Abstract
Quinoline or 1-aza-naphthalene is a weak tertiary base. Quinoline ring has been found to possess antimalarial, anti-bacterial, antifungal, anthelmintic, cardiotonic, anticonvulsant, anti-inflammatory, and analgesic activity. Quinoline not only has a wide range of biological and pharmacological activities but there are several established protocols for the synthesis of this ring. The article aims at highlighting these very diversities of the ring.
Keywords: Quinoline, Synthesis, Biological activity
1. Introduction
Quinoline [1] or 1-aza-napthalene or benzo[b]pyridine is nitrogen containing heterocyclic aromatic compound. It has a molecular formula of C9H7N and its molecular weight is 129.16. The log P value is 2.04 and has an acidic pKb of 4.85 and a basic pKa of 9.5. Quinoline is a weak tertiary base. It can form salt with acids and displays reactions similar to those of pyridine and benzene. It shows both electrophilic and nucleophilic substitution reactions. It is nontoxic to humans on oral absorption and inhalation.
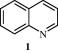
Quinoline nucleus occurs in several natural compounds (Cinchona Alkaloids) and pharmacologically active substances displaying a broad range of biological activity. Quinoline has been found to possess antimalarial, anti-bacterial, antifungal, anthelmintic, cardiotonic, anticonvulsant, anti-inflammatory, and analgesic activity. A few promising compounds [2–6] with quinoline ring system are given in Fig. 1.
Figure 1.
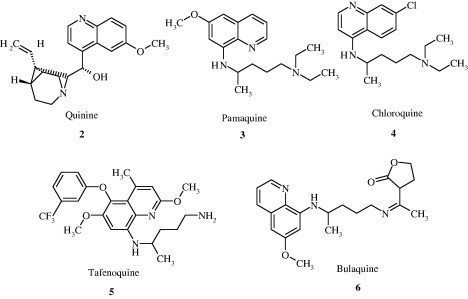
Few promising compounds with quinoline ring system.
2. Synthesis
A number of established protocols are there for the synthesis of quinoline ring, which can be well modified to prepare a number of differently substituted quinolines.
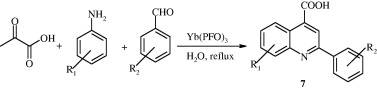
2-Phenylquinoline-4-carboxylic acid [7] has been synthesized by treatment of 2-oxopropionic acid with aniline and benzaldehyde in the presence of rare earth metal catalysts and refluxing in water (Wang et al., 2009a).
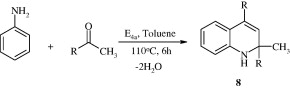
2,4-Diphenyl-2-methyl-1,2 dihydroquinoline [8] has been synthesized by using aniline and acetophenone in the presence of a small pore size E4a zeolite catalyst (Hegedus et al., 2007).
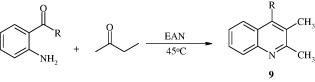
By stirring 2-amino substituted aromatic ketones and carbonyl compounds having a reactive α-methylene group in ethyl ammonium nitrate (EAN) 2,3,4-trisubstituted quinolines [9] have been developed (Zhou et al., 2008).
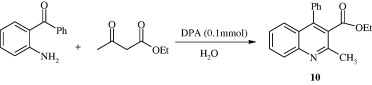
Using 2-aminosubstituted ketone and ketone as reactants poly-substituted quinolines [10] have been synthesized in aqueous media and solvent-free conditions in the presence of dodecylphosphonic acid (DPA) as catalyst (Ghassamipour and Sardarian, 2009).
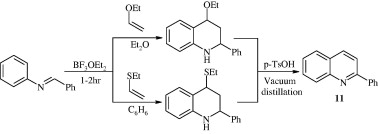
Kouznetsov (2009) synthesized phenyl substituted quinolines [11] by subjecting a mixture of ethyl vinyl ether or ethyl vinyl sulfide and N-arylaldimine to acidic catalysis in the presence of boron trifluoride etherate (BF3.OEt2) to yield 2,4-substituted tetrahydroquinolines, which were then converted to 2-phenyl substituted quinolines under vacuum distillation with tosylic acid (p-TsOH).
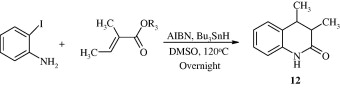
3,4-Dihydroquinolin-2-one [12] has been developed by treating 2-iodoanilines and ethyl acrylate with Azobisisobutyronitrile (AIBN) in presence of tributyltin hydride (n-Bu3SnH) (Zhou et al., 2009).
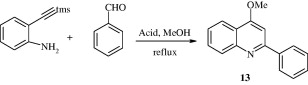
2-Phenyl-4-alkoxy quinoline [13] has been synthesized by condensation and cyclization of 2-(2-trimethylsilyl)ethynyl) aniline with arylaldehydes. The reaction is promoted by sulfuric acid in the presence of methanol as solvent (Wang et al., 2009b).
Certain halogen-substituted quinolines [14] have been synthesized by the condensation and cyclization of two molecules of o-haloacetophenones with urea or primary amines (Qi et al., 2009).
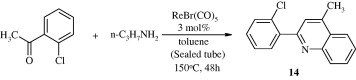
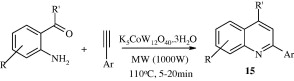
Iraj et al. (2010) synthesized 2,4-disubstituted quinolines [15] through a one-pot reaction of structurally diverse 2-aminoaryl ketones with various arylacetylenes in the presence of potassium dodecatugstocobaltate trihydrate (K5CoW12O40·3H2O) as a reusable and environmentally benign catalyst under microwave irradiation and solvent-free conditions.
Ultrasound promoted synthesis of quinolines [16] using basic ionic liquids (BIL) in aqueous media has been reported by Kowsari and Mallakmohammadi (2011). The advantage of such procedure being that it is simple in operation and high yields are obtained. The reaction involves treating isatin with aromatic methyl ketones at ultrasonic frequencies of 20–50 kHz.
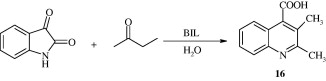
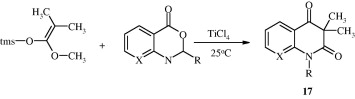
One-step methodology has been introduced for the synthesis of quinoline alkaloid analogues [17] (Zografos et al., 1999). The reaction is based on a modification of the Mukaiyama aldol condensation, making use of the high reactivity of lactones or anhydrides.
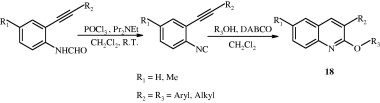
Diversified 2-alkoxy- and 2-aroxy-3-substituted quinolines [18] have been synthesized from o-alkynylaryl isocyanides and alcohols and phenols promoted by 1,4-diazabicyclo[2.2.2]octane (DABCO) (Zhao et al., 2010).
