Summary
Phospholipase A1 activities have been detected in most cells where they have been sought and yet their characterization lags far behind that of the phospholipases A2, C and D. The study presented here details the first cloning and characterization of a cytosolic PLA1 that exhibits preference for phosphatidylcholine (GPCho) substrates. Trypanosoma brucei phospholipase A1 (TbPLA1) is unique from previously identified eukaryotic PLA1 because it is evolutionarily related to bacterial secreted PLA1. A T. brucei ancestor most likely acquired the PLA1 from a horizontal gene transfer of a PLA1 from Sodalis glossinidius, a bacterial endosymbiont of tsetse flies. Nano-electrospray ionization tandem mass spectrometry analysis of TbPLA1 mutants established that the enzyme functions in vivo to synthesize lysoGPCho metabolites containing long-chain mostly polyunsaturated and highly unsaturated fatty acids. Analysis of purified mutated recombinant forms of TbPLA1 revealed that this enzyme is a serine hydrolase whose catalytic mechanism involves a triad consisting of the amino acid residues Ser-131, His-234 and Asp-183. The TbPLA1 homozygous null mutants generated here constitute the only PLA1 double knockouts from any organism.
Introduction
Phospholipase A1 (PLA1) (EC 3.1.1.32) specifically hydrolyses the sn-1 acyl esters from phospholipids (PLs) producing free fatty acids (FAs) and lysophospholipids (lysoPLs). PLA1 activities have been described in numerous cell types and tissues from a diverse range of organisms, but despite their apparent ubiquity and diversity relatively very few PLA1s have been cloned and characterized.
A minority of PLA1s cloned thus far possess a cytosolic subcellular localization, all three of which are homologues of one another and specifically utilize phosphatidic acid (GPA)1 as substrate (Higgs et al., 1998; Tani et al., 1999; Nakajima et al., 2002). PLA1 enzymes whose primary amino acid sequences exhibit homology with neutral lipase sequences can be categorized into a separate subgroup; these PLA1, like their lipase homologues, are secreted from their cell (Soldatova et al., 1993; Hoffman, 1994; King et al., 1996; Sato et al., 1997; Watanabe et al., 1999; Sonoda et al., 2002). The preferred substrates observed for this group of enzymes so far are GPA (Sonoda et al., 2002) and phosphatidylserine (GPSer) (Sato et al., 1997). Secreted PLA1 from bacteria that are not homologous to either lipases or mammalian PA-PLA1 have also been cloned (Givskov et al., 1988; Schmiel et al., 1998). Finally, two unrelated sequences cloned from Arabidopsis thaliana code for PLA1s localized to organelles (Ishiguro et al., 2001; Noiriel et al., 2004); a third (Kato et al., 2002) is homologous to mammalian PA-PLA1 (Higgs et al., 1998).
The biological significance of any of the various PLA1 forms is not well understood and has not been explored in depth. Mounting evidence, however, suggests that some PLA1 act to generate bioactive lipid molecules, a role normally thought to be reserved for the phospholipases A2, C and D. PS-PLA1 secreted from platelets selectively forms the lipid mediator lysoGPSer (Sato et al., 1997) which can effectively stimulate histamine release from mast cells (Hosono et al., 2001). Also, a PLA1 from A. thaliana has been shown to catalyse the release of linoleic acid, the initial and regulatory step in jasmonic acid biosynthesis, a multifunctional growth regulator in plants (Ishiguro et al., 2001). Furthermore, two GPA-utilizing PLA1s are suspected to play a role in the regulation of intracellular membrane transport (Tani et al., 1999; Nakajima et al., 2002; Shimoi et al., 2005). PLA1 have also been theorized to co-ordinate with other phospholipases to form lipid messengers such as lysoGPA, which has been shown to be produced by a PLD/PA-PLA1 concerted mechanism and to activate the cellular receptor EDG7/LPA3 in Sf9 cells (Sonoda et al., 2002).
Likewise, it has been postulated for decades that sn-2 FA cleavage from PLs may sometimes be mediated by concerted sequential PLA1/LysoPLA activities (van den Bosch, 1980; Arthur, 1989; Burgoyne and Morgan, 1990; Badiani and Arthur, 1991; Pete et al., 1996; Pete and Exton, 1996; Wang et al., 1997; Ridgley and Ruben, 2001; Gauster et al., 2005). A relevant consequence of this type of reaction could be, for example, the discharge of arachidonic acid (AA), which can act as a second messenger and precursor of eicosanoids (Gijon et al., 2000).
The PLA1/LysoPLA route towards the release of unsaturated FAs has been supported indirectly by in vitro studies using soluble subcellular fractions from bovine brain (Pete et al., 1996) and from the kinetoplastid protozoan parasite Trypanosoma brucei, the infective agent responsible for African Sleeping Sickness (Ridgley and Ruben, 2001). Both of these studies observed the requirement of sn-1 acyl cleavage prior to the release of AA from sn-1-palmitoyl-sn-2-arachidonoyl-sn-glycero-3-phosphatidylcholine [GPCho(16:0/20:4)]. In support of this, a soluble PLA1 and a soluble LysoPLA, the latter of which selectively deacylates lysoGPCho(−/20:4), have been chromatographically purified from bovine brain (Pete et al., 1994; Pete and Exton, 1996). Moreover, LysoPLA activities in guinea pig microsomes have been observed to show preference for lysoGPCho(−/20:4) and lysoGPEth(−/20:4) (Arthur, 1989; Badiani and Arthur, 1991). Sequence identification of the enzymes responsible for the PLA1 and LysoPLA activities from any of the examples outlined above has not been made.
The fate of unsaturated FAs liberated from lipids in T. brucei is not certain, but free AA has been implicated in regulating calcium mobilization in the cells (Eintracht et al., 1998; Catisti et al., 2000). Alternatively, the acid has been shown to serve as a precursor for prostaglandin biosynthesis in T. brucei (Kubata et al., 2000). Though studies on the utilization of unsaturated FAs in T. brucei have been initiated, none of the phospholipases responsible for FA release from PLs have been identified. However, sn-1 FA cleavage from GPCho by T. brucei homogenates has been observed to be robust and optimal at pH 6.0–8.5; and this activity was attributed to PLA1 activity in this organism (Tizard et al., 1978a; Hambrey et al., 1981; Opperdoes and van Roy, 1982). The homogenates possessed PLA1 activity that was 10-fold greater than its LysoPLA activity, though both activities appeared to originate from the same soluble enzyme fraction (Sage et al., 1981). A 380-fold enrichment in PLA1 activity was achieved and the enzyme was purported to be 26 kDa (Hambrey et al., 1984). Fractions enriched in PLA1 activity were unable to cleave myristate from the glycosylphosphatidylinositol anchor of the variant surface glycoprotein (Hambrey et al., 1986).
To date, the only cloned and characterized phospholipase in T. brucei is GPI-PLC, which is a phospholipase C implicated in the cleavage of the variant surface glycoprotein from its glycosylphosphatidylinositol anchor (Hereld et al., 1988; Carrington et al., 1998; Gruszynski et al., 2006). This report details the identification and characterization of a cytosolic PLA1 from T. brucei that deacylates GPCho containing long polyunsaturated and highly unsaturated FAs.
Results
Identification and cloning of a T. brucei PLA1 gene that has a prokaryotic origin
In order to identify putative PLA1 genes in the T. brucei genome, a comprehensive search for phospholipases and lipases was carried out using the T. brucei genome database (GeneDB, Sanger Institute Pathogen Sequencing Unit, strain 927). Thirteen genes encoding putative lipolytic enzymes were identified, most of which contained the lipase consensus pattern [LIV] – {KG} – [LIVFY] – [LIVMST] – G – [HYWV] – S – {YAG} – G – [GSTAC] in their deduced amino acid sequence. The predicted protein sequence of each gene was subjected to local alignment analysis. Results revealed that one peptide sequence from T. brucei showed sequence similarity to PhlA, a secreted PLA1 from the bacterium Serratia liquefaciens (Givskov et al., 1988). The T. brucei phospholipase A1 (TbPLA1) was only homologous to bacterial PLA1 isoforms, including YplA, a PhlA homologue from Yersinia enterocolitica (Schmiel et al., 1998).
Very interestingly, the homologue with the greatest overall similarity to TbPLA1 is a putative PLA1 from the proteobacterium Sodalis glossinidius (morsitans strain) (Toh et al., 2006), an intracellular endosymbiont of Glossina (tsetse) flies, the insect vector host of T. brucei. There are no eukaryotic homologues of TbPLA1, including in two other kinetoplastid members Trypanosoma cruzi and Leishmania spp., the aetiologic agents of Chagas disease and Leishmaniasis respectively. However, orthologues of TbPLA1 were found in existing GeneDB coding sequences from the livestock-infective Trypanosoma congolense and Trypanosoma vivax. TbPLA1 does not share similarity to neutral lipases nor other previously reported eukaryotic PLA1 sequences, apart from possessing a lipase consensus motif.
Using sequence-specific primers, putative TbPLA1 was amplified from T. brucei genomic DNA (Lister 427 strain) by PCR and the 903 bp open reading frame (ORF) sequenced (GenBank™ accession number AJ716154). An alignment of the TbPLA1 predicted amino acid sequence with those of the other Trypanosoma spp. and bacterial homologues is shown (Fig. 1). The deduced primary sequence of TbPLA1 shares 21.5%, 18% and 18% identity with its homologues from S. glossinidius, S. liquefaciens and Y. enterocolitica respectively; whereas T. congolense and T. vivax possess 41% and 37% identity with TbPLA1 respectively.
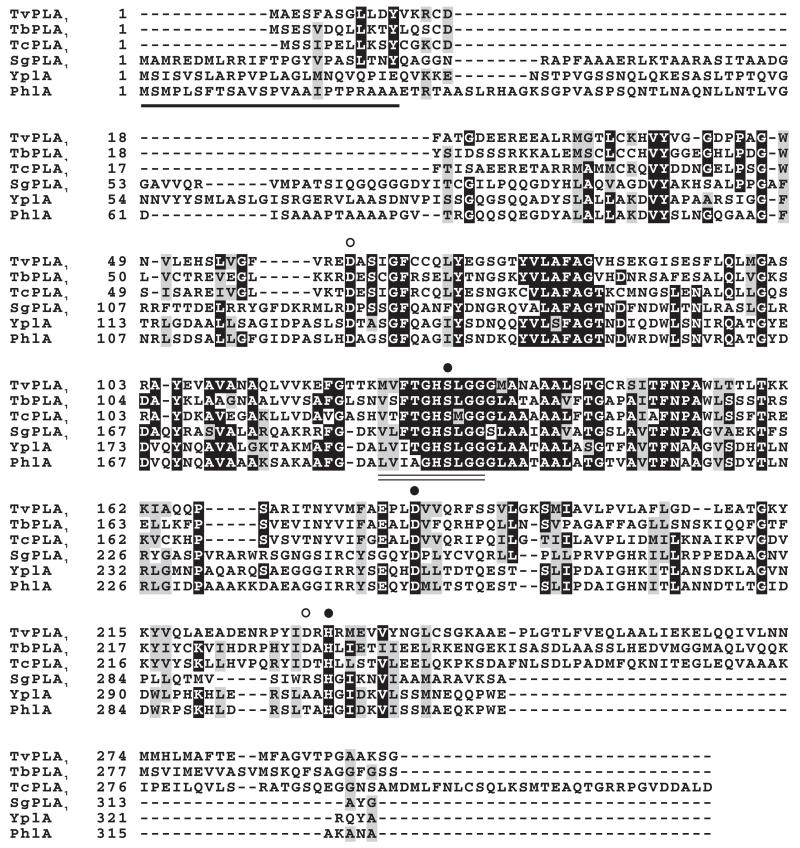
Fig. 1.
Multiple sequence alignment of trypanosome and bacterial PLA1. Amino acid sequences were aligned with MUSCLE (Edgar, 2004) and edited using the BOXSHADE algorithm. Amino acid residues below circles represent those which were mutated to reveal active-site components (closed circles) and non-active-site residues (open circles). The lipase consensus pattern is underscored by a double line and the signal sequence utilized for PhlA secretion (Givskov et al., 1988) is underlined. TcPLA1, Trypanosoma congolense PLA1 (product of GeneDB Systematic name congo50h08.q1k_9); TbPLA1, Trypanosoma brucei PLA1 (Accession Number CAG29794); TvPLA1, Trypanosoma vivax PLA1 (product of GeneDB Systematic name Tviv1355g06.p1k_2); SgPLA1, Sodalis glossinidius PLA1 (Accession Number Q2NQT2); YplA, Yersinia enterocolitica PLA1 (Accession Number O85477); PhlA, Serratia liquefaciens PLA1 (Accession Number P18952).
