Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disorder to date, with no curative or preventive therapy. Histopathological hallmarks of AD include deposition of β-amyloid plaques and formation of neurofibrillary tangles. Extent studies on pathology of the disease have made important discoveries regarding mechanism of disease and potential therapeutic targets. Many cellular changes including oxidative stress, disruption of Ca2+ homeostasis, inflammation, metabolic disturbances, and accumulation of unfolded/misfolded proteins can lead to programmed cell death in AD. Despite intensive research, only five approved drugs are available for the management of AD. Hence, there is a need to look at alternative therapies. Use of natural products and culinary herbs in medicine has gained popularity in recent years. Several natural substances with neuroprotective effects have been widely studied. Most of these compounds have remarkable antioxidant properties and act mainly by scavenging free radical species. Some of them increase cell survival and improve cognition by directly affecting amyloidogenesis and programmed cell death pathways. Further studies on these natural products and their mechanism of action, parallel with the use of novel pharmaceutical drug design and delivery techniques, enable us to offer an addition to conventional medicine. This review discussed some natural products with potential neuroprotective properties against Aβ with respect to their mechanism of action.
Keyword: Alzheimer’s disease, Amyloid β, Apoptosis, Natural products, Neuroprotection, Tau protein.
1. INTRODUCTION
Alzheimer’s disease (AD) is a progressive neuro-degenerative disorder that is characterized by the loss of memory and cognitive impairments [1]. Many biochemical changes within cell have been identified to induce neuronal cell death. Oxidative stress, disruption of Ca2+ homeostasis, inflammation, metabolic disturbances, and accumulation of unfolded/mis-folded proteins are among cellular changes that finally lead to programmed cell death in AD [2].
Histopathological hallmarks of AD include deposition of β-amyloid (Aβ) plaques and formation of neurofibrillary tangles [3]. Indeed, AD is a protein mis-folding disease, called “proteopathy”. Senile plaques are made of insoluble Aβ peptides that are formed by proteolytic fragmentation of amyloid precursor protein (APP), a trans-membrane protein that penetrates through the neuronal membranes [4]. Neurofibrillary tangles are made of aggregates of hyperphosphorylated tau, a microtubule-associated protein [5].
Most of the researches in AD during past two decades have focused on “amyloid hypothesis”. This hypothesis postulates that Aβ deposition is the initial event in neuronal dysfunction in AD. The support given for this hypothesis is the dementia of patient with Down’s syndrome (trisomy 21). As APP genes locate in chromosome 21, it has been proposed that their mutations result in cognitive impairment and dementia [6].
APP cleavage by β-secretase at extracellular domain, and by γ-secretase at the trans-membrane region, leads to formation of Aβ proteins [7]. Gamma-secretase complex consists of at least four proteins, including Presenilin (PS). PS, an aspartyl protease, is the catalytic subunit of the enzyme and its mutation causes alterations in APP processing and increases the amount of toxic Aβ [8]. Although amyloid plaques are the major characteristic of AD pathology, their presence does not necessarily mean the increased amyloid production. Decrease of clearance could also lead to this accumulation. It has been also reported that some soluble species of Aβ are more toxic than Aβ aggregates [9]. Thus, the main focus has been made on preventing the formation of Aβ from its precursor.
Although several compounds have been proposed to decrease neurodegeneration in AD models, limited approved drugs are available for the management of AD patients. A major part of today’s pharmaceutical market is the use of natural products. The use of drug substances derived from natural sources has a long tradition in medicine [10, 11].
This review article describes the mechanism of AD, current medications for AD and existed problems in developing new medications. We also summarize the potential of some natural products for inhibiting Aβ-induced cell death and recent findings on their mechanisms of action. Although Aβ is not the only factor capable of causing cognitive decline in AD, due to extent of compounds and experimental models, in this paper we focused on the active substances reported to inhibit Aβ-induced cognitive impairment or cell death.
2. MECHANISM OF NEURONAL CELL DEATH IN AD
Oxidative stress is the imbalance between prooxidants and antioxidant factors that lead to accumulation of reactive oxygen species (ROS) [12]. This reactive species can lead to cell membrane lipid destruction, DNA cleavage, oxidation of proteins, and finally apoptosis [13]. Apoptosis is the predominant type of neuronal cell loss observed in AD [14]. Apoptotic cell death signaling can be divided into two major pathways; intrinsic (or mitochondrial) pathway and extrinsic (or death receptor- mediated) pathways [15].
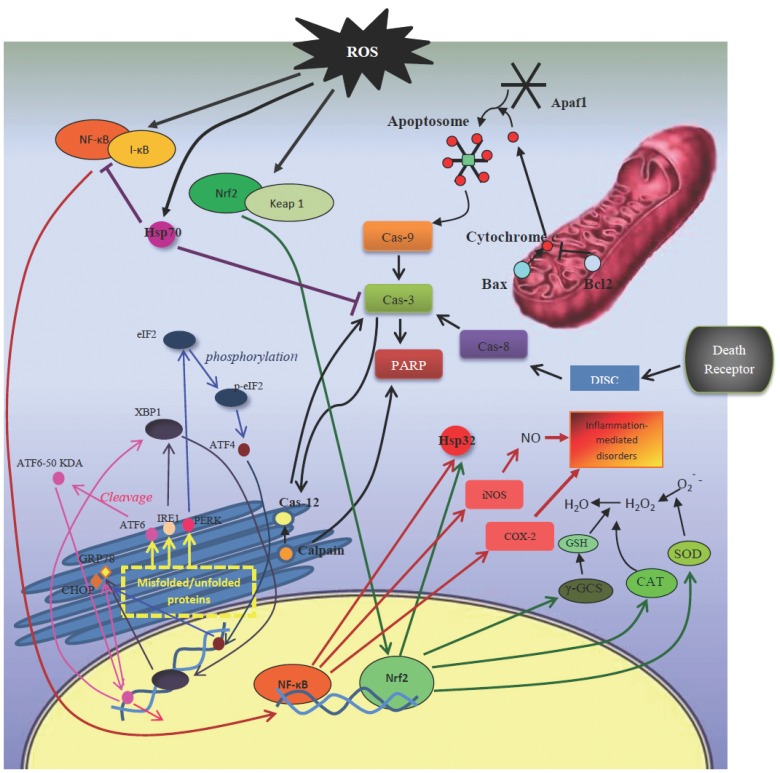
Apoptosis in mammalian cells regulates by a large number of proteins (Fig. 1). In the intrinsic pathway, stimuli acts directly or indirectly on mitochondria and affects mainly Bcl-2 family and caspase [16].
Fig. (1).
Mechanism of apoptotic pathways and ER stress.
Bcl2 superfamily consists of both pro-apoptotic (such as Bax, Bad, and Bak) and anti-apoptotic (such as Bcl-2 and Bcl-xL) proteins. Decrease of anti-apoptotic protein and/or increase of pro-apoptotic factors results in disruption of mitochondria membrane potential, swelling of mitochondrial membrane, and release of cytochrome c to cytoplasm [17]. In cytoplasm, cytochrome c forms a multi-molecular holoenzyme complex with apoptotic protease activating factor 1 (Apaf1), which cleaves procaspase-9 to its active form. Active caspase-9 then cleaves procaspase-3 and initiates the caspase cascades [18].
Extrinsic pathway of apoptosis involves the interaction of death signals, for example tumor necrosis factor (TNF-α) with death receptors, such as tumor necrosis factor receptors 1 (TNFR1), and formation of death-inducible signaling complex that activates caspase-8, which could also cleave procaspase-3 to its active form [19].
Activated caspase-3, in both intrinsic and extrinsic pathways, activates poly (ADP-ribose) polymerase (PARP) and other death substrates, such as APP, PS1 and PS2 proteins [20].
Stress conditions also affect the folding of proteins in endoplasmic reticulum (ER) lumen [21]. Three main ER pathways involved in folding include inositol-requiring enzyme 1 (IRE1), protein kinase RNA-like ER kinase (PERK), and activating transcription factor 6 (ATF6) [22]. ER stress response is mediated via three different signaling pathways: unfold protein response (UPR), which increases the level of chaperones; ER-associated protein degradation (ERAD) that degrades the misfolded proteins by activating ubiquitin/proteosomal pathway; and ER overload response (EOR) which is induced when ER is overload with proteins that are not transported to Golgi complex [23]. Under ER stress conditions, glucose-regulated protein 78 (GRP78) which is an ER chaperone, dissociates from ATF6, PERK, and IRE1 and binds to malfolded proteins to facilitate their folding [24]. C/EBP homologous protein (CHOP) together with caspase-12, which are ER resident caspases, and calpain mediate ER stress-induced apoptosis by affecting executioner caspases, such as caspase-3 [25,26].
While accumulation of unfolded proteins in ER provokes these pathways, accumulation of misfolded proteins in the cytosol leads to increased expression of heat shock proteins (HSPs) which act as molecular chaperons [27]. HSPs expression is induced by several stimuli including heat shock, ischemia damage, infection, and heavy metals [28]. HSPs may protect cells by mechanisms unrelated to their chaperone function through inhibition of apoptosis [29]. Stress-inducible Hsp70 is a prominent cytoprotective factor that protects the sensitive sites of the target proteins and thereby acts as a cytoplasmic “antioxidant” [30].
In addition to mitochondria- and ER-resident proteins, many stress-sensing transcription factors are also activated in AD.
NF-E2 related factor 2 (Nrf2) is a central transcription factor involved in transcriptional activation of phase II detoxifying enzymes via antioxidant response element (ARE) [31]. Release of Nrf2 from its cytoplasmic inhibitor, Kelch-like ECH-associated protein 1 (Keap1), leads to activation of Nrf2 and its translocation to nucleus, where it activates transcription of ARE-driven genes, such as Hsp32 and γ-glutamylcysteine synthetase (γ-GCS) [32].
Nuclear factor-κB (NF-κB) is another transcription factor that is activated by TNF-α, interleukin 1β (IL-1β) and lipopolysaccharide (LPS) (canonical pathway) or by LTα/β, CD40 ligand (non-canonical pathway) [33]. In unstimulated cells, NF-κB is sequestered inactive in cytoplasm by binding to IκBs (Inhibitor of κB). Activation of the NF-κB involves the phosphorylation of two serine residues located on IκB regulatory domain by IκB kinase (IKK) and release of NF-κB [34]. In nucleus, NF-κB induces production of different mediators, like nitric oxide (NO), and regulates a number of inflammation- and oxidative stress-related genes, such as cyclooxygenase 2 (COX-2), superoxide dismutase (SOD), glutamate receptors, growth factors (brain-derived neurotrophic factor (BDNF) and nerve growth factor(NGF)), and cytokines (TNF-α and TNFR) [35,36]. NF-κB signaling is inhibited by peroxisome proliferator-activated receptor γ (PPARγ), a transcription factor of the nuclear hormone receptor superfamily [37]. PPARγ agonists attenuate effectively oxidative stress, inflammation and apoptosis in the central nervous system [38].
Mitogen-activated protein kinase (MAPK) cascades are other major signaling pathways involved in cell proliferation, differentiation and adaptation [39]. The p38 MAPK signaling has been widely accepted as a cascade contributing to neuroinflammation, excitotoxicity, synaptic plasticity and tau phosphorylation [40]. Inhibitors of ERK and p38 MAPK improve spatial learning and memory impairment in Aβ-injected rats by increasing phosphorylated cAMP-response element binding protein (CREB) level [41]. JNK protein is a stress activated protein kinase with several targets including Bcl family members and microtubule associated proteins, such as tau [42].
Another important signaling pathway involved in AD is Wnt pathway. Wnt signaling plays an important role in normal neural development and maintenance of neuronal homeostasis, synaptic plasticity, axonogenesis and establishment of brain polarity [43]. Activation of the Wnt pathway attenuates cytosolic glycogen synthase kinase 3β (GSK-3β) activity via protein kinase C (PKC) enzyme, thereby prevents phosphorylation and degradation of β-catenin and increases its nuclear translocation [44]. In nucleus, β-catenin interacts with TCF/LEF family trans-cription factors to promote specific gene expression [45]. These gene products are important in determining cell’s fate during normal development and in maintaining homeostasis [46]. Several studies have shown that PS-1 protein could form high molecular weight complexes with GSK-3β and β-catenin protein [47]. It has been suggested that PS-1 inherited mutations may affect the levels, trafficking or the phosphorylation state of cytosolic β-catenin [48].
