Abstract
Starches obtained from four Dioscorea species namely Dioscorea dumetorum (Bitter), Dioscorea oppositifolia (Chinese), Dioscorea alata (Water), and Dioscorea rotundata (White) have been evaluated as binding agents in chloroquine phosphate tablet formulations in comparison with official corn starch. The compressional properties of the formulations were analyzed using density measurements and the Heckel and Kawakita equations. The mechanical properties of the tablets were assessed using tensile strength, brittle fracture index (BFI), and friability tests while the drug release properties of the tablets were assessed using disintegration and dissolution times. The results indicate that the four starches vary considerably in their physicochemical properties. The ranking for the tensile strength and the disintegration and dissolution times for the formulations was Chinese > Bitter > Corn > White > Water while the ranking was reversed for BFI and friability. The results suggest that Water, White, and Corn could be useful when faster disintegration time of tablets is desired while Chinese and Bitter could be more useful when bond strength is of concern and in minimizing the problems of lamination and capping in tablet formulation.
Keywords: Starch, Yam, Dioscorea species, Corn starch, Binding agent
1. Introduction
Starch is one of the most widely used as fillers, binders, and disintegrants in the manufacture of solid dosage forms. Although corn starch is one of the most widely used starches in pharmaceutical formulations, starches from other botanical sources have shown different functional properties such as gelling, swelling, and water binding capacity, which are related to their capacity to function effectively as binders and disintegrants in solid dosage forms (Adebayo and Itiola, 1998).
Yams, belonging to the genus Dioscorea, are staple root crops cultivated in many parts of Africa and South East Asia. Their high starch content, ranging from 70% to 80% of the dry weight, and cheap cost have made them potential source of industrial starch which could be explored commercially in the food and pharmaceutical industries (Gebre-Mariam and Schimdt, 1998; Iwuoha, 2004; Odeku and Picker-Freyer, 2007). Moreover, the long history of local consumption among various communities suggests good safety profile and high potential for regulatory acceptance in many countries (Riley et al., 2006). However, yam exists in more than six different species, four of which are widely available in the tropical region. Recent studies have also shown the potential of four different Dioscorea (yam) species, namely White yam (Dioscorea rotundata), Bitter yam (Dioscorea dumetorum), Chinese yam (Dioscorea oppositifolia), and Water yam (Dioscorea alata), as direct compression excipients. The results of the characterization of the starches indicated that the physicochemical and material properties of the starches vary considerably among the four species. Furthermore, Chinese and Bitter yam starches were highly compressible and formed tablets of acceptable crushing force, while White and Water yam starches did not form intact tablets except at high compression pressures (Odeku and Picker-Freyer, 2007). Yam starch obtained from D. rotundata has been evaluated as binding agent and disintegrant in tablet formulations (Odeku et al., 1998). With the proof of the usefulness of the starch obtained from this specie, it is of interest to investigate the starches from the other species with a view to determine their relative usefulness with the aim of maximizing their potentials for pharmaceutical use. Thus in the present study, the suitability of starches obtained from four Dioscorea species namely D. dumetorum (Bitter yam); D. oppositifolia (Chinese yam); D. alata (Water yam), and D. rotundata (White yam), as binding agents in a chloroquine tablet formulation has been investigated in comparison with the widely used corn starch.
Chloroquine phosphate, an important antimalarial drug was used as the model drug. Chloroquine phosphate possesses poor compression properties and requires a binding agent among other excipients to form satisfactorily strong tablets.
2. Materials and methods
2.1. Materials
The materials used were: chloroquine phosphate BP and corn starch BP (BDH Chemicals Ltd., Poole, UK), Lactose BP (DMV Veghel, Netherlands). Tubers of Bitter yam (D. dumetorum (Kunth), Chinese yam (D. oppositifolia (L.), Water yam (D. alata (L. DIAL2) and White yam (D. rotundata (L.). were obtained from local farmers in Ibadan, Nigeria and authenticated. In the following text only the abbreviations Bitter, Chinese, Water, and White will be used respectively for the Dioscorea starches.
2.2. Isolation of starches
Fresh tubers of yam were washed with distilled water, peeled, washed again, and then cut into small pieces. The pieces were then washed with sodium metabisulphite in distilled water to prevent darkening and then milled into a fine paste using a laboratory mill. The slurry was strained through a muslin cloth and the filtrate was left to settle. The supernatant was decanted at 12 h intervals and the starch slurry re-suspended in distilled water. The starch cake was collected after 3 days and dried in a hot air oven at 60 °C for 48 h. The dried mass was pulverized using a laboratory blender and then screened through a 120 μm mesh sieve (Young, 1984).
2.3. Chemical composition
The crude fat and crude fiber contents were determined according to AOAC methods (2000). Crude protein content was estimated from the nitrogen content determined by elemental analysis, using a conversion factor of 6.25 (Gebre-Mariam and Schimdt, 1998). The free sugar content was quantitatively determined for each starch material using the method described by Dubois et al. (1956). All determinations were done in triplicate and results are given as mean and standard deviation.
2.4. Amylose content determination
Amylose content of the starches was determined by using the colorimetric method as described by Williams et al. (1970). Hundred micrograms of sample was weighed and transferred to 100 ml volumetric flasks. One microliters of 95% ethanol and 9 ml of 1 N sodium hydroxide (NaOH) were added carefully. The sample was heated for 10 min in a boiling water bath to gelatinize and then cooled and made up to volume with water. A 5 ml portion of the starch solution was pipetted into a 100 ml volumetric flask, and 1 ml of 1 N acetic acid, then 2 ml of iodine solution was added and then made up to 100 ml with distilled water. The solution was shaken and the absorbance was determined at 620 nm after 20 min. All determinations were done in triplicate and results are given as mean and standard deviation.
2.5. Swelling power and water binding capacity
The swelling power was determined using the method described by Bowen and Vadino (1984). Starch suspension (5% w/w) was prepared at room temperature with shaking for 5 min. The dispersion was allowed to stand for 24 h before the sedimentation volume was measured and the swelling capacity was calculated as:
| (1) |
where V1 refers to the initial volume occupied by starch and V2 refers to the final volume after 24 h. Determinations were done in quadruplicates.
The water binding capacity was determined using the method of Ring (1985). Five grams of starch was placed in a 100 ml measuring cylinder and made up to 100 ml with deionized water with shaking. The dispersion was centrifuged (Centrifuge TDL 80-2, Bombay, India) for 25 min at 5000 rpm. The supernatant was discarded and the residue was weighed (W1). The residue was then dried to constant weight (W2) in a hot air oven. The water binding capacity was calculated as:
| (2) |
Determinations were done in quadruplicates.