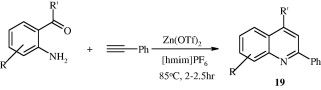
2,4-Disubstituted quinolines [19] have been synthesized according to Meyer-Schuster rearrangement (Sarma and Prajapati, 2008). In this method 2-aminoaryl ketones and phenylacetylenes rearrange in the presence of a catalytic amount of zinc trifluoromethanesulfonate in the ionic liquid 1-hexyl-3-methylilmidazolium hexafluorophosphate [hmim][PF6] resulting in 2,4-disubstituted quinolines. The same product has also been obtained in the presence of indium(III)trifluoromethanesulfonate (In(CF3SO3)3) under microwave irradiation without any solvent (Lekhok et al., 2008).
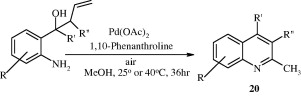
Palladium-catalysed Wacker-type oxidative cyclization has been proposed for the synthesis of 2-methylquinolines [20] with good yields under mild conditions (Wang et al., 2011).
Poly-substituted quinolines [21] have been developed by the reaction of 2-aminobenzylic alcohol derivatives with ketones or alcohols in the presence of base and benzophenone as hydride scavenger (Martinez et al., 2008).
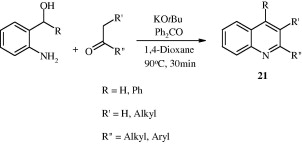
2,4-Disubstituted quinolines [22] have been synthesized by cyclization of 2-iodoanilines with alkynyl aryl ketones in the presence of nickel catalyst (Chen et al., 2006).
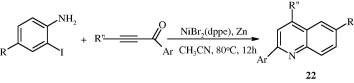
Horn et al. (2008) reported synthesis of quinolines [23] from α,β-unsaturated ketones and o-aminophenylboronic acid derivatives which is a modification of the traditional Skraup-Doebner-von Miller synthesis. The method has an advantage that it can proceed under basic conditions rather than strongly acidic conditions.
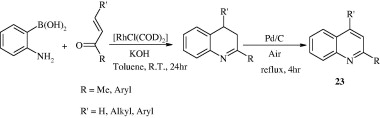
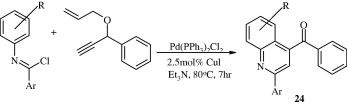
The reaction of benzimidoyl chlorides with 1-(1-(allyloxy)prop-2-ynyl)benzene (1,6-enynes) forms quinoline derivatives [24] via palladium-catalysed Sonogashira coupling and subsequent cyclization (Gao et al., 2010).
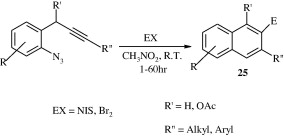
Intramolecular cyclization of 1-azido-2-(2-propynyl)benzene in the presence of electrophilic reagents in nitromethane (CH3NO2) at room temperature or in the presence of catalytic amounts of AuCl3/AgNTf2 in THF at 100 °C gives corresponding quinolines [25] in good yields (Huo et al., 2010).
3. Biological activity
3.1. Antimalarial
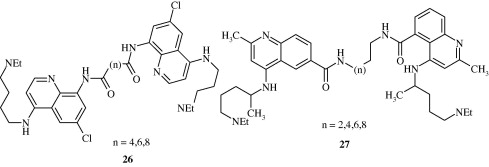
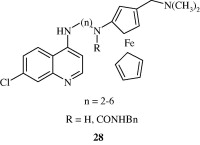
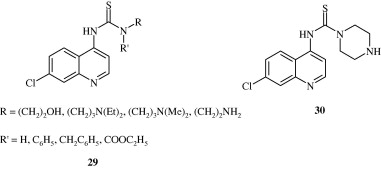
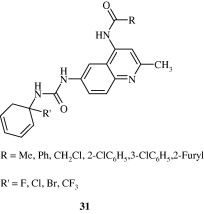
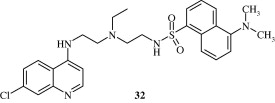
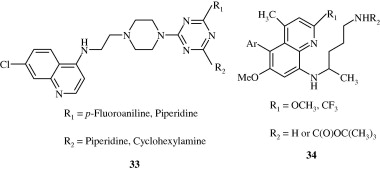
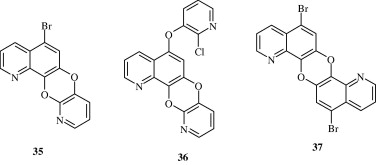
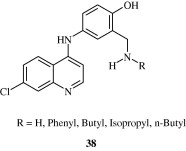
Most important use of the quinoline ring is its antimalarial potential. Bisquinolines [26, 27] developed by Raynes et al. (1996) are found to possess a good degree of antimalarial activity against both chloroquine-resistant and chloroquine-sensitive parasites. Analogues of ferrochloroquine [28] were also found to have antimalarial activity by Chibale et al. (2000). In these analogues carbon chain of chloroquine is replaced by hydrophobic ferrocenyl group. Certain 7-chloroquinolinyl thioureas [29, 30] synthesized by Mahajan et al. (2007) are potential antimalarial agents. Modapa et al. (2009) synthesized few ureido-4-quinolinamides [31] which showed antimalarial effect at MIC of 0.25 mg/mL against chloroquine-sensitive Plasmodium falciparum strain. Chloroquinolyl derivative [32] developed by Kovi et al. (2009) also has a potent antimalarial activity at submicromolar levels. Certain 4-aminoquinoline triazines [33] synthesized by Kumar et al. (2008) also have antimalarial activity screened against chloroquinine (CQ) sensitive strain 3D7 of P. falciparum in an in vitro model. Shiraki et al. (2011) developed certain 5-aryl-8-aminoquinolines [34] with promising antimalarial activity which had lesser haemolytic activity compared to tafenoquine. Acharya et al. (2008) synthesized and evaluated the antimalarial activity of some pyridine–quinoline hybrids [35–37] against chloroquine susceptible strain of P. falciparum. Singh et al. (2011) developed antimalarial agents with 4-anilinoquinoline ring [38]. The compounds showed good activity against chloroquine-sensitive P. falciparum strains as well as against rodent malaria parasite P. yoeii.
3.2. Analgesic activity
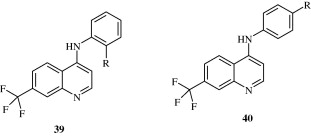
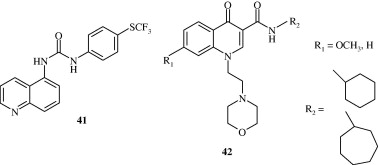
4-Substituted-7-trifluoromethylquinolines [39, 40] synthesized by Abadi et al. (2005) have been found to have a good analgesic activity. The activity is attributed to their nitric oxide releasing properties. Gomtsyan et al. (2005) developed a quinoline [41] based analgesic agent whose activity was attributed to its antagonism at Vanilloid receptors. A few quinoline derivatives [42] developed by Manera et al. (2007) by acting as selective agonists at Cannabinoid CB2 receptors show their analgesic activity.