The bacterial sequences possess a signal sequence used in targeted secretion that appears to be absent or very altered and unrecognizable (by the SIGNALP algorithm) in the trypanosome sequences. This finding was the first suggestion of an intracellular localization for TbPLA1. All in all, these results provide the first evidence that TbPLA1 is a unique eukaryotic PLA1 because it is solely related to PLA1 from bacteria. Furthermore, the high similarity of the TbPLA1 sequence to that of a bacterial endosymbiont of the vector responsible for transmission of T. brucei parasites to mammals strongly suggests that a T. brucei ancestor acquired the PLA1 gene through horizontal gene transfer (HGT) during cohabitation with the bacterium inside an infected tsetse fly.
Expressed and purified recombinant TbPLA1 exhibits sn-1 exclusivity
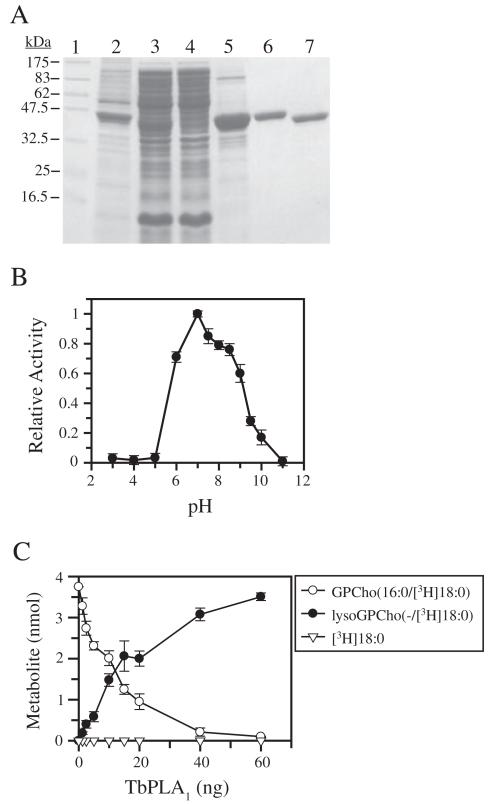
To determine whether TbPLA1 could cleave FAs esterified to the sn-1 position of PLs, a source of pure and active protein with which to carry out in vitro analysis was sought using recombinant protein expression techniques. The entire TbPLA1 ORF was subcloned into the expression vector pET15b. Subsequent transformation of Escherichia coli and induction of TbPLA1 expression by IPTG produced de novo synthesized soluble TbPLA1 with an N-terminal His·tag (Fig. 2A, lane 3). The soluble enzyme was purified to homogeneity using nickel affinity chromatography followed by anion exchange (Fig. 2A, lanes 6 and 7). The pure His·tag-cleaved protein consistently migrated as a larger molecular weight protein by SDS-PAGE than expected from its predicted 34.80 kDa size, though peptide mass fingerprinting and MALDI-TOF mass spectrometry analysis shows that the protein is indeed TbPLA1 with a nominal molecular weight of 34.98 kDa respectively (data not shown).
Fig. 2.
Purification and regiospecificity of recombinant TbPLA1.
A. SDS-PAGE analysis of proteins from TbPLA1-overexpressing E. coli cells during various purification steps as compared with molecular weight standards (lane 1). The various fractions include insoluble proteins (lane 2), the soluble protein fraction containing His·tagged recombinant TbPLA1 (lane 3), nickel column flow through (lane 4), nickel affinity eluate (lane 5), purified His·tagged recombinant TbPLA1 after anion exchange (~3 μg) (lane 6), purified His·tag-cleaved TbPLA1 after thrombin digestion and anion exchange (~3 μg) (lane 7).
B. Phospholipase activity was measured at various pH values using the fluorescent BODIPY®C11-PC assay at a mole fraction of 0.018 with a final concentration of substrate at 0.075 mM. A universal buffer containing 50 mM Tri-sodium citrate, 50 mM Tris and 50 mM NaCl was used to obtain the various pH conditions. Data points represent the activity measured relative to the maximal pH-activity value of pH 7.0. Data represent the average from two experiments performed in triplicate.
C. Metabolism of GPCho(16:0/[3H]18:0) after a 5 min reaction is shown as a function of varying amounts of recombinant TbPLA1. Formation of lysoGPCho(−/[3H]18:0) appears to be dose-dependent, whereas [3H]18:0 acid cleavage from the sn-2 position is undetectable.
The pH dependence for recombinant TbPLA1 was assessed using the Bis-BODIPY® FL-C11-PC assay. Results demonstrated that the enzyme shows highest specific activity between pH 6.0 and 8.5, and that enzyme activity is abolished at low pH (Fig. 2B). The optimal activity was detected at pH 7.0 and this pH was used in all other subsequent measurements of TbPLA1 activity.
The regiospecificity of cleavage for recombinant TbPLA1 was initially examined by incubating it with radiolabelled GPCho(16:0/[3H]18:0). This substrate was presented to the enzyme in the form of TX-100/GPCho mixed micelles. Both the formation of free [3H]18:0 acid and lysoGPCho(−/[3H]18:0) was monitored as a function of TbPLA1 concentration (Fig. 2C). Results clearly show that lysoGPCho(−/[3H]18:0) formation mirrors the metabolism of GPCho(16:0/[3H]18:0), indicating sn-1 activity. TbPLA1 apparently has no LysoPLA2 activity because the resultant product from the first reaction, lysoGPCho(−/[3H]18:0), cannot serve as a substrate for any subsequent reactions, based on the observation that [3H]18:0 acid from position sn-2 was not detected. When Ca2+, sometimes required as a cofactor for soluble PLA activity, was included in the assay, no [3H]18:0 acid was seen to accumulate and no enhancement of sn-1 activity was observed. Taken together, these results indicated that recombinant TbPLA1 hydrolyses FA esters exclusively at the sn-1 position of the glycerol backbone of GPCho.
TbPLA1 most efficiently hydrolyses GPCho molecules
The ability of recombinant enzyme to hydrolyse sn-1 hydrocarbon chains of neutral lipid substrates and PLs containing different headgroups was investigated by quantifying any free FA reaction products by gas chromatograph mass spectrometer (GC-MS) after their derivatization into methyl esters. A summary analysis of the reaction products from an assortment of lipid substrates indicated that purified TbPLA1 deacylated a variety of choline-containing PLs with greater efficiency than PLs containing ethanolamine, inositol, serine, or just phosphorous at the sn-3 position (Table 1). When acyl chain length is held constant with palmitate, substrate preference was observed, in decreasing order, as GPCho(16:0/16:0)> GPSer(16:0/16:0)>>GPA(16:0/16:0)=GPEth(16:0/16:0), the latter two were hydrolysed at around 1/10 the rate of GPCho(16:0/16:0).
Table 1.
Hydrolysis of lipid substrates by TbPLA1
| Substrate | PLA1 activitya (μmol min−1 mg−1 |
|---|---|
| GPCho(16:0/18:1) | 120.2 ± 18.7 |
| GPCho(18:0/20:4) | 118.4 ± 5.4 |
| GPCho(16:0/14:0) | 114.3 ± 6.0 |
| GPCho(14:0/18:0) | 110.9 ± 2.0 |
| GPCho(14:0/14:0)b | 100.4 ± 8.7 |
| GPCho(16:0/16:0)b | 84.8 ± 7.4 |
| GPCho(16:0/18:0) | 78.5 ± 7.9 |
| GPCho(18:0/18:0)b | 54.0 ± 7.6 |
| GPSer(16:0/16:0)b | 49.2 ± 7.6 |
| lysoGPCho(14:0/−) | 16.1 ± 1.0 |
| GPA(16:0/16:0)b | 8.6 ± 0.7 |
| GPEth(16:0/16:0)b | 7.3 ± 2.4 |
| Bovine liver GPInsb,c | 0.7 ± 0.04d |
| DG(16:0/16:0)b | 0.5 ± 0.06d |
| TG(18:0/18:0/18:0) | NDd |
| GPCho(1-0-16:0/16:0) | ND |
Assays were conducted as described under Experimental procedures. Activity is expressed as micromoles of sn-1 FA released per min mg−1 of protein. Values are means ± SD, n = 3 determinations, each performed in triplicate. ND, none detected.
Regiospecificity of FA hydrolysis detected cannot be discerned with this substrate.
The only FAME detected was that from stearic acid (C18:0), which comprises nearly 50% of the FAs in bovine liver GPIns.
1.85 μg of enzyme was added instead of 0.1 μg, and the reaction was extended to 20 min. Less than 10% of substrate was turned over.
TbPLA1 displayed some LysoPLA1 activity towards lysoGPCho(14:0/−) that amounted to 14.5% and 16.0% of its PLA1 activity towards GPCho(14:0/18:0) and GPCho(14:0/14:0) respectively. A variety of molecular species of GPCho were hydrolysed with average rates between 54.0 and 120.2 μmol min−1 mg−1. Diacylglycerol (DG) and bovine liver GPIns showed exceedingly poor substrate capabilities because their rate of hydrolysis was less than 1/100 the rate of GPCho even when 18.5-fold more enzyme was added. No fatty acyl hydrolysis from plasmalogen GPCho or from triacylglycerol (TG) could be detected. These results show that TbPLA1 exhibits robust activity towards GPCho and that GPCho, and to a lesser extent GPSer, is the preferred substrate for the enzyme in vitro.
TbPLA1 activity is mediated by a Ser-His-Asp catalytic triad
Although TbPLA1 is not homologous to neutral lipases, its amino acid sequence contains a lipase consensus pattern that harbours a conserved GXSXG motif (Fig. 1), an indicator that TbPLA1 may be a member of the serine hydrolase superfamily. The catalytic triad in these enzymes is usually occupied by a base residue (His), an acid (Asp) and a nucleophile (Ser), the latter of which is identifiable as the S in the GXSXG motif. The segments of sequence in which the base and acid residues lie are not as obvious due to the regions’ generally lower degree of conservation, but the acid is often located well between the nucleophile and base. Based on this information it was predicted that Asp-183 could be an acid residue involved in TbPLA1 catalysis (Fig. 1).
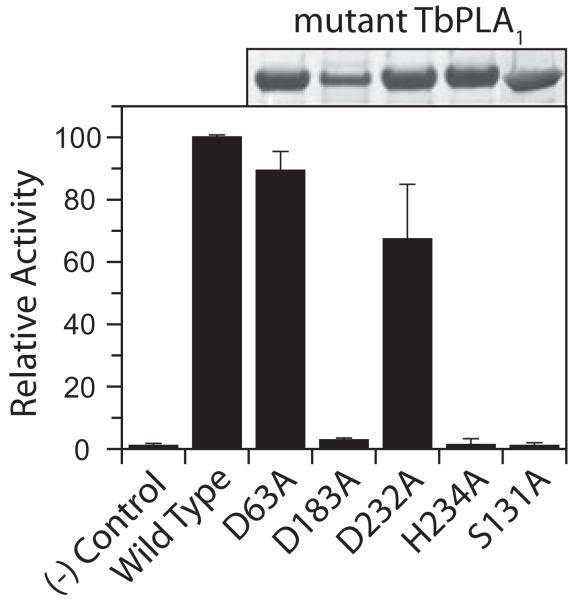
Nevertheless, two conserved aspartic acid residues in recombinant TbPLA1 (Asp-63 and Asp-183), along with a third (Asp-232), which is conserved in the trypanosomes, were mutated to alanine by site-directed mutagenesis in order to compare activity to recombinant wild-type (WT) protein. Mutant enzyme activity was also examined after replacing the T. brucei GXSXG serine (Ser-131) and the only conserved histidine residue (His-234) to alanine. All of the mutant TbPLA1 proteins were expressed and purified at the same levels as WT TbPLA1 with the exception of the D183A mutant, which produced fourfold less protein (Fig. 3). The activity of the D63A and D232A mutants maintained an average of 89% and 67% of recombinant WT activity respectively, implying that the presence of these residues is not obligatory for TbPLA1 activity. The TbPLA1 activity measured for the D183A, H234A and S131A mutants was less than 5% of the activity observed for the WT enzyme, but nearly equal to that of the no-enzyme control (Fig. 3). The negation of activity for three enzymes containing individual serine, histidine and aspartic acid residue mutations suggests that hydrolysis of substrate was prevented due to an interruption of a charge relay system that is known to be invoked by the Ser-His-Asp catalytic triads in serine hydrolases.
Fig. 3.