2.6. Rheological properties of the starches
The viscosity profiles of the starch was studied using a Rapid Visco-Analyzer (RVA, Serial-3, Newport Scientific, NSW, Australia) coupled with Thermocline for Windows software. A 3 g quantity of the starch was dispersed in 25 ml of distilled water in the viscometer test canister. The paddle was carefully lowered into the slurry and the paddle blade was vigorously rotated through the sample until smooth slurry was formed. The paddle and canister assembly were properly placed in the viscometer and the measurement cycle was initiated by depressing the motor tower of the instrument. The test proceeded and terminated automatically. Heating of the slurry in the equipment was done under a constant rate of shear, and the increase in viscosity of material was measured as torque on the spindle and a curve was traced. The time–temperature regimen used was as follows: idle temperature 50 °C for 1 min, heated from 50 °C to 95 °C in 3.75 min, then held at 95 °C for 2.50 min. The sample was subsequently cooled to 50 °C over a 3.75 min period followed by a period of 2 min where the temperature was controlled at 50 °C (RVA Manual, 1990; Thiewes and Steeneken, 1997).
2.7. Preparation of starch paste
Aqueous slurry of each starch was made with distilled water (10% w/v) and then heated over a water bath with stirring until a starch paste is formed. The resultant starch paste was used as a binding agent in the tablet formulations.
2.8. Preparation of granules
Batches (200 g) of a basic formulation of chloroquine phosphate (60% w/w), Lactose (30% w/w) and Corn starch (10% w/w) were dry mixed for 5 min in a planetary mixer (Model A120, Hobart Manufacturing Co., UK) and then moistened with 22 ml of distilled water or appropriate amounts of starch paste to produce granules containing different concentrations of the starches as binder. Massing was continued for 5 min and the wet masses were granulated by passing them manually through a mesh 12 sieve (1400 μm). These were dried in a hot air oven at 50 °C for 18 h. Dried granules were sieved through a mesh 16 sieve (1000 μm) and then stored in air tight container. Particle densities were determined using the Helium pycnometer (Accupyc 1330; Micromeritics, Norcross, GA, USA).
2.9. Determination of pre-compression density
The bulk density of each formulation at zero pressure (loose density) was determined by pouring 30 g of the granules at an angle of 45° through a funnel into a glass measuring cylinder with a diameter of 24 mm and a volume of 50 ml (Itiola, 1991). Determinations were done in triplicate. The relative density, Do, of each formulation was obtained from the ratio of its loose density to its particle density.
2.10. Preparation of tablets
Tablets (500 mg) were prepared from the 500–1000 μm size fractions of granules by compressing them for 30 s with predetermined loads on a Carver hydraulic hand press (Model C, Carver Inc., Menomonee falls, Wisconsin, USA). The 10.5 mm die and flat faced punches were lubricated with a 1% w/v dispersion of magnesium stearate in acetone. After ejection, the tablets were stored over silica gel for 24 h. The weights (w) of 10 tablets and their dimensions were measured within ±1 mg and 0.01 mm, respectively, and their relative densities (D) were calculated using the equation:
| (3) |
where Vt is the volume (cm3) of the tablet and ρs is the particle density (g cm3) of the solid materials.
2.11. Compaction data analysis
The compression equations of Heckel and Kawakita were used to assess the compaction properties of the formulations (Odeku and Itiola, 1998). The Heckel equation is widely used for relating the relative density of a powder bed during compression (D) to the applied pressure (P) (Heckel, 1961a,b). The equation is written as follows:
| (4) |
The slope of the linear region, K, is the reciprocal mean yield pressure, Py, of the material. From the value of the intercept, A, the relative density, DA, can be calculated using the following equation (Lin and Cham, 1995):
| (5) |
DA represents the relative density of the material during densification at the point at which a coherent or intact tablet is just formed.
The relative density of the material before compression, i.e., when no pressure has been applied, Do, is used to describe the initial rearrangement phase of densification as a result of die filling only, and is the loose initial relative density of the material (Odeku and Itiola, 1998). The relative density, DB, describes the phase of densification after application of low pressures due to rearrangement and/or fragmentation of the particles before appreciable deformation of the particles has occurred and is the difference between DA and Do:
| (6) |
The Kawakita equation is used to study powder compression using the degree of volume reduction, C, (Kawakita and Ludde, 1970/71) and is written as:
| (7) |
In practice, the equation can be rearranged and written as:
| (8) |
where Vo is the powder’s initial bulk volume and Vp is the volume after compression. The constant ‘a’ is the minimum porosity of the material before compression and the constant ‘b’ relates to the plasticity of the material. The reciprocal of ‘b’ defines a pressure term, Pk, which is the pressure required to reduce the powder bed by 50% (Odeku and Itiola, 1998).
2.12. Mechanical properties of tablets
The tensile strengths of the normal tablets (T) and apparent tensile strengths of tablets containing a hole (To) were determined at room temperature by diametral compression (Odeku and Itiola, 1998) using a hardness tester (Ketan Scientific and Chemicals, Ahmadabad, India) and by applying the equation:
| (9) |
where T (or To) is the tensile strength of the tablet (MNm−2), F is the load (MN) needed to cause fracture, d is the tablet diameter (m), and t is the tablet thickness (m). Results were taken only from tablets which split cleanly into two halves without any sign of lamination. All measurements were made in triplicate or more and the results given are the means of several determinations.
The BFI of the tablets was calculated using the following equation:
| (10) |
The friability of the tablets was determined using a friabilator (Veego Scientific devices, Mumbai, Maharashtra, India) operated at 25 rpm for 4 min. Determinations were done in quadruplicates.
2.13. Disintegration and dissolution tests
The disintegration time of the tablets was determined in distilled water at 37 ± 0.5 °C using a disintegration tester (Veego Scientific devices, Mumbai, Maharashtra, India).
Dissolution test was carried out on the tablets using the USP XXIII basket method (Hanson Model 72RL, USA) rotated at 100 rpm in 900 ml of 0.1 M HCl, maintained at 37 ± 0.5 °C. Samples (5 ml) were withdrawn and replaced with equal amounts of fresh medium. The sample was diluted and the amount of chloroquine phosphate released was determined at wavelength of 255 nm, using a uv–visible spectrophotometer (Phillips Pye Unicam, PU 8610 Kinetics, Sarose Scientific Instruments, Cambridge, UK). Determinations were done in quadruplicates.
2.14. Statistical analysis
Statistical analysis was done to compare the effects of the starches on the tablet properties using the analysis of variance (ANOVA) on a computer software GraphPad Prism© 4 (Graphpad Software Inc. San Diego, CA, USA). Tukey–Kramer multiple comparison tests were used to compare the differences between the starches. At 95% confidence interval, probability, p values less than or equal to 0.05 were considered significant.