3.3. Anti-inflammatory activity
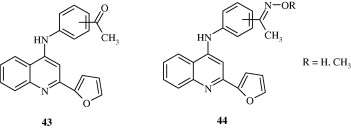
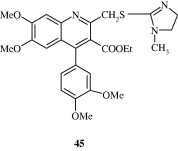
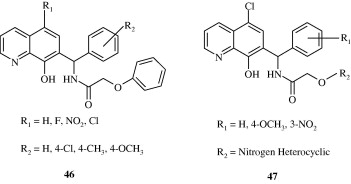
2-(Furan-2-yl)-4-phenoxy-quinoline [43, 44] derivatives developed by Chen et al. (2006) are found to be inhibitors of lysozyme and β-glucuronidase release. Baba et al. (1996) developed a quinoline derivative [45] with potent anti-inflammatory effect in adjuvant arthritis rat model. Certain quinoline derivatives [46, 47] have been developed for treating osteoarthritis by Gilbert et al. (2008). These are amino-acetamide inhibitors of Aggrecanase-2.
3.4. Antineoplastic
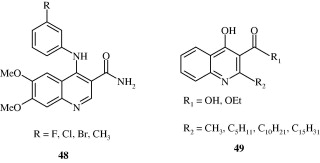
Some of the amido-anilinoquinolines [48] developed by Scott et al. (2009) act as anti-tumour agents by inhibiting CSF-1R kinase. Novel 4-hydroxyquinolines [49] synthesized by Mai et al. (2009) are histone acetyltransferase (HAT) inhibitors. Miller et al. (2009) developed a few 3-cyanoquinolines [50] as inhibitors of insulin like growth factor receptors (IGF-1R) for the treatment of cancer.
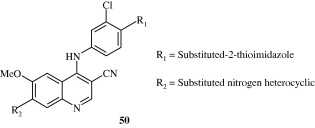
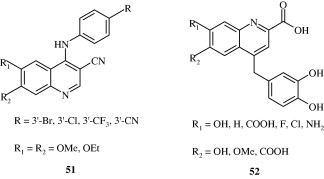
A few 4-anilinoquinolines [51] developed by Assefa et al. (2003) have been found to be tyrosine kinase inhibitors. Potent quinoline carboxylic acids [52] have been developed by Chen et al. (2009) which act by inhibiting insulin like growth factors. Linomide [53] has been found to have action against androgen responsive cancer and rat prostatic cancer by Vukanovic et al. (1993). c-Met kinase inhibitory quinolines [54] with IC50 less than 1 nM have been developed by Wang et al. (2011). It produces the inhibition of c-Met phosphorylation in c-Met dependent cell lines.
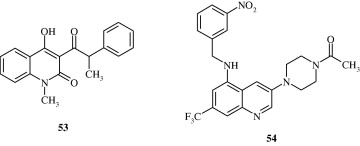
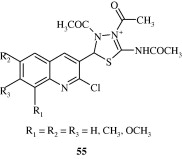
Marganakop et al. (2012) developed certain 6,7,8-substituted thiosemicarbazones of 2-chloro-3-formyl-quinoline derivatives [55] which had anticancer activities. The compounds had a better drug score and c log P values.
3.5. Antibacterial
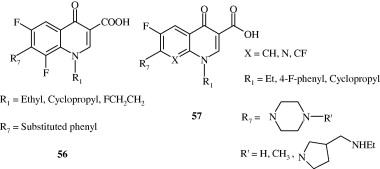
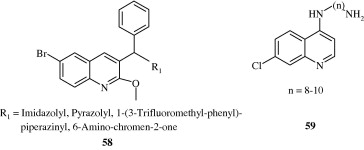
Ma et al. (2009) synthesized phenoxy, phenylthio and benzyloxy substituted quinolones [56] with a fair amount of anti-bacterial activity. Sanchez et al. (1988) developed certain 8-substituted quinoline carboxylic acids [57] with anti-bacterial activity. Upadhayaya et al. (2009) developed quinoline derivatives [58] through molecular modelling techniques which were found to be active against Mycobacterium tuberculosis H37Rv strain. These were derivatives of 3-benzyl-6-bromo-2-methoxy quinolines. De Souza et al. (2009) developed 7-chloro quinoline derivatives [59] effective against multi-drug resistant tuberculosis. Lilienkampf et al. (2009) developed quinoline based compound bearing an isoxazole containing side chain [60] active against Mycobacterium tuberculosis. Some novel anti-tubercular quinolines [61] have been developed by Eswaran et al. (2010) using mefloquine as the lead, wherein active pharmacophores viz. hydrazones, ureas, thioureas and pyrazoles have been attached at the 4th position.
3.6. Antifungal
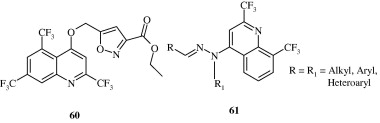
Gholap et al. (2007) developed certain tetrahydroquinolines [62] which are found to have a good degree of activity against fungi Candida albicans, Fusarium oxysporum and Mucor sp. Kharkar et al. (2009) developed a series of quinoline derivatives [63] using terbenafine as lead as antifungal agents. The developed compounds contained different bulky aromatic rings in the side chain. The compounds were designed using LeapFrog drug design program. Kumar et al. (2011) developed certain secondary amines [64] containing 2-chloroquinoline and evaluated them for their antimycotic activity against Aspergillus niger, A. flavus, Monascus purpureus and Penicillium citrinum. These are non-azole antimycotic agents.
3.7. Antiviral
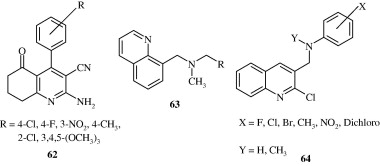
Anilidoquinoline [65] derivatives synthesized by Ghosh et al. (2008) are found to have a good degree of in vitro activity against Japanese encephalitis virus. Certain quinoline derivatives [66] synthesized by Chen et al. (2009) act by behaving as HIV-1 Tat–TAR interaction inhibitors. Massari et al. (2009) developed certain desfluoroquinolines [67] for the treatment of HIV infection. Certain mono and polysubstituted quinolines [68–70] synthesized by Fakhfakh et al. (2003) have activity against HIV-1.
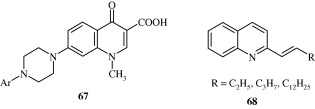
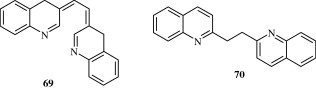
3.8. Anthelmintic
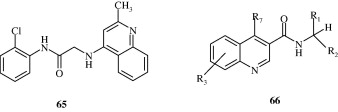
Rossiter et al. (2005) synthesized substituted 2,4-arylquinolines [71–74] which have a good degree of activity against the nematode Haemonchus contortus. These arylquinolines maintain their activity against levamisole, ivermectin and thiabendazole resistant strains of H. contortus.