Probing for active-site residues. Mutant TbPLA1 proteins were expressed and purified from E. coli in parallel as stated in the Experimental procedures and 10 μl of the final eluate was analysed by SDS-PAGE (above graph). The radiolabelled PLA1 assay was used with 5 ng of purified WT and mutant TbPLA1 to compare substrate hydrolysis after 10 min; a no-enzyme negative control was tested in parallel. Mutant activities are shown as relative percentages of WT activity (26 μmol min−1 mg−1).
Generation of TbPLA1 mutants
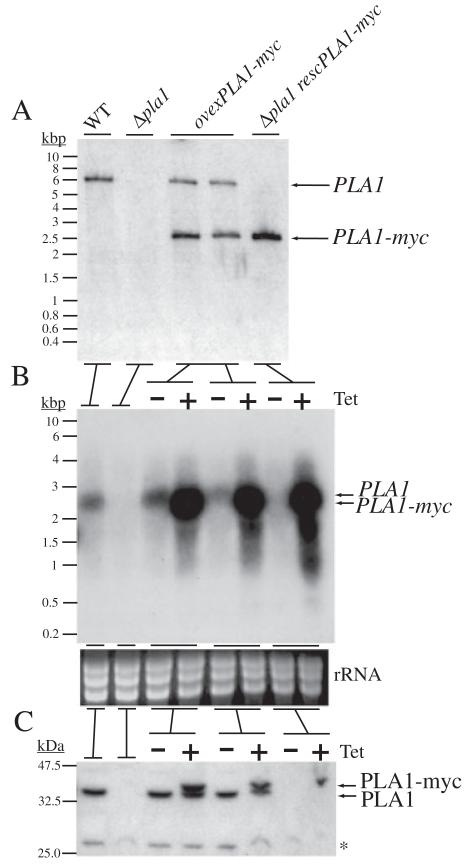
As no PLA1 has ever before been directly studied in vivo in its native cell, the next line of experiments sought to do just that in order to gain insight into TbPLA1 function in the trypanosome itself. To set conditions for which to accomplish this, experiments which led to the creation of a double knockout of TbPLA1 were performed. Promoterless deletion constructs (5′-puroR-3′, 5′-bsdR-3′) targeted to the 5′ and 3′ flanking regions of the TbPLA1 ORF were generated and genes for antibiotic resistance were cloned between the flanking regions. These constructs were used to stably transform PCF stage ‘WT’ cell lines, which are in fact transgenic clones that constitutively express T7 RNA polymerase and the tetracycline repressor protein (Wirtz et al., 1994). TbPLA1 is a single copy gene (data not shown) and therefore two successive rounds of gene replacement by UTR-targeted homologous recombination with 5′-puroR-3′ and 5′-bsdR-3′ were sufficient in this case to replace the two alleles of TbPLA1. Evidence for successful generation of the null mutant cell line Δpla1 is shown (Fig. 4A).
Fig. 4.
Validation and characterization of TbPLA1 mutant cell lines.
A. TbPLA1 null mutants were generated in the procyclic form of T. brucei after sequential TbPLA1 UTR-targeted homologous recombination with UTR-flanked puromycin and blasticidin resistance genes. Cell lines were analysed by Southern blot after digesting genomic DNA with EcoRV. EcoRV-digested T. brucei WT gDNA reveals one size of DNA fragment detectable by fluorescein-labelled TbPLA1 ORF probe. In TbPLA1 null mutant cells (Δpla1) both TbPLA1 alleles are absent. A tetracycline (Tet)-inducible myc-tagged recombinant ectopic copy of TbPLA1 (PLA1-myc) cloned into a phleomycin-resistant pLEW100 cassette was introduced into a different locus in WT gDNA to produce transgenic TbPLA1 overexpression cell lines (ovexPLA1-myc), or introduced in the null mutant gDNA to produce a rescue cell line (Δpla1 rescPLA1-myc).
B. Northern blot analysis of TbPLA1 mutants. Total RNA extracted from WT and TbPLA1 mutant trypanosomes were hybridized with TbPLA1 (top panels), loading controls are also presented (bottom panels).
C. Western blot analysis of cell lysates of TbPLA1 mutants. Both native PLA1 (theoretically 32.4 kDa) and tagged PLA1-myc (theoretically 33.9 kDa) proteins were detected by antibodies raised against the purified recombinant enzyme. * = background bands that show loading in the null mutant lanes.
Genetic transformation was also carried out that served to generate cell lines which overexpressed TbPLA1 using the T. brucei tetracycline-inducible overexpression vector pLEW100 (Wirtz et al., 1999). pLEW100/PLA1-myc was engineered by cloning a recombinant form of TbPLA1 into pLEW100. Upon tetracycline induction, trypanosomes transformed with pLEW100/PLA1-myc produce an ectopic copy of TbPLA1 with a C-terminal myc tag (PLA1-myc). In PCF cells, both the WT and Δpla1 cell lines were stably transformed with pLEW100/PLA1-myc (Fig. 4A). The respective ovexPLA1-myc overexpression and Δpla1 rescPLA1-myc rescue clones, under tetracycline induction, produced recombinant ectopic TbPLA1 mRNA transcripts that were, on average, > 23-fold over the level of TbPLA1 transcripts in both their WT and respective uninduced counterpart cells (Fig. 4B). In culture, the TbPLA1 null mutants and tetracycline-induced TbPLA1 overexpression cell lines have no apparent phenotype in morphology or in growth rate (data not shown).
The various overexpression clones were examined for their ability to translate the overexpressed TbPLA1-myc mRNA. A Western blot probed with anti-TbPLA1 antibodies revealed that overexpression of PLA1-myc was minimal when compared with the level of mRNA overexpression (Fig. 4C). Compared with uninduced ovexPLA1-myc cells, its induced counterpart cells produced, on average, only 2.2 times the amount of TbPLA1 protein (native PLA1 + PLA1-myc). This observed level of regulation at the level of mRNA production to protein translation is not surprising considering the potential damage a membrane-lytic enzyme such as TbPLA1 could invoke if expressed at uncontrollable levels in the cell.
TbPLA1 synthesizes unsaturated lysoGPCho metabolites in vivo
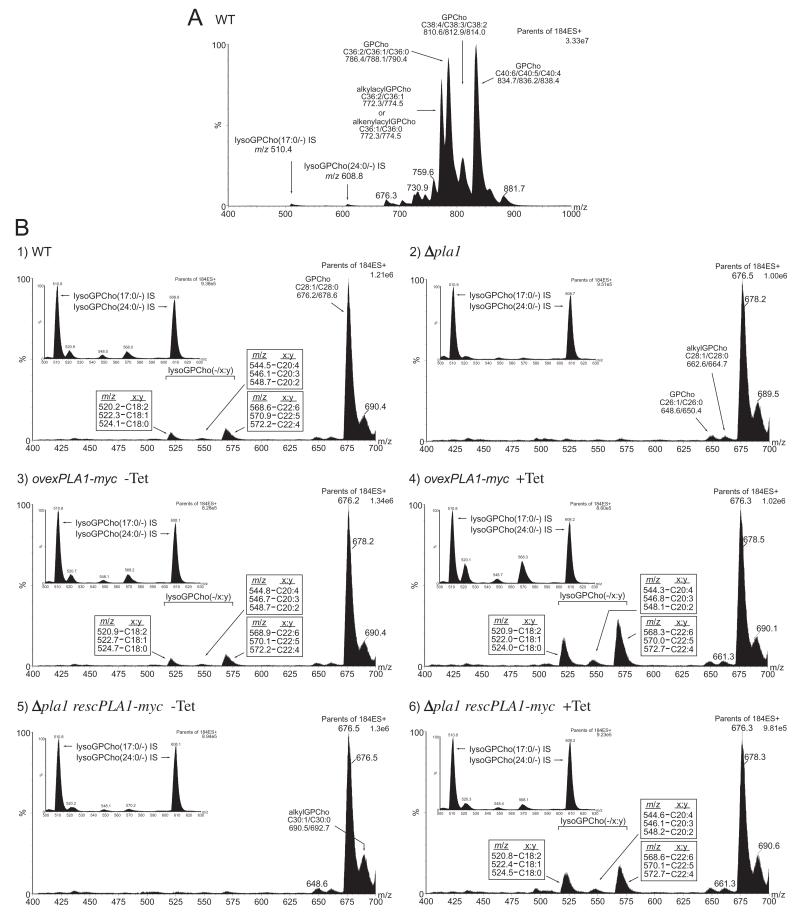
To be able to capture metabolites created via PLA1 action in vivo, a highly sensitive detection method which enabled identification and characterization of global changes in T. brucei PLs was needed. To this end, nano-electrospray ionization tandem mass spectrometry (nano-ESI-MS-MS) was employed to analyse individual PL classes present in WT, Δpla1, ovexPLA1-myc and Δpla1 rescPLA1-myc cells. In positive ion mode, collision-induced dissociation of PLs containing a choline head group results in the production of a unique fragment ion of m/z 184 (Kerwin et al., 1994). Precursor ion scanning for m/z 184 from total lipids extracted from WT cells selectively detected [M+H]+ ions of GPCho and sphingomyelin (Fig. 5A). Ions in the precursor m/z 184 ion scan spectra were annotated based on their [M+H-NMe3(+)]+ daughter ion masses compared with that of their theoretical values.
Fig. 5.
TbPLA1 functions to synthesize long-chain unsaturated lysoGPCho intermediates.
A. Positive ion ESI-MS-MS spectrum of m/z 184 lipid precursors in total lipid extracts from T. brucei WT cells. Identities of the major [M+H]+ ions are indicated as well as internal standard lysoGPCho [M+H]+ peaks.
B. Positive ion ESI-MS-MS short-range spectra of the parents of the m/z 184 ion from total lipid extracts from WT and TbPLA1 mutant cell lines. The major sets of lysoGPCho [M+H]+ metabolites detected are boxed and annotated next to the m/z peak from which they are derived. The numbers in place of x:y refer to the total number of sn-2 FA carbon atoms (x) and their degree of unsaturation (y). Panel insets are similar short-range spectra from lipid extracts spiked with lysoGPCho internal standards used for quantitative purposes (Table 2).
The major peak areas in the m/z 184 precursor ion spectrum represent GPCho with the following number of FA carbons and their degrees of saturation (represented as their sum): m/z 834, C40:6; m/z 836, C40:5; m/z 838, C40:4; m/z 786, C36:2; m/z 788, C36:1; m/z 790, C36:0; m/z 774, alkylacyl C36:1 and/or alkenylacyl C36:0; m/z 772, alkylacyl C36:2 and/or alkenylacyl C36:1; m/z 810, C38:4; m/z 812, C38:3; m/z 814, C38:2.
Detection of lysoGPCho metabolites formed from either PLA1 or PLA2 activity was facilitated from m/z 184 precursor ion scanning of total lipids in a mass range (m/z 400–700) which excluded the dominating diacyl and alkylacyl/alkenylacyl GPCho species. Peak analysis of short range spectra from PCF WT cell lipids resulted in the identification of a set of [M+H]+ lysoGPCho metabolites with the following FA constituents: m/z 568, C22:6; m/z 570, C22:5; m/z 572, C22:4; m/z 520, C18:2; m/z 522, C18:1; m/z 524, C18:0; m/z 548, C20:2; m/z 546, C20:3; m/z 544, C20:4 (Fig. 5B, panel 1). The abundance of these long-chain mostly polyunsaturated and highly unsaturated lysoGPCho metabolites is markedly lower than nearly all of the diacyl GPCho species as shown by its relative proportion to the minor diacyl species GPCho(28:1) and GPCho(28:0).
To be able to quantify the amount of long-chain unsaturated lysoGPCho intermediates synthesized by TbPLA1 activity, lysoGPCho series peak intensities were also compared with internal standard peak intensities of non-natural lysoGPCho(17:0/−) and lysoGPCho(24:0/−) added to each sample prior to lipid extraction (Fig. 5B, panel 1 inset). The calculated amounts of lysoGPCho metabolites from WT cellular lipids are tabulated as shown (Table 2). A permeating feature of the lysoGPCho molecules synthesized in T. brucei is that their acyl moiety is composed of a long and highly unsaturated chain of 22 carbons in nearly 50% of the metabolite population. This observation is consistent with the notion of the suspected presence of these FAs in some of the most abundant diacyl GPCho species (m/z 834–836), if the sn-1 position was occupied by C18:0 (Fig. 5A) (Patnaik et al., 1993).
Table 2.