3. Results and discussion
3.1. Physicochemical and rheological properties
The proximate compositions of the starches from the different Dioscorea species are presented in Table 1. The chemical composition of the starches varied considerably. The ranking of the amount of crude protein was Bitter > Water > Chinese > White > Corn, while the ranking for crude fat content was Corn > Bitter > Chinese > Water > White. The ranking for the crude fiber content was Bitter > Chinese > Corn > Water > White while the ranking was reversed for the amylose content. The results show that Bitter had the highest amount of non-carbohydrate constituent, while White had the lowest values. The results also showed that the amylose content varied significantly (p < 0.05) among the various species. White and Water showed higher amylose content than Corn while Chinese and Bitter had lower values. The amylose/amylopectin contents of starches have been shown to vary depending on the botanical source of the starch and are affected by the climatic conditions and soil type during growth (Brunnschweiler et al., 2005).
Table 1.
The proximate composition of Dioscorea and corn starches (mean and SD, n = 3).
| Starch | Crude fat (%) | Crude fiber (%) | Crude protein (%) | Free sugar (%) | Amylose content (%) |
|---|---|---|---|---|---|
| Bitter | 0.34 ± 0.02 | 0.81 ± 0.01 | 2.89 ± 0.23 | 3.86 ± 0.02 | 18.76 ± 0.66 |
| Chinese | 0.26 ± 0.01 | 0.72 ± 0.02 | 0.75 ± 0.14 | 3.52 ± 0.04 | 21.61 ± 0.50 |
| Water | 0.31 ± 0.01 | 0.61 ± 0.00 | 1.71 ± 0.08 | 4.04 ± 0.03 | 27.33 ± 0.77 |
| White | 0.29 ± 0.01 | 0.58 ± 0.01 | 0.28 ± 0.08 | 3.23 ± 0.02 | 28.83 ± 0.65 |
| Corn | 0.38 ± 0.02 | 0.67 ± 0.00 | 0.12 ± 0.01 | 3.16 ± 0.02 | 27.20 ± 0.08 |
The results of the swelling power and water binding capacity of the Dioscorea starches are presented in Table 2. The ranking of the swelling capacity of the starches was Water > White > Corn > Chinese > Bitter while the ranking generally reversed for the water binding capacity. Hence, Bitter and Chinese showed lower swelling and water binding capacity than Corn, while Water and White showed higher swelling and water retention capacity. The difference in the swelling and water binding capacity of the starches may be attributed to the different intensities of molecular association forces inside the granules. The force is governed by factors such as the amylose/amylopectin content, molecular weight, conformation, degree of polymerization, and degree of branching of amylopectin (Mélo et al., 2003). Thus, Bitter and Chinese are relatively resistant to swelling than the other starches (Odeku and Picker-Freyer, 2007). Generally, the intrinsic swelling power and water binding capacity have been recognized as qualitative assessments of potential disintegrant effects of starches (Adebayo and Itiola, 1998).
Table 2.
Swelling power and water binding capacity of the starches at room temperature 25 °C (mean ± SD, n = 4).
| Starch | Swelling power | Water binding capacity |
|---|---|---|
| Bitter | 0.80 ± 0.03 | 1.20 ± 0.02 |
| Chinese | 0.82 ± 0.04 | 1.15 ± 0.01 |
| Water | 1.29 ± 0.06 | 0.69 ± 0.02 |
| White | 1.22 ± 0.03 | 0.59 ± 0.02 |
| Corn | 1.20 ± 0.03 | 0.95 ± 0.03 |
The rheological properties of starches are important since they are first converted into pastes before being used as binding agents in tablet formulations. The viscosity profiles of the starches are presented in Fig. 1. The viscosity profile is largely a reflection of changes which occurs in native starches during the heating and cooling cycle in the Rapid Visco Analyser (RVA). During the initial heating stage, there is a rise in viscosity as the granules begins to swell, which gradually registered a rapid rise after 70 °C, apparently due to the onset of gelatinization (Yadav et al., 2006). The viscosity reaches a peak at which point majority of the granules are swollen, and thereafter a clear breakdown due to rupture of the swollen granules during the cooling phase. Subsequently, there is a rise in viscosity during the cooling phase due to gelling on account of retrogradation (reassociation) of starch molecule (Guha et al., 1998).
Figure 1.
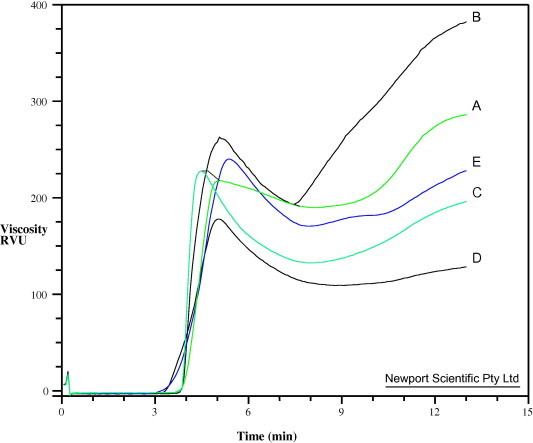
Viscosity profile of the starches: A, Bitter; B, Chinese; C, White; D, Water; E, Corn.
The viscosity parameters of the starches are shown in Table 3. The peak viscosity is the maximum viscosity achieved during or soon after the heating portion of the test and it occurs at the equilibrium point between swelling, rupture and alignment. The peak viscosity values of the starches were in the rank order of Chinese > Corn > White > Bitter > Water. The peak time is the time at which peak viscosity occurred and is an inverse relative sensitivity of the starches to heat. The ranking of the pasting time was Bitter > Chinese > Corn > Water > White. Larger granules are known to gelatinize faster than smaller ones. Hence, White with the largest granule size and Bitter with the smallest granule size (Odeku and Picker-Freyer, 2007) had the lowest and highest peak times, respectively. The pasting temperature (°C), gives the temperature at which a perceptible increase in viscosity occurs and is always higher than the gelatinization temperature (Moorthy, 2002). The ranking of the pasting temperature which is the temperature at the onset of the rise in viscosity was Bitter > Corn > Water > White > Chinese. The pasting temperature was slightly higher than the gelatinization temperature of the starches measured by Differential Scanning Calorimetry (DSC) earlier reported (Odeku and Picker-Freyer, 2007). The trough viscosity is the minimum viscosity value in the constant-temperature phase of the RVA profile. Low values of trough viscosity indicate that the materials are resistant to changes during the heating and cooling cycle. The ranking of the trough viscosity was Chinese > Bitter > Corn > White > Water. The ranking of the final viscosity which is a direct measure of the viscosity of the gel formed after retrogradation was in the same order as the trough viscosity. Thus, Chinese showed the highest viscosity values and Water the lowest values. Furthermore, the final viscosity was higher than the peak viscosity for Chinese and Bitter while the reverse is the case for Corn, Water, and White. This indicates that Chinese and Bitter showed higher tendency to retrograde than the other starches. The values of the final viscosity of the starches could be useful in the evaluation of their potentials as binders in tablet formulations.
Table 3.