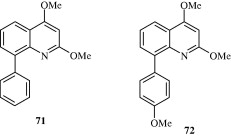
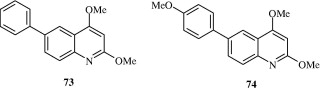
3.9. Anti-protozoal
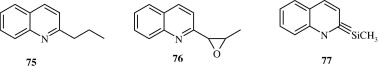
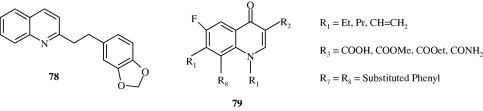
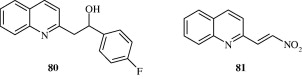
Fournet et al. (1993) found that 2-substituted quinoline alkaloids isolated from G. longiflora plant used for the treatment of new world cutaneous leishmaniasis have in vitro antileishmanial activity against the extracellular forms of Leishmania spp. These include 2-substituted 3-carbon chain quinoline alkaloids and 2-substituted aryl quinoline alkaloids [75, 76]. Alkenyl and alkynyl quinolines [77, 78] developed by Fakhfakh et al. (2003) show activity against the causal agents of cutaneous leishmaniasis, visceral leishmaniasis, African trypanosomiasis and Chagas’ disease. Ma et al. (2009) developed certain quinolones [79] which had activity against Trypanosoma cruzi. Franck et al. (2004) developed quinoline derivatives [80, 81] which showed activity against T. cruzi.
3.10. Cardiovascular activity
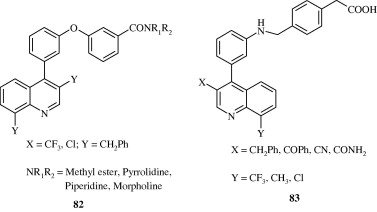
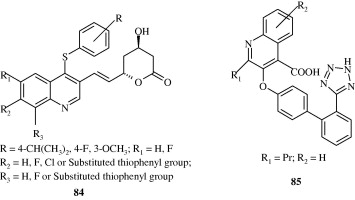
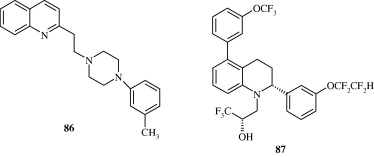
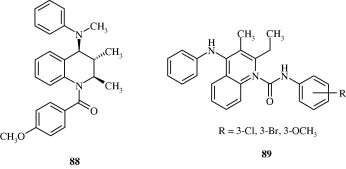
Certain biarylether amide quinolines [82] developed by Bernotas et al. (2009) act as liver X receptor agonists and are useful in conditions of dyslipidaemia. These agents also reverse the conditions of arteriosclerosis. A few phenyl acetic acid based quinolines [83] developed by Hu et al. (2007) also act as agonists at liver X receptors. These agents have good binding affinity for LXRβ and LXRα receptors. 4-Thiophenyl quinolines [84] developed by Cai et al. (2007) are HMG-CoA reductase inhibitors and have utility as hypocholesterolaemic agents. Quinoline-4-carboxylic acids [85] synthesized by Lloyd et al. (1994) are angiotensin II receptor antagonists and hence act as hypotensive agents. Hypotensive activity of centhaquin [86] has been demonstrated by Srimal et al. (1990) and it has been shown to reduce the blood pressure in cat in a dose dependent manner. Tetrahydroquinolines [87] which inhibit cholesteryl ester transfer protein have been developed by Rano et al. (2009). Tetrahydroquinolinamines [88, 89] developed by Ramos et al. (2008) have been found to be inhibitors of platelet aggregation.
3.11. CNS effects
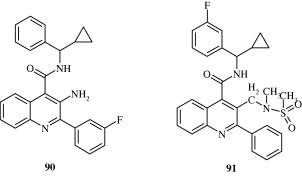
Quinoline based NK3 receptor antagonists [90, 91] with CNS activity have been developed by Smith et al. (2009).
3.12. Hypoglycaemic activity
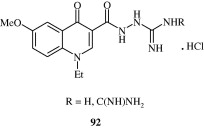
Quinoline carboxyguanides [92] prepared by Edmont et al. (2000) are hypoglycaemic agents.
3.13. Reproductive System
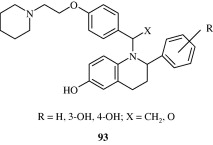
Certain tetrahydroquinolines [93] developed by Wallace et al. (2003) are selective oestrogen receptor modulators.
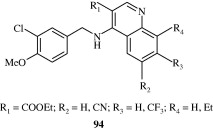
Bi et al. (2004) developed certain quinolines [94] which act as potent PDE5 inhibitors thus having utility in the treatment of erectile dysfunction.
3.14. Miscellaneous
Quinolines have been found to possess a number of other activities as well.
Evans et al. (1991) developed certain quinoline based leukotriene synthesis inhibitors [95].

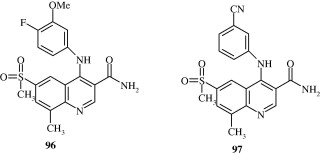
Selective PDE4 inhibitor quinolines [96, 97] have been developed by Lunniss et al. (2009) with utility in chronic obstructive pulmonary disorder.
Quinoline 3-carboxamide [98] is used for the treatment of chronic relapsing autoimmune encephalitis. This activity was studied by Karussis et al. (1993).
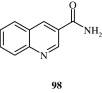
Bachiller et al. (2010a,b) have developed some novel tacrine–8-hydroxyquinoline hybrids [99] with activity against Alzheimer’s. Tacrine has cholinesterase inhibition action while 8-hydroxyquinoline derivatives have metal-chelating, neuroprotective and anti-oxidant properties.
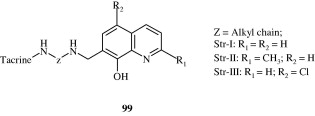
Tetrahydroquinolin-6-yloxy propanes [100] have been developed by Shakya et al. (2009) which are β-3 agonists.
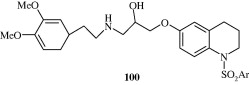
Certain aminoalkoxyquinolines [101] as somatostatin receptor subtype-2 agonists have been reported by Wolkenberg et al. (2011) which have utility in proliferative diabetic retinopathy and exudative age related macular degeneration.