3. CURRENT TREATMENTS
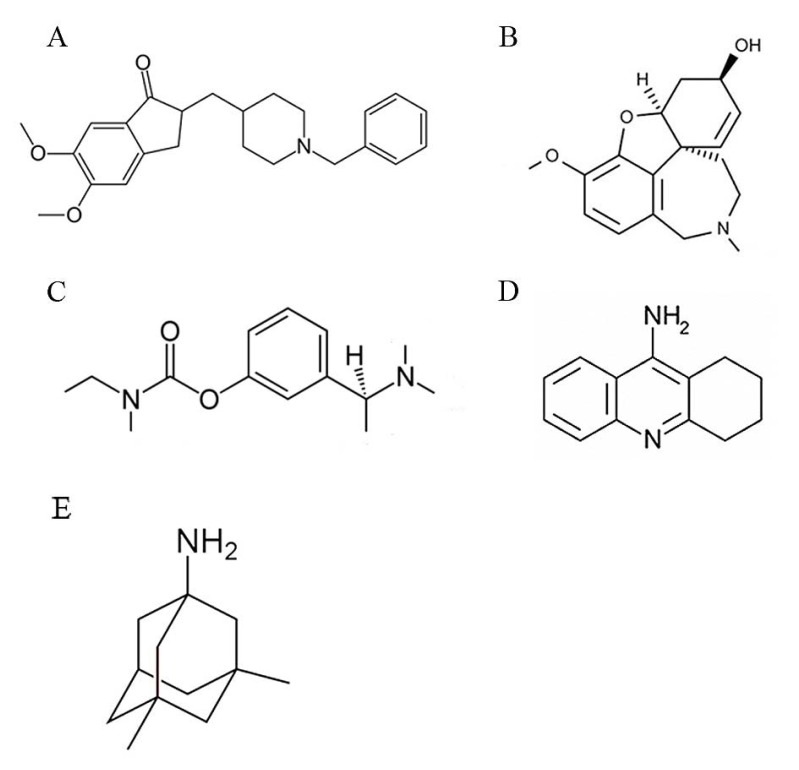
To date, there is no curative or preventive therapy for AD. Five medications have FDA approve for management of AD, all of them offer symptomatic benefits; tacrine, rivastagmine, donepezil, and galantamine are acetyl cholinesterase inhibitor (AChEI), while memantine is an N-methyl-D-aspartate (NMDA) receptor antagonist [49] (Fig. 2).
Fig. (2).
Chemical structure of FDA-approved drugs for AD. A) Donepezil; B) Galantamine; C) Rivastigmine; D) Tacrine; E) Memantine.
It has been found that AD is associated with reduction of cholinergic neurons activity [50]. AChEIs reduce the rate of acetylcholine degradation, and thereby increase its concentration in the brain. All of these drugs are used in mild to moderate AD [51]. Donepezil is approved for the treatment of advanced stages of AD [52]. Memantine is also used in moderate to severe AD. This NMDA receptor antagonist blocks overstimulation of receptor by glutamate that could lead to excitotoxicity in AD [53].
As mentioned above, none of these drugs could delay the onset of AD or halting its progression [54]. Several problems exist in development of new therapeutics. First of all, there are mixed causes of dementia and neuropathology in many patients, particularly those who are older than 80 years [55]. Besides, in patients with different stages of the disease, it might be too early or too advanced for a disease-modifying effect of specific drugs [56]. Another problem in development of AD therapy is that several compounds with positive results in preclinical studies fail at clinical trials because of their low penetration across blood brain barrier (BBB), which limits their targeting. In recent years, several compounds have been reported for their neuroprotective effects in cellular and/or animal models of AD [57,58]. Although many of them were failed in different phases of clinical trials, some of them are promising candidates for AD treatment [59,60].
4. NATURAL PRODUCTS
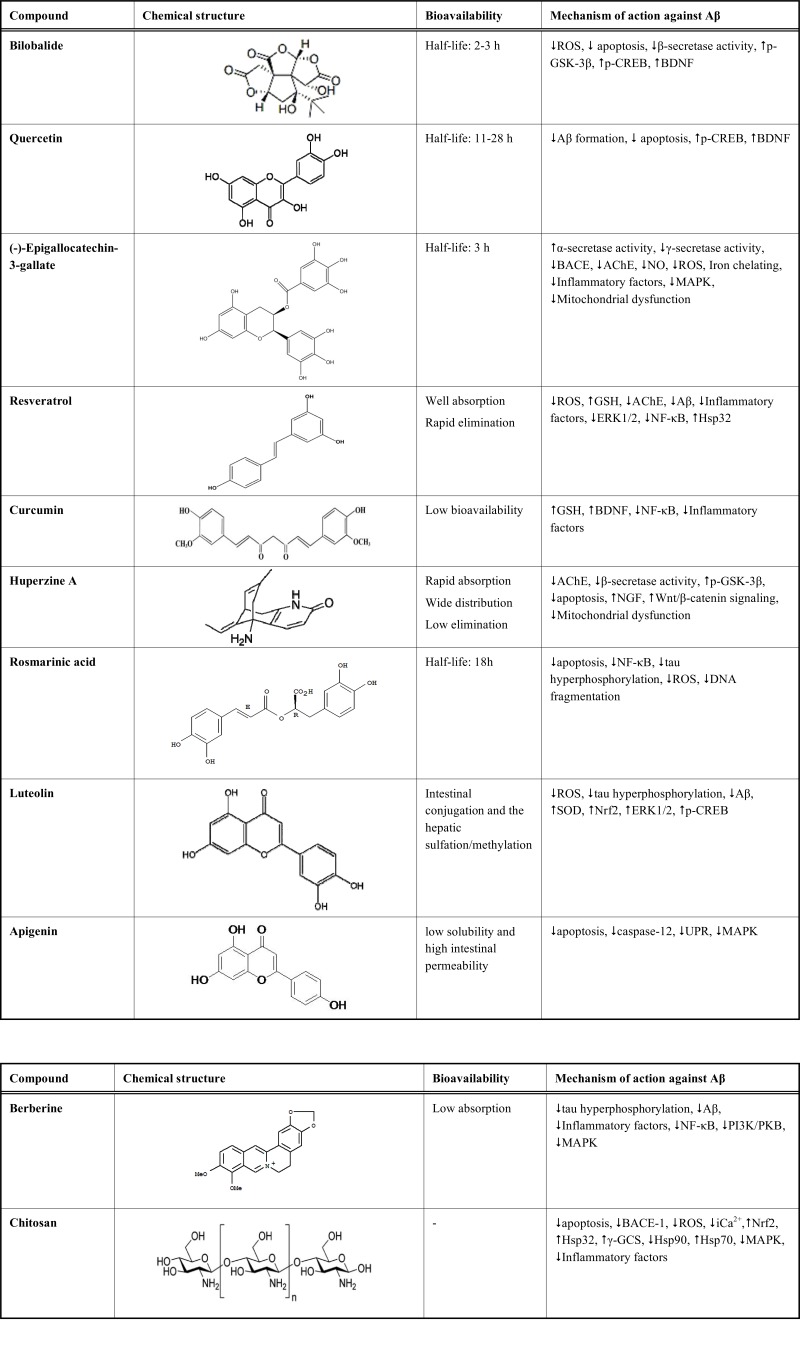
Medicinal plants are important source of protective compounds against AD. Using the structure of these active substances as templates for synthetic drugs provides a wide range of potential neuroprotective compounds [61]. In the past few decades, several researches attempted to assess the effect of total plant extract on AD and to isolate the active substance responsible for the protective effects [62,63]. In this section, we review the most studied neuroprotective natural substances with focus on their mechanism of action (Table 1).
Table 1.
Natural Compounds with Protective Effect Against Aβ
|
4.1. Bilobalide
Bilobalide (BB) is the main terpenoid of Ginkgo biloba leaves with potent protective effects on neurons and Schwann cells [64]. This sesquiterpene trilactone induces liver enzymes CYP3A1 and 1A2, which may be partially responsible for interactions between Ginkgo and other herbal medicines or pharmaceutical drugs [65].
In rats, oral administration of Ginkgo extracts and/or pure BB caused dose dependent increase of BB plasma levels [66]. The plasma half-life of BB in human is about 2-3 h [67].
BB (25-100 µM) blocked ROS-induced apoptosis in early stages and decreased the elevated levels of p53, Bax and caspase-3 in PC12 cells [68]. BB (50 µl, 0.14 g/ml) also affected mitochondrial function by upregulating cytochrome c oxidase subunit I [69].
BB (10 µM) inhibited the β-secretase activity of cathepsin B and reduced generation of two β-secretase cleavage products of APP, Aβ and soluble APPβ, via PI3K-dependent pathway [70]. Additionally, GSK 3β signaling might be involved in BB (10 µM)-induced Aβ reduction as a downstream target of the activated PI3K pathway [71]. In hippocampal neurons, BB stimulated neurogenesis and synaptogenesis by increasing the levels of pCREB and BDNF [72]. Chen et al. reported that the capacity of BB to potentiate neurite outgrowth in developing nerves can be suppressed as its concentration is boosted to 400 µM. They suggested that an excessive dosage of BB could provoke adverse responses to the recovery of regenerated nerves [73].
4.2. Quercetin
Quercetin (QCT) is a polyphenolic flavonoid found in a wide variety of foods including capers, apples, onions, berries, green and black tea, and red wine [74]. This flavonol-type flavonoid acts as a bioactive compound, mainly by scavenging ROS and showing antioxidant properties [75]. In addition, it exerts several pharmacological effects, such as anti-cancer, antiviral, anti-inflammatory, and anti-amyloidogenic activities [76, 77]. In human studies, QCT (doses up to 1,000 mg/day) had no adverse effects on blood parameters of liver and kidney function, hematology, or serum electrolytes [74].
One characteristic feature of QCT is that the elimination of its metabolites from plasma is quite slow, with reported half-lives ranging from 11 to 28 h, which makes QCT accumulation in body in daily uptake [78]. Recently, solid lipid nanoparticles (SLNs) of QCT were prepared in order to improve its penetration via BBB. Behavioral studies confirmed that SLN-encapsulated QCT shows a better neuroprotective effect [79]. Most animal studies reported no toxicity/carcinogenicity, which may be due to extensive QCT metabolism by the intestinal and the liver cells. Liposomal preparations or higher BBB permeability conditions, by increasing the amount of QCT aglycone reaching the CNS parenchyma, may elevate the risk of neurotoxicity. Thus, further toxicological studies are needed to investigate risk/beneficial effects of liposomal preparations [80].
QCT (10 µM) showed anti-amyloidogenic effects by inhibiting the formation of Aβ fibrils [81]. Lower doses of QCT (5-20 µM) significantly attenuated Aβ-induced apoptosis in hippocampal cultures; however it induced cytotoxicity at high doses (40 µM) [82]. QCT combination with BB could significantly enhance phosphorylation of CREB and the level of BDNF in mice brain [83].
4.3. (-)-Epigallocatechin-3-gallate
(-)-Epigallocatechin-3-gallate (EGCG) is the most abundant catechin in green tea leaves (Camellia sinensis). Carob flour, a cocoa-like substance derived from the ground pods of the carob plant or Ceratonia siliqua has also a high content of EGCG. Apples, blackberries, strawberries, nuts, peaches, avocados, plums, onions and raspberries have lower amounts of EGCG [84]. During fermentation, many catechins are oxidized to theaflavin and thearubigen which provide darker colors of the black tea [85]. EGCG is a potent antioxidant flavonoid and has been the subject of many studies in cancer, atherosclerosis, and neurodegenerative diseases, such as AD [86].
Absorption of EGCG from the small intestine is largely by passive diffusion; however, at high concentrations, the small intestinal and colonic tissues become saturated [87]. After oral absorption, tea catechins undergo extensive methylation, glucuronidation, and sulfation. The elimination half-life of EGCG is about 3 h [88]. It has been shown that EGCG nanolipids oral bioavailability was two folds more than free EGCG and they show a better α-secretase enhancing effect [89].
Orally administered EGCG (10 mg/kg) could reduce AChE activity, glutathione peroxidase activity, NO metabolites and ROS content in streptozotocin-model of dementia [90]. In mutant PS2 AD mice, EGCG (3 mg/kg in drinking water) enhanced memory formation and α-secretase activity, and suppressed γ-secretase activity [91]. Rezai-zadeh et al. (2008) showed that EGCG administration, i.p. (20 mg/kg) and p.o. (50 mg/kg in drinking water), modulated tau profile and provided cognitive benefits to Tg mice [92]. Besides, EGCG (5-10 µM) exhibited potent iron-chelating activities and decreased both immature and full length cellular holo-APP [93].
EGCG (1.5 and 3 mg/kg in drinking water) also prevented LPS-induced memory impairment and apoptosis via non-amyloidogenic proteolysis by decreasing APP expression, beta-site APP cleaving enzyme 1 (BACE-1) activity, and Aβ levels. In this model of AD, EGCG (1, 10, and 100 µM) also prevented astrocytes activation and inflammatory factors including TNF-α, IL-1β, macrophage colony-stimulating factor, soluble intracellular adhesion molecule-1, IL-6, inducible nitric oxide synthase (iNOS) and COX-2 [94,95]. Pae et al. reported that dietary supplementation with high dose of EGCG (1 % w/w) promotes adverse response by inducing TNF-α, IL-6, and IL-1β and lipid inflammatory mediator PGE2 in mice splenocytes and macrophages [96].
EGCG (2 and 20 µM) also inhibited vascular endothelial growth factor, prostaglandin E2, p38, JNK, and MAPK phosphatase-1 [97]. Higher concentrations of EGCG (200 µM) induced cellular toxicity in human astrocytoma, U373MG cells [97].
EGCG (3 mg/kg in drinking water) inhibited apoptosis in Aβ1-42- injected mice brain by inhibiting ERK and NF-κB [91]. EGCG (10 µM) could prevent Aβ-induced impairment of NMDA, calcium influx, ROS production, and mitochondrial dysfunction in primary cortical neurons [98]. In vitro and in vivo studies done by Dragicevic et al. (2011) showed that EGCG and luteolin (Lu) were the top two mitochondrial restoration compounds among 25 tested flavonoids [99].
In addition to mentioned effects, EGCG could be also used as an enhanced supplement for Huperzine A (HupA). EGCG (10-300 mg/kg) enhanced the inhibitory effect of HupA on AChE by increasing its affinity for serum albumin. Upon addition of EGCG to HupA, a remarkably enhanced and prolonged inhibitory effect was detected [100].