Rheological properties of the starches (mean ± SD, n = 4).
| Starch | Peak viscosity (RVU) | Peak time (min) | Pasting temperature (°C) | Trough viscosity (RVU) | Final viscosity (RVU) |
|---|---|---|---|---|---|
| Bitter | 217.67 ± 2.40 | 5.76 ± 0.04 | 88.80 ± 0.87 | 189.83 ± 1.19 | 286.08 ± 1.14 |
| Chinese | 262.92 ± 2.97 | 5.74 ± 0.05 | 84.90 ± 0.96 | 193.25 ± 1.39 | 382.33 ± 1.04 |
| Water | 177.92 ± 3.40 | 5.44 ± 0.04 | 87.50 ± 0.66 | 109.00 ± 1.07 | 128.33 ± 1.56 |
| White | 227.75 ± 2.02 | 4.94 ± 0.04 | 85.75 ± 0.92 | 132.58 ± 1.53 | 196.08 ± 1.11 |
| Corn | 240.00 ± 1.08 | 5.43 ± 0.03 | 88.55 ± 0.87 | 170.50 ± 1.60 | 227.83 ± 1.61 |
3.2. Compression properties
Representative Heckel plots for chloroquine phosphate formulations containing 5% w/w of the various starches are shown in Fig. 2. Py was calculated from the portion of the plots showing the highest correlation coefficient = 0.990 for all the formulations (usually between 42.42 and 169.69 MNm−2). The intercept, A, was determined from the extrapolation of the linear portion. The values of the mean yield pressure, Py, Do, DA, and DB for the formulations are presented in Table 4. The Do values, which represent the degree of initial packing in the die as a result of die filling for the formulations increased with increase in starch concentration. The ranking of the Do values for the formulations was Bitter > Chinese > White > Corn > Water. This indicates that formulations containing Bitter exhibited the highest degree of packing in the die as a result of die filling while formulations containing Water exhibited the lowest values.
Figure 2.
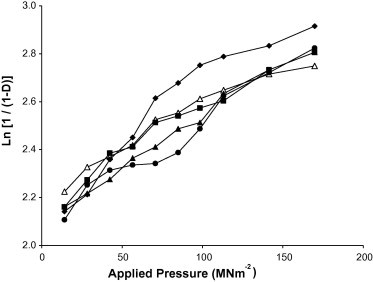
Heckel plots for chloroquine phosphate tablet formulations containing 5.00% w/w starch binder: ♦, Bitter; ■, Chinese; ●, Water; ▴, White; △, Corn.
Table 4.
Parameters derived from density measurements and from the Heckel and Kawakita plots.
| Starch | Starch concentration (% w/w) | Do | Heckel plots |
Kawakita plots |
|||
|---|---|---|---|---|---|---|---|
| Py (MPa) | DA | DB | D1 | Pk (MPa) | |||
| 0.00 | 0.291 | 217.39 | 0.870 | 0.579 | 0.504 | 3.085 | |
| Bitter | 2.50 | 0.333 | 400.00 | 0.914 | 0.580 | 0.394 | 1.332 |
| 5.00 | 0.344 | 344.83 | 0.912 | 0.568 | 0.410 | 1.285 | |
| 7.50 | 0.366 | 256.41 | 0.897 | 0.530 | 0.426 | 1.261 | |
| 10.00 | 0.383 | 238.10 | 0.887 | 0.504 | 0.445 | 1.197 | |
| Chinese | 2.50 | 0.314 | 416.67 | 0.899 | 0.585 | 0.412 | 1.384 |
| 5.00 | 0.319 | 303.03 | 0.894 | 0.575 | 0.420 | 1.362 | |
| 7.50 | 0.343 | 256.41 | 0.901 | 0.558 | 0.425 | 1.128 | |
| 10.00 | 0.355 | 243.90 | 0.909 | 0.554 | 0.444 | 1.096 | |
| Water | 2.50 | 0.299 | 303.03 | 0.886 | 0.587 | 0.484 | 2.388 |
| 5.00 | 0.304 | 238.10 | 0.880 | 0.576 | 0.504 | 2.362 | |
| 7.50 | 0.313 | 185.19 | 0.862 | 0.549 | 0.516 | 2.353 | |
| 10.00 | 0.318 | 166.67 | 0.866 | 0.548 | 0.530 | 2.305 | |
| White | 2.50 | 0.294 | 322.58 | 0.883 | 0.589 | 0.483 | 2.200 |
| 5.00 | 0.318 | 270.27 | 0.888 | 0.570 | 0.532 | 2.039 | |
| 7.50 | 0.325 | 217.39 | 0.881 | 0.556 | 0.548 | 2.025 | |
| 10.00 | 0.329 | 188.68 | 0.882 | 0.553 | 0.558 | 1.976 | |
| Corn | 2.50 | 0.314 | 384.62 | 0.900 | 0.586 | 0.481 | 2.105 |
| 5.00 | 0.317 | 277.78 | 0.893 | 0.576 | 0.492 | 2.023 | |
| 7.50 | 0.319 | 243.90 | 0.890 | 0.571 | 0.508 | 2.004 | |
| 10.00 | 0.325 | 222.22 | 0.892 | 0.567 | 0.549 | 1.983 | |
The DA values which represent the total degree of packing at zero and low pressures also increased with increase in the concentration of starch binders. The ranking of DA for the formulations was Bitter > Chinese > Corn > White > Water. Thus, formulations containing Bitter exhibited the highest degree of total packing in the die at zero and low pressures while formulations containing Water had the lowest values.
The DB value represents the particle rearrangement phase in the early compression stages and tends to indicate the extent of fragmentation particles or granules, although fragmentation may occur concurrently with plastic and elastic deformation of constituent particle. The value of DB decreased with increase in concentration of starch binders. This indicates that fragmentation of granules decreased with increase in the concentration of starch binders. The ranking of the DB values was generally Corn > Chinese > White > Water > Bitter.
The mean yield pressure, Py, is inversely related to the ability of the formulation to deform plastically under pressure. The values of Py decreased with increase in starch concentration. The ranking of Py was Chinese > Bitter > Corn > White > Water. This result indicates that the formulations containing Water exhibited the fastest onset of plastic deformation during compression while those containing Chinese exhibited the slowest.
Representative Kawakita plots for chloroquine phosphate formulations containing 5% w/w of the starches are shown in Fig. 3. A linear relationship was obtained at all compression pressures used with a correlation coefficient of 0.999 for all starches. Values of ‘a’ and ‘ab’ were obtained from the slope and intercept of the plots, respectively. Values of 1 − a yield the initial relative density of the starches (DI) while the Pk values were obtained from the reciprocal of b (Table 4). The DI value, which is a measure of the packed initial relative density of the starches with the application of small pressures or tapping (Podczeck and Sharma, 1996), increased with increase in starch content. The ranking of DI was generally White > Water > Corn > Chinese > Bitter. Thus, formulations containing White showed the highest degree of packing with the application of small pressure while formulations containing Bitter showed the lowest values.