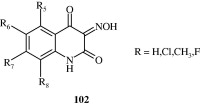
The 1,2,3,4-tetrahydroquinoline-2,2,4-trione oximes developed by Cai et al. (1996) [102] act as antagonists of NDMA in glycine receptors. These compounds can be used as agents against neurodegenerative diseases (e.g., Alzheimer’s disease).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abadi A.H., Hegazy G.H., Zaher A.A.E. Synthesis of novel 4-substituted-7-trifluoromethylquinoline derivatives with nitric oxide releasing properties and their evaluation as analgesic and anti-inflammatory agents. Bioorg. Med. Chem. 2005;13:5759–5765. doi: 10.1016/j.bmc.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Acharya B.N., Thavaselvam D., Kaushik M.B. Synthesis and antimalarial evaluation of novel pyridine quinoline hybrids. Med. Chem. Res. 2008;17:487–494. [Google Scholar]
- Assefa H., Kamath S., Buolamwini J.K. 3D-QSAR and docking studies on 4-anilinoquinazoline and 4-anilinoquinoline epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors. J. Comput. Aided Mol. Des. 2003;17:475–493. doi: 10.1023/b:jcam.0000004622.13865.4f. [DOI] [PubMed] [Google Scholar]
- Baba A., Kawamura N., Makino H., Ohta Y., Taketomi S., Sohda T. Studies on disease modifying antirheumatic drugs: synthesis of novel quinoline and quinazoline derivatives and their anti-inflammatory effect. J. Med. Chem. 1996;39:5176–5182. doi: 10.1021/jm9509408. [DOI] [PubMed] [Google Scholar]
- Bachiller M.I.F., Perez C., Munoz G.C.G., Conde S., Lopez M.G., Villarroya M., Garcia A.G., Franco M.I.R. Novel tacrine–8-hydroxyquinoline hybrids as multifunctional agents for the treatment of Alzheimer’s disease, with neuroprotective, cholinergic, antioxidant, and copper complexing properties. J. Med. Chem. 2010;53:4927–4937. doi: 10.1021/jm100329q. [DOI] [PubMed] [Google Scholar]
- Bachiller M.I.F., Perez C., Munoz G.C.G., Conde S., Lopez M.G., Villarroya M., Garcia A.G., Franco M.I.R. Novel tacrine–8-hydroxyquinoline hybrids as multifunctional agents for the treatment of Alzheimer’s disease, with neuroprotective, cholinergic, antioxidant, and copper-complexing properties. J. Med. Chem. 2010;53:4927–4937. doi: 10.1021/jm100329q. [DOI] [PubMed] [Google Scholar]
- Bernotas R.C., Singhaus R.R., Kaufman D.H., Ullrich J., Fletcher H., Quinet E., Nambi P., Unwalla R., Wilhelmsson A., Nilsson A.G., Farnegardh M., Wrobel J. Biarylether amide quinolines as liver X receptor agonists. Bioorg. Med. Chem. 2009;17:1663–1670. doi: 10.1016/j.bmc.2008.12.048. [DOI] [PubMed] [Google Scholar]
- Bi Y., Stoy P., Adam L., He B., Krupinski J., Normandin D., Pongrac R., Seliger L., Watson A., Macor J.E. Quinolines as extremely potent and selective PDE5 inhibitors as potential agents for treatment of erectile dysfunction. Bioorg. Med. Chem. Lett. 2004;14:1577–1580. doi: 10.1016/j.bmcl.2003.12.090. [DOI] [PubMed] [Google Scholar]
- Cai S.X., Zhou Z.L., Huang J.C., Whittemore E.R., Egbuwoku Z.O., Lu Y., Hawkinson J.E., Woodward R.M., Weber E., Keana J.F.W. Synthesis and structure activity relationships of 1,2,3,4-tetrahydroquinoline-2,3,4-trione 3-oximes: novel and highly potent antagonists for NMDA receptor glycine site. J. Med. Chem. 1996;39:3248–3255. doi: 10.1021/jm960214k. [DOI] [PubMed] [Google Scholar]
- Cai Z., Zhou W., Sun L. Synthesis and HMG CoA reductase inhibition of 4-thiophenyl quinolines as potential hypocholesterolemic agents. Bioorg. Med. Chem. 2007;15:7809–7829. doi: 10.1016/j.bmc.2007.08.044. [DOI] [PubMed] [Google Scholar]
- Chen S., Chen R., He M., Pang R., Tan Z., Yang M. Design, synthesis, and biological evaluation of novel quinoline derivatives as HIV-1 Tat–TAR interaction inhibitors. Bioorg. Med. Chem. 2009;17:1948–1956. doi: 10.1016/j.bmc.2009.01.038. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhao Y., Lu C., Tzeng C., Wang J.P. Synthesis, cytotoxicity, and antiinflammatory evaluation of 2-(furan-2-yl)-4-(phenoxy)quinoline derivatives. Part 4. Bioorg. Med. Chem. 2006;14:4373–4378. doi: 10.1016/j.bmc.2006.02.039. [DOI] [PubMed] [Google Scholar]
- Chibale K., Moss J.R., Blackie M., Schalkwyk D., Smith P.J. New amine and urea analogs of ferrochloroquine: synthesis, antimalarial activity in vitro and electrochemical studies. Tetrahedron Lett. 2000;41:6231–6235. [Google Scholar]
- Edmont D., Rocher R., Plisson C., Chenault J. Synthesis and evaluation of quinoline carboxyguanidines as antidiabetic agents. Bioorg. Med. Chem. Lett. 2000;10:1831–1834. doi: 10.1016/s0960-894x(00)00354-1. [DOI] [PubMed] [Google Scholar]
- Eswaran S., Adhikari A.V., Chowdhury I.H., Pal N.K., Thomas K.D. New quinoline derivatives: synthesis and investigation of antibacterial and antituberculosis properties. Eur. J. Med. Chem. 2010;45:3374–3383. doi: 10.1016/j.ejmech.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Evans J.F., Leveille C., Mancini J.A., Prasit P., Therien M., Zamboni R., Gauthier J.Y., Fortin R., Charleson P., Maclntyre D.E. 5-lipooxygenase-activating protein is the target of a quinoline class of leukotriene synthesis inhibitors. Mol. Pharm. 1991;40:22–27. [PubMed] [Google Scholar]