Quantitative analysis of lysoGPCho metabolites in TbPLA1 mutants.a
| lysoGPCho seriesb | |||||
|---|---|---|---|---|---|
|
|
|||||
| Cell line | Tet | (−/18:y) | (−/20:y) | (−/22:y) | Total |
| WT | 53 | 31 | 72 | 156 | |
| Δ pla1 | 15 | 8 | 7 | 30 | |
| ovexPLA1-myc | − | 56 | 22 | 81 | 159 |
| + | 159 | 42 | 231 | 432 | |
| Δ plal rescPLAI-myc | − | 29 | 14 | 23 | 66 |
| + | 52 | 22 | 54 | 128 | |
Total lipid extracts from 108 PCF T. brucei cells spiked with 500 pmol of each internal standard [lysoGPCho(17:0/−) and lysoGPCho(24:0/−)] were examined by ESI-MS-MS. lysoGPCho molecules were detected from precursor ion scanning for m/z 184 (see insets from Fig. 5B), and they were quantified by comparison with the internal standards. Values are from a typical analysis and are presented as pmol per 108 cells. Tet, tetracycline.
Integration of peaks to obtain peak areas was performed by grouping lysoGPCho into various series of peaks whereby one series comprises both the major and minor lysoGPCho molecules, and their isotopes, containing the same FA chain length but with varying degrees of unsaturation, represented by ‘y’.
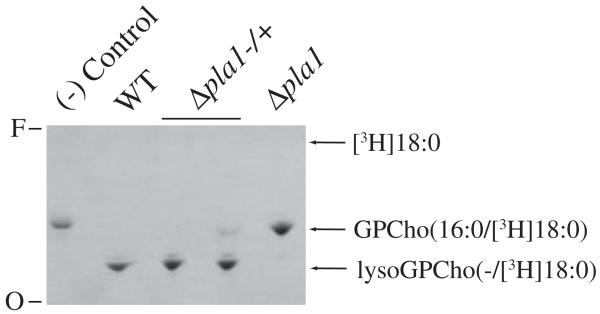
Equivalent systematic ESI-MS-MS analysis of PCF Δpla1 cells revealed that the levels of lysoGPCho metabolites in the null mutants decreased on average by 81% when compared with WT levels (Table 2) (Fig. 5B, panel 2 and inset). There was a 90% decrease in the number of moles of lysoGPCho(−/22:y), whereas lysoGPCho(−/18:y) and lysoGPCho(−/20:y) levels decreased by 72% and 74% in the TbPLA1 null mutants respectively. The variety and abundance of GPCho(28:1)/GPCho(28:0) (Fig. 5B, panel 2) and the other diacyl GPCho molecular species (data not shown) in the null mutant remained unaltered. Overall, these results suggest that in vivo lysoGPCho biosynthesis in T. brucei is largely dependent upon TbPLA1 activity. Furthermore, due to the sn-1 exclusivity of TbPLA1 (Fig. 2 and Table 1), the FA of each lysoGPCho is esterified at the sn-2 position of these metabolites. It is unknown whether the small amount of lysoGPCho species still present in the null mutants is due to enzymatic synthesis in vivo by an alternative PLA, or whether they represent background levels of fragment ions randomly and mechanically formed from diacyl GPCho during the sample preparation and/or the ionization process. However, the observation that lysates of Δpla1 cells could not produce any lysoGPCho supports the idea that these cells are most likely totally devoid of unsaturated lysoGPCho molecules (Fig. 6).
Fig. 6.
Phospholipase activity from lysates of T. brucei cells. PCF phospholipase activity from 106 cell equivalents was examined after 20 min incubation with GPCho(16:0/[3H]18:0). The TLC autoradiograph shows the metabolism of GPCho(16:0/[3H]18:0) into lysoGPCho(−/[3H]18:0) by PLA1 in WT and single knockout (Δpla1−/+) lysate. PLA1 activity is absent in the cell-free control and the Δpla1 double knockout cell line. To stimulate any possible PLA2 activity in the lysates 10 mM CaCl2 was added to each assay. Even with the dominant PLA1 activity being absent from Δpla1 clones, no PLA2 activity was observed in their lysate. Free [3H]18:0 was used as a standard to monitor PLA2 activity and its Rf is indicated. O, origin; F, front.
Confirmation that TbPLA1 mediates lysoGPCho metabolite synthesis in vivo comes from GPCho analysis in ovexPLA1-myc cells. Tetracycline-induced overexpression of TbPLA1 in these cells resulted in an elevated abundance of lysoGPCho compared with either WT or non-induced ovexPLA1-myc cells (Fig. 5B, panels 3 and 4 and their respective insets). The levels of lysoGPCho intermediates from the (−/18:y) and (−/22:y) series in the overexpression cell line reached 3.0 and 3.2 times higher than normal PCF levels of these metabolites respectively (Table 2). Interestingly, the (−/20:y) series intermediates only showed a modest (1.4-fold) increase after tetracycline induction.
TbPLA1-mediated synthesis of T. brucei long-chain polyunsaturated and highly unsaturated lysoGPCho was also established using the PCF Δpla1 rescPLA1-myc rescue clones. The Δpla1 ablated lysoGPCho(−/18:y) metabolite levels were nearly restored (98%) to WT levels upon induction with tetracycline in Δpla1 rescPLA1-myc cells, whereas the non-induced cells continued to display the Δpla1 phenotype (Fig. 5B, panels 5 and 6 and their respective insets) (Table 2). The number of moles of lysoGPCho(−/20:y) and lysoGPCho(−/22:y) were brought back to 71% and 75% of normal levels in the PCF rescue cell line.
Data from ESI-MS-MS analysis of TbPLA1 mutants collectively revealed a range of lysoGPCho metabolites whose production is at least partly regulated by the levels of TbPLA1 in the cell. A comprehensive short range search for lysoGPEth, lysoGPSer, lysoGPIns and lysoGPA yielded negative results; this supports previous findings that lysoGPCho in T. brucei is the predominant lysoPL synthesized by this parasite (Dixon and Williamson, 1970; Patnaik et al., 1993). The robust activity of TbPLA1 towards GPCho both in vitro and in vivo strongly suggests that GPCho is the preeminent substrate for this enzyme.
The global PL composition of the TbPLA1 mutants generated in this study was also examined, but the high mass range spectrums of diacyl GPCho, GPEth, GPSer and GPIns from the TbPLA1 mutants remained unchanged compared with the composition of these lipids in WT cells (data not shown).
TbPLA1 is constitutively expressed in T. brucei and its gene product is located in the cytosol
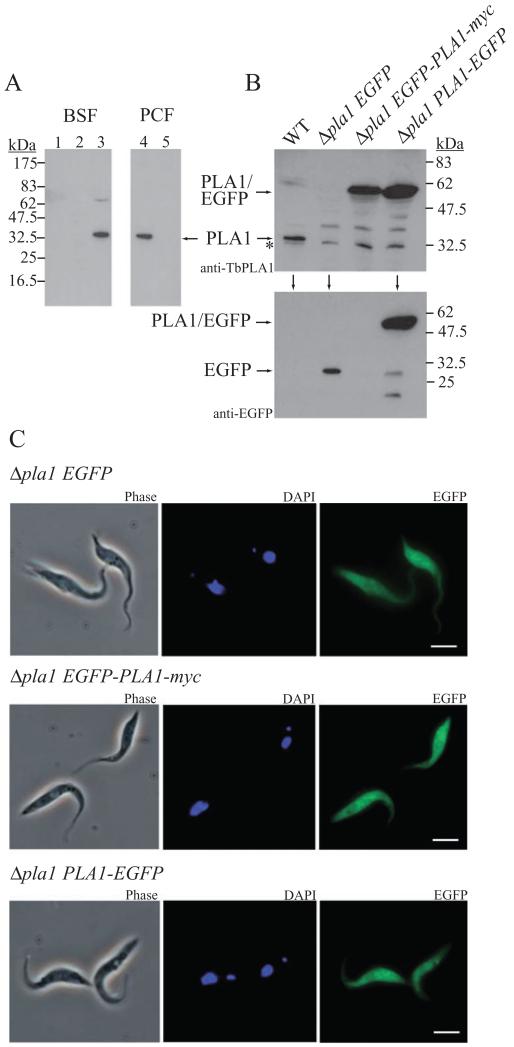
The first empirical indication that TbPLA1 may be a cytosolic protein came from observing that a high-speed soluble fraction (obtained from centrifuging lysate at 100 000 g for 1 h) from WT cells, but not lysate from TbPLA1 null mutant cells, could quickly form lysoGPCho in our radiolabelled assay (data not shown). The observations that optimal TbPLA1 activity occurs at a neutral pH and that sequence analysis suggests that this protein has lost its signal peptide also support the notion that TbPLA1 resides in the cytosol. Therefore, to confirm the intracellular location of the native enzyme, the presence of TbPLA1 in subcellular fractions of T. brucei was investigated. A Western blot of subcellular fractions probed with antibodies raised against the purified recombinant enzyme reveals that native TbPLA1 is recognizable as a soluble protein that localizes entirely in the cytosolic protein fraction in both bloodstream (BSF) and PCF WT T. brucei (Fig. 7A, lanes 3 and 4). No TbPLA1 is detected in the cytosolic fraction of PCF Δpla1 cells (Fig. 7A, lane 5), nor in the large granular or microsomal fractions of BSF WT cells (Fig. 7A, lanes 1 and 2).
Fig. 7.
Cellular distribution of TbPLA1.
A. Subcellular fractions were prepared as described in Experimental procedures and used in immunoblotting with anti-TbPLA1 antibodies. TbPLA1 is constitutively expressed and was detected in the cytosolic fraction of both BSF and PCF WT cells (lane 3 and lane 4 respectively) but absent from PCF TbPLA1 null mutants (lane 5). TbPLA1 is also absent from the large granular and small granular (microsomal) fractions from BSF WT cells (lane 1 and lane 2 respectively).
B. PCF TbPLA1 null mutant cells transformed with an ectopic copy of an N-terminal EGFP-PLA1 gene fusion (Δpla1 EGFP-PLA1-myc) or a C-terminal PLA1-EGFP gene fusion (Δpla1 PLA1-EGFP) were analysed after tetracycline induction by anti-TbPLA1 (top panel) or anti-EGFP (lower panel) immunoblotting for the presence of expressed fusion protein (~60 kDa). WT cells and null mutants transformed with an ectopic copy of EGFP only (Δpla1 EGFP) serve as negative controls for fusion protein expression. * = background bands just smaller than native TbPLA1 that show loading in the null mutant lane.
C. Analysis of the subcellular localization by immunofluorescence microscopy of both PLA1/EGFP fusion proteins (right panels) compared with EGFP only confirms a cytoplasmic distribution for soluble TbPLA1. PCF transgenic mutants are also shown in phase contrast (left panels) and after DAPI staining (middle panels).
In a further gesture to pinpoint TbPLA1 subcellular location, Δpla1 cells transformed with an altered pLEW100/PLA1-myc engineered to contain EGFP fused at either the N-terminus (Δpla1 EGFP-PLA1-myc) or C-terminus (Δpla1 PLA1-EGFP) of TbPLA1 were generated. TbPLA1 expressed as a fusion protein with EGFP upon tetracycline induction of Δpla1 PLA1-EGFP and Δpla1 EGFP-PLA1-myc cells. Evidence that full-length fusion proteins were produced by these cell lines was obtained by Western blot analysis. Anti-TbPLA1 antibodies detected the fusion proteins in the expected 60 kDa range, whereas the WT TbPLA1 was detected in its native 35 kDa range (Fig. 7B, upper panel). A copy of pLEW100 that contained only EGFP was also introduced into the null mutants as a control cell line (Δpla1 EGFP). Western blot analysis using anti-EGFP antibodies showed that Δpla1 EGFP cells expressed non-fused EGFP (27 kDa) after tetracycline induction (Fig. 7B, lower panel). Both Δpla1 EGFP-PLA1-myc and Δpla1 PLA1-EGFP induced cells expressing the PLA1/EGFP fusion proteins fluoresced upon UV exposure in a pattern very similar to cells expressing EGFP only, which is known to be a soluble protein localized to the cytosol of cells in which it is expressed (Fig. 7C). The localization studies taken together thus suggest that TbPLA1 functions by hydrolysing sn-1 FAs of GPCho molecules exposed to the neutral environment of the cytoplasm.
Discussion
This report describes the identification and characterization of a PLA1 from T. brucei, the first phospholipase of type A to be identified from this parasite, and the only PLA1 cloned from any protist. TbPLA1 should be considered a true PLA1 because it possesses high and specific activity towards sn-1 esters of diacyl PLs, exhibits LysoPLA1 activity that is a fraction of its PLA1 activity, and shows very little activity towards neutral lipid substrates.