Figure 3.
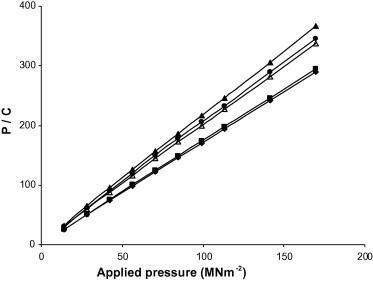
Kawakita plots for chloroquine phosphate tablet formulations containing 5% w/w starch binder. ♦, Bitter; ■, Chinese; ●, Water; ▴, White; △, Corn.
The value of Pk, an inverse measure of the amount of plastic deformation occurring during the compression process (Odeku and Itiola, 1998), decreased with increase in the starch content. The ranking of the Pk values was Water < White < Corn < Bitter < Chinese. Thus formulations containing Chinese and Bitter exhibited higher amount of total plastic deformation than Corn while Water and White exhibited lower values. It has been shown that the lower the Pk value, the more the total plastic deformation occurring during compression (Podczeck and Sharma, 1996; Odeku and Itiola, 1998). Thus, formulations containing Water and White which showed faster onset of plastic deformation than corn starch during compression as indicated by the low Py values, also exhibited the lowest amount of plastic deformation as indicated by the high Pk values.
3.3. Mechanical properties
The results of the tensile tests on the chloroquine tablets fit the general equation:
| (11) |
with a correlation coefficient >0.980. A and B were constants for each formulation and depended on whether the tablet had a hole in it or not. Fig. 4 shows representative plots of log tensile strength versus relative density for formulations containing 5% w/w of the starches as binder. The tensile strength of tablet with hole was lower than that of the same without a hole, the hole acting as a stress concentrator (Hiestand et al., 1977).
Figure 4.
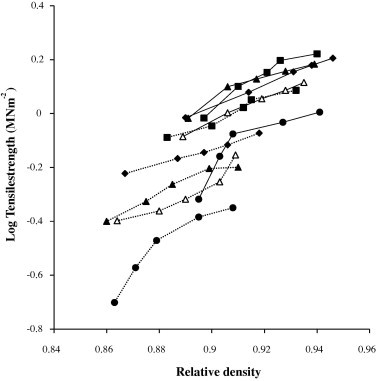
Log tensile strength versus relative density for chloroquine phosphate tablets containing 5% w/w of binders with hole (……) and without a hole ( ______ ) at their center: ♦, Bitter; ■, Chinese; ●, Water; ▴, White; △, Corn.
The tensile strength and BFI of the tablets at relative density of 0.90, which is representative of commercial tablets, are shown in Table 5. The values of the tensile strength increased while BFI decreased with an increase in relative density and concentration of starch binder. High concentration of a plasto-elastic starch binder leads to an increase in plastic deformation of the formulation and subsequently to the formation of more solid bonds resulting in tablets with more resistance to fracture and abrasion (Odeku and Itiola, 2003). The ranking of the values of the tensile strength of the tablets was Chinese > Bitter > Corn > White > Water while the rankings for the BFI was in the reverse order. Thus, Chinese and Bitter would be more useful than corn, White, and Water starches when high bond strength is desired and in minimizing the problems of lamination and capping especially on high speed tabletting machine with short dwell time for the plastic deformation of materials.
Table 5.
Tensile strength (MNm−2), brittle fracture index (BFI) and friability (%) at relative density, D, of 0.90 for chloroquine phosphate tablets (mean ± SD, n = 4).
| Binder | Binder concentration (% w/w) | T (MNm−2) | BFI | Friability (%) |
|---|---|---|---|---|
| 0.00 | 0.525 ± 0.021 | 0.466 ± 0.009 | 3.25 ± 0.13 | |
| Bitter | 2.50 | 0.804 ± 0.010 | 0.370 ± 0.029 | 0.78 ± 0.01 |
| 5.00 | 1.059 ± 0.009 | 0.215 ± 0.022 | 0.63 ± 0.02 | |
| 7.50 | 1.150 ± 0.018 | 0.200 ± 0.017 | 0.62 ± 0.01 | |
| 10.00 | 1.272 ± 0.020 | 0.147 ± 0.010 | 0.55 ± 0.01 | |
| Chinese | 2.50 | 0.942 ± 0.011 | 0.136 ± 0.011 | 0.70 ± 0.01 |
| 5.00 | 1.171 ± 0.016 | 0.123 ± 0.009 | 0.64 ± 0.02 | |
| 7.50 | 1.248 ± 0.010 | 0.106 ± 0.004 | 0.60 ± 0.01 | |
| 10.00 | 1.287 ± 0.013 | 0.100 ± 0.010 | 0.55 ± 0.02 | |
| Water | 2.50 | 0.656 ± 0.007 | 0.615 ± 0.010 | 3.75 ± 0.13 |
| 5.00 | 0.801 ± 0.010 | 0.453 ± 0.021 | 3.00 ± 0.02 | |
| 7.50 | 1.045 ± 0.012 | 0.406 ± 0.019 | 2.63 ± 0.02 | |
| 10.00 | 1.155 ± 0.019 | 0.367 ± 0.013 | 2.25 ± 0.03 | |
| White | 2.50 | 0.601 ± 0.016 | 0.437 ± 0.024 | 2.40 ± 0.05 |
| 5.00 | 1.057 ± 0.012 | 0.383 ± 0.009 | 2.10 ± 0.03 | |
| 7.50 | 1.207 ± 0.009 | 0.314 ± 0.029 | 1.70 ± 0.03 | |
| 10.00 | 1.249 ± 0.017 | 0.313 ± 0.023 | 0.96 ± 0.02 | |
| Corn | 2.50 | 0.929 ± 0.010 | 0.399 ± 0.012 | 2.00 ± 0.10 |
| 5.00 | 1.016 ± 0.018 | 0.363 ± 0.016 | 1.52 ± 0.03 | |
| 7.50 | 1.131 ± 0.014 | 0.349 ± 0.003 | 1.45 ± 0.03 | |
| 10.00 | 1.155 ± 0.011 | 0.307 ± 0.002 | 0.90 ± 0.02 | |
Friability is an important test that often form part of a manufacturer’s specification (Odeku and Itiola, 2003). Friability is especially important because the tablets are likely to be subjected to various abrasive motions during production and subsequent use. Conventional compressed tablets that lose less than 1% of their weight during the friability test are generally considered acceptable (Itiola and Pilpel, 1991).