- Fakhfakh M.A., Fournet A., Prina E., Mouscadet J.F., Franck X., Hocquemiller R., Figadere B. Synthesis and biological evaluation of substituted quinolines: potential treatment of protozoal and retroviral co-infections. Bioorg. Med. Chem. 2003;11:5013–5023. doi: 10.1016/j.bmc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Fournet A., Barrios A.A., Munoz V., Hocquemiller R., Cave A., Bruneton J. 2-substituted quinoline alkaloids as potential antileishmanial drugs. Antimicrob. Agents Chemother. 1993;37:859–863. doi: 10.1128/aac.37.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck X., Fournet A., Prina E., Mahieux R., Hocquemiller R., Figadere B. Biological evaluation of substituted quinolines. Bioorg. Med. Chem. Lett. 2004;14:3635–3638. doi: 10.1016/j.bmcl.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Gao G.L., Niu Y.N., Yan Z.Y., Wang H.L., Wang G.W., Shaukat A., Liang Y.M. Unexpected domino reaction via Pd-catalysed sonogashira coupling of benzimidoyl chlorides with 1,6-enynes and cyclization to synthesize quinoline derivatives. J. Org. Chem. 2010;75:1305–1308. doi: 10.1021/jo9026116. [DOI] [PubMed] [Google Scholar]
- Ghassamipour S., Sardarian A.R. Friedländer synthesis of poly-substituted quinolines in the presence of dodecylphosphonic acid (DPA) as a highly efficient, recyclable and novel catalyst in aqueous media and solvent-free conditions. Tetrahedron Lett. 2009;50:514–519. [Google Scholar]
- Gholap A.R., Toti K.S., Shirazi F., Kumari R., Bhat M.K., Deshpande M.V., Srinivasan K.V. Synthesis and evaluation of antifungal properties of a series of the novel 2-amino-5-oxo-4-phenyl-5,6,7,8-tetrahydroquinoline-3-carbonitrile and its analogues. Bioorg. Med. Chem. 2007;15:6705–6715. doi: 10.1016/j.bmc.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Ghosh J, Swarup V, Saxena A, Das S, Hazra A, Paira P, Banerjee S, Mondal NB, Basu A., 2008. Therapeutic effect of a novel anilidoquinoline derivative, 2-(2-methyl-quinoline-4ylamino)-N-(2-chlorophenyl)-acetamide, in Japanese encephalitis: correlation with in vitro neuroprotection. Int. J. Antimicrob. Agents 32, 349–354. [DOI] [PubMed]
- Gilbert A.M., Bursavich M.G., Lombardi S., Georgiadis K.E., Reifenberg E., Flannery C., Morris E.A. N-((8-Hydroxy-5-substituted-quinolin-7-yl)(phenyl)methyl)-2-phenyloxy/amino-acetamide inhibitors of ADAMTS-5 (Aggrecanase-2) Bioorg. Med. Chem. Lett. 2008;18:6454–6457. doi: 10.1016/j.bmcl.2008.10.065. [DOI] [PubMed] [Google Scholar]
- Gomtsyan A., Bayburt E.K., Schmidt R.G., Zheng G.Z., Perner P.J., Didomenico S., Koenig J.R., Turner S., Jinkerson T., Drizin I., Hannick S.M., Macri B.S., McDonald H.A., Honore P., Wismer C.T., Marsh K.C., Wetter J., Stewart K.D., Oie T., Jarvis M.F., Surowy C.S., Faltynek C.R., Lee C.H. Novel transient receptor potential vanilloid 1 receptor antagonists for the treatment of pain: structure–activity relationships for ureas with quinoline, isoquinoline, quinazoline, phthalazine, quinoxaline, and cinnoline moieties. J. Med. Chem. 2005;48:744–752. doi: 10.1021/jm0492958. [DOI] [PubMed] [Google Scholar]
- Hegedus A., Hell Z., Vargadi T., Potor A., Gresits I. A new, simple synthesis of 1,2-dihydroquinolines via cyclocondensation using zeolite catalyst. Catal. Lett. 2007;117:99–101. [Google Scholar]
- Horn J., Marsden S.P., Nelson A., House D., Weingarten G.G. Convergent, regiospecific synthesis of quinolines from o-aminophenylboronates. Org. Lett. 2008;10:4117–4120. doi: 10.1021/ol8016726. [DOI] [PubMed] [Google Scholar]
- Hu B., Jetter J., Kaufman D., Singhaus R., Bernotas R., Unwalla R., Quinet E., Savio D., Halpern A., Basso M., Keith J., Clerin V., Chen L., Liu Q.Y., Feingold I., Huselton C., Azam F., Nilsson A.G., Wilhelmsson A., Nambi P., Wrobel J. Further modification on phenyl acetic acid based quinolines as liver X receptor modulators. Bioorg. Med. Chem. 2007;15:3321–3333. doi: 10.1016/j.bmc.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Huo Z., Gridnev I.D., Yamamoto Y. A method for the synthesis of substituted quinolines via electrophilic cyclization of 1-azido-2-(2-propynyl)benzene. J. Org. Chem. 2010;75:1266–1270. doi: 10.1021/jo902603v. [DOI] [PubMed] [Google Scholar]
- Iraj M.B., Shahram T., Majid M., Valiollah M., Salma A., Arsalan M. Microwave-promoted alkynylation-cyclization of 2-aminoaryl ketones: a green strategy for the synthesis of 2,4-disubstituted quinolines. Synlett. 2010;20:3104–3112. [Google Scholar]
- Karussis D.M., Lehmann D., Slavin S., Karussis U.V., Koll R.M., Ovadia H., Kalland T., Abramsky O. Treatment of chronic-relapsing experimental autoimmune encephalomyelitis with the synthetic immunomodulator linomide (quinoline-3-carboxamide) Proc. Natl. Acad. Sci. USA. 1993;90:6400–6404. doi: 10.1073/pnas.90.14.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkar P.S., Deodhar M.N., Kulkarni V.M. Design, synthesis, antifungal activity, and ADME prediction of functional analogues of terbinafine. Med. Chem. Res. 2009;18:421–432. [Google Scholar]
- Kouznetsov V.V. Recent synthetic developments in a powerful imino Diels–Alder reaction (Povarov reaction): application to the synthesis of N-polyheterocycles and related alkaloids. Tetrahedron. 2009;65:2721–2750. [Google Scholar]
- Kovi K.E., Yearick K., Iwaniuk D.P., Natarajan J.K., Alumasa J., de Dois A.C., Roepe P.D., Wolf C. Search for new pharmacophores for antimalarial activity. Part I: synthesis and antimalarial activity of new 2-methyl-6-ureido-4-quinolinamides. Bioorg. Med. Chem. 2009;17:270–283. doi: 10.1016/j.bmc.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Kowsari E., Mallakmohammadi M. Ultrasound promoted synthesis of quinolines using basic ionic liquids in aqueous media as a green procedure. Ultrason. Sonochem. 2011;18:447–454. doi: 10.1016/j.ultsonch.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Kumar A., Srivastava K., Kumar S.R., Puri S.K., Chauhan P.M.S. Synthesis and bioevaluation of hybrid 4-aminoquinoline triazines as a new class of antimalarial agents. Bioorg. Med. Chem. Lett. 2008;18:6530–6533. doi: 10.1016/j.bmcl.2008.10.049. [DOI] [PubMed] [Google Scholar]