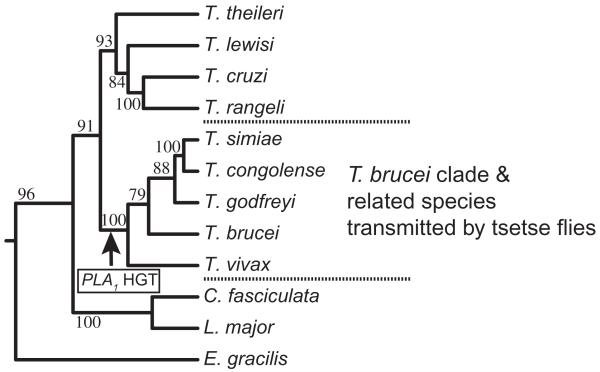
TbPLA1 is distinctive from previously identified PLA1 from other eukaryotes because it is only homologous to prokaryotic extracellular PLA1 enzymes. Outside of its own Genus the TbPLA1 peptide sequence most resembles a PLA1 homologue from S. glossinidius, a recently established bacterial secondary endosymbiont of the Glossina (tsetse) fly (Aksoy et al., 1995). This finding together with an analysis of trypanosomatid phylogeny allows us to propose that a T. brucei ancestor acquired the S. glossinidius PLA1 gene through HGT after/during its adaptation to a parasitic lifestyle in its tsetse host. The PLA1 HGT is predicted to have occurred before the species in the ‘T. brucei’ clade of lineages diverged from one another, but after they diverged from the species in the clade containing T. cruzi, T. lewisi and T. theileri (Fig. 8). This hypothesis is supported by several observations and deductions. First, TbPLA1 homologues are absent in T. cruzi or the Leishmania spp. These two kinetoplastids do not use Glossina as a host and it is unlikely that they acquired PLA1 through vertical gene transfer from an early kinetoplastid ancestor and then subsequently lost it. Second, an S. glossinidius PLA1 homologue is present in T. congolense and T. vivax, whose lineages diverge within the same clade as T. brucei. Third, T. brucei, T. congolense and T. vivax all infect Glossina flies. Last, both T. lewisi and T. theileri, which have been reported to possess very little PLA1 activity (Tizard et al., 1977a; 1980), are rooted to a clade outside of T. brucei and T. congolense, trypanosomes that have high PLA1 activity (this study) (Tizard et al., 1978b,c; Hambrey et al., 1980). Unlike prokaryote–prokaryote HGT, HGT between prokaryote and eukaryote is still regarded as more rare and atypical, and it is most common in protists (Boucher and Doolittle, 2000; Field et al., 2000; de Koning et al., 2000). On the whole, the probable HGT that created trypanosome PLA1 from a proteobacteria contributed to the evolution of the T. brucei lineage, and it supports the idea of the coevolution of trypanosomes with their hosts.
Fig. 8.
Proposed HGT point for S. glossinidius PLA1. The arrow indicates the proposed acquisition of S. glossinidius PLA1 by an ancestor of T. brucei. The maximum likelihood tree was calculated using the Phylip v3.6 Dnamlk algorithm and based on a MUSCLE alignment of glycosomal glyceraldehyde 3-phosphate dehydrogenase (gGAPDH) genes from various kinetoplastids. Values at nodes are maximum likelihood bootstrap values (%) obtained from 100 replicates (Seqboot). The tree is rooted to the corresponding gene of Euglena gracilis. The tree phylogenies agree very well with the more detailed and exact gGAPDH trees previously published (Hamilton et al., 2004; 2005). The gGAPDH sequences were obtained from GenBank and their accession numbers are: Trypanosoma theileri, AJ620282; Trypanosoma lewisi, AJ620272; Trypanosoma cruzi, AJ620269; Trypanosoma rangeli, AF053742; Trypanosoma simiae, AJ620293; Trypanosoma congolense, AJ620291; Trypanosoma godfreyi, AJ620292; Trypanosoma brucei, AJ620284; Trypanosoma vivax, AJ620295; Crithidia fasciculata, AF047493; Leishmania major, AF047497; Euglena gracilis, L21903.
This study successfully employed both in vitro and in vivo analytical techniques to establish a correlation that GPCho is the preferred substrate class upon which TbPLA1 acts. The results from this study also provide evidence that TbPLA1 is an intracellular enzyme localized to the cytosol, which is in stark contrast to the fate of its secreted bacterial homologues. Therefore, in addition to its distinct evolution, TbPLA1 is also a novel PLA1 because it is the only cytosolic PLA1 thus far discovered whose preferred substrates are GPCho molecules. In fact, of all the PLA1 cloned and characterized across taxa, only the two organelle-associated PLA1s from A. thaliana have been shown to be able to hydrolyse GPCho to some extent (Ishiguro et al., 2001; Noiriel et al., 2004). Just three other cytosolic PLA1 enzymes have been cloned previously from other organisms, all of which are mammalian in origin and preferentially hydrolyse GPA (Higgs et al., 1998; Tani et al., 1999; Nakajima et al., 2002). TbPLA1, on the other hand, shows very little activity towards GPA.
GPCho is the major PL in T. brucei, constituting approximately 57% of all the PL species present in T. brucei PCF parasites. The entire GPCho pool is not, however, a potential source of substrate for TbPLA1; roughly a quarter of the GPCho pool consists of non-hydrolysable plasmanyl/plasmenyl species (Patnaik et al., 1993). Furthermore, in most eukaryotic cells GPCho is thought to be the major PL of the outer leaflet of the plasma membrane, a location which would render many GPCho substrates inaccessible to TbPLA1. Though TbPLA1 presumably has access to PLs on the inner leaflet of the plasma membrane, the presence of GPSer and GPEth, which are widely accepted to reside in large part on the inner leaflet of the plasma membrane in their plasmanyl/plasmenyl form, could also naturally limit to a certain extent the availability of GPCho to TbPLA1 in this location. Though the amount of GPCho distributed along cytosolic surfaces in the cell is unknown, it is quite possible that the majority of TbPLA1 substrates are part of the outer leaflets of organelles and vesicles, and this includes the ER, where GPCho is de novo synthesized.
An important component in this study was the desire to answer some fundamental questions concerning the nature of T. brucei PLA1 activity. The absence of a crystal structure for any true PLA12 enzyme precludes making deductions on their mechanisms of action without empirical evidence. As such, to better comprehend the structural basis for the functional characteristics observed for TbPLA1, the identification of the catalytic residues involved in sn-1 ester hydrolysis were sought. Sitedirected mutagenesis of recombinant TbPLA1 provided evidence that the catalytic mechanism employed by TbPLA1 is similar to that of the serine hydrolases, in that its activity appears to rely on the concerted action of a catalytic triad composed of Ser-131, Asp-183 and His-234. In theory, loss of activity from any of the mutants could have been due to conformational changes or protein misfolding engendered by the substituted alanine during protein expression. However, due to the good understanding of the catalytic mechanisms used by hydrolases, and the residues involved, it is unlikely that the complete loss of activity from these soluble and well-expressed mutant proteins was caused by unintentional structural anomalies. Also, the orientation of the catalytic triad residues of TbPLA1 is consistent with that of the other serine hydrolases where the residues appear in the order Ser-Asp-His. Accordingly, TbPLA1 is the newest member of the serine hydrolase family.
The central discovery from this report is that TbPLA1 mediates the synthesis of long-chain polyunsaturated and highly unsaturated lysoGPCho metabolites in T. brucei. Detecting TbPLA1-dependent fluctuations of these metabolites was reliant upon creating TbPLA1 double knockout and overexpression transgenic cells. This is the only PLA1 double knockout from any organism, a poignant fact which explains why not much is understood about this class of enzymes. As such, there is no precedent study which directly detects and manipulates in vivo PLA1 activity in its native cell as is described here. Biochemical analysis of the TbPLA1 null mutants and various transgenic TbPLA1 overexpression cell lines showed that depleted or increased levels of TbPLA1 provoked lysoG-PCho metabolite ablation and elevation respectively, whereas alterations in other PL pools were not detected. Interestingly, the levels of the lysoGPCho(−/20:y) intermediates did not rise as much as the lysoGPCho(−/18:y) and lysoGPCho(−/22:y) intermediates during TbPLA1 overexpression. Importantly, however, it should be acknowledged that although certain lysoGPCho species are more abundant than others in the mass spectra, this does not necessarily mean that they are synthesized at greater rates by TbPLA1 because both LysoPLA and acyltransferases could show preference to further metabolize a particular series of lysoPL intermediate (Arthur, 1989; Pete and Exton, 1996). A consequence of this type of scenario would be that very little if any global increase in metabolites would be detected despite their increased synthesis.
Unsurprisingly, TbPLA1 null mutants and overexpression cell lines maintained normal cell growth and revealed no apparent phenotype in culture. In Leishmania major, another trypanosomatid, the inability to de novo synthesize sphingolipids and ether PLs, principle components of membranes, were also viable and did not result in growth defects (Zufferey et al., 2003; Denny et al., 2004). Furthermore, the abundance of lysoGPCho in eukaryotic cells is very well regulated and normally very low (0.5–1.0% of total lipids), and their loss does not seem to affect viability in other cells (van den Bosch, 1980). The high specific activity fostered by TbPLA1, coupled with its cytoplasmic localization, poses a threat to the cell if high levels of the enzyme were allowed to freely circulate and activate because it could potentially lyse the cell from within by producing excess FAs and lysoGPCho. Besides some protection provided by the plasmanyl/plasmenyl lipids present in T. brucei, tight regulation of lysoGPCho levels should be crucial for the normal functioning of T. brucei. It is shown here that tetracycline induction of both the overexpression and rescue cell lines resulted in very abundant overexpression of TbPLA1 mRNA, but not substantial PLA1 overexpression. This result suggests that lysoGPCho levels in T. brucei are at least in part regulated at the level of TbPLA1 mRNA translation and protein synthesis. Regulation of lysoGPCho levels is also most likely mediated downstream of their formation by regulating the enzymes that can further metabolize lysoPLs. As part of a Lands cycle, a T. brucei acyltransferase could remodel lysoGPCho intermediates to form a new diacyl GPCho, or alternatively a LysoPLA could remove the remaining FA. A search of the T. brucei database does highlight several putative acyltransferase and LysoPLA sequences, whose activities may account for the T. brucei LysoPLA and acyltransferase activities observed by us and others (Tizard et al., 1977b; Samad et al., 1988; Bowes et al., 1993; Werbovetz and Englund, 1996).
One of the lysoGPCho metabolites detected in this study, lysoGPCho(−/20:4), has previously been shown to be an intermediate in a proposed sequential PLA1/LysoPLA mechanism for the liberation of AA in T. brucei, an organism which is thought to lack PLA2 activity (Ridgley and Ruben, 2001). Cytosolic PLA2 enzymes, which have a marked specificity for sn-2 AA liberation from certain PLs, are quite identifiable at the sequence level by their C2 and α/β hydrolase domains (Six and Dennis, 2000), yet a search of the T. brucei genome database does not reveal any cytosolic PLA2 homologues. It is worth mentioning that a soluble enzyme activity from T. brucei that may release sn-2 FAs has been purported to be due to a PLA2 (Shuaibu et al., 2001), but it is curious that the properties of this activity are very similar to the properties presented here for recombinant TbPLA1. Other authors have initially reported PLA2 activity in T. brucei before later proposing that the activity was due to a concerted PLA1/LysoPLA mechanism (Eintracht et al., 1998; Ridgley and Ruben, 2001). Thus, with long-chain PLA2 activity possibly absent in T. brucei, the parasite potentially could have evolved a PLA1/LysoPLA sn-2 deacylation system which could utilize its adapted TbPLA1.
If TbPLA1 is part of an alternative mechanism to release sn-2 FAs, its loss potentially could have been compensated for by other lipolytic activities. However, this study showed that redundancy at the level of lysoGPCho synthesis is not supported by T. brucei; both the lysate PLA1 activity and ESI-MS-MS experiments indicated that lysoGPCho synthesis is severely and most likely totally compromised in TbPLA1 null mutants. Redundancy in lysoGPCho formation could, however, exist at undetectable levels, levels that could be sufficient to supply the necessary levels of AA to the cell, if this is a critical activity. It is possible that a lysosomal PLA1 activity is present in T. brucei (Opperdoes and van Roy, 1982), but this would most likely serve to degrade endocytosed PLs and not serve to recover lost cytosolic PLA1 activity.