4.4. Resveratrol
Resveratrol (RSV) belongs to a class of polyphenolic compounds called Stilbenes [101]. Stilbenes are produced by several plants after exposure to stress, injury, fungal infection, or UV radiation [102]. RSV is one of the main flavonoids of red wine and can be found in the skin of grapes and other fruits and nuts [103]. Several studies reported anti-cancer, anti-inflammatory, cardiovascular benefits, lowering blood glucose, and neuroprotective effects of RSV [104,105]. In short term study of repeated dose of RSV, no severe adverse effect of RSV was reported. Only 12.5% of the participants experienced frontal headache [106, 107].
RSV is well-absorbed from gastrointestinal lumen but it has low bioavailability due to its rapid metabolism and elimination [108]. RSV loaded lipid core nanocapsules increased RSV concentration in brain tissue, compared to free RSV [109].
RSV (10 and 20 mg/kg, p.o.) acts mainly as a potent antioxidant by scavenging ROS, increasing GSH level and ameliorating antioxidant capacity of the cell [110]. RSV (10 µM) also increases intracellular Ca2+ in cortical neurons via modulating secondary messengers, cGMP, cAMP, and NO. This enhancement of Ca2+ promotes cellular glucose utilization by inducing calcium dependent AMP-activated protein kinase [111].
RSV decreased the level of Aβ by inducing non-amyloidogenic cleavage of APP and increasing the clearance of Aβ [112]. Indeed, RSV (50 µM) bound to Aβ in different states; its binding response to fibrillar Aβ1-42 was higher than its monomer, but it bound to monomeric Aβ1-40 stronger than its fibrillar form [113]. Its high affinity to Aβ (at the concentration of 10-4 M in AD human brain) led researchers to design a new method of amyloid detection [113]. RSV specifically stained Aβ plaques and can be used as a reliable probe for amyloids. RSV (15, 45, and 135 mg/kg) can also inhibit AChE activity within cells [114].
RSV (2.5-40 µg/ml) inhibited LPS-induced inflammatory response by decreasing inflammatory factors, such as NO, TNF-α, IL-1β, and IL-6 in astrocytes [115]. RSV (100 and 200 µM) can also inhibit CRP protein and ERK1/2 MAPK [116]. Its inhibitory effect on LPS-induced NF-κB activation (50 µM) is mainly mediated by inhibiting IKK and IκB phosphorylation. NF-κB suppression resulted in decrease of downstreams levels, TNF-α and IL-6 [117, 118].
Combinational use of melatonin (1, 10, 50, 100, and 500 μM) with RSV (0.1, 1, 5, 10, and 20 μM) potentiated RSV effects by increasing Hsp32, reducing ROS level, restoration of mitochondrial membrane potential (MMP), increasing GSH, and phosphorylation of AMPK [119]. Co-treatment with low concentrations of melatonin (1-10 µM) and RSV (0.1-1 μM) that did not prevent Aβ1-42-induced cytotoxicity alone, synergistically protected HT22 hippocampal neuronal cells against Aβ1-42-induced toxicity [119].
4.5. Curcumin
Curcumin (CUR) is the principal curcuminoid of turmeric, popular Indian spice derived from the rhizomes of Curcuma longa, which is a member of the ginger family [120]. Curcuminoids are polyphenolic compounds that give turmeric its yellow color and can be used as a food coloring [121].
Many preclinical studies suggest that CUR may be useful for the prevention and/or treatment of several diseases, such as colorectal cancer, cystic fibrosis, inflammatory diseases and AD [122]. In phase I clinical studies, CUR (up to 8,000 mg/day, p.o.) did not result in significant side effects except mild nausea and diarrhea [123]. However, excess use of CUR can damage the gut microbiota, interfering with the normal physiology and immune response [124]. Bioavailability of orally administered CUR is relatively low and mostly metabolites of CUR, instead of CUR itself, are detected in plasma following oral consumption [125]. Its conjugation with aminoacids (such as isoleucine, phenylalanine, and valine) increases its α-secretase activation [126]. In addition, several drug delivery systems have been studied for better targeting of CUR, such as liposomes, polymeric nanoparticles, SLNs, micelles, nanogels, and complexes with dendrimer/dimer [127-132]. SLN CUR showed a great recovery in membrane lipids, as well as AChE activity, in AlCl3-treated mice and the results were comparable with those achieved by rivastigmine [133]. CUR (10 µM) also inhibited aluminum-induced oxidative stress and mitochondrial dysfunction in rat brain [134]. It can also directly bind to metals, especially iron and cupper, and thereby acts as a chelator [135].
CUR (5-10 µM) protected PC12 cells against Aβ-induced neurotoxicity by inhibiting oxidative damage, intracellular calcium influx, and tau hyperphosphorylation. Higher concentrations of CUR (50 µM) induced cellular toxicity in PC12 cells [136]. This polyphenol inhibited Aβ-induced mitochondrial depolarization of membrane potential and suppressed apoptotic factors cytochrome c, Bax and caspase-3. CUR (1-10 µM) also inhibited activated cyclin D1 protein level in Aβ-treated neurons [137]. It can also modulate cellular antioxidant enzymes, SOD and CAT. Both total and phosphorylated GSK-3β decreased in CUR-treated cells [138]. Inhibiting GSK-3β activated Wnt/β-catenin signaling and induced β-catenin (5-20 µM of CUR) [139]. CUR (5-20 µM) also inhibited GSK-3β-mediated PS1 activation and thereby decreased Aβ production [140]. Another study reported that CUR (10-4-10-2 %) promoted Aβ fibril conversion by reducing the pre-fibrillar/ oligomeric form of Aβ, resulting in reduction of neurotoxicity in Drosophila [141]. Mustuga et al. (2001) examined the binding of CUR (0.009 %) to senile plaques in brain species of several aged animals and human AD patients. Interestingly, they found that CUR specifically bound to aggregated amyloids in various animals, and further to phosphorylated tau protein, probably according to its conformational nature [142]. This binding affinity led to design of a bivalent ligand of CUR and cholesterol, BMAOI 14 that can rapidly cross BBB and stains monomeric, oligomeric and fibrillar Aβ [143,144]. Having many properties required for optical imaging and good BBB penetration made BMAOI 14 a good candidate of Aβ-imaging agent. Another group designed 1-(4-[18F]fluoroethyl)-7-(4’-methyl)curcumin as a PET radioligand for Aβ plaque imaging [144].
CUR (1 µM) ameliorated cognitive deficit by stimulating BDNF and increasing GSH levels in hippocampi and modulating NMDA receptor levels [145]. CUR (50mg/kg, i.p.) treatment also protected cultured neurons against glutamate induced cytotoxicity by mechanisms required TNFR2 [146]. Interestingly, CUR can act as a PPAR-γ agonist and reduces inflammatory responses by inhibiting NF-κB nuclear translocation and decreasing COX-2 levels in Aβ-treated astrocytes, which results in decrease of IL-1, IL-6, and TNF-α level [147,148].
Another interesting approach was provided by Gomez et al. (2007) that designed a CUR -based small molecule catalyst template for accelerating oxidative protein folding in ER through novel non-redox chemistry and by this (10-50 µM), reduced the misfolding/unfolding abnormalities within cell [149].
4.6. Huperzine A
HupA is a naturally occurring sesquiterpene alkaloid found in the firmoss Huperzia serrate [150]. HupA has been used in China for the treatment of fever and swelling. HupA has been also used as a dietary supplement for improving memory [151].
HupA displays a good pharmacokinetic profile with rapid absorption, wide distribution in body and low to moderate rate of elimination [152]. Encapsulated and micosphered formulations of HupA were designed to control the release of HupA and thereby increase its efficacy [153-157]. HupA is a strong AChEI and acts by a mechanism similar to rivastigmine, donepezil, and galantamine. New tacrine-HupA hybrids (Huprines) are potent AChEI and significantly attenuate Aβ-induced oxidative injury [158-160]. Clinical trials showed minimal adverse cholinergic effects of HupA such as dizziness, nausea, gastroenteric symptoms, headaches, and depressed heart rate. These minimal cholinergic side effects would be an advantage of HupA compared to other AChEIs for the treatment of AD [161].
HupA potentially beneficial actions include inhibiting apoptotic factors, such as caspase-3, Bax and p53, and regulating the expression and secretion of NGF [162].
HupA (0.1 mg/kg) improved learning and memory of Tg mice in Morris water maze test, mainly by activating PKC/MAPK pathway, α-secretases, BACE, and increasing phospho GSK-3β [158,163]. HupA (167 and 500 µg/kg, nasal gel) also activated Wnt/β-catenin signaling pathway. HupA neuroprotection was associated with reduced amyloid plaques and oligomeric Aβ level in the cortex and hippocampus [164]. HupA (0.1 and 1 µM) could also antagonize NMDA receptor and potassium current [165].
HupA also affected mitochondrial function by restoring enzymatic activity of respiratory chain complexes and preventing Aβ-induced ATP reduction and mitochondrial swelling [166].
4.7. Rosmarinic Acid
Rosmarinic acid (RA) is a polyphenol antioxidant carboxylic acid existed in many Lamiaceae herbs used commonly as culinary herbs, such as lemon balm (Melissa officinalis), rosemary (Rosmarinus officinalis), oregano (Origanum vulgare), sage (Salvia officinalis), thyme and peppermint [167,168]. RA possesses many biological activities including antioxidant, anti-inflammatory, anticancer, antiviral, antibacterial, and neuroprotective effects [169,170]. No severe side effect has been reported for RA.
Orally administered RA undergoes cleavage of ester bonds, selective para-dehydroxylation, methylation, and sulfate-conjugation. Approximately 83% of the total amount of these metabolites excretes in urine, 8 to 18 h after RA administration to rats [171,172].
RA (0.25-4 mg/kg, i.p.) significantly prevented Aβ-induced memory impairments, mainly by targeting NF-κB and TNF-α [173,174]. RA also reduced tau hyperphosphorylation [175]. RA (1-10 µM) could also inhibit apoptotic pathways by inhibiting ROS formation, DNA fragmentation, and caspase-3 activation [175].
4.8. Luteolin
Lu is a yellow crystalline flavonoid widely distributed in plant families of Bryophyta, Pteridophyta, Pinophyta and Magnoliophyta. Dietary sources of Lu include carrots, peppers, celery, olive oil, peppermint, thyme, rosemary and oregano [176]. Lu possesses a variety of pharmacological activities, including antioxidant, anti-inflammatory, antimicrobial, anticancer, and neuroprotective activities [177].
Pharmacokinetic analysis showed that Lu converts to glucuronides during passing through the intestinal mucosa. Some Lu could escape the intestinal conjugation and the hepatic sulfation/methylation and presented in plasma as a free Lu [178].
It has been shown that Lu (20-100 µM) efficiently attenuated zinc-induced tau hyperphosphorylation, not only by its antioxidant activity, but also through the regulation of tau phosphatase/kinase system [179]. Moreover, it down-regulated the expression of APP and lowered the secretion of Aβ [180]. Lu inhibited caspase-dependent apoptosis by reducing intracellular ROS generation, increasing SOD activity and reversing mitochondrial membrane potential dissipation [180]. Lu (10-20 µM) concentration-dependently enhanced neuronal cell survival with efficacy higher than and potency similar to vitamin E, in ROS-insulted primary neurons [181]. Lu also activated Nrf2 pathway and induced ERK1/2 activation in neuronal cells [182].
Lu modulation of long-term potentiation formation was mediated not only directly, but also by protecting synapses from the detrimental effects of chronic cerebral hypoperfusion. This effect of Lu (150-450 mg/kg) on learning and memory may be due to the activation of CREB [183].
4.9. Apigenin
Apigenin (AP) is a yellow crystalline nonmutagenic bioflavonoid presented in leafy plants and vegetables such as parsley, artichoke, basil and celery [184]. AP possesses anti-inflammatory, antioxidant, and anticancer properties [184]. There is very little evidence to date to suggest that AP promotes adverse metabolic reactions in vivo when consumed as part of a normal diet [184, 185].
AP has low solubility and high intestinal permeability. AP could be well absorbed in the whole intestine by different transport mechanisms but its main absorption site is duodenum [186]. It is also a potent inhibitor of CYP450, an enzyme responsible for the metabolism of many pharmaceutical drugs in the body [187].
AP (10-50 µM) reduced apoptotic cell death induced by thapsigargin and brefeldin A, two representative ER stress inducers, by suppressing ROS accumulation and blocking activation of caspase-12 and -3 and cleavage of PARP. It could also reduce ER stress markers, including CHOP, GRP78 and GRP94, the cleavage of ATF6, the phosphorylation of eIF2α and IRE1α. Suppression of MAPK pathway was also observed in AP-treated cells [188].
4.10. Berberine
Berberine (BBR) is a quaternary ammonium salt from the protoberberine, group of isoquinoline alkaloids found in Berberis aquifolium (Oregon grape), Berberis vulgaris (barberry), Berberis aristata (tree turmeric), Hydrastis canadensis (goldenseal), Phellodendron amurense (Amur cork tree), Coptis chinensis (Chinese goldthread), and Tinospora cordifolia [189].