- Kumar S., Bawa S., Drabu S., Panda B.P. Design and synthesis of 2-chloroquinoline derivatives as non-azoles antimycotic agents. Med. Chem. Res. 2011;20:1340–1348. [Google Scholar]
- Lekhok K.C., Prajapati D., Boruah R.C. Indium(III) trifluoromethanesulfonate: an efficient reusable catalyst for the alkynylation–cyclization of 2-aminoaryl ketones and synthesis of 2,4-disubstituted quinolines. Synlett. 2008;5:655–658. [Google Scholar]
- Lilienkampf A., Mao J., Wan B., Wang Y., Franzblau S.G., Kozikowski A.P. Structure activity relationships for a series of quinoline-based compounds active against replicating and nonreplicating mycobacterium tuberculosis. J. Med. Chem. 2009;52:2109–2118. doi: 10.1021/jm900003c. [DOI] [PubMed] [Google Scholar]
- Lloyd J., Ryono D.E., Bird J.E., Buote J., Delaney C.L., Dejneka T., Dickinson K.E.J., Moreland S., Normandin D.E., Skwish S., Spitzmiller E.R., Waldron T.L. Quinoline-4 carboxylic acids as angiotensin II receptor antagonists. Bioorg. Med. Chem. Lett. 1994;4:195–200. [Google Scholar]
- Lunniss C.J., Cooper A.W.J., Eldred C.D., Kranz M., Lindvall M., Lucas F.S., Neu M., Preston A.G.S., Ranshaw L.E., Redgrave A.J., Robinson J.E., Shipley T.J., Solanke Y.E., Somers D.O., Wiseman J.O. Quinolines as a novel structural class of potent and selective PDE4 inhibitors: optimisation for oral administration. Bioorg. Med. Chem. Lett. 2009;19:1380–1385. doi: 10.1016/j.bmcl.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Ma X., Zhou W., Brun R. Synthesis, in vitro antitrypanosomal and antibacterial activity of phenoxy, phenylthio or benzyloxy substituted quinolones. Bioorg. Med. Chem. Lett. 2009;19:986–989. doi: 10.1016/j.bmcl.2008.11.078. [DOI] [PubMed] [Google Scholar]
- Mahajan A., Yeh S., Nell M., Rensburg C.E.J., Chibale K. Synthesis of new 7-chloroquinolinyl thioureas and their biological investigation as potential anti-malarial and anticancer agents. Bioorg. Med. Chem. Lett. 2007;17:5683–5685. doi: 10.1016/j.bmcl.2007.07.049. [DOI] [PubMed] [Google Scholar]
- Mai A., Rotili D., Tarantino D., Nebbioso A., Castellano S., Sbardella G., Tini M., Altucci L. Identification of 4-hydroxyquinolines inhibitors of p300/CBP histone acetyltransferases. Bioorg. Med. Chem. Lett. 2009;19:1132–1135. doi: 10.1016/j.bmcl.2008.12.097. [DOI] [PubMed] [Google Scholar]
- Manera C., Cascio M.G., Benetti V., Allara M., Tuccinardi T., Martinelli A., Saccomanni G., Vivoli E., Ghelardini C., Marzo V.D., Ferrarini P.L. New 1,8-naphthyridine and quinoline derivatives as CB2 selective agonists. Bioorg. Med. Chem. Lett. 2007;17:6505–6510. doi: 10.1016/j.bmcl.2007.09.089. [DOI] [PubMed] [Google Scholar]
- Marganakop S.B., Kamble R.R., Taj T., Kariduraganvar M.Y. An efficient one-pot cyclization of quinoline thiosemicarbazones to quinolines derivatized with 1,3,4-thiadiazole as anticancer and anti-tubercular agents. Med. Chem. Res. 2012;21:185–191. [Google Scholar]
- Martinez R., Ramon D.J., Yus M. Transition metal free indirect Friedlander synthesis of quinolines from alcohols. J. Org. Chem. 2008;73:9778–9780. doi: 10.1021/jo801678n. [DOI] [PubMed] [Google Scholar]
- Massari S., Daelemans D., Manfroni G., Sabatini S., Tabarrini O., Pannecouque C., Cecchetti V. Studies on anti-HIV quinolones: new insights on the C-6 position. Bioorg. Med. Chem. 2009;17:667–674. doi: 10.1016/j.bmc.2008.11.056. [DOI] [PubMed] [Google Scholar]
- Miller L.M., Mayer S.C., Berger D.M., Boschelli D.H., Boschelli F., Di L., Du X., Dutia M., Floyd M.B., Johnson M., Kenny C.H., Krishnamurthy G., Moy F., Petusky S., Tkach D., Torres N., Wu B., Xu W. Lead identification to generate 3-cyanoquinoline inhibitors of insulin-like growth factor receptor (IGF-1R) for potential use in cancer treatment. Bioorg. Med. Chem. Lett. 2009;19:62–66. doi: 10.1016/j.bmcl.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Modapa S., Tusi Z., Sridhar D., Kumar A., Siddiqi M.I., Srivastava K., Rizvi A., Tripathi R., Puri S.K., Keshava G.B.S., Shukla P.K., Batra S. Search for new pharmacophores for antimalarial activity. Part I: synthesis and antimalarial activity of new 2-methyl-6-ureido-4-quinolinamides. Bioorg. Med. Chem. 2009;17:203–221. doi: 10.1016/j.bmc.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Qi C., Zheng Q., Hua R. A domino three-component condensation of ortho-haloacetophenones with urea or amines: a novel one-pot synthesis of halogen-substituted quinolines. Tetrahedron. 2009;65:1316–1320. [Google Scholar]
- Ramos A.I.M., Mecom J.S., Kiesow T.J., Graybill T.L., Brown G.D., Aiyar N.V., Davenport E.A., Kallal L.A., Reed B.A.K., Li P., Londregan A.T., Morrow D.M., Senadhi S., Thalji R.K., Zhao S., Kurtis C.L.B., Marino J.P. Tetrahydro-4-quinolinamines identified as novel P2Y1 receptor antagonists. Bioorg. Med. Chem. Lett. 2008;18:6222–6226. doi: 10.1016/j.bmcl.2008.09.102. [DOI] [PubMed] [Google Scholar]
- Rano T.A., McMaster E.S., Pelton P.D., Yang M., Demarest K.T., Kuo G.H. Design and synthesis of potent inhibitors of cholesteryl ester transfer protein (CETP) exploiting a 1,2,3,4-tetrahydroquinoline platform. Bioorg. Med. Chem. Lett. 2009;19:2456–2460. doi: 10.1016/j.bmcl.2009.03.051. [DOI] [PubMed] [Google Scholar]
- Raynes K., Foley M., Tilley L., Deady L.W. Novel bisquinoline antimalarials: synthesis, antimalarial activity and inhibition of haem polymerisation. Biochem. Pharmacol. 1996;52:551–559. doi: 10.1016/0006-2952(96)00306-1. [DOI] [PubMed] [Google Scholar]
