Key Points
CMV reactivation after HCT is associated with a reduced risk of early relapse in patients with AML but not other disease groups.
The benefit, however, is offset by an increased risk of nonrelapse mortality.
Abstract
The association between cytomegalovirus (CMV) reactivation and relapse was evaluated in a large cohort of patients with acute myeloid leukemia (AML) (n = 761), acute lymphoblastic leukemia (ALL) (n = 322), chronic myeloid leukemia (CML) (n = 646), lymphoma (n = 254), and myelodysplastic syndrome (MDS) (n = 371) who underwent allogeneic hematopoietic cell transplantation (HCT) between 1995 and 2005. In multivariable models, CMV pp65 antigenemia was associated with a decreased risk of relapse by day 100 among patients with AML (hazard ratio [HR] = 0.56; 95% confidence interval [CI], 0.3-0.9) but not in patients with ALL, lymphoma, CML, or MDS. The effect appeared to be independent of CMV viral load, acute graft-versus-host disease, or ganciclovir-associated neutropenia. At 1 year after HCT, early CMV reactivation was associated with reduced risk of relapse in all patients, but this did not reach significance for any disease subgroup. Furthermore, CMV reactivation was associated with increased nonrelapse mortality (HR = 1.31; 95% CI, 1.1-1.6) and no difference in overall mortality (HR = 1.05; 95% CI, 0.9-1.3). This report demonstrates a modest reduction in early relapse risk after HCT associated with CMV reactivation in a large cohort of patients without a benefit in overall survival.
Introduction
The interplay between cytomegalovirus (CMV) infection and disease recurrence in patients with hematologic malignancies after allogeneic hematopoietic cell transplantation (HCT) has been an area of ongoing scientific interest for several years. An effect of CMV infection on relapse was first suggested in an analysis of a small cohort of patients by Lönnqvist et al,1 who observed that patients with CMV infection had less relapse compared with patients who had no CMV infection. Several groups subsequently observed an association between donor or recipient CMV serostatus and decreased risk of relapse,2-4 although larger observational cohorts did not reproduce these findings.5-9 More recently, observational data from Elmaagacli et al2 suggested that CMV reactivation is associated with a reduced risk of disease relapse in patients with acute myeloid leukemia (AML), and Ito et al10 have observed a similar association in a cohort of patients with chronic myelogenous leukemia. Given these data, the aim of our study was to evaluate a potential protective effect of early CMV reactivation in a larger cohort of patients undergoing HCT at a single transplant center.
Methods
Cohort eligibility
We evaluated all patients who received their first allogeneic HCT for treatment of a hematologic malignancy at our institution from 1995 to 2005, except for those patients who received umbilical cord blood as the stem cell source. After 2005, CMV surveillance using a polymerase chain reaction method was introduced. This era was chosen to represent a time when the protocols for CMV surveillance and preemptive treatment were uniform. The institutional review board of the Fred Hutchinson Cancer Research Center approved this protocol for accessing and analyzing these retrospective data. This study was conducted in accordance with the Declaration of Helsinki.
Data sources
Demographic, clinical, and laboratory data from patients and their donors were prospectively collected in a research database. This database also contained data from pertinent prior studies performed at outside hospitals.
Transplantation technique
Patients received a variety of pretransplant conditioning regimens, including myeloablative regimens consisting of high-dose cyclophosphamide with 12.0 to 13.2 Gy of total-body irradiation or busulfan or reduced-intensity regimens containing 2.0 Gy of total-body irradiation with or without fludarabine. To prevent graft-versus-host disease (GVHD), patients received immunosuppressive therapy with a calcineurin inhibitor and either methotrexate (MTX) or mycophenolate mofetil (MMF) (Table 1).
Table 1.