Many pharmacological activities have been reported for BBR, including antioxidant activity, AChE and butyrylcholinesterase inhibition, monoamine oxidase inhibition, and cholesterol-lowering activity [190]. Gastrointestinal symptoms such as diarrhea, constipation, flatulence and abdominal pain were the main reported side effects of BBR treatment in human subjects [191, 192]. Under ultraviolet light, BBR (1.8 mM) shows a strong yellow fluorescence [193]. It has been reported that the intestinal absorption of BBR is very low. The in vivo studies indicated that the bioavailability of the BBR-loaded microemulsion formulation was significantly greater than that of the BBR tablet suspensions, suggesting the microemulsion as a promising oral drug delivery system for BBR [194].
BBR treatment (100 mg/kg, p.o.) significantly ameliorated learning, as well as long-term spatial memory retention in Tg mice [195]. Significantly decrease at the levels of C-terminal fragments of APP and the hyper-phosphorylation of APP and tau was observed in N2a mouse neuroblastoma [195]. These results were gained by the effect of BBR on Akt/GSK3 signaling pathway that regulates APP processing. BBR (1-20 µM) also attenuated tau hyperphosphorylation and Aβ production in HEK293 cells [196,197]. BBR (20 mg/kg, i.p.) could also promote the survival and differentiation of hippocampal precursor cells and axonal regeneration in injured nerves of the peripheral nervous system [198].
In addition, BBR (50 mg/kg, p.o.) significantly inhibited Aβ-stimulated production of inflammatory factors including IL-6, COX-2 and iNOS in BV2 microglia cells [199]. BBR (1-5 µM) strongly inhibited NF-κB by blocking the PI3K/protein kinase B and MAPK pathways [200].
4.11. Chitosan and its Derivatives
Chitosan is a linear polysaccharide composed of randomly distributed β-(1-4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit) [201]. Chitosan is produced commercially by deacetylation of chitin, which is the structural element in the exoskeleton of crustaceans (such as crabs and shrimp) and cell walls of fungi [202]. Most common chitosan side effects are gastric upsets but it’s not severe. No serious adverse effects were reported in clinical studies of chitosan [203, 204].
Chitosan rapidly clots blood and has recently gained approval in the United States and Europe for use in bandages and other hemostatic agents [205]. It is widely used in pharmaceutical science for targeted drug delivery [206]. Chitosan nanoparticles have been studied for delivering rivastigmine and tacrine to CNS for the treatment of AD [207-210]. More interestingly, a chitosan nanocarrier is designed as a nano-vaccine for delivering Aβ into brain [211].
Recent studies suggested that chitosan itself could protect neurons against H2O2-induced apoptosis by preventing Aβ formation and blocking intrinsic apoptosis pathway. Chitosan (0.1 and 0.5 % w/v) also upregulated Nrf2 (and its downstreams, HO-1 and γ-GCS) and inhibited NF-κB in NT2 neural cells [212]. Chitooligosaccharides (COS; 50-150 µg/ml) showed the same pattern of protection in PC12 cells by decreasing intracellular ROS and Ca2+ levels and suppressing apoptosis. It could also increase protective Hsp70 levels while down-regulated Hsp90 expression. Moreover, COS could inhibit the phosphorylation of different MAPKs, whose aberrant phosphorylation has been implicated in AD [213]. COS (25-1000 µg/ml) also had inhibitory effects on BACE-1 and inhibited Aβ-induced AChE activity [214,215].
Water soluble chitosan (10 µg/ml) also prevented inflammatory responses in Aβ-stimulated human astrocytoma cells by decreasing TNF-α and IL-6 levels, and suppressing iNOS expression [216].
5. CONCLUSION
Several natural products are used alone or in combination with other neuroprotective compounds to improve memory and cognition in AD patients. The healing power of culinary herbs and medicinal plants attracted researchers’ attention to study natural products as a potentially valuable resource for drug discovery against AD. This review discussed some natural products with potential neuroprotective properties against Aβ with respect to their mechanism of action. Although neuroprotective compounds derived from natural sources are attractive therapeutic alternatives in the treatment of AD, poor bioavailability and low clinical efficacy are the major problems. Using novel pharmaceutical technologies and medicinal chemistry to prepare novel formulations or design new compounds based on natural templates, opens up new window into using natural therapeutics agents against AD.
ACKNOWLEDGEMENTS
This work was supported by Shahid Beheshti University of Medical Sciences Research Funds.
CONFLICT OF INTEREST
The authors have no conflict of interest.
ABBREVIATIONS
- Aβ
= β-amyloid
- AChE
= acetyl cholinesterase
- AChEI
= acetyl cholinesterase inhibitor
- AD
= Alzheimer’s disease
- AP
= apigenin
- Apaf1
= apoptotic protease activating factor 1
- APP
= amyloid precursor protein
- ARE
= antioxidant response element
- BACE1
= beta-site APP cleaving enzyme 1
- BB
= bilobalide
- BBB
= blood brain barrier
- BBR
= berberine
- BDNF
= brain-derived neurotrophic factor
- CAT
= catalase
- CHOP
= C/EBP homologous protein
- COS
= chitooligosaccharides
- CREB
= cAMP-response element binding protein
- COX
= cyclooxygenase
- CUR
= curcumin
- eIF2α
= eukaryotic initiation factor 2α
- EGCG
= (-)-Epigallocatechin-3-gallate
- EOR
= ER overload response
- ER
= endoplasmic reticulum
- ERAD
= ER-associated protein degradation
- γ-GCS
= γ-glutamylcysteine synthetase
- GRP
= glucose-regulated protein
- GSK-3β
= glycogen synthase kinase 3β
- HSP
= heat shock protein
- IκB
= inhibitor of κB
- IKK
= IκB kinase
- IL
= interleukin
- IP3
= inositol 1,4,5-triphosphate receptor
- IRE1
= inositol-requiring protein 1
- Keap1
= Kelch-like ECH-associated protein 1
- LPS
= lipopolysaccharide
- Lu
= luteolin
- MAPK
= mitogen-activated protein kinase
- MDA
= malondialdehyde
- NF-κB
= nuclear factor-κB
- NGF
= nerve growth factor
- Nrf2
= NF-E2 related factor 2
- NF-κB
= nuclear factor- κB
- NMDA
= N-methyl-D-aspartate
- NO
= nitric oxide
- iNOS
= inducible nitric oxide synthase
- PARP
= poly (ADP-ribose) polymerase
- PERK
= protein kinase RNA-like ER kinase
- PKC
= protein kinase C
- PPAR-γ
= peroxisome proliferator-activated receptor γ
- PS
= presenilin
- QCT
= quercetin
- RA
= rosmarinic acid
- ROS
= ractive oxygen species
- RSV
= Resveratrol
- SOD
= superoxide dismutase
- SLN
= solid lipid nanoparticles
- TNF-α
= tumor necrosis factor
- TNFR1
= tumor necrosis factor receptor 1
- UPR
= unfolded protein response
REFERENCES
- 1.Casey G. Alzheimer's and other dementias. Nurs. N. Z. 2012;18(6): 20–4. [PubMed] [Google Scholar]
- 2.Marchbanks RM. Biochemistry of Alzheimer's dementia. J. Neurochem. 1982;39(1):9–15. doi: 10.1111/j.1471-4159.1982.tb04695.x. [DOI] [PubMed] [Google Scholar]
- 3.Blass JP, Ko L, Wisniewski HM. Pathology of Alzheimer's disease. Psychiatr. Clin. North. Am. 1991;14(2):397–420. [PubMed] [Google Scholar]
- 4.Zetterberg H, Blennow K, Hanse E. Amyloid beta and APP as biomarkers for Alzheimer's disease. Exp. Gerontol. 2010;45(1): 23–9. doi: 10.1016/j.exger.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Obulesu M, Venu R, Somashekhar R. Tau mediated neurodegeneration an insight into Alzheimer's disease pathology. Neurochem. Res. 2011;36(8):1329–35. doi: 10.1007/s11064-011-0475-5. [DOI] [PubMed] [Google Scholar]
- 6.Heston LL. Down's syndrome and Alzheimer's dementia defining an association. Psychiatry Dev. 1984; 2(4): 287–94. [PubMed] [Google Scholar]
- 7.Zhang YW, Thompson R, Zhang H, Xu H. APP processing in Alzheimer's disease. Mol. Brain . 2011;4:3. doi: 10.1186/1756-6606-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verdile G, Gandy SE, Martins RN. The role of presenilin and its interacting proteins in the biogenesis of Alzheimer's beta amyloid. Neurochem. Res. 2007;32(4-5):609–23. doi: 10.1007/s11064-006-9131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Resende R, Ferreiro E, Pereira C, Resende de Oliveira C. Neurotoxic effect of oligomeric and fibrillar species of amyloid-beta peptide 1-42: involvement of endoplasmic reticulum calcium release in oligomer-induced cell death. Neuroscience . 2008;155(3):725–37. doi: 10.1016/j.neuroscience.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 10.Farver D. The use of "natural products" in clinical medicine. S. D. J. Med. 1996;49(4):129–30. [PubMed] [Google Scholar]
- 11.Anekonda TS, Reddy PH. Can herbs provide a new generation of drugs for treating Alzheimer's disease. Brain Res. Brain Res. Rev. 2005;50(2):361–76. doi: 10.1016/j.brainresrev.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Sultana R, Butterfield DA. Role of oxidative stress in the progression of Alzheimer's disease. J. Alzheimers Dis. 2010;19(1):341–53. doi: 10.3233/JAD-2010-1222. [DOI] [PubMed] [Google Scholar]
- 13.Axelsen PH, Komatsu H, Murray IV. Oxidative stress and cell membranes in the pathogenesis of Alzheimer's disease. Physiology (Bethesda) . 2011; 26(1):54–69. doi: 10.1152/physiol.00024.2010. [DOI] [PubMed] [Google Scholar]
- 14.Wyllie AH. Apoptosis cell death in tissue regulation. J. Pathol. 1987;153:313–6. doi: 10.1002/path.1711530404. [DOI] [PubMed] [Google Scholar]
- 15.Shimohama S. Apoptosis in Alzheimer's disease--an update. Apoptosis. Source Journal. . 2000;5(1):9–16. doi: 10.1023/a:1009625323388. [DOI] [PubMed] [Google Scholar]
- 16.Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J. Neurochem. 2003;85:1026–36. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- 17.Donovan M, Cotter TG. Control of mitochondrial integrity by Bcl-2 family members and caspase-independent cell death. Biochim. Biophys. Acta . 2004;1644(2-3):133–47. doi: 10.1016/j.bbamcr.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1 a human protein homologous to C. elegans . ED-4;participates in cytochrome c-dependent activation of caspase-3. Cell 1997, 90:405–13. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 19.Putcha GV, Harris CA, Moulder KL, Easton RM, Thompson CB, Johnson EMJr. Intrinsic and extrinsic pathway signaling during neuronal apoptosis lessons from the analysis of mutant mice. J. Cell Biol. 2002;157(3):441–53. doi: 10.1083/jcb.200110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6(2):99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 21.Rapoport TA. Protein transport across the endoplasmic reticulum membrane facts, models, mysteries. FASEB. J. 1991;5: 2792–8. doi: 10.1096/fasebj.5.13.1916103. [DOI] [PubMed] [Google Scholar]
- 22.Ansari N, Khodagholi F. Molecular mechanism aspect of ER stress in Alzheimer's disease: Current approaches and future strategies. Curr. Drug Targets . 2012;[Epub ahead of print]:0. doi: 10.2174/138945013804806532. [DOI] [PubMed] [Google Scholar]
- 23.Hampton RY. ER stress response getting the UPR hand on misfolded proteins. Curr. Biol. 2000;10:R518–21. doi: 10.1016/s0960-9822(00)00583-2. [DOI] [PubMed] [Google Scholar]
- 24.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell . 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 25.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–9. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 26.Farimani MM, Sarvestani NN, Ansari N, Khodagholi F. Calycopterin promotes survival and outgrowth of neuron-like PC12 cells by attenuation of oxidative- and ER-stress-induced apoptosis along with inflammatory response. Chem. Res. Toxicol. 2011; 24(12): 2280–92. doi: 10.1021/tx200420a. [DOI] [PubMed] [Google Scholar]