- Rossiter S., Peron S.J., Whitfield P.J., Jones K. Synthesis and anthelmintic properties of arylquinolines with activity against drug-resistant nematodes. Bioorg. Med. Chem. Lett. 2005;15:4806–4808. doi: 10.1016/j.bmcl.2005.07.044. [DOI] [PubMed] [Google Scholar]
- Sanchez J.P., Domagala J.M., Hagen S.E., Heifetz C.L., Hutt M.P., Nichols J.B., Trehan A.K. Quinolone antibacterial agents. Synthesis and structure-activity relationships of 8-substituted quinoline-3-carboxylic acids and 1,8-naphthyridine-3-carboxylic acids. J. Med. Chem. 1988;31:983–991. doi: 10.1021/jm00400a016. [DOI] [PubMed] [Google Scholar]
- Sarma R., Prajapati D. Ionic liquid – an efficient recyclable system for the synthesis of 2,4-disubstituted quinolines via Meyer–Schuster rearrangement. Synlett. 2008;19:3001–3005. [Google Scholar]
- Scott D.A., Balliet C.L., Cook D.J., Davies A.M., Gero T.W., Omer C.A., Poondru S., Theoclitou M.E., Tyurin B., Zinda M.J. Identification of 3-amido-4-anilinoquinolines as potent and selective inhibitors of CSF-1R kinase. Bioorg. Med. Chem. Lett. 2009;19:697–700. doi: 10.1016/j.bmcl.2008.12.046. [DOI] [PubMed] [Google Scholar]
- Shakya N., Roy K.K., Saxena A.K. Substituted 1,2,3,4-tetrahydroquinolin-6-yloxypropanes as β3-adrenergic receptor agonists: design, synthesis, biological evaluation and pharmacophore modeling. Bioorg. Med. Chem. 2009;17:830–847. doi: 10.1016/j.bmc.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Shiraki H., Kozar M.P., Melendez V., Hudson T.H., Ohrt C., Magill A.J., Lin A.J. Antimalarial activity of novel 5-aryl-8-aminoquinoline derivatives. J. Med. Chem. 2011;54:131–142. doi: 10.1021/jm100911f. [DOI] [PubMed] [Google Scholar]
- Singh B., Chetia D., Puri S.K., Srivastava K., Prakash A. Synthesis and in vitro and in vivo antimalarial activity of novel 4-anilinoquinoline Mannich base derivatives. Med. Chem. Res. 2011;20:1523–1529. [Google Scholar]
- Smith P.W., Wymana P.A., Lovell P., Goodacre C., Serafinowska H.T., Vong A., Harrington F., Flynn S., Bradley D.M., Porter R., Coggon S., Murkitt G., Searle K., Thomas D.R., Watson J.M., Martin W., Wu Z., Dawson L.A. New quinoline NK3 receptor antagonists with CNS activity. Bioorg. Med. Chem. Lett. 2009;19:837–840. doi: 10.1016/j.bmcl.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Souza M.V.N.D., Pais K.C., Kaiser C.R., Peralta M.A., Ferreira M.D.L., Lourenco M.C.S. Synthesis and in vitro antitubercular activity of a series of quinoline derivatives. Bioorg. Med. Chem. 2009;17:1474–1480. doi: 10.1016/j.bmc.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Srimal R.C., Gulati K., Nityanand S., Dhawan B.N. Pharmacological studies on 2-(2-(4-(3-methylphenyl)-1-piperazinyl)ethyl) quinoline (centhaquin). I. Hypotensive activity. Pharmacol. Res. 1990;22:319–329. doi: 10.1016/1043-6618(90)90729-w. [DOI] [PubMed] [Google Scholar]
- Upadhayaya R.S., Vandavasi J.K., Vasireddy N.R., Sharma V., Dixit S.S., Chattopadhyaya J. Design, synthesis, biological evaluation and molecular modelling studies of novel quinoline derivatives against Mycobacterium tuberculosis. Bioorg. Med. Chem. 2009;17:2830–2841. doi: 10.1016/j.bmc.2009.02.026. [DOI] [PubMed] [Google Scholar]
- Vukanovic J., Passaniti A., Hirata T., Traystman R.J., Asp B.H., Isaacs J.T. Antiangiogenic effects of the quinoline-3-carboxamide linomide. Cancer Res. 1993;53:1833–1837. [PubMed] [Google Scholar]
- Wallace O.B., Lauwers K.S., Jones S.A., Dodge J.A. Tetrahydroquinoline based selective estrogen receptor modulators (SERMs) Bioorg. Med. Chem. Lett. 2003;13:1907–1910. doi: 10.1016/s0960-894x(03)00306-8. [DOI] [PubMed] [Google Scholar]
- Wang L.M., Hu L., Chen H.J., Sui Y.Y., Shen W. One-pot synthesis of quinoline-4-carboxylic acid derivatives in water: ytterbium perfluorooctanoate catalysed Doebner reaction. J. Fluorine Chem. 2009;130:406–409. [Google Scholar]
- Wang Y., Peng C., Liu L., Zhao J., Su L., Zhu Q. Sulfuric acid promoted condensation cyclization of 2-(2-(trimethylsilyl) ethynyl)anilines with arylaldehydes in alcoholic solvents: an efficient one-pot synthesis of 4-alkoxy-2-arylquinolines. Tetrahedron Lett. 2009;50:2261–2265. [Google Scholar]
- Wang Y., Ai J., Wang Y., Chen Y., Wang L., Liu G., Geng M., Zhang A. Synthesis and c-Met kinase inhibition of 3,5-disubstituted and 3,5,7-trisubstituted quinolines: identification of 3-(4 acetylpiperazin-1-yl)-5-(3-nitrobenzylamino)-7 (trifluoromethyl)quinoline as a novel anticancer agent. J. Med. Chem. 2011;54:2127–2142. doi: 10.1021/jm101340q. [DOI] [PubMed] [Google Scholar]
- Wolkenberg S.E., Zhao Z., Thut C., Maxwell J.W., McDonald T.P., Kinose F., Reilly M., Lindsley C.W., Hartman G.D. Design, synthesis, and evaluation of novel 3,6-diaryl-4-aminoalkoxyquinolines as selective agonists of somatostatin receptor subtype 2. J. Med. Chem. 2011;54:2351–2358. doi: 10.1021/jm101501b. [DOI] [PubMed] [Google Scholar]
- Zhao J., Peng C., Liu L., Wang Y., Zhu Q. Synthesis of 2-alkoxy(aroxy)-3-substituted quinolines by DABCO promoted cyclization of o-alkynylaryl isocyanides. J. Org. Chem. 2010;75:7502–7504. doi: 10.1021/jo1017525. [DOI] [PubMed] [Google Scholar]
- Zhou T., Lin J., Chen Z. A convenient synthesis of quinolines via ionic liquid-catalysed friedlander annulation. Lett. Org. Chem. 2008;5:47–50. [Google Scholar]
- Zhou W., Zhang L., Jiao N. The tandem reaction combining radical and ionic processes: an efficient approach to substituted 3,4-dihydroquinolin-2-ones. Tetrahedron. 2009;65:1982–1987. [Google Scholar]
- Zografos A.L., Mitsos C.A., Markopoulou O.I. One step synthesis for the preparation of quinoline alkaloid analogues. Org. Lett. 1999;1:1953–1955. [Google Scholar]