Patient characteristics according to disease group
| Characteristic, n (%) | Disease group | |||||
|---|---|---|---|---|---|---|
| Combined* | AML | ALL | Lymphoma | CML | MDS | |
| n | 2566 | 761 | 322 | 254 | 646 | 371 |
| Age (y), median (range) | 42.4 (0.6-74.5) | 43.2 (1.0-74.5) | 23.7 (0.6-63.8) | 39.6 (0.9-67.0) | 43.9 (3.0-69.6) | 50.7 (1.2-72.6) |
| Age (y) | ||||||
| 0-18 | 260 (10%) | 74 (10%) | 124 (39%) | 5 (2%) | 32 (5%) | 23 (6%) |
| 19-40 | 853 (33%) | 219 (29%) | 135 (42%) | 92 (36%) | 298 (46%) | 64 (17%) |
| ≥41 | 1453 (57%) | 443 (58%) | 63 (20%) | 157 (62%) | 316 (49%) | 284 (77%) |
| Donor age (y) | ||||||
| 0-40 | 1028 (40%) | 293 (39%) | 168 (52%) | 110 (43%) | 295 (46%) | 117 (32%) |
| ≥41 | 1070 (42%) | 317 (42%) | 82 (25%) | 117 (46%) | 235 (36%) | 190 (51%) |
| Unknown | 468 (18%) | 151 (20%) | 72 (22%) | 27 (11%) | 116 (18%) | 64 (17%) |
| Race | ||||||
| White | 2130 (83%) | 622 (82%) | 250 (78%) | 223 (88%) | 535 (83%) | 317 (85%) |
| Other/unknown | 436 (17%) | 139 (18%) | 72 (22%) | 31 (12%) | 111 (17%) | 54 (15%) |
| Donor race | ||||||
| White | 1504 (59%) | 414 (54%) | 175 (54%) | 146 (57%) | 410 (63%) | 222 (60%) |
| Other | 300 (12%) | 91 (12%) | 41 (13%) | 23 (9%) | 91 (14%) | 32 (9%) |
| Unknown | 762 (30%) | 256 (34%) | 106 (33%) | 85 (33%) | 145 (22%) | 117 (32%) |
| Male sex | 1482 (58%) | 403 (53%) | 202 (63%) | 152 (60%) | 382 (59%) | 206 (56%) |
| Donor male sex | 1439 (56%) | 429 (56%) | 170 (53%) | 134 (53%) | 363 (56%) | 216 (58%) |
| Sex mismatch | ||||||
| Female donor/male recipient | 622 (24%) | 168 (22%) | 98 (30%) | 68 (27%) | 154 (24%) | 82 (22%) |
| Other | 1944 (76%) | 593 (78%) | 224 (70%) | 186 (73%) | 492 (76%) | 289 (78%) |
| HLA | ||||||
| Matched, related | 1117 (44%) | 333 (44%) | 100 (31%) | 143 (56%) | 244 (38%) | 164 (44%) |
| Mismatched, related | 170 (7%) | 64 (8%) | 31 (10%) | 13 (5%) | 37 (6%) | 21 (6%) |
| Matched, unrelated | 999 (39%) | 288 (38%) | 146 (45%) | 69 (27%) | 271 (42%) | 160 (43%) |
| Mismatched, unrelated | 244 (10%) | 63 (8%) | 39 (12%) | 17 (7%) | 92 (14%) | 26 (7%) |
| Haploidentical | 32 (1%) | 12 (2%) | 6 (2%) | 10 (4%) | 1 (0%) | 0 (0%) |
| Unknown | 4 (0%) | 1 (0%) | 0 (0%) | 2 (1%) | 1 (0%) | 0 (0%) |
| Myeloablative conditioning | 2138 (83%) | 659 (87%) | 303 (94%) | 110 (43%) | 646 (100%) | 349 (94%) |
| CMV risk | ||||||
| D−/R− | 897 (35%) | 236 (31%) | 122 (38%) | 93 (37%) | 257 (40%) | 126 (34%) |
| D+/R− | 365 (14%) | 109 (14%) | 51 (16%) | 38 (15%) | 86 (13%) | 51 (14%) |
| D−/R+ | 632 (25%) | 215 (28%) | 80 (25%) | 62 (24%) | 134 (21%) | 84 (23%) |
| D+/R+ | 672 (26%) | 201 (26%) | 69 (21%) | 61 (24%) | 169 (26%) | 110 (30%) |
| Cell source PBSC | 1330 (52%) | 460 (60%) | 145 (45%) | 211 (83%) | 117 (18%) | 220 (59%) |
| Ex vivo T-cell depletion | 115 (4%) | 39 (5%) | 10 (3%) | 4 (2%) | 19 (3%) | 37 (10%) |
| Transplantation year | ||||||
| January 1995 to November 1998 | 931 (36%) | 241 (32%) | 130 (40%) | 61 (24%) | 349 (54%) | 111 (30%) |
| December 1998 to May 2002 | 821 (32%) | 220 (29%) | 93 (29%) | 81 (32%) | 210 (33%) | 128 (35%) |
| June 2002 to December 2005 | 814 (32%) | 300 (39%) | 99 (31%) | 112 (44%) | 87 (13%) | 132 (36%) |
| Disease risk | ||||||
| Low | 1136 (44%) | 899 (43%) | 217 (67%) | 234 (92%) | 77 (12%) | 87 (23%) |
| Intermediate | 245 (10%) | 99 (5%) | — | — | 94 (15%) | — |
| High | 1175 (46%) | 1070 (52%) | 105 (33%) | 17 (7%) | 475 (74%) | 284 (77%) |
| Missing | 10 (0%) | 9 (0%) | 0 (0%) | 3 (1%) | 0 (0%) | 0 (0%) |
| AML cytogenetic risk | ||||||
| Favorable | — | 62 (8%) | — | — | — | — |
| Intermediate | — | 393 (52%) | — | — | — | — |
| Adverse | — | 154 (20%) | — | — | — | — |