Representative plots of the friability of the tablets versus relative density for tablets containing 5% w/w of the starch binders are presented in Fig. 5. The values of friability of the tablets at relative density of 0.90 are also shown in Table 5. Tablet formulations containing Water failed the friability test at all concentrations employed in the formulations while White and Corn only passed the friability test at a concentration of 10% w/w. On the other hand, tablet formulations containing Chinese and Bitter met the official requirements at all concentrations employed. This suggests that Chinese and Bitter would provide better protection for tablets against abrasive motion during handling and subsequent use when compared to Corn, White and Water.
Figure 5.
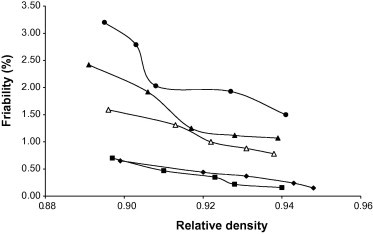
Friability versus relative density for chloroquine phosphate tablet formulations containing 5.00% w/w of starch binder: ♦, Bitter; ■, Chinese; ●, Water; ▴, White; △, Corn.
It is notable that the results of the Pk values were inversely related to those of the tensile strength of the formulations. Thus, formulations containing Chinese and Bitter which showed low Pk values also showed significantly (p < 0.001) higher mechanical strength as indicated by the higher tensile strength and lower friability values. This supports the assertion that Pk provides a measure of the total amount of plastic deformation occurring during compression as has been previously established by Odeku and Itiola (1998). It is also noteworthy that the final viscosity values of all the starches showed the same ranking as the tensile strength values. Thus, Chinese and Bitter starch pastes which showed higher viscosity values formed tablets with stronger mechanical properties than the other starches. Hence the viscosity of the starch paste appears to have a significant effect on the binding properties of the starches.
3.4. Drug release properties
Representative plots of disintegration time versus relative density for tablets containing 5% w/w of the starch binders are shown in Fig 6. The disintegration time (DT) of the tablets at a relative density of 0.90 is presented in Table 6. DT generally increased with increase in relative density of the tablets and with concentration of starch binder. The ranking of DT for the tablets containing the starches was Chinese > Bitter > Corn > White > Water. Tablets containing Chinese and Bitter exhibited higher DT while those containing Water and White exhibited lower DT compared to Corn. Statistical analysis showed that there were significant (p < 0.001) differences in the disintegration time for the various formulations. Furthermore, formulations containing Chinese and Bitter did not conform to the British Pharmacopoeia requirement on disintegration of uncoated tablets (i.e., disintegration within 15 min) at concentrations 5 and 10% w/w, respectively. This emphasizes the need to optimize the level of starch binders used in tablet formulations to obtain tablets with the desired disintegration time.
Figure 6.
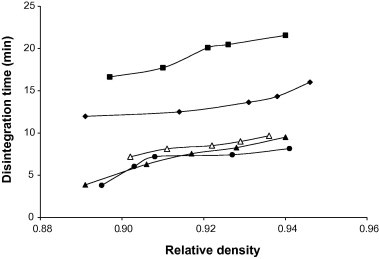
Disintegration time (min) versus relative density for chloroquine phosphate tablet formulations containing 5.00% w/w of starch binder: ♦, Bitter; ■, Chinese; ●, Water; ▴, White; △, Corn.
Table 6.
Disintegration (DT) and dissolution characteristics of chloroquine phosphate tablets at relative density = 0.90 (mean ± SD, n = 4).
| Starch | Starch concentration (%) | DT (min) | t50 (min) | t80 (min) | t1 (min) | k1 | k2 |
|---|---|---|---|---|---|---|---|
| 0.00 | 1.80 ± 0.09 | 14.00 ± 0.50 | 25.00 ± 0.50 | 15.00 ± 0.50 | 0.05 ± 0.00 | 0.08 ± 0.00 | |
| Bitter | 2.50 | 6.75 ± 0.06 | 16.50 ± 0.53 | 30.00 ± 0.25 | 30.00 ± 0.18 | 0.05 ± 0.00 | 0.09 ± 0.00 |
| 5.00 | 12.10 ± 0.11 | 22.50 ± 0.51 | 40.00 ± 0.50 | 30.50 ± 0.50 | 0.04 ± 0.00 | 0.08 ± 0.00 | |
| 7.50 | 14.10 ± 0.06 | 25.50 ± 0.50 | 42.50 ± 0.50 | 40.00 ± 0.43 | 0.04 ± 0.00 | 0.07 ± 0.00 | |
| 10.00 | 22.50 ± 0.08 | 40.00 ± 0.23 | 59.00 ± 0.20 | 41.00 ± 0.66 | 0.03 ± 0.00 | 0.06 ± 0.00 | |
| Chinese | 2.50 | 9.25 ± 0.05 | 20.00 ± 0.25 | 36.25 ± 0.25 | 30.00 ± 1.00 | 0.04 ± 0.00 | 0.10 ± 0.00 |
| 5.00 | 16.50 ± 0.09 | 32.50 ± 0.50 | 49.00 ± 0.25 | 38.75 ± 0.25 | 0.03 ± 0.00 | 0.09 ± 0.00 | |
| 7.50 | 24.60 ± 0.05 | 39.00 ± 0.15 | 57.50 ± 0.50 | 40.50 ± 0.66 | 0.02 ± 0.00 | 0.06 ± 0.00 | |
| 10.00 | 26.25 ± 0.05 | 46.50 ± 0.50 | 83.50 ± 0.25 | 50.00 ± 1.00 | 0.02 ± 0.00 | 0.04 ± 0.01 | |
| Water | 2.50 | 4.70 ± 0.07 | 15.50 ± 0.15 | 28.75 ± 0.25 | 30.00 ± 1.32 | 0.06 ± 0.00 | 0.12 ± 0.00 |
| 5.00 | 5.10 ± 0.19 | 16.25 ± 0.27 | 30.50 ± 0.50 | 30.40 ± 0.22 | 0.05 ± 0.00 | 0.10 ± 0.00 | |
| 7.50 | 5.30 ± 0.02 | 19.00 ± 0.18 | 36.00 ± 0.25 | 40.00 ± 0.60 | 0.05 ± 0.00 | 0.17 ± 0.00 | |
| 10.00 | 6.90 ± 0.08 | 20.00 ± 0.23 | 37.00 ± 0.25 | 40.10 ± 0.89 | 0.04 ± 0.00 | 0.15 ± 0.00 | |
| White | 2.50 | 4.60 ± 0.07 | 15.50 ± 0.50 | 28.00 ± 0.50 | 30.00 ± 0.10 | 0.05 ± 0.00 | 0.10 ± 0.00 |
| 5.00 | 5.20 ± 0.25 | 18.00 ± 0.20 | 32.50 ± 0.50 | 30.20 ± 0.72 | 0.05 ± 0.00 | 0.09 ± 0.01 | |
| 7.50 | 5.90 ± 0.05 | 22.50 ± 0.35 | 41.00 ± 0.66 | 40.00 ± 0.50 | 0.04 ± 0.00 | 0.10 ± 0.00 | |
| 10.00 | 7.50 ± 0.04 | 28.00 ± 0.25 | 47.50 ± 0.40 | 40.00 ± 1.00 | 0.03 ± 0.00 | 0.08 ± 0.01 | |
| Corn | 2.50 | 6.60 ± 0.07 | 17.00 ± 0.25 | 31.00 ± 0.50 | 40.00 ± 1.50 | 0.05 ± 0.00 | 0.16 ± 0.00 |
| 5.00 | 6.80 ± 0.26 | 18.00 ± 0.25 | 33.00 ± 0.39 | 40.20 ± 0.40 | 0.04 ± 0.00 | 0.15 ± 0.00 | |
| 7.50 | 7.25 ± 0.04 | 21.00 ± 0.50 | 36.50 ± 0.50 | 40.30 ± 0.27 | 0.04 ± 0.00 | 0.11 ± 0.00 | |
| 10.00 | 7.30 ± 0.05 | 20.50 ± 0.50 | 37.00 ± 0.15 | 40.00 ± 0.87 | 0.04 ± 0.00 | 0.12 ± 0.00 | |
The amount of chloroquine phosphate released was plotted against time and representative plots for tablets containing 5% w/w starch binders are presented in Fig. 7. The values of t50 and t80 (i.e., time required for 50% and 80% of chloroquine phosphate to be released respectively) were calculated. The integrated form of the Noyes and Whitney Equation (1897) was employed:
| (12) |
where Cs is the concentration of the solute at saturation, C is the concentration at time t, and k is a dissolution rate constant. Values of Ln [Cs/(Cs − C) were plotted versus t (Kitazawa et al., 1975) and typical plots for tablets containing 5% w/w of starch binders are shown in Fig. 8. In all cases, two straight regression lines of slopes k1 and k2 were obtained. The time at which the lines intersect is denoted by t1.