- 27.Khodagholi F, Ansari N, Amini M, Tusi SK. Involvement of molecular chaperones and the transcription factor Nrf2 in neuroprotection mediated by para-substituted-4,5-diaryl-3-thiomethyl-1,2,4-triazines. Cell Stress Chaperones . 2012;17(4):409–22. doi: 10.1007/s12192-011-0316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: essential proteins for apoptosis regulation. J. Cell Mol. Med. 2008;12:743–61. doi: 10.1111/j.1582-4934.2008.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li CY, Lee JS, Ko YG, Kim JI, Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J. Biol. Chem. 2000; 275: 25665–71. doi: 10.1074/jbc.M906383199. [DOI] [PubMed] [Google Scholar]
- 30.Papp E, Nardai G, Söti C, Csermely P. Molecular chaperones, stress proteins and redox homeostasis. Biofactors . 2003;17: 249–57. doi: 10.1002/biof.5520170124. [DOI] [PubMed] [Google Scholar]
- 31.Ansari N, Khodagholi F, Amini M. 2-Ethoxy-4,5-diphenyl-1,3-oxazine-6-one activates the Nrf2/HO-1 axis and protects against oxidative stress-induced neuronal death. Eur. J. Pharmacol. 2011;658(2-3):84–90. doi: 10.1016/j.ejphar.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 32.Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011;85(4): 241–72. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 33.Rothwarf DM, Karin M. The NF-kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. Sci. STKE. 1999;1999(5):0. doi: 10.1126/stke.1999.5.re1. [DOI] [PubMed] [Google Scholar]
- 34.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat. Rev. Drug Discov. 2004;3(1):17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 35.Ansari N, Khodagholi F, Amini M, Shaerzadeh F. Attenuation of LPS-induced apoptosis in NGF-differentiated PC12 cells via NF-?B pathway and regulation of cellular redox status by an oxazine derivative. Biochimie . 2011;93(5):899–908. doi: 10.1016/j.biochi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Camandola S, Mattson MP. NF-kappa B as a therapeutic target in neurodegenerative diseases. Expert. Opin. Ther. Targets . 2007;11(2):123–32. doi: 10.1517/14728222.11.2.123. [DOI] [PubMed] [Google Scholar]
- 37.Okamoto H, Iwamoto T, Kotake S, Momohara S, Yamanaka H, Kamatani N. Inhibition of NF-kappaB signaling by fenofibrate, a peroxisome proliferator-activated receptor-alpha ligand, presents a therapeutic strategy for rheumatoid arthritis. Clin. Exp. Rheumatol. 2005; 23(3):323–30. [PubMed] [Google Scholar]
- 38.Bordet R, Ouk T, Petrault O, Gelé P, Gautier S, Laprais M, Deplanque D, Duriez P, Staels B, Fruchart JC, Bastide M. PPAR a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem. Soc. Trans. 2006;34(Pt 6):1341–6. doi: 10.1042/BST0341341. [DOI] [PubMed] [Google Scholar]
- 39.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol. Rev. 2012;92(2):689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 40.Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta . 2011;1813(9):1619–33. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Ashabi G, Ramin M, Azizi P, Taslimi Z, Alamdary SZ, Haghparast A, Ansari N, Motamedi F, Khodagholi F. ERK and p38 inhibitors attenuate memory deficits and increase CREB phosphorylation and PGC-1a levels in Aß-injected rats. Behav. Brain Res. 2012; 232(1):165–73. doi: 10.1016/j.bbr.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Mielke K, Herdegen T. JNK and p38 stresskinases--degenerative effectors of signal-transduction-cascades in the nervous system. Prog. Neurobiol. 2000;61(1):45–60. doi: 10.1016/s0301-0082(99)00042-8. [DOI] [PubMed] [Google Scholar]
- 43.Freese JL, Pino D, Pleasure SJ. Wnt signaling in development and disease. Neurobiol. Dis. 2010;38(2):148–53. doi: 10.1016/j.nbd.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Ferrari GV, Inestrosa NC. Wnt signaling function in Alzheimer's disease. Brain Res. Brain Res. Rev. 2000;33(1):1–12. doi: 10.1016/s0165-0173(00)00021-7. [DOI] [PubMed] [Google Scholar]
- 45.Cadigan KM. TCFs and Wnt/ß-catenin signaling: more than one way to throw the switch. Curr. Top. Dev. Biol. 2012;98:1–34. doi: 10.1016/B978-0-12-386499-4.00001-X. [DOI] [PubMed] [Google Scholar]
- 46.Valenta T, Hausmann G, Basler K. The many faces and functions of ß-catenin. EMBO J. 2012;31(12): 2714–36. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palacino JJ, Murphy MP, Murayama O, Iwasaki K, Fujiwara M, Takashima A, Golde TE, Wolozin B. Presenilin 1 regulates beta-catenin-mediated transcription in a glycogen synthase kinase-3-independent fashion. J. Biol. Chem. 2001; 276(42):38563–9. doi: 10.1074/jbc.M105376200. [DOI] [PubMed] [Google Scholar]
- 48.Killick R, Pollard CC, Asuni AA, Mudher AK, Richardson JC, Rupniak HT, Sheppard PW, Varndell IM, Brion JP, Levey AI, Levy OA, Vestling M, Cowburn R, Lovestone S, Anderton BH. Presenilin 1 independently regulates beta-catenin stability and transcriptional activity. J. Biol. Chem. 2001; 276(51):48554–61. doi: 10.1074/jbc.M108332200. [DOI] [PubMed] [Google Scholar]
- 49.Haas C. Strategies development and pitfalls of therapeutic options for Alzheimer's disease. J. Alzheimers Dis. 2012; 28(2): 241–81. doi: 10.3233/JAD-2011-110986. [DOI] [PubMed] [Google Scholar]
- 50.Rees TM, Brimijoin S. The role of acetylcholinesterase in the pathogenesis of Alzheimer's disease. Drugs Today (Barc). . 2003;39(1):75–83. doi: 10.1358/dot.2003.39.1.740206. [DOI] [PubMed] [Google Scholar]
- 51.Muñoz-Torrero D. Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimer's disease. Curr. Med. Chem. 2008;15(24): 2433–55. doi: 10.2174/092986708785909067. [DOI] [PubMed] [Google Scholar]
- 52.Winblad B. Donepezil in severe Alzheimer's disease. Am. J. Alzheimers Dis. Other Demen. 2009; 24(3):185–92. doi: 10.1177/1533317509332094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McKeage K. Memantine a review of its use in moderate to severe Alzheimer's disease. CNS Drugs . 2009; 23(10):881–97. doi: 10.2165/11201020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 54.Bond M, Rogers G, Peters J, Anderson R, Hoyle M, Miners A, Moxham T, Davis S, Thokala P, Wailoo A, Jeffreys M, Hyde C. The effectiveness and cost-effectiveness of donepezil galantamine rivastigmine and memantine for the treatment of Alzheimer's disease (review of Technology Appraisal No. 111) a systematic review and economic model. Health Technol. Assess . 2012;16(21):1–470. doi: 10.3310/hta16210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Becker RE, Greig NH. Alzheimer's disease drug development: old problems require new priorities. CNS Neurol. Disord. Drug Targets . 2008;7(6):499–511. doi: 10.2174/187152708787122950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Becker RE, Greig NH. Alzheimer's disease drug development in 2008 and beyond: problems and opportunities. Curr. Alzheimer Res. 2008;5(4):346–57. doi: 10.2174/156720508785132299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Irannejad H, Amini M, Khodagholi F, Ansari N, Tusi SK, Sharifzadeh M, Shafiee A. Synthesis and in vitro evaluation of novel 1,2,4-triazine derivatives as neuroprotective agents. Bioorg. Med. Chem. 2010;18(12):4224–30. doi: 10.1016/j.bmc.2010.04.097. [DOI] [PubMed] [Google Scholar]
- 58.Zhao B. Natural antioxidants protect neurons in Alzheimer's disease and Parkinson's disease. Neurochem. Res. 2009;34(4):630–8. doi: 10.1007/s11064-008-9900-9. [DOI] [PubMed] [Google Scholar]
- 59.Winblad B, Andreasen N, Minthon L, Floesser A, Imbert G, Dumortier T, Maguire RP, Blennow K, Lundmark J, Staufenbiel M, Orgogozo JM, Graf A. Safety, tolerability, and antibody response of active Aß immunotherapy with CAD106 in patients with Alzheimer's disease randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol. 2012;11(7):597–604. doi: 10.1016/S1474-4422(12)70140-0. [DOI] [PubMed] [Google Scholar]
- 60.Frakey LL, Salloway S, Buelow M, Malloy P. A randomized, double-blind, placebo-controlled trial of modafinil for the treatment of apathy in individuals with mild-to-moderate Alzheimer's disease. J. Clin. Psychiatry . 2012;73(6):796–801. doi: 10.4088/JCP.10m06708. [DOI] [PubMed] [Google Scholar]
- 61.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012;75(3):311–35. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asadi S, Ahmadiani A, Esmaeili MA, Sonboli A, Ansari N, Khodagholi F. In vitro antioxidant activities and an investigation of neuroprotection by six Salvia species from Iran: a comparative study. Food Chem. Toxicol. 2010;48(5):1341–9. doi: 10.1016/j.fct.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 63.Kuruuzum-Uz A, Suleyman H, Cadirci E, Guvenalp Z, Demirezer LO. Investigation on anti-inflammatory and antiulcer activities of Anchusa azurea extracts and their major constituent rosmarinic acid. Z. Naturforsch. C. 2012;67(7-8):360–6. doi: 10.1515/znc-2012-7-802. [DOI] [PubMed] [Google Scholar]
- 64.Defeudis FV. Bilobalide and neuroprotection. Pharmacol. Res. 2002;46(6):565–8. doi: 10.1016/s1043-6618(02)00233-5. [DOI] [PubMed] [Google Scholar]
- 65.Umegaki K, Taki Y, Endoh K, Taku K, Tanabe H, Shinozuka K, Sugiyama T. Bilobalide in Ginkgo biloba extract is a major substance inducing hepatic CYPs. J. Pharm. Pharmacol. 2007;59(6):871–7. doi: 10.1211/jpp.59.6.0014. [DOI] [PubMed] [Google Scholar]
- 66.Lang D, Ude C, Wurglics M, Schubert-Zsilavecz M, Klein J. Brain permeability of bilobalide as probed by microdialysis before and after middle cerebral artery occlusion in mice. J. Pharm. Pharm. Sci. 2010;13(4):607–14. doi: 10.18433/j31c7q. [DOI] [PubMed] [Google Scholar]
- 67.Biber A. Pharmacokinetics of Ginkgo biloba extracts. Pharmacopsychiatry . 2003;36 Suppl 1:S32–S37. doi: 10.1055/s-2003-40446. [DOI] [PubMed] [Google Scholar]
- 68.Zhou LJ, Zhu XZ. Reactive oxygen species-induced apoptosis in PC12 cells and protective effect of bilobalide. J. Pharmacol. Exp. Ther. 2000; 293(3):982–8. [PubMed] [Google Scholar]
- 69.Shi C, Zou J, Li G, Ge Z, Yao Z, Xu J. Bilobalide protects mitochondrial function in ovariectomized rats by up-regulation of mRNA and protein expression of cytochrome c oxidase subunit I. J. Mol. Neurosci. 2011;45(2):69–75. doi: 10.1007/s12031-010-9388-z. [DOI] [PubMed] [Google Scholar]
- 70.Shi C, Wu F, Xu J, Zou J. Bilobalide regulates soluble amyloid precursor protein release via phosphatidyl inositol 3 kinase-dependent pathway. Neurochem. Int. 2011;59(1):59–64. doi: 10.1016/j.neuint.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 71.Shi C, Zheng DD, Wu FM, Liu J, Xu J. The phosphatidyl inositol 3 kinase-glycogen synthase kinase 3ß pathway mediates bilobalide-induced reduction in amyloid ß-peptide. Neurochem. Res. 2012;37(2): 298–306. doi: 10.1007/s11064-011-0612-1. [DOI] [PubMed] [Google Scholar]
- 72.Tchantchou F, Lacor PN, Cao Z, Lao L, Hou Y, Cui C, Klein WL, Luo Y. Stimulation of neurogenesis and synapto- genesis by bilobalide and quercetin via common final pathway in hippocampal neurons. Alzheimers Dis. 2009;18(4):787–98. doi: 10.3233/JAD-2009-1189. [DOI] [PubMed] [Google Scholar]
- 73.Chen YS, Liu CJ, Cheng CY, Yao CH. Effect of bilobalide on peripheral nerve regeneration. Biomaterials. 2004; 25(3):509–14. doi: 10.1016/s0142-9612(03)00548-9. [DOI] [PubMed] [Google Scholar]
- 74.Kelly GS. Quercetin. Monograph. Altern. Med. Rev. 2011;16(2):172–94. [PubMed] [Google Scholar]
- 75.Ossola B, Kääriäinen TM, Männistö PT. The multiple faces of quercetin in neuroprotection. Expert. Opin. Drug Saf. 2009;8(4):397–409. doi: 10.1517/14740330903026944. [DOI] [PubMed] [Google Scholar]
- 76.Russo M, Spagnuolo C, Tedesco I, Bilotto S, Russo GL. The flavonoid quercetin in disease prevention and therapy facts and fancies. Biochem. Pharmacol. 2012;83(1):6–15. doi: 10.1016/j.bcp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 77.Bischoff SC. Quercetin potentials in the prevention and therapy of disease. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11(6):733–40. doi: 10.1097/MCO.0b013e32831394b8. [DOI] [PubMed] [Google Scholar]
- 78.Hollman PC, van Trijp JM, Buysman MN, vanderGaag MS, Mengelers MJ, deVries JH, Katan MB. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997;418(1-2):152–6. doi: 10.1016/s0014-5793(97)01367-7. [DOI] [PubMed] [Google Scholar]