Though the results reported here bring us a step closer, it is still not proven that TbPLA1 functions in a pathway to release AA from GPCho, nor if this AA release is essential for the cell. However, if redundancy is a reason why TbPLA1 null mutants are still viable and show no growth phenotype, it most likely does not exist at the level of endogenous lysoGPCho(−/20:4) synthesis, but at the level of AA production and/or liberation from other lipids. In higher eukaryotes, both endogenous and exogenous linoleic acid (C18:2Δ9,12) can be desaturated, elongated and desaturated again to produce AA (C20:4Δ5,8,11,14). Both elongation and desaturation of FAs in trypanosomes has been observed (Dixon et al., 1971). But, it has recently been shown that T. brucei uses three of its elongases (elongases 1–3) in BSF and PCF parasites to elongate pre-existing FAs to myristate (C14:0) and stearate (C18:0) respectively (Lee et al., 2006); therefore, elongase 4, shown to elongate polyunsaturated FAs, may be the only elongase which may play a role in AA synthesis. Of course, AA can also be taken up directly by the cell from LDL-linked or albumin-linked lysoGPCho and non-esterified FAs in the blood, whose AA derivatives amount to 6.98% and 0.97% of the AA composition in human plasma respectively (Croset et al., 2000). T. brucei LysoPLA activity has been shown to efficiently degrade exogenous lysoGPCho (Samad et al., 1988; Werbovetz and Englund, 1996). Last, AA could conceivably be freed from neutral lipids by lipases.
It is evident that PL degradation by intracellular phospholipases and lysophospholipases is an exceedingly complex matter. This study has contributed significantly to the understanding of how TbPLA1 functions at a biochemical and molecular level in T. brucei. Equally important is the fact that TbPLA1 has emerged as a unique example of a PLA1 enzyme.
Experimental procedures
Cloning of TbPLA1
blast analysis identified a putative PLA1 in the T. brucei genome. NdeI-containing forward (5′-atagtacggcatATGTCGGAATCCGTTGATCAGT-3′) and BamHI-containing reverse (5′-gacattaggatcctcTGAAGAACCGAACCCTCCG-3′) primers were used to PCR amplify TbPLA1 from T. brucei genomic DNA using Pfu polymerase (small letters in primer sequences denote here and elsewhere an engineered restriction enzyme site and/or linker sequence). The PCR product was gel purified (Qiagen), ligated into a blunt-ended TOPO cloning vector (Invitrogen) and sequenced on both strands. Some purified PCR product was used as a probe in Southern blotting after fluorescein-labelling by random priming (Amersham Biosciences).
Expression and purification of TbPLA1
TbPLA1 excised from TOPO using NdeI and BamHI was subcloned into the pET15b expression vector (Novagen), resequenced and transformed into E. coli BL21 Gold cells (Novagen). The cells were grown at 37°C to an OD600 of 0.8 in 3.0 l of Luria–Bertani broth containing 50 μg ml−1 ampicillin, transferred to a 15°C shaking water bath, and induced overnight with 1.0 mM IPTG. Cells were harvested by centrifugation and resuspended in 40 ml of binding buffer (50 mM NaH2PO4 pH 8.4, 150 mM NaCl, 50 mM imidazole, 5% glycerol), lysed by a One-shot Cell Disrupter (Constant Systems), and the lysate was clarified by centrifugation at 10 000 g, 4°C for 25 min. The resultant supernatant was filtered (0.45 μm) and loaded onto a charged Hi-Trap nickel column (Amersham Biosciences) equilibrated with binding buffer. The column was washed extensively with binding buffer and recombinant protein was eluted in one step with 1.0 ml elution buffer (50 mM NaH2PO4 pH 8.4, 150 mM NaCl, 500 mM imidazole, 5% glycerol). Half of the eluate was immediately diluted 30-fold with anion exchange binding buffer (20 mM Tris-HCl pH 8.0, 5% glycerol) and loaded onto a Resource™ Q anion exchange column (Amersham Biosciences) equilibrated with binding buffer. The remaining half was digested with thrombin for 4 h at room temperature to cleave the His·tag from TbPLA1 and then diluted and loaded onto the column. Gradient elution was achieved by using an AKTA Purifier pump (Amersham Biosciences) at 0.5 ml min−1 using anion exchange elution buffer (20 mM Tris-HCl pH 8.0, 0.5 M NaCl, 5% glycerol), and aliquots from the major peak were snap frozen. This 1 day procedure yields greater than 15.0 mg l−1 of highly active purified TbPLA1. If needed, buffer exchange by dialysis was performed before certain experiments. Protein concentration was determined by the Bio-Rad protein assay using bovine serum albumin as a standard. His·tagcleaved TbPLA1 was used in all experiments except where noted.
Radiolabelled PLA1 assay
PLA1 activity was determined by measuring the formation of lysoGPCho(−/[3H]18:0) from GPCho(16:0/[3H]18:0) (American Radiolabelled Chemicals, St Louis, MO) that was presented to the enzyme as mixed micelles. Mixed micelles were prepared as an 8× stock solution containing 1.2 mM GPCho(16:0/18:0) (specific activity 26.6 μCi μmol−1) and 65.9 mM TX-100 (0.018 mole fraction) in 50 mM Tris-HCl, pH 7.0. The final assay mixture contained 50 mM Tris-HCl, pH 7.0, 75 mM NaCl, 150 μm GPCho substrate, and 0.63–60.0 ng of TbPLA1 recombinant protein in a total volume of 40 μl. Enzyme was omitted from control samples. Reactions were initiated by the addition of substrate and incubated at 22°C for 5 min unless stated otherwise. TbPLA1 reaction rates were linear up to 38 min. Lipids were extracted by the method of Bligh and Dyer (1959) and the lower phase was dried under nitrogen and redissolved in chloroform. Samples were applied to HPTLC Silica 60 plates (Merck) and developed in chloroform/methanol/water (10:10:3, v/v). The HPTLC plates were exposed to a BAS-TR 2040 imaging plate and the radiolabelled lipids detected with a FLA-5100 scanner (all from Fujifilm) and quantified using Advanced Image Data Analyzer (AIDA) densitometry software (Raytest).
Substrate specificity by GC-MS
An 80 μl reaction mixture containing 50 mM Tris-HCl pH 7.0, 100 mM NaCl and 3.75 mM substrate at a mole fraction of 0.036 with TX-100 was incubated with 100 ng enzyme at 22°C for 10 min. Reaction rates were linear with respect to time up to 35 min. Enzyme was omitted from control samples. Heptadecanoic acid (C17:0) internal standard (50 nmol) was added to each reaction tube before extraction of free FAs by the method of Dole (Dole and Meinertz, 1960). Fatty acid methyl esters (FAMEs) were prepared using diazomethane in diethyl ether and let to evaporate overnight before redissolving in 30 μl dichloromethane. Sample (1.0 μl) was injected onto a 30 m HP-5 column (J & W Scientific) and analysed using an Agilent 6890N GC-MS. Individual FAMEs were identified according to retention times and mass spectra of standards, and quantified by comparison of peak areas with that of the internal standard.
Site-directed mutagenesis
Five pairs of complimentary primers were synthesized to mutate selected amino acids from TbPLA1 to alanine and they are the following: D63A, 5′-GAGGGACTTAAAAAAAGGGCCGAGAGTTGCGG-3′ and 5′-CCGCAACTCTCGGCCCTTTTTTTAAGTCCCTC-3′; D183A, 5′-GCCGAAGCGCTTGCTGTGTTCCAACGACATCCG-3′ and 5′-CGGATGTCGTTGGAACACAGCAAGCGCTTCGGC-3′; D232A 5′-CAGGCCACATTATATTGCTGCTCACCTCATAGAGAC-3′ and 5′-GTCTCTATGAGGTGAGCAGCAATATAATGTGGCCTG-3′; H234A, 5′-CATTATATTGATGCTGCCCTCATAGAGACAATTATTG-3′ and 5′-CAATAATTGTCTCTATGAGGGCAGCATCAATATA ATG-3′; S131A, 5′-GTTTCATTCACGGGGCATGCGCTTGGCGGTGGTTTG-3′ and 5′-CAAACCACCGCCAAGCGcATGCCCCGTGAATGAAAC-3′. The primers and the vector template, pET15b/TbPLA1, were used with the QuickChange Site-directed Mutagenesis kit (Stratagene). Plasmids harbouring the altered TbPLA1 were sequenced to confirm the mutation.
Creation of TbPLA1 mutants
The 5′- and 3′-UTR sequences immediately upstream and downstream from the TbPLA1 gene, respectively, were obtained from the database. 5′-UTR (270 bp) and 3′-UTR (410 bp) fragments were amplified from genomic DNA using the 5′-UTR sense primer (5′-ataagaatgcggccgcTCACCATTTGGCACGTGAGGGA-3′) and antisense primer (5′-cgtttaaacttacggaccgtcaagcttGATTCCTTATAAAATGATTCCACTTG-3′), and the 3′-UTR sense primer (5′-tgacggtccgtaagtttaaacggatccACTCATAACGCAAAA-3′) and antisense primer (5′-gtaagcggccgcTCACAAACAAATATTACAGACAGGAAT-3′). These two products, with 5′-NotI sites, were linked (via the complimentary underlined primer sequence) in a linking PCR reaction, amplified by PCR, NotI digested and ligated into the NotI site of pGEM5Zf (Promega). The linker region was excised by HindIII/BamHI digestion and the puromycin (puroR) and blasticidin (bsdR) drug resistance genes with HindIII/BamHI sticky ends were ligated individually into the cloning site. The resultant plasmids were NotI digested to release their 5′-puroR-3′ and 5′-bsdR-3′ DNA inserts, precipitated with ethanol, and redissolved in sterile water prior to being used in the electroporation and selection of T. brucei transgenic populations, performed as described elsewhere (Martin and Smith, 2006a,b).
Ectopic copies of TbPLA1 were engineered into pLEW100/PLA1-myc by inserting the ORF of TbPLA1 into the HindIII/PacI cloning site of pLEW100 (Wirtz et al., 1999) by using the HindIII-containing ORF forward (5′-atagtacaagcttATGTCGGAATCCGTTGATCAGT-3′) and PacI-containing reverse primers (5′-gatagcttaattaaTGAAGAACCGAACCCTCCG-3′). Mid-log cells were electroporated with 10 μg of NotI-linearized pLEW100/PLA1-myc plasmids in a total volume of 450 μl of cytomix buffer. The transfected parasites were selected in medium containing phleomycin (2.5 μg ml−1). When tetracycline was added to the media during biochemical analysis, a final concentration of 1 μg ml−1 was used. Cells were counted each day and were passaged only when the density was between 2 × 106 and 3 × 106 (normally every second day).
EGFP fused to TbPLA1 was engineered in both a C- and N-terminal orientation to produce the plasmids pLEW100/EGFP-TbPLA1-myc (N-terminal) and pLEW100/TbPLA1–EGFP (C-terminal). N-terminal EGFP was cloned into the HindIII cloning site of pLEW100/PLA1-myc using HindIII-containing forward (5′-tagtacaagcttATGGTGAGCAAGGGCGAGG-3′) and reverse (5′-tagtacaagcttCTTGTACAGCTCGTCCATGCC-3′) primers. C-terminal EGFP was cloned into the PacI/BamHI cloning site (displacing the myc tag) of pLEW100/PLA1-myc using PacI-containing forward (5′-gatagcttaattaacATGGTGAGCAAGGGCGAGG-3′) and BamHI-containing reverse (5′-atagtacggatccaTTACTTGTACAGCTCGTCCATGCC-3′) primers. The fusion proteins were tetracycline induced overnight, washed twice in PBS, and fixed in 4% paraformaldehyde. The cells were mounted on polysine slides, washed and stained with DAPI before visualizing EGFP with exposure to UV light on a Zeiss Axiovert 200 imaging microscope.
Southern and Northern blotting
For Southern blotting, T. brucei genomic DNA (2 μg/lane) was digested with EcoRI and the fragments separated on a 0.7% agarose gel, transferred to nitrocellulose and UV-cross-linked. For Northern blotting, total RNA was extracted with an RNA Mini Kit (Qiagen) and 12 μg was separated on 1% MOPS/Formaldehyde, 0.7% agarose gel before transfer and linking to nitrocellulose. The blots were blocked with ULTRAhyb™ (Ambion) for 1 h at 42°C before hybridization with fluorescein-labelled TbPLA1 (southern) or [32P]-CTP-labelled TbPLA1 probe (northern) overnight at 42°C. The blots were washed twice with 2× SSC plus 0.1% SDS for 20 min each, and washed twice with 0.5× SSC plus 0.1% SDS for 20 min each at 42°C. Northern blots were directly exposed to film whereas bound probe on the Southern blots were detected using horseradish peroxidase-conjugated antifluorescein antibody with the Gene Images CDP-Star kit (Amersham Biosciences). The bands on the Northern blot-transferred images were analysed by densitometry using AIDA.