Figure 7.
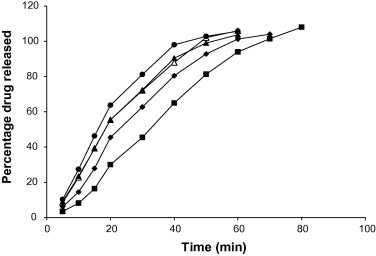
Percentage drug released versus time for chloroquine phosphate tablet formulations containing 5.00% w/w of starch binder: ♦, Bitter; ■, Chinese; ●, Water; ▴, White; △, Corn.
Figure 8.
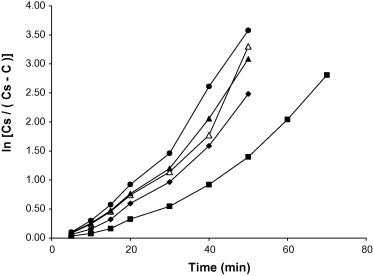
Ln [Cs/(Cs − C)] versus time plots for chloroquine phosphate tablet formulations containing 5.00% w/w of starch binder: ♦, Bitter; ■, Chinese; ●, Water; ▴, White; △, Corn.
Values of t50, t80, t1, k1 and k2 for all the samples at the relative density of 0.90 are presented in Table 6. It can be observed that the values of t50, t80 and t1 increased with an increase in binder concentration while the values of k1 and k2 generally decreased. The ranking of values of t50, t80 and t1 for the tablets containing the different starch binders was Chinese > Bitter > Corn > White > Water. Tablets containing Chinese and Bitter had significantly (p < 0.001) higher disintegration and dissolution times than tablets containing the other starches. In addition, the values of k1 were lower than the values of k2, implying a faster dissolution rate of the drug after t1. The change from k1 to k2 at time t1 is attributable to a change in the surface area due to the break up of the tablets into fragments (Kitazawa et al., 1975). It was also observed that t1 values were generally higher than the disintegration time values, probably resulting from the greater agitation employed in the disintegration test than in dissolution tests (Itiola and Pilpel, 1991; Odeku and Itiola, 2003).
It is well known that disintegration of tablets plays a vital role in the dissolution process since it determines to a large extent the area of contact between the solid and liquid (Odeku and Itiola, 2003). The most widely reported mechanisms of disintegrant action of starches are believed to depend on swelling and wicking (Lowenthal, 1973; Wan and Prasad, 1989). Thus, the swelling power and water binding capacity of starch powders have been shown to have significant effects on their disintegrant properties (Adebayo and Itiola, 1998). However, when starch is wetted and converted into mucilages or pastes and added intragranularly, it loses most of its swelling property but may still effect tablet disintegration within acceptable limits probably due to the capillary action (wicking) of the starch, which is believed to depend on the porosity of the tablets (Lowenthal, 1973; Odeku and Alabi, 2007). Thus, the faster disintegration and dissolution times observed for tablets containing Water, White, and Corn could be as a result of their higher swelling power which facilitates disintegration. This result is similar to previous findings where starches obtained from D. rotundata have been shown to possess dual role of binder and disintegrant in tablet formulations (Odeku et al., 1998). In addition, the higher values of viscosity of Chinese and Bitter which accentuated their binding capacity may also retard the entry of disintegration fluid into the pores of the tablets leading to slower release of the drug (Esezobo et al., 1998). This suggests that Water, White, and Corn could be useful when faster disintegration time of tablets is desired while Chinese and Bitter could be more useful when bond strength is of concern.
4. Conclusions
The results of the present study provide some insight into the relative effectiveness of the four Dioscorea starches and corn starch as binders in chloroquine tablet formulations. The results show that formulations containing Water had the fastest onset but lowest amount of plastic deformation under compression pressure. The formulations containing Chinese and Bitter however showed a slower onset of plastic deformation but higher amount of total plastic deformation when compared with those of corn starch. Chinese and Bitter yam starches also produced tablets with stronger mechanical properties and longer disintegration and dissolution times than White, Water and Corn starches. This suggests that Chinese and Bitter could be more useful than Corn as binding agent especially when higher mechanical strength and slower dissolution rates are desired. On the other hand, White and Water yam would be more useful for tablets where faster disintegration and dissolution are desired. The results thus suggest that the experimental starches can be further developed as excipients for commercial purposes.