- 79.Dhawan S, Kapil R, Singh B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. J. Pharm. Pharmacol. 2011;63(3):342–51. doi: 10.1111/j.2042-7158.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- 80.Ossola B, Kääriäinen TM, Männistö PT. The multiple faces of quercetin in neuroprotection. Expert Opin Drug Saf . 2009;8(4):397–409. doi: 10.1517/14740330903026944. [DOI] [PubMed] [Google Scholar]
- 81.Jiménez-Aliaga K, Bermejo-Bescós P, Benedí J, Martín-Aragón S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci. 2011;89(25-26):939–45. doi: 10.1016/j.lfs.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 82.Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Abeta(1-42): relevance to Alzheimer's disease. J. Nutr. Biochem. 2009; 20(4): 269–75. doi: 10.1016/j.jnutbio.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tchantchou F, Lacor PN, Cao Z, Lao L, Hou Y, Cui C, Klein WL, Luo Y. Stimulation of neurogenesis and synaptogenesis by bilobalide and quercetin via common final pathway in hippocampal neurons. J. Alzheimers Dis. 2009;18(4):787–98. doi: 10.3233/JAD-2009-1189. [DOI] [PubMed] [Google Scholar]
- 84.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011;82(12):1807–21. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanaka T, Miyata Y, Tamaya K, Kusano R, Matsuo Y, Tamaru S, Tanaka K, Matsui T, Maeda M, Kouno I. Increase of theaflavin gallates and thearubigins by acceleration of catechin oxidation in a new fermented tea product obtained by the tea-rolling processing of loquat ( Eriobotrya japonica ) and green tea leaves. J. Agric. Food Chem . 2009;57(13):5816–22. doi: 10.1021/jf900963p. [DOI] [PubMed] [Google Scholar]
- 86.Mandel SA, Amit T, Kalfon L, Reznichenko L, Weinreb O, Youdim MB. Cell signaling pathways and iron chelation in the neurorestorative activity of green tea polyphenols special reference to epigallocatechin gallate (EGCG) J. Alzheimers Dis. 2008;15(2): 211–22. doi: 10.3233/jad-2008-15207. [DOI] [PubMed] [Google Scholar]
- 87.Gawande S, Kale A, Kotwal S. Effect of nutrient mixture and black grapes on the pharmacokinetics of orally administered (-) epigallocatechin-3-gallate from green tea extract a human study. Phytother. Res. 2008; 22(6):802–8. doi: 10.1002/ptr.2372. [DOI] [PubMed] [Google Scholar]
- 88.Lambert JD, Lee MJ, Lu H, Meng X, Hong JJ, Seril DN, Sturgill MG, Yang CS. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J. Nutr . 2003;133(12):4172–7. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- 89.Smith A, Giunta B, Bickford PC, Fountain M, Tan J, Shytle RD. Nanolipidic particles improve the bioavailability and alpha-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer's disease. Int. J. Pharm. 2010;389(1-2): 207–12. doi: 10.1016/j.ijpharm.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Biasibetti R, Tramontina AC, Costa AP, Dutra MF, Quincozes-Santos A, Nardin P, Bernardi CL, Wartchow KM, Lunardi PS, Gonçalves CA. Green tea (-)epigallocatechin-3-gallate reverses oxidative stress and reduces acetylcholinesterase activity in a streptozotocin-induced model of dementia. Behav. Brain Res. 2012; 236C:186–193. doi: 10.1016/j.bbr.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 91.Lee JW, Lee YK, Ban JO, Ha TY, Yun YP, Han SB, Oh KW, Hong JT. Green tea (-)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-kappaB pathways in mice. J. Nutr. 2009;139(10):1987–93. doi: 10.3945/jn.109.109785. [DOI] [PubMed] [Google Scholar]
- 92.Rezai-Zadeh K, Arendash GW, Hou H, Fernandez F, Jensen M, Runfeldt M, Shytle RD, Tan J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008;1214:177–87. doi: 10.1016/j.brainres.2008.02.107. [DOI] [PubMed] [Google Scholar]
- 93.Reznichenko L, Amit T, Zheng H, Avramovich-Tirosh Y, Youdim MB, Weinreb O, Mandel S. Reduction of iron-regulated amyloid precursor protein and beta-amyloid peptide by (-)-epigallocatechin-3-gallate in cell cultures: implications for iron chelation in Alzheimer's disease. J. Neurochem . 2006;97(2):527–36. doi: 10.1111/j.1471-4159.2006.03770.x. [DOI] [PubMed] [Google Scholar]
- 94.Li R, Huang YG, Fang D, Le WD. (-)-Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J. Neurosci. Res. 2004;78(5):723–31. doi: 10.1002/jnr.20315. [DOI] [PubMed] [Google Scholar]
- 95.Lee YJ, Choi DY, Yun YP, Han SB, Oh KW, Hong JT. Epigallocatechin-3-gallate prevents systemic inflammation-induced memory deficiency and amyloidogenesis via its anti-neuroinflammatory properties. J. Nutr. Biochem. 2012; 24(1): 298–310. doi: 10.1016/j.jnutbio.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 96.Pae M, Ren Z, Meydani M, Shang F, Smith D, Meydani SN, Wu D. Dietary supplementation with high dose of epigallocatechin-3-gallate promotes inflammatory response in mice. J. Nutr. Biochem. 2012; 23(6):526–31. doi: 10.1016/j.jnutbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 97.Kim SJ, Jeong HJ, Lee KM, Myung NY, An NH, Yang WM, Park SK, Lee HJ, Hong SH, Kim HM, Um JY. Epigallocatechin-3-gallate suppresses NF-kappaB activation and phosphorylation of p38 MAPK and JNK in human astrocytoma U373MG cells. J. Nutr. Biochem. 2007;18(9):587–96. doi: 10.1016/j.jnutbio.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 98.He Y, Cui J, Lee JC, Ding S, Chalimoniuk M, Simonyi A, Sun AY, Gu Z, Weisman GA, Wood WG, Sun GY. Prolonged exposure of cortical neurons to oligomeric amyloid-ß impairs NMDA receptor function via NADPH oxidase-mediated ROS production protective effect of green tea (-)-epigallocatechin-3-gallate. ASN Neuro. 2011;3(1):e00050. doi: 10.1042/AN20100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dragicevic N, Smith A, Lin X, Yuan F, Copes N, Delic V, Tan J, Cao C, Shytle RD, Bradshaw PC. Green tea epigallocatechin-3-gallate (EGCG) and other flavonoids reduce Alzheimer's amyloid-induced mitochondrial dysfunction. J. Alzheimers Dis. 2011; 26(3):507–21. doi: 10.3233/JAD-2011-101629. [DOI] [PubMed] [Google Scholar]
- 100.Zhang L, Cao H, Wen J, Xu M. Green tea polyphenol (-)-epigallocatechin-3-gallate enhances the inhibitory effect of huperzine A on acetylcholinesterase by increasing the affinity with serum albumin. Nutr. Neurosci. 2009;12(4):142–8. doi: 10.1179/147683009X423283. [DOI] [PubMed] [Google Scholar]
- 101.Bhat KPL, Kosmeder JW 2nd, Pezzuto JM. Biological effects of resveratrol. Antioxid. Redox Signal. 2001;3(6):1041–64. doi: 10.1089/152308601317203567. [DOI] [PubMed] [Google Scholar]
- 102.Shen T, Wang XN, Lou HX. Natural stilbenes an overview. Nat. Prod. Rep. 2009; 26(7):916–35. doi: 10.1039/b905960a. [DOI] [PubMed] [Google Scholar]
- 103.Mullin GE. Red wine, grapes, and better health--resveratrol. Nutr. Clin. Pract. 2011; 26(6):722–3. doi: 10.1177/0884533611423927. [DOI] [PubMed] [Google Scholar]
- 104.Li H, Xia N, Förstermann U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide . 2012; 26(2):102–10. doi: 10.1016/j.niox.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 105.Li F, Gong Q, Dong H, Shi J. Resveratrol, a neuroprotective supplement for Alzheimer's disease. Curr. Pharm. Des. 2012;18(1): 27–33. doi: 10.2174/138161212798919075. [DOI] [PubMed] [Google Scholar]
- 106.Waheed A. [No authors listed] Resveratrol. Monograph. Altern Med Rev. 2010;15(2):152–8. [PubMed] [Google Scholar]
- 107.Almeida L, Vaz-da-Silva M, Falcão A, Soares E, Costa R, Loureiro AI, Fernandes-Lopes C, Rocha JF, Nunes T, Wright L, Soares-da-Silva P. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009;53 Suppl 1:S7–15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 108.Neves AR, Lucio M, Lima JL, Reis S. Resveratrol in medicinal chemistry a critical review of its pharmacokinetics, drug-delivery, and membrane interactions. Curr. Med. Chem. 2012;19(11):1663–81. doi: 10.2174/092986712799945085. [DOI] [PubMed] [Google Scholar]
- 109.Frozza R.L. Bernardi, A. Paese, K. Hoppe, J.B. da Silva, T. Battastini, A.M. Pohlmann, A.R. Guterres, S.S. Salbego C. Characterization of trans-resveratrol-loaded lipid-core nanocapsules and tissue distribution studies in rats. J. Biomed. Nanotechnol. 2010;6(6):694–703. doi: 10.1166/jbn.2010.1161. [DOI] [PubMed] [Google Scholar]
- 110.Kumar A, Naidu PS, Seghal N, Padi SS. Neuroprotective effects of resveratrol against intracerebroventricular colchicine-induced cognitive impairment and oxidative stress in rats. Pharmacology . 2007;79(1):17–26. doi: 10.1159/000097511. [DOI] [PubMed] [Google Scholar]
- 111.Zhang JQ, Wu PF, Long LH, Chen Y, Hu ZL, Ni L, Wang F, Chen JG. Resveratrol promotes cellular glucose utilization in primary cultured cortical neurons via calcium-dependent signaling pathway. J. Nutr. Biochem. 2013; 24(4): 629–37. doi: 10.1016/j.jnutbio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 112.Li F, Gong Q, Dong H, Shi J. Resveratrol, a neuroprotective supplement for Alzheimer's disease. Curr. Pharm. Des. 2012;18(1): 27–33. doi: 10.2174/138161212798919075. [DOI] [PubMed] [Google Scholar]
- 113.Ge JF, Qiao JP, Qi CC, Wang CW, Zhou JN. The binding of resveratrol to monomer and fibril amyloid beta. Neurochem. Int. 2012;61(7):1192–201. doi: 10.1016/j.neuint.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 114.Luo L, Huang YM. [Effect of resveratrol on the cognitive ability of Alzheimeros mice] Zhong. Nan. Da. Xue. Xue. Bao. Yi. Xue. Ban. 2006;31(4):566–9. [PubMed] [Google Scholar]
- 115.Wight RD, Tull CA, Deel MW, Stroope BL, Eubanks AG, Chavis JA, Drew PD, Hensley LL. Resveratrol effects on astrocyte function. Relevance to neurodegenerative diseases. Biochem. Biophys. Res. Commun. 2012;426(1):112–5. doi: 10.1016/j.bbrc.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee EO, Park HJ, Kang JL, Kim HS, Chong YH. Resveratrol reduces glutamate-mediated monocyte chemotactic protein-1 expression via inhibition of extracellular signal-regulated kinase 1/2 pathway in rat hippocampal slice cultures. J. Neurochem. 2010;112(6):1477–87. doi: 10.1111/j.1471-4159.2009.06564.x. [DOI] [PubMed] [Google Scholar]
- 117.Pallàs M, Casadesús G, Smith MA, Coto-Montes A, Pelegri C, Vilaplana J, Camins A. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr. Neurovasc. Res. 2009;6(1):70–81. doi: 10.2174/156720209787466019. [DOI] [PubMed] [Google Scholar]
- 118.Capiralla H, Vingtdeux V, Zhao H, Sankowski R, Al-Abed Y, Davies P, Marambaud P. Resveratrol mitigates lipopolysaccharide- and Aß-mediated microglial inflammation by inhibiting the TLR4/NF-?B/STAT signaling cascade. J. Neurochem. 2012;120(3):461–72. doi: 10.1111/j.1471-4159.2011.07594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kwon KJ, Kim HJ, Shin CY, Han SH. Melatonin Potentiates the Neuroprotective Properties of Resveratrol Against Beta-Amyloid-Induced Neurodegeneration by Modulating AMP-Activated Protein Kinase Pathways. J. Clin. Neurol. 2010;6(3):127–37. doi: 10.3988/jcn.2010.6.3.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Esatbeyoglu T, Huebbe P, Ernst IM, Chin D, Wagner AE, Rimbach G. Curcumin--from molecule to biological function. Angew. Chem. Int. Ed. Engl. 2012;51(22):5308–32. doi: 10.1002/anie.201107724. [DOI] [PubMed] [Google Scholar]
- 121.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin the Indian solid gold. Adv. Exp. Med. Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 122.Srivastava RM, Singh S, Dubey SK, Misra K, Khar A. Immunomodulatory and therapeutic activity of curcumin. Int. Immunopharmacol. 2011;11(3):331–41. doi: 10.1016/j.intimp.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 123.Hsu CH, Cheng AL. Clinical studies with curcumin. Adv. Exp. Med. Biol. 2007;595:471–80. doi: 10.1007/978-0-387-46401-5_21. [DOI] [PubMed] [Google Scholar]
- 124.Marathe SA, Dasgupta I, Gnanadhas DP, Chakravortty D. Multifaceted roles of curcumin: two sides of a coin! Expert Opin. Biol. Ther. 2011;11(11):1485–99. doi: 10.1517/14712598.2011.623124. [DOI] [PubMed] [Google Scholar]