Electrospray tandem mass spectrometry
Total lipids from 108 mid-log PCF trypanosomes were extracted by the method of Bligh and Dyer (1959) and the lower chloroform phase was washed once with fresh upper phase. The resultant lower phase lipid extract was dried under nitrogen and redissolved in 20 μl of chloroform/methanol (1:2). For samples containing internal standards, lysoGPCho(17:0/−) and lysoGPCho(24:0/−) (0.5 nmol/standard) were added prior to lipid extraction. An aliquot of the samples was analysed with a Micromass Quattro Ultima triple quadrupole mass spectrometer equipped with a nano-electrospray source. Samples were loaded into thin-wall nanoflow capillary tips (Waters) and analysed by ESI-MS-MS in both positive (GPCho, GPSer and GPEth) and negative ion mode (GPIns, GPSer and GPEth) under capillary/cone voltages of 0.7 kV/50 V and 0.9 kV/50 V respectively. Tandem mass spectra were obtained using argon as the collision gas (~3.0 mTorr) with collision offset energies as follows: 35 V, positive ion precursors of m/z 184 for GPCho; 45 V, negative ion precursors of m/z 241 for GPIns; 25 V, neutral loss scanning of m/z 141 in positive mode for GPEth; 22 V, neutral loss scanning of m/z 185 in positive mode for GPSer; 50 V, negative ion precursors of m/z 196 for GPEth; and 28 V, neutral loss scanning of m/z 87 for GPSer. Each spectrum encompasses at least 50 repetitive scans of 4 s duration.
Subcellular fractionation and Western blotting
Log phase bloodstream or PCF form parasites [strain 427, MITat 1.2, cultured as previously described in SDM-79 media at pH 7.4 which contained 15% heat-inactivated fetal calf serum as a lipid source (Martin and Smith, 2006b)] were osmotically lysed at 106 cell equivalents ml−1 using cold H2O containing 2 μg ml−1 leupeptin and 0.1 mM TLCK. Cell lysis was confirmed by microscopy. Lysate was centrifuged at 1000 g to pellet nuclei and unbroken cells. The resultant supernatant was then centrifuged at 14 500 g for 10 min to pellet the large granular fraction, which was resuspended with PBS to an equivalent volume of aspirated supernatant. The 14 500 supernatant was centrifuged again at 140 000 g for 1 h. The resultant pellet, the small granular fraction, was resuspended in PBS after aspirating the supernatant, the cytosolic protein fraction. The proteins from 10 μl of each fraction were resolved on a 15% SDS-PAGE gel and analysed by Western blot. The blot was developed using an ECL detection kit according to the manufacturers’ instructions (Amersham Biosciences). Purified recombinant TbPLA1 was used to obtain rabbit antiserum (Eurogentec). Anti-rabbit TbPLA1 antibodies were affinity purified using a recombinant TbPLA1-coupled CNBr-activated Sepharose column and following the protocol outlined by the manufacture (Amersham Biosciences). If needed, the bands on the Western blottransferred images were analysed by densitometry using AIDA.
Bis-BODIPY® FL-C11-PC assay
Bis-BODIPY® FL-C11-PC (Molecular Probes) was used as a GPCho fluorescent substrate at a 1:100 molar ratio with GPCho(16:0/16:0), and TX-100/lipid micelles were made. In its diacyl state, the proximity of the BODIPY fluorophores on adjacent PL acyl chains causes an intramolecular self-quenching of fluorescence. Hydrolysis and release of an acyl chain by a phospholipase A alleviates quenching and an increase in fluorescence results. The fluorophores are situated at the ends of two 11 carbon chains thus mimicking a GPCho with an approximate length near to that of GPCho(16:0/16:0).
The assay was developed for use in a 96 well plate. The assay was performed at a mole fraction of 0.018 [mole fraction = (lipid)/(lipid + detergent)]. Micelles were made as an 8× working solution which was then diluted accordingly for use in an 80 μl reaction per well. Each well initially contained 40 μl of buffer (50 mM Tris-HCl, pH 7.0) containing the micelle substrate (0.075 mM final). The reaction was started by the addition of 40 μl reaction buffer (50 mM Tris-HCl, 100 mM NaCl, pH 7.0) containing 400 pg (11.4 pmol) of recombinant TbPLA1. The reaction was left to proceed at 22°C for 10 min before quenching with 170 μl of 100% methanol. Liberated acyl chains containing a fluorophore were excited at 485 nm and detected through a 520 nm centred bandpass filter using the FLX 800 Microplate Reader (Bio-Tek Instruments). All experiments were performed at least three times in triplicate and data points varied by less than one standard deviation. Velocities obtained were initial velocities.
Acknowledgements
This work was supported by a Wellcome Trust Senior Fellowship Grant 067441 and a Wellcome Trust Prize Studentship (to G.S.R).
Footnotes
The lipid nomenclature system used closely follows the new comprehensive classification, nomenclature, and chemical representation system for lipids recently developed by an international consortium (Fahy et al., 2005).
Structures exist for two enzymes that can cleave sn-1 esters to some extent. Outer membrane phospholipase A, however, can cleave at both positions sn-1 and sn-2 of diacyl- or lysoPLs and thus is considered a phospholipase B (Nishijima et al., 1977). The guinea pig pancreatic lipase-related protein 2 displays both triglyceride lipase and phospholipase A1 activities, and shares 63% sequence identity to human pancreatic lipase (Withers-Martinez et al., 1996).
References
- Aksoy S, Pourhosseini AA, Chow A. Mycetome endosymbionts of tsetse flies constitute a distinct lineage related to Enterobacteriaceae. Insect Mol Biol. 1995;4:15–22. doi: 10.1111/j.1365-2583.1995.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Arthur G. Lysophospholipase A2 activity in guinea-pig heart microsomal fractions displaying high activities with 2-acylglycerophosphocholines with linoleic and arachidonic acids. Biochem J. 1989;261:581–586. doi: 10.1042/bj2610581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani K, Arthur G. 2-acyl-sn-glycero-3-phosphoethanolamine lysophospholipase A2 activity in guinea-pig heart microsomes. Biochem J. 1991;275(Part 2):393–398. doi: 10.1042/bj2750393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Intracellular phospholipases A. Biochim Biophys Acta. 1980;604:191–246. doi: 10.1016/0005-2736(80)90574-x. [DOI] [PubMed] [Google Scholar]
- Boucher Y, Doolittle WF. The role of lateral gene transfer in the evolution of isoprenoid biosynthesis pathways. Mol Microbiol. 2000;37:703–716. doi: 10.1046/j.1365-2958.2000.02004.x. [DOI] [PubMed] [Google Scholar]
- Bowes AE, Samad AH, Jiang P, Weaver B, Mellors A. The acquisition of lysophosphatidylcholine by African trypanosomes. J Biol Chem. 1993;268:13885–13892. [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. The control of free arachidonic acid levels. Trends Biochem Sci. 1990;15:365–366. doi: 10.1016/0968-0004(90)90227-3. [DOI] [PubMed] [Google Scholar]
- Carrington M, Carnall N, Crow MS, Gaud A, Redpath MB, Wasunna CL, Webb H. The properties and function of the glycosylphosphatidylinositol-phospholipase C in Trypanosoma brucei. Mol Biochem Parasitol. 1998;91:153–164. doi: 10.1016/s0166-6851(97)00190-4. [DOI] [PubMed] [Google Scholar]
- Catisti R, Uyemura SA, Docampo R, Vercesi AE. Calcium mobilization by arachidonic acid in trypanosomatids. Mol Biochem Parasitol. 2000;105:261–271. doi: 10.1016/s0166-6851(99)00186-3. [DOI] [PubMed] [Google Scholar]
- Croset M, Brossard N, Polette A, Lagarde M. Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem J. 2000;345(Part 1):61–67. [PMC free article] [PubMed] [Google Scholar]
- Denny PW, Goulding D, Ferguson MA, Smith DF. Sphingolipid-free Leishmania are defective in membrane trafficking, differentiation and infectivity. Mol Microbiol. 2004;52:313–327. doi: 10.1111/j.1365-2958.2003.03975.x. [DOI] [PubMed] [Google Scholar]
- Dixon H, Williamson J. The lipid composition of blood and culture forms of Trypanosoma lewisi and Trypanosoma rhodesiense compared with that of their environment. Comp Biochem Physiol. 1970;33:111–128. doi: 10.1016/0010-406x(70)90487-1. [DOI] [PubMed] [Google Scholar]
- Dixon H, Ginger CD, Williamson J. The lipid metabolism of blood and culture forms of Trypanosoma lewisi and Trypanosoma rhodesiense. Comp Biochem Physiol B. 1971;39:247–266. doi: 10.1016/0305-0491(71)90168-4. [DOI] [PubMed] [Google Scholar]
- Dole VP, Meinertz H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960;235:2595–2599. [PubMed] [Google Scholar]
- Edgar R. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eintracht J, Maathai R, Mellors A, Ruben L. Calcium entry in Trypanosoma brucei is regulated by phospholipase A2 and arachidonic acid. Biochem J. 1998;336:659–666. doi: 10.1042/bj3360659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy E, Subramaniam S, Brown HA, Glass CK, Merrill AH, Jr, Murphy RC, et al. A comprehensive classification system for lipids. J Lipid Res. 2005;46:839–861. doi: 10.1194/jlr.E400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Field J, Rosenthal B, Samuelson J. Early lateral transfer of genes encoding malic enzyme, acetyl-CoA synthetase and alcohol dehydrogenases from anaerobic prokaryotes to Entamoeba histolytica. Mol Microbiol. 2000;38:446–455. doi: 10.1046/j.1365-2958.2000.02143.x. [DOI] [PubMed] [Google Scholar]
- Gauster M, Rechberger G, Sovic A, Horl G, Steyrer E, Sattler W, Frank S. Endothelial lipase releases saturated and unsaturated fatty acids of high density lipoprotein phosphatidylcholine. J Lipid Res. 2005;46:1517–1525. doi: 10.1194/jlr.M500054-JLR200. [DOI] [PubMed] [Google Scholar]
- Gijon MA, Spencer DM, Siddiqi AR, Bonventre JV, Leslie CC. Cytosolic phospholipase A2 is required for macrophage arachidonic acid release by agonists that Do and Do not mobilize calcium. Novel role of mitogen-activated protein kinase pathways in cytosolic phospholipase A2 regulation. J Biol Chem. 2000;275:20146–20156. doi: 10.1074/jbc.M908941199. [DOI] [PubMed] [Google Scholar]
- Givskov M, Olsen L, Molin S. Cloning and expression in Escherichia coli of the gene for extracellular phospholipase A1 from Serratia liquefaciens. J Bacteriol. 1988;170:5855–5862. doi: 10.1128/jb.170.12.5855-5862.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruszynski AE, van Deursen FJ, Albareda MC, Best A, Chaudhary K, Cliffe LJ, et al. Regulation of surface coat exchange by differentiating African trypanosomes. Mol Biochem Parasitol. 2006;147:211–223. doi: 10.1016/j.molbiopara.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Hambrey PN, Tizard IR, Mellors A. Accumulation of phospholipase A1 in tissue fluid of rabbits infected with Trypanosoma brucei. Tropenmed Parasitol. 1980;31:439–443. [PubMed] [Google Scholar]
- Hambrey PN, Mellors A, Tizard IR. The phospholipases of pathogenic and non-pathogenic Trypanosoma species. Mol Biochem Parasitol. 1981;2:177–186. doi: 10.1016/0166-6851(81)90098-0. [DOI] [PubMed] [Google Scholar]
- Hambrey PN, Forsberg CM, Mellors A, Tizard IR, Werchola GM. Isolation of phospholipase A1 from Trypanosoma brucei. Tropenmed Parasitol. 1984;35:15–19. [PubMed] [Google Scholar]
- Hambrey PN, Forsberg CM, Mellors A. The phospholipase A1 of Trypanosoma brucei does not release myristate from the variant surface glycoprotein. J Biol Chem. 1986;261:3229–3232. [PubMed] [Google Scholar]
