Kim DH, Yoon BH, Kim YW, Lee S, Shin BY, Jung JW, Kim HJ, Lee YS, Choi JS, Kim SY, Lee KT, Ryu JH. The seed extract of Cassia obtusifolia ameliorates learning and memory impairments induced by scopolamine or transient cerebral hypoperfusion in mice. J. Pharmacol. Sci. . 2007; 105:82–93. doi: 10.1254/jphs.fp0061565. [DOI] [PubMed] [Google Scholar]
- 104.Lin HQ, Ho MT, Lau LS, Wong KK, Shaw PC, Wan DC. Anti-acetylcholinesterase activities of traditional Chinese medicine for treating Alzheimer’s disease. Chem. Biol. Interact. . 2008; 175:352–354. doi: 10.1016/j.cbi.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 105.Rauter AP, Martins A, Lopes R, Ferreira J, Serralheiro LM, Araújo ME, Borges C, Justino J, Silva FV, Goulart M, Thomas-Oates J, Rodrigues JA, Edwards E, Noronha JP, Pinto R, Mota-Filipe H. Bioactivity studies and chemical profile of the antidiabetic plant Genista tenera. J. Ethnopharmacol. . 2009; 122:384–393. doi: 10.1016/j.jep.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 106.Satheeshkumar N, Mukherjee PK, Bhadra S, Saha BP. Acetylcholinesterase enzyme inhibitory potential of standardized extract of Trigonella foenum graecum L and its constituents. Phytomedicine . 2010; 17: 292–295. doi: 10.1016/j.phymed.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 107.Bhadra S, Mukherjee PK, Kumar NS, Bandyopadhyay A. Anticholinesterase activity of standardized extract of Illicium verum Hook.f. fruits. Fitoterapia . 2011;82:342–346. doi: 10.1016/j.fitote.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 108.Orhan I, Senol FS, Gülpinar AR, Kartal M, Sekeroglu N, Deveci M, Kan Y, Sener B. Acetylcholinesterase inhibitory and antioxidant properties of Cyclotrichium niveum, Thymus praecox subsp.caucasicus var. caucaicusEchinacea purpurea and E. pallida. Food Chem. Toxicol. 2009; 47: 1304–1310. doi: 10.1016/j.fct.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 109.Costa P. Inhibitory effect of Lavandula viridis on Fe2+-induced lipid peroxidation, antioxidant and anti-cholinesterase properties. Food Chem. 2011; , 126: 1779–1786. doi: 10.1016/j.foodchem.2010.12.085. [DOI] [PubMed] [Google Scholar]
- 110.Orhan IE, Belhattab R, Senol FS, Gülpinar AR, Hosbas S. Profiling of cholinesterase inhibitory and antioxidant activities of Artemisia absinhiumA. herba-alba, A. fragrans, Marrubium vulgare, M. astranicum, Origanum vulgare subsp. glandulossum and essential oil analysis of two Artemisia species. Ind. Crop. Prod. . 2010; 32:566–571. [Google Scholar]
- 111.Loizzo MR. Menichini, F.Chemical analysis, antioxidant, antiinflammatory and anticholinesterase activities of Origanum ehrenbergii Boiss and Origanum syriacum L. essential oils. Food Chem. 2009; 117: 174–180. [Google Scholar]
- 112.Mossa AT, Nawwar GA. Free radical scavenging and antiacetylcholinesterase activities of Origanum majorana L. essential oil. Hum. Exp. Toxicol. . 2011;30: 1501–1513. doi: 10.1177/0960327110391686. [DOI] [PubMed] [Google Scholar]
- 113.Tel G, Oztürk M, Duru ME, Harmandar M, Topçu G. Chemical composition of the essential oil and hexane extract of Salvia chionantha and their antioxidant and anticholinesterase activities. Food Chem. Toxicol. . 2010; 48:3189–3193. doi: 10.1016/j.fct.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 114.Senol FS. Survey of 55 Turkish Salvia taxa for their acetylcholinesterase inhibitory and antioxidant activities. Food Chem. 2010; 120:34–43. [Google Scholar]
- 115.Loizzo MR, Menichini F, Tundis R, Bonesi M, Conforti F, Nadjafi F, Statti GA, Frega NG, Menichini F. In vitro biological activity of Salvia leriifolia benth essential oil relevant to the treatment of Alzheimer's disease. J. Oleo Sci. . 2009;58: 443–446. doi: 10.5650/jos.58.443. [DOI] [PubMed] [Google Scholar]