- 125.Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. Adv. Exp. Med. Biol. 2007;595:453–70. doi: 10.1007/978-0-387-46401-5_20. [DOI] [PubMed] [Google Scholar]
- 126.Narasingapa RB, Jargaval MR, Pullabhatla S, Htoo HH, Rao JK, Hernandez JF, Govitrapong P, Vincent B. Activation of a-secretase by curcumin-aminoacid conjugates. Biochem. Biophys. Res. Commun. 2012;424(4):691–6. doi: 10.1016/j.bbrc.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 127.Sun M, Su X, Ding B, He X, Liu X, Yu A, Lou H, Zhai G. Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine (Lond) . 2012;7(7):1085–100. doi: 10.2217/nnm.12.80. [DOI] [PubMed] [Google Scholar]
- 128.Doggui S, Sahni JK, Arseneault M, Dao L, Ramassamy C. Neuronal uptake and neuroprotective effect of curcumin-loaded PLGA nanoparticles on the human SK-N-SH cell line. J. Alzheimers Dis. 2012;30(2):377–92. doi: 10.3233/JAD-2012-112141. [DOI] [PubMed] [Google Scholar]
- 129.Mathew A, Fukuda T, Nagaoka Y, Hasumura T, Morimoto H, Yoshida Y, Maekawa T, Venugopal K, Kumar DS. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer's disease. PLoS One . 2012;7(3):e32616. doi: 10.1371/journal.pone.0032616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kakkar V, Kaur IP. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem. Toxicol. 2011;49(11): 2906–13. doi: 10.1016/j.fct.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 131.Taylor M, Moore S, Mourtas S, Niarakis A, Re F, Zona C, La Ferla B, Nicotra F, Masserini M, Antimisiaris SG, Gregori M, Allsop D. Effect of curcumin-associated and lipid ligand-functionalized nanoliposomes on aggregation of the Alzheimer's Aß peptide. Nanomedicine . 2011;7(5):541–50. doi: 10.1016/j.nano.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 132.Ray B, Bisht S, Maitra A, Maitra A, Lahiri DK. Neuroprotective and neurorescue effects of a novel polymeric nanoparticle formulation of curcumin (NanoCurc™) in the neuronal cell culture and animal model: implications for Alzheimer's disease. J. Alzheimers Dis. 2011; 23(1):61–77. doi: 10.3233/JAD-2010-101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alam S, Panda JJ, Chauhan VS. Novel dipeptide nanoparticles for effective curcumin delivery. Int. J. Nanomed. 2012;7:4207–21. doi: 10.2147/IJN.S33015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jiang T, Zhi XL, Zhang YH, Pan LF, Zhou P. Inhibitory effect of curcumin on the Al(III)-induced Aß42 aggregation and neurotoxicity in vitro. Biochim. Biophys. Acta . 2012;1822(8):1207–15. doi: 10.1016/j.bbadis.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 135.Mishra S, Palanivelu K. The effect of curcumin (turmeric) on Alzheimer's disease: An overview. Ann. Indian Acad. Neurol. 2008;11(1):13–9. doi: 10.4103/0972-2327.40220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Park SY, Kim HS, Cho EK, Kwon BY, Phark S, Hwang KW, Sul D. Curcumin protected PC12 cells against beta-amyloid-induced toxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Food Chem. Toxicol. 2008;46(8): 2881–7. doi: 10.1016/j.fct.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 137.Wang J, Zhang YJ, Du S. The protective effect of curcumin on Aß induced aberrant cell cycle reentry on primary cultured rat cortical neurons. Eur. Rev. Med. Pharmacol. Sci. 2012;16(4):445–54. [PubMed] [Google Scholar]
- 138.Huang HC, Xu K, Jiang ZF. Curcumin-Mediated Neuroprotection Against Amyloid-ß-Induced Mitochondrial Dysfunction Involves the Inhibition of GSK-3ß. J. Alzheimers Dis. 2012;32(4):981–96. doi: 10.3233/JAD-2012-120688. [DOI] [PubMed] [Google Scholar]
- 139.Zhang X, Yin WK, Shi XD, Li Y. Curcumin activates Wnt/ß-catenin signaling pathway through inhibiting the activity of GSK-3ß in APPswe transfected SY5Y cells. Eur. J. Pharm. Sci. 2011;42(5):540–6. doi: 10.1016/j.ejps.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 140.Xiong Z, Hongmei Z, Lu S, Yu L. Curcumin mediates presenilin-1 activity to reduce ß-amyloid production in a model of Alzheimer's disease. Pharmacol. Rep. 2011;63(5):1101–8. doi: 10.1016/s1734-1140(11)70629-6. [DOI] [PubMed] [Google Scholar]
- 141.Caesar I, Jonson M, Nilsson KP, Thor S, Hammarström P. Curcumin promotes A-beta fibrillation and reduces neurotoxicity in transgenic Drosophila. PLoS One . 2012;7(2):e31424. doi: 10.1371/journal.pone.0031424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mutsuga M, Chambers JK, Uchida K, Tei M, Makibuchi T, Mizorogi T, Takashima A, Nakayama H. Binding of curcumin to senile plaques and cerebral amyloid angiopathy in the aged brain of various animals and to neurofibrillary tangles in Alzheimer's brain. J. Vet. Med. Sci. 2012;74(1):51–7. doi: 10.1292/jvms.11-0307. [DOI] [PubMed] [Google Scholar]
- 143.Lenhart JA, Ling X, Gandhi R, Guo TL, Gerk PM, Brunzell DH, Zhang S. Clicked" bivalent ligands containing curcumin and cholesterol as multifunctional abeta oligomerization inhibitors: design, synthesis, and biological characterization. J. Med. Chem. 2010;53(16):6198–209. doi: 10.1021/jm100601q. [DOI] [PubMed] [Google Scholar]
- 144.Liu K, Guo TL, Chojnacki J, Lee HG, Wang X, Siedlak SL, Rao W, Zhu X, Zhang S. Bivalent ligand containing curcumin and cholesterol as fluorescence probe for Aß plaques in Alzheimer's disease. ACS Chem. Neurosci. 2012;3(2):141–146. doi: 10.1021/cn200122j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lin MS, Hung KS, Chiu WT, Sun YY, Tsai SH, Lin JW, Lee YH. Curcumin enhances neuronal survival in N-methyl-d-aspartic acid toxicity by inducing RANTES expression in astrocytes via PI-3K and MAPK signaling pathways. Prog. Neuropsychopharmacol. Biol. Psychiatry . 2011;35(4):931–8. doi: 10.1016/j.pnpbp.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 146.Kawamoto EM, Scavone C, Mattson MP, Camandola S. Curcumin Requires Tumor Necrosis Factor a Signaling to Alleviate Cognitive Impairment Elicited by Lipopolysaccharide. Neurosignals . 2013; 21(1-2):75–88. doi: 10.1159/000336074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang HM, Zhao YX, Zhang S, Liu GD, Kang WY, Tang HD, Ding JQ, Chen SD. PPARgamma agonist curcumin reduces the amyloid-beta-stimulated inflammatory responses in primary astrocytes. J. Alzheimers Dis. 2010; 20(4):1189–99. doi: 10.3233/JAD-2010-091336. [DOI] [PubMed] [Google Scholar]
- 148.Sikora E, Scapagnini G, Barbagallo M. Curcumin, inflammation, ageing and age-related diseases. Immun. Ageing . 2010;7(1):1. doi: 10.1186/1742-4933-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gomez G, Mansouraty G, Gardea J, Narayan M. Acceleration of oxidative protein folding by curcumin through novel non-redox chemistry. Biochem. Biophys. Res. Commun. 2007;364(3):561–6. doi: 10.1016/j.bbrc.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 150.Pepping J, Huperzine A. Am J Health Syst. Pharm Source. 2000;57(6):530: 533–4. doi: 10.1093/ajhp/57.6.530. [DOI] [PubMed] [Google Scholar]
- 151.Ha GT, Wong RK, Zhang Y. Huperzine a as potential treatment of Alzheimer's disease an assessment on chemistry, pharmacology, and clinical studies. Chem. Biodivers. 2011;8(7):1189–204. doi: 10.1002/cbdv.201000269. [DOI] [PubMed] [Google Scholar]
- 152.Qian BC, Wang M, Zhou ZF, Chen K, Zhou RR, Chen GSl. Pharmacokinetics of tablet huperzine A in six volunteers. Zhongguo. Yao. Li. Xue. Bao. 1995;16(5):396–8. [PubMed] [Google Scholar]
- 153.Gao P, Xu H, Ding P, Gao Q, Sun J, Chen D. Controlled release of huperzine A from biodegradable microspheres: In vitro and in vivo studies. Int. J. Pharm. 2007;330(1-2):1–5. doi: 10.1016/j.ijpharm.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 154.Fu X, Ping Q, Gao Y. Effects of formulation factors on encapsulation efficiency and release behaviour in vitro of huperzine A-PLGA microspheres. J. Microencapsul. 2005; 22(7):705–14. doi: 10.1080/02652040500162196. [DOI] [PubMed] [Google Scholar]
- 155.Gao P, Ding P, Xu H, Yuan Z, Chen D, Wei J, Chen D. In vitro and in vivo characterization of huperzine a loaded microspheres made from end-group uncapped poly(d,l-lactide acid) and poly(d,l-lactide-co-glycolide acid) Chem. Pharm. Bull. (Tokyo) . 2006;54(1):89–93. doi: 10.1248/cpb.54.89. [DOI] [PubMed] [Google Scholar]
- 156.Liu WH, Song JL, Liu K, Chu DF, Li YX. Preparation and in vitro and in vivo release studies of Huperzine A loaded microspheres for the treatment of Alzheimer's disease. J. Control Release . 2005;107(3):417–27. doi: 10.1016/j.jconrel.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 157.Fu X, Ping Q, Gao Y. Effects of formulation factors on encapsulation efficiency and release behaviour in vitro of huperzine A-PLGA microspheres. J. Microencapsul. 2005; 22(1):57–66. doi: 10.1080/02652040400026509. [DOI] [PubMed] [Google Scholar]
- 158.Ratia M, Giménez-Llort L, Camps P, Muñoz-Torrero D, Pérez B, Clos MV, Badia A, Huprine X, Huperzine A. Improve Cognition and Regulate Some Neurochemical Processes Related with Alzheimer's Disease in Triple Transgenic Mice. (3xTg-AD). Neurodegener. Dis. 2012;11(3):129–40. doi: 10.1159/000336427. [DOI] [PubMed] [Google Scholar]
- 159.Camps P, El Achab R, Morral J, Muñoz-Torrero D, Badia A, Baños JE, Vivas NM, Barril X, Orozco M, Luque FJ. New tacrine-huperzine A hybrids (huprines): highly potent tight-binding acetylcholinesterase inhibitors of interest for the treatment of Alzheimer's disease. J. Med. Chem. 2000;43(24):4657–66. doi: 10.1021/jm000980y. [DOI] [PubMed] [Google Scholar]
- 160.Camps P, Muñoz-Torrero D. Tacrine-huperzine A hybrids (huprines) a new class of highly potent and selective acetylcholinesterase inhibitors of interest for the treatment of Alzheimer's disease. Mini Rev. Med. Chem. 2001;1(2):163–74. doi: 10.2174/1389557013406972. [DOI] [PubMed] [Google Scholar]
- 161.Ha GT, Wong RK, Zhang Y. Huperzine a as potential treatment of Alzheimer's disease an assessment on chemistry, pharmacology, and clinical studies. Chem Biodivers. 2011;8(7):1189–204. doi: 10.1002/cbdv.201000269. [DOI] [PubMed] [Google Scholar]
- 162.Zhang HY, Tang XC. Neuroprotective effects of huperzine A: new therapeutic targets for neurodegenerative disease. Trends Pharmacol. Sci. 2006; 27(12):619–25. doi: 10.1016/j.tips.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 163.Wang Y, Tang XC, Zhang HY. Huperzine A alleviates synaptic deficits and modulates amyloidogenic and nonamyloidogenic pathways in APPswe/PS1dE9 transgenic mice. J. Neurosci. Res. 2012;90(2):508–17. doi: 10.1002/jnr.22775. [DOI] [PubMed] [Google Scholar]
- 164.Wang CY, Zheng W, Wang T, Xie JW, Wang SL, Zhao BL, Teng WP, Wang ZY. Huperzine A activates Wnt/ß-catenin signaling and enhances the nonamyloidogenic pathway in an Alzheimer transgenic mouse model. Neuropsychopharmacology . 2011;36(5):1073–89. doi: 10.1038/npp.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gordon RK, Nigam SV, Weitz JA, Dave JR, Doctor BP, Ved HS. The NMDA receptor ion channel: a site for binding of Huperzine A. J. Appl. Toxicol. 2001; 21 Suppl 1:S47–51. doi: 10.1002/jat.805. [DOI] [PubMed] [Google Scholar]
- 166.Yang L, Ye CY, Huang XT, Tang XC, Zhang HY. Decreased accumulation of subcellular amyloid-ß with improved mitochondrial function mediates the neuroprotective effect of huperzine A. J. Alzheimers Dis. 2012;31(1):131–42. doi: 10.3233/JAD-2012-120274. [DOI] [PubMed] [Google Scholar]
- 167.Shekarchi M, Hajimehdipoor H, Saeidnia S, Gohari AR, Hamedani MP. Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharmacogn. Mag. 2012;8(29):37–41. doi: 10.4103/0973-1296.93316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Grzegorczyk I, Królicka A, Wysokinska H. Establishment of Salvia officinalis L hairy root cultures for the production of rosmarinic acid. Z. Naturforsch. C. 2006;61(5-6):351–6. doi: 10.1515/znc-2006-5-609. [DOI] [PubMed] [Google Scholar]
- 169.Petersen M, Simmonds MS. Rosmarinic acid. Phytochemistry . 2003;62(2):121–5. doi: 10.1016/s0031-9422(02)00513-7. [DOI] [PubMed] [Google Scholar]