| Missing | — | 151 (20%) | — | — | — | — |
| GVHD prophylaxis | ||||||
| Calcineurin + MTX | 38 (1%) | 9 (1%) | 2 (1%) | 12 (5%) | 6 (1%) | 2 (1%) |
| Calcineurin only | 489 (19%) | 146 (19%) | 25 (8%) | 133 (52%) | 6 (1%) | 39 (11%) |
| Calcineurin + MMF | 1951 (76%) | 581 (76%) | 280 (87%) | 88 (35%) | 617 (96%) | 324 (87%) |
| Other | 88 (3%) | 25 (3%) | 15 (5%) | 21 (8%) | 17 (3%) | 6 (2%) |
| Acute GVHD | ||||||
| Grade 0-1 | 565 (22%) | 173 (23%) | 48 (15%) | 73 (29%) | 131 (20%) | 87 (23%) |
| Grade 2 | 1412 (55%) | 385 (51%) | 198 (61%) | 122 (48%) | 387 (60%) | 204 (55%) |
| Grade 3-4 | 570 (22%) | 197 (26%) | 75 (23%) | 56 (22%) | 127 (20%) | 80 (22%) |
| Missing | 19 (1%) | 6 (1%) | 1 (0%) | 3 (1%) | 1 (0%) | 0 (0%) |
| Chronic GVHD | 1371 (53%) | 353 (46%) | 167 (52%) | 140 (55%) | 377 (58%) | 210 (57%) |
| Cumulative incidence of CMV by day 100 (95% CI) | ||||||
| Any AG | 36.1 (34-38) | 35.9 (33-39) | 29.9% (25-35) | 34.3% (28-40) | 38.1% (34- 42) | 38.3 (33-43) |
| AG ≥ 10 | 11.6 (10-13) | 9.9 (8-12) | 9.9% (7-13) | 10.6% (7-14) | 16.9% (14-20) | 9.4 (7-12) |
| 2 consecutive AG ≥ 10 | 6.0 (5-7) | 4.9 (3-6) | 4.7% (2-7) | 4.3% (2-7) | 9.0% (7-11) | 5.1 (3-7) |
AG, antigenemia.
Combined cohort includes all patients with AML, ALL, lymphoma, CML, and MDS in addition to patients with MM (n = 113), CLL (n = 58), CML receiving reduced intensity conditioning (n = 18), acute leukemia not otherwise specified (n = 20), plasma cell leukemia (n = 2), and acute promyelocytic leukemia (n = 1).
CMV surveillance, treatment, and antiviral prophylaxis
All patients underwent weekly surveillance by pp65 antigenemia from the time of engraftment and until at least day 100 after HCT. Preemptive therapy with either induction-dose ganciclovir (5 mg/kg intravenously every 12 hours) or foscarnet (90 mg/kg intravenously every 12 hours) in case of neutropenia was initiated for ≥1 pp65-antigen–positive cell per 2 slides with >2.0 × 105 white blood cells per slide.4,11,12 Induction dosing was given for at least 7 days. If subsequent testing demonstrated a decrease in pp65 antigenemia, then the therapy was changed to maintenance-dose ganciclovir (5 mg/kg intravenously daily) or foscarnet (90 mg/kg intravenously daily) for at least 2 weeks or until the pp65 antigenemia assay was negative. Acyclovir (250 mg/m2 or 800 mg orally twice daily) or valacyclovir (500 mg orally twice daily) was given to all patients for herpes simplex virus type 1, herpes simplex virus type 2, or varicella-zoster virus prophylaxis.
Outcomes and definitions
The primary outcome of this study was morphologic relapse or, for patients receiving reduced-intensity conditioning regimens, relapse if transplanted in complete remission (CR) or progression of underlying disease if transplanted when not in CR, by 100 days and 1 year after HCT. Secondary outcomes were nonrelapse mortality (NRM) and overall mortality by 1 year after HCT.
Disease risk was categorized as follows: low: chronic lymphocytic leukemia (CLL) in CR, low-grade non-Hodgkin lymphoma (NHL), high-grade NHL in CR, multiple myeloma (MM) in CR, CML in first chronic phase (CP), and acute lymphoblastic leukemia (ALL) in first CR; intermediate: CLL not in CR, MM not in CR, AML in CR; and high: AML evolved from myelodysplastic disease (MDS), high-grade NHL not in CR, Hodgkin disease, secondary MDS, AML not in CR, CML in second CP or accelerated phase/blast crisis, and ALL not in first CR. Cytogenetic risk for patients with AML was classified according to the revised Medical Research Council prognostic classification.13 Only results from cytogenetic studies performed before initial treatment were included.