References
- Adebayo A.S., Itiola O.A. Evaluation of breadfruit and cocoyam starches as exodisintegrants in a paracetamol tablet formulation. Pharm. Pharmacol. Commun. 1998;4:385–389. [Google Scholar]
- AOAC . 17th ed. Association of Official Analysis Chemists (A.O.A.C.); Washington, DC, USA: 2000. Official Methods of Analysis. [Google Scholar]
- Bowen F.E., Vadino W.A. A simple method for differentiating sources. Drug Dev. Ind. Pharm. 1984;10:505–511. [Google Scholar]
- Brunnschweiler J., Luethi D., Handschin S., Farah Z., Escher F., Conde-Petit B. Isolation, physicochemical characterization and application of yam (Dioscorea spp.) starch as thickening and gelling agent. Starch/Stärke. 2005;57:107–117. [Google Scholar]
- Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric methods for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- Esezobo S., Zubair S., Pilpel N. Effects of Tapioca obtained from cassava (Manihot utilissima) on the disintegration and dissolution rates of paracetamol tablets. J. Pharm. Pharmacol. 1998;41:7–10. doi: 10.1111/j.2042-7158.1989.tb06319.x. [DOI] [PubMed] [Google Scholar]
- Gebre-Mariam T., Schimdt P.C. Some physicochemical properties of Dioscorea starch from Ethiopia. Starch/Stärke. 1998;50:241–246. [Google Scholar]
- Guha M., Ali S.Z., Bhattacharya S. Effect of barrel temperature and screw speed on rapid viscoanalyser pasting behaviour of rice extrudate. Int. J. Food Sci. Technol. 1998;33:259–266. [Google Scholar]
- Heckel R.W. Density–pressure relationships in powder compaction. Trans. Metall. Soc. AIME. 1961;221:671–675. [Google Scholar]
- Heckel R.W. An analysis of powder compaction phenomena. Trans. Metall. Soc. AIME. 1961;221:1001–1008. [Google Scholar]
- Hiestand E.N., Wells J.E., Poet C.B., Ochs J.F. Physical processes of tabletting. J. Pharm. Sci. 1977;66:510–519. doi: 10.1002/jps.2600660413. [DOI] [PubMed] [Google Scholar]
- Itiola O.A. Compression characteristics of three starches and the mechanical properties of their tablets. Pharm. World J. 1991;8:91–94. [Google Scholar]
- Itiola O.A., Pilpel N. Formulation effects on the mechanical properties of metronidazole tablets. J. Pharm. Pharmacol. 1991;43:145–147. doi: 10.1111/j.2042-7158.1991.tb06655.x. [DOI] [PubMed] [Google Scholar]
- Iwuoha C.I. Comparative evaluation of physico-chemical characteristics of flours from steeped tubers of white yam (Dioscorea rotundata Poir), Water Yam (Dioscorea alata L.) and Yellow Yam (Dioscorea cayenensis Lam) Tropicultura. 2004;22:56–63. [Google Scholar]
- Kawakita K., Ludde K.H. Some considerations on powder compression equations. Powder Technol. 1970/71;4:61–68. [Google Scholar]
- Kitazawa S., Johno I., Ito Y., Temura S., Okada J. Effects of hardness on disintegration times and dissolution rate of uncoated caffeine tablets. J. Pharm. Pharmacol. 1975;27:765–770. doi: 10.1111/j.2042-7158.1975.tb09397.x. [DOI] [PubMed] [Google Scholar]
- Lin C., Cham T. Compression behaviour and tensile strength of heat-treated polyethylene glycols. Int. J. Pharm. 1995;118:169–179. [Google Scholar]
- Lowenthal W. Mechanisms of action of tablet disintegrants. Pharm. Acta Helv. 1973;48:589–609. [PubMed] [Google Scholar]
- Mélo E.A., Stamford T.L.M., Silva M.P.C., Krieger N., Stamford N.P. Functional properties of yam bean (Pachyrhizus erosus) starch. Bioresour. Technol. 2003;89:103–106. doi: 10.1016/s0960-8524(02)00313-9. [DOI] [PubMed] [Google Scholar]
- Moorthy S.N. Physicochemical and functional properties of tropical tuber starches: a review. Starch/Stärke. 2002;54:559–592. [Google Scholar]
- Noyes A.A., Whitney W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897;19:930–934. [Google Scholar]
- Odeku O.A., Alabi C.O. Evaluation of native and modified forms of Pennisetum glaucum (millet) starch as disintegrant in chloroquine tablet formulations. J. Drug Del. Sci. Technol. 2007;17:155–157. [Google Scholar]
- Odeku O.A., Itiola O.A. Evaluation of khaya gum as a binder in a paracetamol tablet formulation. Pharm. Pharmacol. Commun. 1998;4:183–188. doi: 10.1081/ddc-120002848. [DOI] [PubMed] [Google Scholar]
- Odeku O.A., Itiola O.A. Evaluation of the effects of khaya gum on the mechanical properties and release properties of paracetamol tablets. Drug Dev. Ind. Pharm. 2003;29:311–320. doi: 10.1081/ddc-120018205. [DOI] [PubMed] [Google Scholar]
- Odeku O.A., Picker-Freyer K.M. Analysis of the material and tablet formation properties of four Dioscorea starches. Starch/Stärke. 2007;59:430–444. [Google Scholar]
- Odeku, O.A., Itiola, O.A., Adeniran, A.A., 1998. Effects of yam and corn starches on the mechanical and disintegration of paracetamol tablets. In: Proceedings of the 1st International Workshop on Herbal Medicinal Products, Omoade Press, Ibadan, Nigeria, pp. 193–200.
- Podczeck F., Sharma M. The influence of particle size and particle shape of components of binary powder mixtures on the maximum volume reduction due to packing. Int. J. Pharm. 1996;137:41–47. [Google Scholar]
- Rapid Visco-Analyzer Manual, 1990. Interpretation of results in Newport Scientific Australia, Model RVA Super3 Serial 3, Section 5, pp. 25–28.
- Riley C.K., Wheatley A.O., Asemota H.N. Isolation and characterization of starches from eight Dioscorea alata cultivars grown in Jamaica. Afr. J. Biotechnol. 2006;5:1528–1536. [Google Scholar]
- Ring S.G. Some studies on gelatin. Starch/Stärke. 1985;37:80–87. [Google Scholar]
- Thiewes H.J., Steeneken P.A.M. Comparison of the Barbender Viscograph and the Rapid Visco-Analyser. 1. A statistical evaluation of pasting profiles. Starch/Stärke. 1997;49:85–92. [Google Scholar]
- Wan L.S.C., Prasad K.P.P. Uptake of water into tablets with low substituted carboxymethylcellulose sodium as disintegrant. Int. J. Pharm. 1989;55:155–161. [Google Scholar]
- Williams P.C., Kuzina F.D., Hlynka I.A. Rapid calorimetric procedure for estimating the amylose content of starches and flours. Cereal Chem. 1970;47:411–420. [Google Scholar]
- Yadav A.R., Guha M., Tharanathan R.N., Ramteke R.S. Changes in characteristics of sweet potato flour prepared by different drying techniques. LWT – Food Science and Technology. 2006;39:20–26. [Google Scholar]
- Young A.H. Fractionation of starch. In: Whistler R.L., Demiller J.N., Pashalls E.F., editors. Starch Chemistry and Technology. second ed. Academic Press; London: 1984. pp. 249–283. [Google Scholar]