- Hamilton PB, Stevens JR, Gaunt MW, Gidley J, Gibson WC. Trypanosomes are monophyletic: evidence from genes for glyceraldehyde phosphate dehydrogenase and small subunit ribosomal RNA. Int J Parasitol. 2004;34:1393–1404. doi: 10.1016/j.ijpara.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Hamilton PB, Stevens JR, Gidley J, Holz P, Gibson WC. A new lineage of trypanosomes from Australian vertebrates and terrestrial bloodsucking leeches (Haemadipsidae) Int J Parasitol. 2005;35:431–443. doi: 10.1016/j.ijpara.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Hereld D, Hart GW, Englund PT. cDNA encoding the glycosyl-phosphatidylinositol-specific phospholipase C of Trypanosoma brucei. Proc Natl Acad Sci USA. 1988;85:8914–8918. doi: 10.1073/pnas.85.23.8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs HN, Han MH, Johnson GE, Glomset JA. Cloning of a phosphatidic acid-preferring phospholipase A1 from bovine testis. J Biol Chem. 1998;273:5468–5477. doi: 10.1074/jbc.273.10.5468. [DOI] [PubMed] [Google Scholar]
- Hoffman DR. Allergens in hymenoptera venom. XXVI: the complete amino acid sequences of two vespid venom phospholipases. Int Arch Allergy Immunol. 1994;104:184–190. doi: 10.1159/000236728. [DOI] [PubMed] [Google Scholar]
- Hosono H, Aoki J, Nagai Y, Bandoh K, Ishida M, Taguchi R, et al. Phosphatidylserine-specific phospholipase A1 stimulates histamine release from rat peritoneal mast cells through production of 2-acyl-1-lysophosphatidylserine. J Biol Chem. 2001;276:29664–29670. doi: 10.1074/jbc.M104597200. [DOI] [PubMed] [Google Scholar]
- Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K. The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell. 2001;13:2191–2209. doi: 10.1105/tpc.010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Morita MT, Fukaki H, Yamauchi Y, Uehara M, Niihama M, Tasaka M. SGR2, a phospholipase-like protein, and ZIG/SGR4, a SNARE, are involved in the shoot gravitropism of Arabidopsis. Plant Cell. 2002;14:33–46. doi: 10.1105/tpc.010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin JL, Tuininga AR, Ericsson LH. Identification of molecular species of glycerophospholipids and sphingomyelin using electrospray mass spectrometry. J Lipid Res. 1994;35:1102–1114. [PubMed] [Google Scholar]
- King TP, Lu G, Gonzalez M, Qian N, Soldatova L. Yellow jacket venom allergens, hyaluronidase and phospholipase: sequence similarity and antigenic cross-reactivity with their hornet and wasp homologs and possible implications for clinical allergy. J Allergy Clin Immunol. 1996;98:588–600. doi: 10.1016/s0091-6749(96)70093-3. [DOI] [PubMed] [Google Scholar]
- de Koning AP, Brinkman FS, Jones SJ, Keeling PJ. Lateral gene transfer and metabolic adaptation in the human parasite Trichomonas vaginalis. Mol Biol Evol. 2000;17:1769–1773. doi: 10.1093/oxfordjournals.molbev.a026275. [DOI] [PubMed] [Google Scholar]
- Kubata BK, Duszenko M, Kabututu Z, Rawer M, Szallies A, Fujimori K, et al. Identification of a novel prostaglandin f (2alpha) synthase in Trypanosoma brucei. J Exp Med. 2000;192:1327–1338. doi: 10.1084/jem.192.9.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Stephens JL, Paul KS, Englund PT. Fatty acid synthesis by elongases in trypanosomes. Cell. 2006;126:691–699. doi: 10.1016/j.cell.2006.06.045. [DOI] [PubMed] [Google Scholar]
- Martin KL, Smith TK. Phosphatidylinositol synthesis is essential in bloodstream form Trypanosoma brucei. Biochem J. 2006a;396:287–295. doi: 10.1042/BJ20051825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KL, Smith TK. The glycosylphosphatidylinositol (GPI) biosynthetic pathway of bloodstream-form Trypanosoma brucei is dependent on the de novo synthesis of inositol. Mol Microbiol. 2006b;61:89–105. doi: 10.1111/j.1365-2958.2006.05216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Sonoda H, Mizoguchi T, Aoki J, Arai H, Nagahama M, et al. A novel phospholipase A1 with sequence homology to a mammalian Sec23p-interacting protein, p125. J Biol Chem. 2002;277:11329–11335. doi: 10.1074/jbc.M111092200. [DOI] [PubMed] [Google Scholar]
- Nishijima M, Nakaike S, Tamori Y, Nojima S. Detergent-resistant phospholipase A of Escherichia coli K-12. Purification and properties. Eur J Biochem. 1977;73:115–124. doi: 10.1111/j.1432-1033.1977.tb11297.x. [DOI] [PubMed] [Google Scholar]
- Noiriel A, Benveniste P, Banas A, Stymne S, Bouvier-Nave P. Expression in yeast of a novel phospholipase A1 cDNA from Arabidopsis thaliana. Eur J Biochem. 2004;271:3752–3764. doi: 10.1111/j.1432-1033.2004.04317.x. [DOI] [PubMed] [Google Scholar]
- Opperdoes FR, van Roy J. The phospholipases of Trypanosoma brucei bloodstream forms and cultured procyclics. Mol Biochem Parasitol. 1982;5:309–319. doi: 10.1016/0166-6851(82)90038-x. [DOI] [PubMed] [Google Scholar]
- Patnaik PK, Field MC, Menon AK, Cross GA, Yee MC, Butikofer P. Molecular species analysis of phospholipids from Trypanosoma brucei bloodstream and procyclic forms. Mol Biochem Parasitol. 1993;58:97–105. doi: 10.1016/0166-6851(93)90094-e. [DOI] [PubMed] [Google Scholar]
- Pete MJ, Exton JH. Purification of a lysophospholipase from bovine brain that selectively deacylates arachidonoyl-substituted lysophosphatidylcholine. J Biol Chem. 1996;271:18114–18121. doi: 10.1074/jbc.271.30.18114. [DOI] [PubMed] [Google Scholar]
- Pete MJ, Ross AH, Exton JH. Purification and properties of phospholipase A1 from bovine brain. J Biol Chem. 1994;269:19494–19500. [PubMed] [Google Scholar]
- Pete MJ, Wu DW, Exton JH. Subcellular fractions of bovine brain degrade phosphatidylcholine by sequential deacylation of the sn-1 and sn-2 positions. Biochim Biophys Acta. 1996;1299:325–332. doi: 10.1016/0005-2760(95)00225-1. [DOI] [PubMed] [Google Scholar]
- Ridgley EL, Ruben L. Phospholipase from Trypanosoma brucei releases arachidonic acid by sequential sn-1, sn-2 deacylation of phospholipids. Mol Biochem Parasitol. 2001;114:29–40. doi: 10.1016/s0166-6851(01)00234-1. [DOI] [PubMed] [Google Scholar]
- Sage L, Hambrey PN, Werchola GM, Mellors A, Tizard IR. Lysophospholipase 1 in Trypanosoma brucei. Tropenmed Parasitol. 1981;32:215–220. [PubMed] [Google Scholar]
- Samad A, Licht B, Stalmach ME, Mellors A. Metabolism of phospholipids and lysophospholipids by Trypanosoma brucei. Mol Biochem Parasitol. 1988;29:159–169. doi: 10.1016/0166-6851(88)90071-0. [DOI] [PubMed] [Google Scholar]
- Sato T, Aoki J, Nagai Y, Dohmae N, Takio K, Doi T, et al. Serine phospholipid-specific phospholipase A that is secreted from activated platelets. A new member of the lipase family. J Biol Chem. 1997;272:2192–2198. doi: 10.1074/jbc.272.4.2192. [DOI] [PubMed] [Google Scholar]
- Schmiel DH, Wagar E, Karamanou L, Weeks D, Miller VL. Phospholipase A of Yersinia enterocolitica contributes to pathogenesis in a mouse model. Infect Immun. 1998;66:3941–3951. doi: 10.1128/iai.66.8.3941-3951.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoi W, Ezawa I, Nakamoto K, Uesaki S, Gabreski G, Aridor M, et al. p125 is localized in endoplasmic reticulum exit sites and involved in their organization. J Biol Chem. 2005;280:10141–10148. doi: 10.1074/jbc.M409673200. [DOI] [PubMed] [Google Scholar]
- Shuaibu MN, Kanbara H, Yanagi T, Ameh DA, Bonire JJ, Nok AJ. Phospholipase A2 from Trypanosoma brucei gambiense and Trypanosoma brucei brucei: inhibition by organotins. J Enzyme Inhib. 2001;16:433–441. doi: 10.1080/14756360109162392. [DOI] [PubMed] [Google Scholar]
- Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- Soldatova L, Kochoumian L, King TP. Sequence similarity of a hornet (D. maculata) venom allergen phospholipase A1 with mammalian lipases. FEBS Lett. 1993;320:145–149. doi: 10.1016/0014-5793(93)80080-e. [DOI] [PubMed] [Google Scholar]
- Sonoda H, Aoki J, Hiramatsu T, Ishida M, Bandoh K, Nagai Y, et al. A novel phosphatidic acid-selective phospholipase A1 that produces lysophosphatidic acid. J Biol Chem. 2002;277:34254–34263. doi: 10.1074/jbc.M201659200. [DOI] [PubMed] [Google Scholar]
- Tani K, Mizoguchi T, Iwamatsu A, Hatsuzawa K, Tagaya M. p125 is a novel mammalian Sec23p-interacting protein with structural similarity to phospholipidmodifying proteins. J Biol Chem. 1999;274:20505–20512. doi: 10.1074/jbc.274.29.20505. [DOI] [PubMed] [Google Scholar]
- Tizard IR, Holmes WL, York DA, Mellors A. The generation and identification of the hemolysin of Trypanosoma congolense. Experientia. 1977a;33:901–902. doi: 10.1007/BF01951271. [DOI] [PubMed] [Google Scholar]
- Tizard IR, Nielsen K, Mellors A, Assoku RK. Free fatty acids, lysophospholipases, and the pathogenesis of African trypanosomiasis. Lancet. 1977b;2:91. doi: 10.1016/s0140-6736(77)90097-6. [DOI] [PubMed] [Google Scholar]
- Tizard IR, Sheppard J, Neilsen K. The characterization of a second class of haemolysins from Trypanosoma brucei. Trans R Soc Trop Med Hyg. 1978a;72:198–200. doi: 10.1016/0035-9203(78)90063-9. [DOI] [PubMed] [Google Scholar]
- Tizard IR, Nielsen KH, Seed JR, Hall JE. Biologically active products from African trypanosomes. Microbiol Rev. 1978b;42:664–681. doi: 10.1128/mr.42.4.664-681.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizard IR, Mellors A, Holmes WL, Nielsen K. The generation of phospholipase A and hemolytic fatty acids by autolysing suspensions of Trypanosoma congolense. Tropenmed Parasitol. 1978c;29:127–133. [PubMed] [Google Scholar]
- Tizard IR, Gorski J, Sheppard J, Mellors A, Hambrey P. Studies on the generation of biologically active substances by Trypanosoma theileri in vitro. Res Vet Sci. 1980;28:178–184. [PubMed] [Google Scholar]
- Toh H, Weiss BL, Perkin SA, Yamashita A, Oshima K, Hattori M, Aksoy S. Massive genome erosion and functional adaptations provide insights into the symbiotic lifestyle of Sodalis glossinidius in the tsetse host. Genome Res. 2006;16:149–156. doi: 10.1101/gr.4106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Loo R, Chen Z, Dennis EA. Regiospecificity and catalytic triad of lysophospholipase I. J Biol Chem. 1997;272:22030–22036. doi: 10.1074/jbc.272.35.22030. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Koishi R, Yao Y, Tsuji T, Serizawa N. Molecular cloning and expression of the gene encoding a phospholipase A1 from Aspergillus oryzae. Biosci Biotechnol Biochem. 1999;63:820–826. doi: 10.1271/bbb.63.820. [DOI] [PubMed] [Google Scholar]
- Werbovetz KA, Englund PT. Lipid metabolism in Trypanosoma brucei: utilization of myristate and myristoyllysophosphatidylcholine for myristoylation of glycosyl phosphatidylinositols. Biochem J. 1996;318:575–581. doi: 10.1042/bj3180575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz E, Hartmann C, Clayton C. Gene expression mediated by bacteriophage T3 and T7 RNA polymerases in transgenic trypanosomes. Nucleic Acids Res. 1994;22:3887–3894. doi: 10.1093/nar/22.19.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz E, Leal S, Ochatt C, Cross GA. A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol Biochem Parasitol. 1999;99:89–101. doi: 10.1016/s0166-6851(99)00002-x. [DOI] [PubMed] [Google Scholar]
- Withers-Martinez C, Carriere F, Verger R, Bourgeois D, Cambillau C. A pancreatic lipase with a phospholipase A1 activity: crystal structure of a chimeric pancreatic lipase-related protein 2 from guinea pig. Structure. 1996;4:1363–1374. doi: 10.1016/s0969-2126(96)00143-8. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Allen S, Barron T, Sullivan DR, Denny PW, Almeida IC, et al. Ether phospholipids and glycosylinositolphospholipids are not required for amastigote virulence or for inhibition of macrophage activation by Leishmania major. J Biol Chem. 2003;278:44708–44718. doi: 10.1074/jbc.M308063200. Epub 42003 Aug 44727. [DOI] [PubMed] [Google Scholar]