Statistical analysis
The cumulative incidence of CMV reactivation, morphologic relapse, and NRM was estimated, treating death (CMV reactivation and morphologic relapse) and relapse (NRM) as competing risk events.14 Cox proportional hazards models were used to evaluate risk factors for morphologic relapse, NRM, and overall mortality. Clinical and demographic factors evaluated as risk factors were age (≥41 years vs 0-40 years), donor age (≥41 years vs 0-40 years), race (other/unknown vs white), donor race (other/unknown vs white), sex, donor sex, sex mismatch (female donor/male recipient vs other), HLA matching and donor relation, conditioning regimen (reduced intensity vs myeloablative), CMV donor (D) recipient (R) serology (D+/R−, D−/R+, D+/R+ vs D−/R−), hematopoietic cell source (peripheral blood stem cell [PBSC] vs bone marrow), ex vivo T-cell depletion (yes/no), transplantation year (January 1995 to November 1998, December 1998 to May 2002, or June 2002 to December 2005), disease risk (low, intermediate, or high), AML cytogenetic risk (favorable, intermediate, or high), and GVHD prophylaxis (calcineurin inhibitor + MTX, calcineurin inhibitor only, calcineurin inhibitor + MMF, or other). CMV reactivation was evaluated as a time-dependent covariate (any positive antigenemia, ≥10 antigen-positive cells, or ≥10 antigen-positive cells for 2 consecutive weeks [the level similar to reported as the only CMV reactivation end point by Elmaagacli et al2]). CMV-associated neutropenia (absolute neutrophil count < 500 per mm3 within 60 days after CMV reactivation) was also evaluated as a time-dependent risk factor compared with patients who had CMV reactivation without subsequent neutropenia.15 Peak acute GVHD (grades 0-1, 2, or 3-4) was graded according to standard definitions and analyzed in a time-dependent manner.16
Multivariable regression models were constructed in a stepwise manner. Risk factors in univariate models that were associated with the outcome at P ≤ .05 were included in the final model. CMV reactivation was forced into the multivariable models. Statistical analysis was performed using Statistical Analysis Software procedures (SAS version 9.3).
Results
The cohort included a total of 2566 patients. In addition to the distinct groups of patients shown with underlying diseases of AML, ALL, lymphoma, CML, or MDS, there were 212 patients with other diseases, including MM and CLL (Table 1). Due to the small numbers of patients in these groups, they were not analyzed separately and are included only in the combined cohort. Patient characteristics of each disease group and the combined cohort are presented in Table 1. There were 2 patients who were lost to follow-up in the first year after HCT.
CMV reactivation
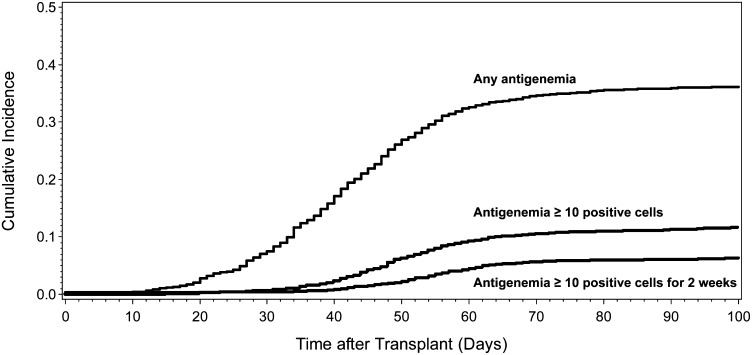
The donor and recipient CMV serostatus were similar across the disease groups, with approximately 65% of patients at significant risk of CMV reactivation (50% R+ and 15% D+/R−). The cumulative incidence of CMV reactivation at any level of pp65 antigenemia by day 100 was 36.1% (95% CI, 34-38) in the combined cohort (Figure 1). Higher levels of antigenemia occurred in a smaller proportion of patients. The cumulative incidence of antigenemia ≥ 10 positive cells was 11.6% and 6.0% for ≥10 positive cells for 2 consecutive weeks. These estimates were similar across the disease groups analyzed (Table 1).
Figure 1.
Cumulative incidence of CMV antigenemia by day 100 after HCT (n = 2566).