- 116.Ahmad B, Mukarram Shah SM, Khan H, Hassan Shah SM. Enzyme inhibition activities of Teucrium royleanum. J. Enzym. Inhib. Med. Ch. . 2007; 22:730–732. doi: 10.1080/14756360701306271. [DOI] [PubMed] [Google Scholar]
- 117.Tappayuthpijarn P, Itharat A, Makchuchit S. Acetylcholinesterase inhibitory activity of Thai traditional nootropic remedy and its herbal ingredients. J. Med. Assoc. Thai. . 2011;94:S183–S189. [PubMed] [Google Scholar]
- 118.Vinutha B, Prashanth D, Salma K, Sreeja SL, Pratiti D, Padmaja R, Radhika S, Amit A, Venkateshwarlu K, Deepak M. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J. Ethnopharmacol. . 2007; 109:359–363. doi: 10.1016/j.jep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 119.Ashraf M, Ahmad K, Ahmad I, Ahmad S, Arshad S, Shah SM, Nasim F. Acetylcholinesterase and NADH oxidase inhibitory activity of some medicinal plants. J. Med. Plant. Res. . 2011;5: 2086–2089. [Google Scholar]
- 120.Adsersen A, Gauguin B, Gudiksen L, Jäger AK. Screening of plants used in Danish folk medicine to treat memory dysfunction for acetylcholinesterase inhibitory activity. J. Ethnopharmacol. . 2006; 104: 418–422. doi: 10.1016/j.jep.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 121.Ustun O, Senol F, Kurkcuoglu M, Orhan I, Kartal M, Baser K. Investigation on chemical composition, anticholinesterase and antioxidant activities of extracts and essential oils of Turkish Pinus species and pycnogenol. Ind. Crop. Prod. . 2012;38: 115–123. [Google Scholar]
- 122.Bonesi M, Menichini F, Tundis R, Loizzo MR, Conforti F, Passalacqua NG, Statti GA, Menichini F. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of Pinus species essential oils and their constituents. J. Enzym. Inhib. Med. Ch. . 2010; 25: 622–628. doi: 10.3109/14756360903389856. [DOI] [PubMed] [Google Scholar]
- 123.Khadri A, Serralheiro MLM, Nogueira JMF, Neffati M, Smiti S, Araújo MEM. Antioxidant and antiacetylcholinesterase activities of essential oils from Cymbopogon schoenanthus L.Spreng. Determination of chemical composition by GC mass spectrometry and 13C NMR. Food Chem. . 2008; 109: 630–637. [Google Scholar]
- 124.Khadri A, Neffati M. Antioxidant, antiacetylcholinesterase and antimicrobial activities of Cymbopogon schoenanthus L. Spreng (lemon grass) from Tunisia. LWT-Food Sci. Technol. . 2010; 43:331–336. [Google Scholar]
- 125.Aremu AO, Amoo SO, Ndhlala AR, Finnie JF, Stadenm JV. Antioxidant activity, acetylcholinesterase inhibition, iridoid content and mutagenic evaluation of Leucosidea sericea. Food Chem. Toxicol. . 2011; 49: 1122–1128. doi: 10.1016/j.fct.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 126.Pachauri SD, Tota S, Khandelwal K, Verma PR, Nath C, Hanif K, Shukla R, Saxena JK, Dwivedi AK. Protective effect of fruits of Morinda citrifolia L.on scopolamine induced memory impairment in mice. A behavoralbiochemical and cerebral blood flow study. J. Ethnopharmacol. 2012; 139:34–41. doi: 10.1016/j.jep.2011.09.057. [DOI] [PubMed] [Google Scholar]
- 127.Chaiyana W, Okonogi S. Inhibition of cholinesterase by essential oil from food plant. Phytomedicine . 2012; 19:836–839. doi: 10.1016/j.phymed.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 128.Menichini F, Tundis R, Bonesi M, de Cindio B, Loizzo MR, Conforti F, Statti GA, Menabeni R, Bettini R, Menichini F. Chemical composition and bioactivity of Citrus medica L.cv. Diamante essential oil obtained by hydrodistilltioncold-pressing and supercritical carbon dioxide extraction. Nat. Prod. Res. 2011; 25:789–799. doi: 10.1080/14786410902900085. [DOI] [PubMed] [Google Scholar]