- 170.Shaerzadeh F, Ahmadiani A, Esmaeili MA, Ansari N, Asadi S, Tusi SK, Sonboli A, Ghahremanzamaneh M, Khodagholi F. Antioxidant and antiglycating activities of Salvia sahendica and its protective effect against oxidative stress in neuron-like PC12 cells. J. Nat. Med. 2011;65(3-4):455–65. doi: 10.1007/s11418-011-0519-9. [DOI] [PubMed] [Google Scholar]
- 171.Nakazawa T, Ohsawa K. Metabolism of rosmarinic acid in rats. J. Nat. Prod. 1998;61(8):993–6. doi: 10.1021/np980072s. [DOI] [PubMed] [Google Scholar]
- 172.Baba S, Osakabe N, Natsume M, Terao J. Orally administered rosmarinic acid is present as the conjugated and/or methylated forms in plasma, and is degraded and metabolized to conjugated forms of caffeic acid, ferulic acid and m-coumaric acid. Life Sci. 2004;75(2):165–78. doi: 10.1016/j.lfs.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 173.Bulgakov VP, Inyushkina YV, Fedoreyev SA. Rosmarinic acid and its derivatives: biotechnology and applications. Crit. Rev. Biotechnol. 2012;32(3): 203–17. doi: 10.3109/07388551.2011.596804. [DOI] [PubMed] [Google Scholar]
- 174.Alkam T, Nitta A, Mizoguchi H, Itoh A, Nabeshima T. A natural scavenger of peroxynitrites, rosmarinic acid, protects against impairment of memory induced by Abeta(25-35) Behav. Brain Res. 2007;180(2):139–45. doi: 10.1016/j.bbr.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 175.Iuvone T, De Filippis D, Esposito G, D'Amico A, Izzo AA. The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-beta peptide-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2006;317(3):1143–9. doi: 10.1124/jpet.105.099317. [DOI] [PubMed] [Google Scholar]
- 176.López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009;9(1):31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- 177.Seelinger G, Merfort I, Schempp CM. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta. Med. 2008;74(14):1667–77. doi: 10.1055/s-0028-1088314. [DOI] [PubMed] [Google Scholar]
- 178.Shimoi K, Okada H, Furugori M, Goda T, Takase S, Suzuki M, Hara Y, Yamamoto H, Kinae N. Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans. FEBS Lett. 1998;438(3): 220–4. doi: 10.1016/s0014-5793(98)01304-0. [DOI] [PubMed] [Google Scholar]
- 179.Zhou F, Chen S, Xiong J, Li Y, Qu L. Luteolin Reduces Zinc-Induced Tau Phosphorylation at Ser262/356 in an ROS-Dependent Manner in SH-SY5Y Cells. Biol. Trace Elem. Res. 2012;149(2): 273–9. doi: 10.1007/s12011-012-9411-z. [DOI] [PubMed] [Google Scholar]
- 180.Liu R, Meng F, Zhang L, Liu A, Qin H, Lan X, Li L, Du G. Luteolin isolated from the medicinal plant Elsholtzia rugulosa (Labiatae) prevents copper-mediated toxicity in ß-amyloid precursor protein Swedish mutation overexpressing SH-SY5Y cells. Molecules . 2011;16(3): 2084–96. doi: 10.3390/molecules16032084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhao G, Yao-Yue C, Qin GW, Guo LH. Luteolin from Purple Perilla mitigates ROS insult particularly in primary neurons. Neurobiol. Aging . 2012;33(1):176–86. doi: 10.1016/j.neurobiolaging.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 182.Wruck CJ, Claussen M, Fuhrmann G, Römer L, Schulz A, Pufe T, Waetzig V, Peipp M, Herdegen T, Götz ME. Luteolin protects rat PC12 and C6 cells against MPP+ induced toxicity via an ERK dependent Keap1-Nrf2-ARE pathway. J. Neural. Transm. Suppl. 2007;(72):57–67. doi: 10.1007/978-3-211-73574-9_9. [DOI] [PubMed] [Google Scholar]
- 183.Xu B, Li XX, He GR, Hu JJ, Mu X, Tian S, Du GH. Luteolin promotes long-term potentiation and improves cognitive functions in chronic cerebral hypoperfused rats. Eur. J. Pharmacol. 2010;627(1-3):99–105. doi: 10.1016/j.ejphar.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 184.Shukla S, Gupta S. Apigenin a promising molecule for cancer prevention. Pharm. Res. 2010; 27(6):962–78. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hollman PC, Katan MB. Health effects and bioavailability of dietary flavonols. Free Radic Res. 1999;31 Suppl:S75–80. doi: 10.1080/10715769900301351. [DOI] [PubMed] [Google Scholar]
- 186.Zhang J, Liu D, Huang Y, Gao Y, Qian S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int. J. Pharm. 2012;436(1-2):311–7. doi: 10.1016/j.ijpharm.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 187.Gradolatto A, Canivenc-Lavier MC, Basly JP, Siess MH, Teyssier C. Metabolism of apigenin by rat liver phase I and phase ii enzymes and by isolated perfused rat liver. Drug Metab. Dispos. 2004;32(1):58–65. doi: 10.1124/dmd.32.1.58. [DOI] [PubMed] [Google Scholar]
- 188.Choi AY, Choi JH, Lee JY, Yoon KS, Choe W, Ha J, Yeo EJ, Kang I. Apigenin protects HT22 murine hippocampal neuronal cells against endoplasmic reticulum stress-induced apoptosis. Neurochem. Int. 2010;57(2):143–52. doi: 10.1016/j.neuint.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 189.Kulkarni SK, Dhir A. Berberine a plant alkaloid with therapeutic potential for central nervous system disorders. Phytother. Res. 2010; 24(3):317–24. doi: 10.1002/ptr.2968. [DOI] [PubMed] [Google Scholar]
- 190.Vuddanda PR, Chakraborty S, Singh S. Berberine a potential phytochemical with multispectrum therapeutic activities. Expert Opin. Investig Drugs . 2010;19(10):1297–307. doi: 10.1517/13543784.2010.517745. [DOI] [PubMed] [Google Scholar]
- 191.Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712–717. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang Y, Li X, Zou D, Liu W, Yang J, Zhu N, Huo L, Wang M, Hong J, Wu P, Ren G, Ning G. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J. Clin. Endocrinol. Metab. 2008;93: 2559–2565. doi: 10.1210/jc.2007-2404. [DOI] [PubMed] [Google Scholar]
- 193.Reyes R, Ramírez G, Delgado NM. Fluorescent berberine binding as a marker of internal glycosaminoglycans sulfate in bovine oocytes and sperm cells. Arch. Androl. 2004;50(5):327–32. doi: 10.1080/01485010490474733. [DOI] [PubMed] [Google Scholar]
- 194.Gui SY, Wu L, Peng DY, Liu QY, Yin BP, Shen JZ. Preparation and evaluation of a microemulsion for oral delivery of berberine. Pharmazie. 2008;63(7):516–9. [PubMed] [Google Scholar]
- 195.Durairajan SS, Liu LF, Lu JH, Chen LL, Yuan Q, Chung SK, Huang L, Li XS, Huang JD, Li M. Berberine ameliorates ß-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer's disease transgenic mouse model. Neurobiol. Aging . 2012;33(12): 2903–19. doi: 10.1016/j.neurobiolaging.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 196.Zhu F, Wu F, Ma Y, Liu G, Li Z, Sun Y, Pei Z. Decrease in the production of ß-amyloid by berberine inhibition of the expression of ß-secretase in HEK293 cells. BMC Neurosci. 2011;12:125. doi: 10.1186/1471-2202-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yu G, Li Y, Tian Q, Liu R, Wang Q, Wang JZ, Wang X. Berberine attenuates calyculin A-induced cytotoxicity and Tau hyperphosphorylation in HEK293 cells. J. Alzheimers Dis. 2011; 24(3):525–35. doi: 10.3233/JAD-2011-101779. [DOI] [PubMed] [Google Scholar]
- 198.Han AM, Heo H, Kwon YK. Berberine promotes axonal regeneration in injured nerves of the peripheral nervous system. J. Med. Food . 2012;15(4):413–7. doi: 10.1089/jmf.2011.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhu F, Qian C. Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin-1beta and inducible nitric oxide synthase in the rat model of Alzheimer's disease. BMC Neurosci. 2006;7:78. doi: 10.1186/1471-2202-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jia L, Liu J, Song Z, Pan X, Chen L, Cui X, Wang M. berine suppresses amyloid-beta-induced inflammatory response in microglia by inhibiting nuclear factor-kappaB and mitogen-activated protein kinase signaling pathways. J. Pharm. Pharmacol. 2012;64(10):1510–21. doi: 10.1111/j.2042-7158.2012.01529.x. [DOI] [PubMed] [Google Scholar]
- 201.Chandy T, Sharma CP. Chitosan--as a biomaterial. Biomater. Artif. Cells Artif. Organs . 1990;18(1):1–24. doi: 10.3109/10731199009117286. [DOI] [PubMed] [Google Scholar]
- 202.Pacheco N, Garnica-Gonzalez M, Gimeno M, Bárzana E, Trombotto S, David L, Shirai K. Structural characterization of chitin and chitosan obtained by biological and chemical methods. Biomacromolecules . 2011;12(9):3285–90. doi: 10.1021/bm200750t. [DOI] [PubMed] [Google Scholar]
- 203.Pittler MH, Abbot NC, Harkness EF, Ernst E. Randomized, double-blind trial of chitosan for body weight reduction. Eur. J. Clin. Nutr. 1999;53(5):379–81. doi: 10.1038/sj.ejcn.1600733. [DOI] [PubMed] [Google Scholar]
- 204.Bokura H, Kobayashi S. Chitosan decreases total cholesterol in women a randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2003;57(5):721–5. doi: 10.1038/sj.ejcn.1601603. [DOI] [PubMed] [Google Scholar]
- 205.Chou CP, Wang YC, Chang SJ, Liu PH, Kuo SM. Evaluation of the Effects of Chitosan Hemostasis Dressings on Hemorrhage Caused by Breast Biopsy. Breast Care (Basel) . 2012;7(3): 220–224. doi: 10.1159/000339687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mengatto LN, Helbling IM, Luna JA. Recent advances in chitosan films for controlled release of drugs. Recent Pat. Drug Deliv. Formul. 2012;6(2):156–70. doi: 10.2174/187221112800672967. [DOI] [PubMed] [Google Scholar]
- 207.Fazil M, Md S, Haque S, Kumar M, Baboota S, Sahni JK, Ali J. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur. J. Pharm. Sci. 2012;47(1):6–15. doi: 10.1016/j.ejps.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 208.Wilson B, Samanta MK, Muthu MS, Vinothapooshan G. Design and evaluation of chitosan nanoparticles as novel drug carrier for the delivery of rivastigmine to treat Alzheimer's disease. Ther. Deliv. 2011; 2(5):599–609. doi: 10.4155/tde.11.21. [DOI] [PubMed] [Google Scholar]
- 209.Wilson B, Samanta MK, Santhi K, Kumar KP, Ramasamy M, Suresh B. Chitosan nanoparticles as a new delivery system for the anti-Alzheimer drug tacrine. Nanomedicine . 2010;6(1):144–52. doi: 10.1016/j.nano.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 210.Wilson B, Samanta MK, Santhi K, Sampath Kumar KP, Ramasamy M, Suresh B. Significant delivery of tacrine into the brain using magnetic chitosan microparticles for treating Alzheimer's disease. J. Neurosci. Methods . 2009;177(2):427–33. doi: 10.1016/j.jneumeth.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 211.Songjiang Z, Lixiang W. Amyloid-beta associated with chitosan nano-carrier has favorable immunogenicity and permeates the BBB. AAPS Pharm. Sci. Tech. 2009;10(3):900–5. doi: 10.1208/s12249-009-9279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Khodagholi F, Eftekharzadeh B, Maghsoudi N, Rezaei PF. Chitosan prevents oxidative stress-induced amyloid beta formation and cytotoxicity in NT2 neurons: involvement of transcription factors Nrf2 and NF-kappaB. Mol. Cell Biochem. 2010;337(1-2):39–51. doi: 10.1007/s11010-009-0284-1. [DOI] [PubMed] [Google Scholar]
- 213.Joodi G, Ansari N, Khodagholi F. Chitooligosaccharide-mediated neuroprotection is associated with modulation of Hsps expression and reduction of MAPK phosphorylation. Int. J. Biol. Macromol. 2011;48(5):726–35. doi: 10.1016/j.ijbiomac.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 214.Eom TK, Ryu B, Lee JK, Byun HG, Park SJ, Kim SK. ß-secretase inhibitory activity of phenolic acid conjugated chitooligosaccharides. J. Enzyme Inhib. Med. Chem. 2012; 28(1): 214–7. doi: 10.3109/14756366.2011.629197. [DOI] [PubMed] [Google Scholar]
- 215.Lee SH, Park JS, Kim SK, Ahn CB, Je JY. Chitooligosaccharides suppress the level of protein expression and acetylcholinesterase activity induced by Abeta25-35 in PC12 cells. Bioorg. Med. Chem. Lett. 2009;19(3):860–2. doi: 10.1016/j.bmcl.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 216.Kim MS, Sung MJ, Seo SB, Yoo SJ, Lim WK, Kim HM. Water-soluble chitosan inhibits the production of pro-inflammatory cytokine in human astrocytoma cells activated by amyloid beta peptide and interleukin-1beta. Neurosci. Lett. 2002;321(1-2):105–9. doi: 10.1016/s0304-3940(02)00066-6. [DOI] [PubMed] [Google Scholar]