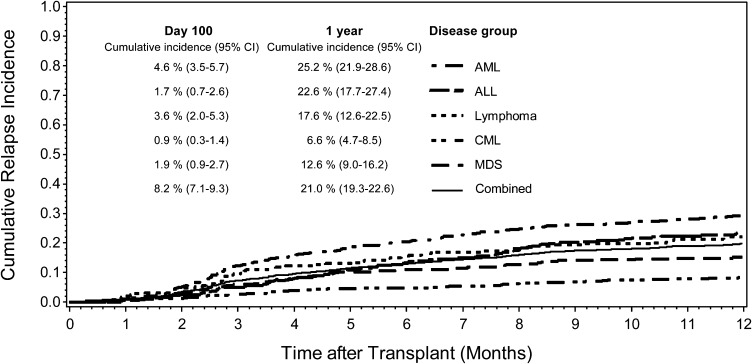
Morphologic relapse
The cumulative incidence of relapse in the first year after HCT was highest for AML (25.2%) and ALL (22.6%). In the lymphoma group, the cumulative incidence for relapse was 17.6%. Relapse was less common in CML (6.6%) and MDS (12.6%) (Figure 2). In univariate regression models, CMV reactivation, defined as any positive pp65 antigenemia by day 100, was not significantly associated with a decreased risk of relapse by day 100 in any of the disease groups (supplemental Tables 1 and 2). We also evaluated whether an association might be apparent in patients who had higher levels of antigenemia, such as ≥10 positive cells or ≥10 positive cells for 2 consecutive weeks (equivalent to the definition of CMV replication used by Elmaagacli et al2) and found no significant association (supplemental Tables 1 and 2).
Figure 2.
Cumulative relapse incidence in the first year after HCT by disease group. Cumulative incidence estimates with 95% CI at day 100 and 1 year for each disease group.
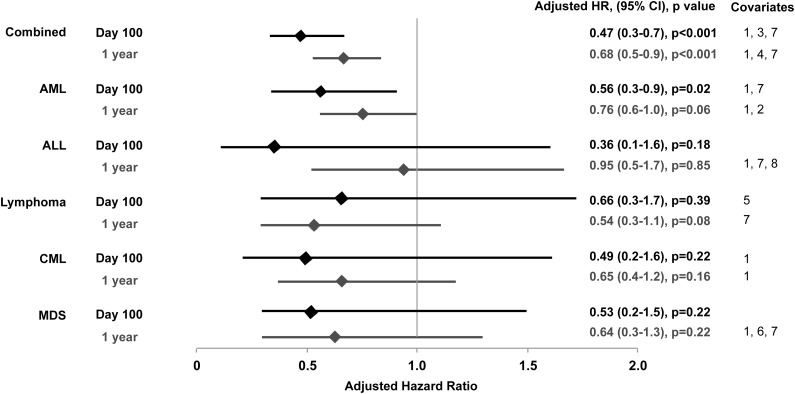
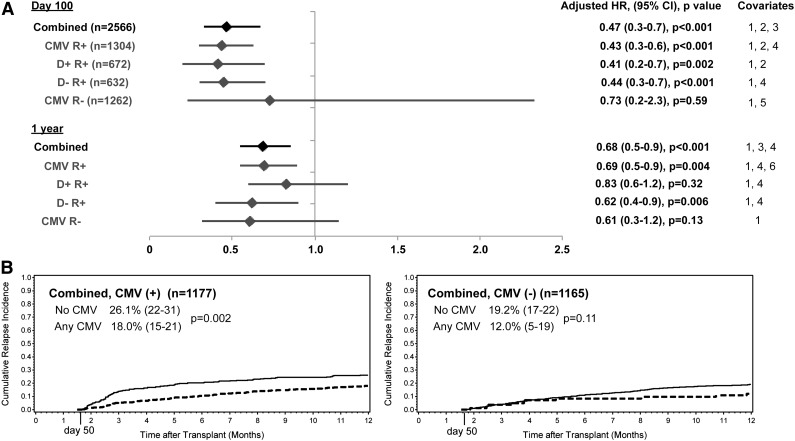
After adjusting for underlying disease risk, donor and recipient serostatus, and other significant variables, CMV reactivation (any positive pp65 antigenemia) was significantly associated with a decreased risk of relapse by day 100 only in patients with AML (adjusted HR = 0.56; 95% CI, 0.3-0.9; P = .02) (Figure 3). Controlling for acute GVHD (grades 0-2 vs 3-4) did not change the association between CMV reactivation and relapse in any of the disease groups (data not shown). The overall effect of early CMV reactivation in the combined cohort was a 53% decrease in the risk of relapse by day 100 (95% CI, 0.3-0.7; P < .001) (Figure 3).
Figure 3.
Adjusted HR and 95% CI from multivariable models evaluating CMV reactivation by day 100 as a risk factor for relapse at day 100 and 1 year after HCT. Covariates: 1, disease risk (low vs high, intermediate vs high); 2, cytogenetic risk (adverse vs intermediate and favorable vs intermediate); 3, patient race (other/unknown vs white); 4, cell source (bone marrow vs PBSC); 5, donor sex (female vs male); 6, conditioning regimen (reduced intensity vs myeloablative); 7, donor and recipient CMV serostatus (D+/R− vs D−/R−, D−/R+ vs D−/R−, or D+/R+ vs D−/R−); 8, acute GVHD (grade 3-4 vs 0-2).
In the multivariable models, higher levels of antigenemia were not associated with incremental protection from relapse. In the combined cohort, the adjusted HR for the risk of relapse by day 100 with antigenemia ≥ 10 positive cells was 0.62 (95% CI, 0.4-1.1; P = .10) and for patients with antigenemia ≥ 10 positive cells for 2 weeks the adjusted HR was 0.48 (95% CI, 0.2-1.2; P = .11). No evidence of incremental protection for higher levels was seen in the multivariable models of any of the individual disease groups (data not shown).
To evaluate whether CMV reactivation might protect against relapse via ganciclovir-associated myelosuppression, CMV-associated neutropenia, defined as an absolute neutrophil count < 500 per mm3 occurring within 60 days after CMV reactivation, was analyzed as a risk factor for relapse. In patients with AML, CMV-associated neutropenia was not associated with decreased risk of relapse (adjusted HR = 2.35; 95% CI, 0.6-10.0; P = .25). Furthermore, no association between CMV-associated neutropenia and decreased relapse was observed in any of the disease groups or in the combined cohort (data not shown).
The protective effect of CMV reactivation on the risk of relapse was more pronounced within the first 100 days after HCT compared with beyond 100 days after HCT (Figure 3). In multivariable models, CMV reactivation before day 100 was associated with a 32% decreased risk of relapse by 1 year in the combined cohort (95% CI, 0.5-0.9; P < .001). However, CMV reactivation was not significantly associated with decreased relapse risk by 1 year in any of the individual disease groups.
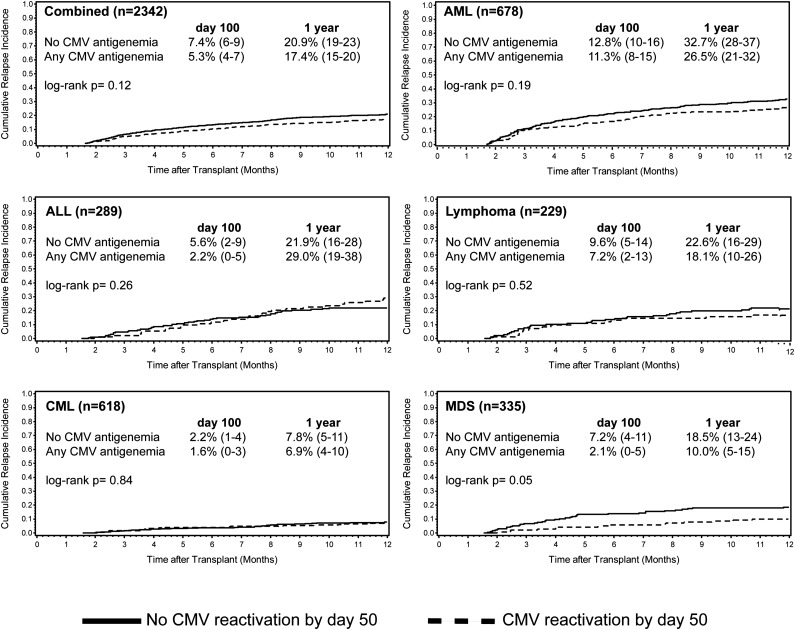
A landmark analysis was performed at day 50 to compare the cumulative incidence of relapse among patients who experienced CMV reactivation before day 50 and those who did not, accounting for the competing risk of death (Figure 4). Day 50 was chosen as an optimal time point because, as illustrated in Figures 1 and 2, the majority of CMV reactivations, but very few relapse events, have occurred by day 50. There were 56 relapse events before day 50 in the combined cohort, representing 11% of the relapse events occurring in the first year after HCT. Among patients who survived to day 50 without experiencing relapse, CMV reactivation before day 50 was associated with a significant decrease in the cumulative incidence of relapse by 1 year only in patients with MDS (P = .05). No significant difference was observed for any of the other disease groups or the combined cohort (Figure 4).
Figure 4.
Cumulative relapse incidence by CMV antigenemia occurring day 0 to 50 after HCT among patients surviving free of relapse to day 50. Cumulative relapse incidence estimates and 95% CI at day 100 and 1 year after HCT.
Paradoxically, pretransplant CMV seropositivity was an independent risk factor for relapse by day 100 in patients with AML, ALL, lymphoma, and in the combined cohort. In the combined cohort, after adjustment for disease risk and race, recipient CMV seropositivity was associated with a 2-fold increase in the risk of relapse. Compared with D−/R− patients, the adjusted HR for D+/R+ patients was 2.05 (95% CI, 1.4-3.1; P < .001) and 2.77 (95% CI, 1.9-4.0; P < .001) for D−/R+ patients, whereas the adjusted HR for D+/R− patients was 0.94 (95% CI, 0.6-1.6; P = .81). In the univariate analyses, donor CMV serostatus was not significantly associated with relapse in any of the disease groups (supplemental Tables 1 and 2).
After evaluating the association between CMV reactivation and relapse within the separate strata of CMV R+ and CMV R− patients, there was a trend toward increased protection from relapse in CMV R+ patients with CMV reactivation compared with CMV R− patients with CMV infection (Figure 5A). In the combined cohort, the adjusted HR for CMV reactivation was 0.43 (95% CI, 0.3-0.6; P < .001) among CMV R+ patients and 0.73 (95% CI, 0.2-2.3; P = .59) among CMV R− patients (test for interaction P = .44). The association between CMV reactivation and decreased relapse among CMV-seropositive (R+) patients did not differ by donor CMV serostatus (Figure 5a). Among patients who survived to day 50 without relapse, the cumulative incidence of relapse by 1 year was highest in CMV R+ patients who did not have early CMV reactivation (26.1%; 95% CI, 22-31) compared with 18.0% (95% CI, 15-21) among CMV R+ patients who had early reactivation (Figure 5B).
Figure 5.
Results of relapse analyses for all patients stratified by pretransplant CMV serology. (A) Adjusted HR and 95% CI from multivariable models evaluating CMV antigenemia by day 100 as a risk factor for relapse at day 100 and 1 year after HCT among CMV-seropositive patients (CMV R+) and CMV-seronegative patients (CMV R−). Covariates: 1, disease risk (low, standard, or high); 2, patient race (unknown/other or white); 3, donor CMV serostatus (D− vs D+); 4, cell source (bone marrow vs PBSC); 5, patient age (0-40 years or ≥41 years); 6, conditioning regimen (myeloablative vs reduced intensity). (B) Cumulative relapse incidence at 1 year after HCT by CMV antigenemia occurring before day 50 among patients surviving relapse-free to day 50 after HCT.
Mortality
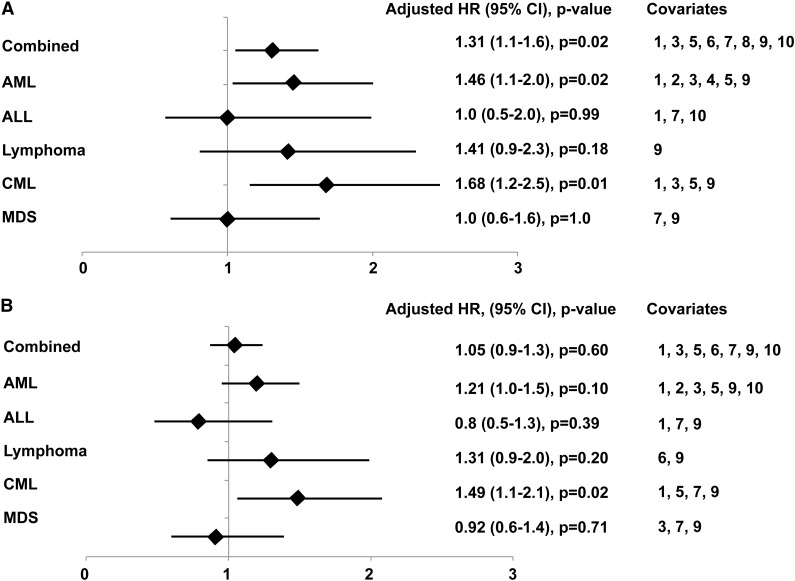
In our cohort, any CMV reactivation by day 100 was an independent risk factor for NRM in the first year after HCT in AML and CML patients (Figure 6A). In the combined cohort, CMV reactivation was associated with a 31% increase in the risk of death without relapse (adjusted HR = 1.31; 95% CI, 1.1-1.6; P = .02). Despite evidence of decreased relapse in the first year after HCT among patients with early CMV reactivation, we did not observe any associated benefit in overall mortality. The adjusted HR for the effect of any CMV reactivation on overall mortality in the combined cohort was 1.05 (95% CI, 0.9-1.3; P = .60).
Figure 6.
Adjusted HR and 95% CI from multivariable models evaluating CMV antigenemia before day 100 as a risk factor for nonrelapse mortality at 1 year and overall mortality at 1 year. Covariates: 1, disease risk (low, standard, or high); 2, cytogenetic risk (low, intermediate, or high); 3, age (0-40 years vs ≥40 years); 4, cell source (bone marrow vs PBSC); 5, HLA matching (matched vs mismatched); 6, conditioning regimen (reduced intensity vs myeloablative); 7, donor and recipient CMV serostatus (D−/R−, D+/R−, D−/R+, D+/R+); 8, GVHD prophylaxis (calcineurin inhibitor alone, calcineurin + methotrexate, or calcineurin inhibitor + mycophenolate mofetil); 9, acute GVHD (grades 3-4 vs 0-2); 10, transplant year (January 1995 to November 1998, December 1998 to May 2002, or June 2002 December 2005).
Discussion
In this large cohort of allogeneic HCT recipients, our data indicate that CMV reactivation in the first 100 days after transplantation is associated with a modest decrease in the risk of early relapse independent of acute GVHD in patients with AML. This extends the findings of Elmaaglaci et al2 to a less-restricted cohort of AML patients that included pediatric patients and patients receiving reduced-intensity conditioning regimens or HLA-mismatched donor cells. Yet, in our cohort, the difference in relapse risk between AML patients with and without early CMV reactivation was not statistically significant by 1 year after transplantation. In contrast, CMV reactivation was not associated with relapse protection in multivariable models either at day 100 or 1 year in patients with ALL, lymphoma, CML, or MDS. Yet when all disease groups were combined, we observed a 53% decreased risk of relapse by day 100 and a 32% decreased risk of relapse by 1 year. It is possible that despite the large size of this cohort, we did not have sufficient statistical power to observe differences in all of the disease groups.
Our observed associations between CMV reactivation and decreased risk of relapse in patients with AML was much less striking than described by Elmaagacli et al.2 We evaluated CMV antigenemia at higher levels to approximate the definition of CMV replication used in that study (≥25 pp65-positive cells per 5 × 105 white blood cells at 2 consecutive time points) to determine if there might be incremental protection from relapse with high-degree antigenemia. We were unable to demonstrate such an association (data not shown). However, this was also an exceedingly rare occurrence in our cohort. Because of an institutional approach to initiate preemptive antiviral therapy at any level of antigenemia, antigenemia levels as high as those described by Elmaagacli et al occurred in <10% of patients.
Our results also differed from those recently published by Ito et al,10 who observed that early CMV reactivation was associated with decreased relapse in a cohort of 110 patients with CML. In contrast to our CML cohort, the great majority of patients in the Ito study received ex vivo T-cell–depleted grafts, which typically results in robust natural killer (NK) cell reconstitution after transplant. Thomson et al17 found no association between CMV reactivation and relapse risk in 100 patients with AML who received alemtuzumab, which depletes a variety of immune cells, including NK cells, and persists in vivo for prolonged periods. It is unclear whether these differences can provide insight into distinct mechanism or simply reflect other variables, including sample size.
Interestingly, the patient’s CMV serostatus at the time of transplantation was an independent risk factor for increased risk of relapse in almost all disease groups studied. Our stratified analyses of CMV reactivation as a risk factor for relapse in CMV-seropositive and CMV-seronegative patients indicated a trend toward increased protection from relapse in CMV-seropositive patients experiencing CMV reactivation compared with CMV-seronegative patients with primary CMV infection.
The principal question that is posed by our findings and those of others is, what biological mechanism(s) underlie the different associations between CMV reactivation and decreased early relapse observed in allogeneic HCT recipients within very different hematologic malignancies? To test whether CMV replication results in a decrease in relapse through ganciclovir-mediated cytotoxicity, we analyzed CMV reactivation with subsequent neutropenia as a risk factor for relapse. If cytotoxic effects played a role, then one might expect a lower relapse risk in patients with CMV infection and subsequent neutropenia compared with those with CMV infection without ensuing neutropenia. We did not observe such an association, which suggests that there is no major direct cytotoxic effect of ganciclovir involved. We recognize that the absolute neutrophil count is a crude surrogate for events occurring in the marrow and the levels of neutropenia at which such an effect would become apparent is unknown.
One promising hypothesis is that the upregulation of NKG2C+ NK cells that has been shown to occur early after CMV infection and involve activating killer immunoglobulin-like receptors may increase NK cell activity against tumor cells expressing HLA-E.18-22 Foley et al18 recently demonstrated increased populations of interferon-γ–producing NKG2C+ NKG2A− NK cells in HCT recipients as soon as 2 weeks after CMV viremia was detected. NK cells are one of the first lymphocyte populations to recover after HCT, reaching normal population numbers months before recovery of memory and effector CD8+ lymphocytes, which would agree with the timing of relapse protection observed in our cohort.23 Moreover, graft-versus-leukemia effects of NK cells are more prominent for AML than for ALL or lymphomas that are intrinsically resistant to NK recognition,24,25 consistent with our finding that the effect of CMV reactivation is most significant for reducing relapse in AML.
Despite the findings affirming a decreased risk of relapse in AML patients with CMV reactivation after HCT, we continue to observe an association between CMV reactivation and NRM in the first year after HCT and no significant positive association with overall survival. While it might be argued that this cohort ending in 2005 might not be reflective of current practices in the detection and management of CMV, these results are in accord with findings from other cohorts that included patients transplanted through 2010.26-28
In conclusion, this report confirms that CMV reactivation after allogeneic HCT is independently associated with a modest reduction in the risk of early relapse in patients with AML but not other hematologic malignancies. The pathogenic principles underlying this association are unclear, and further laboratory studies will be required to better understand the mechanism leading to protection from recurrent malignancy. However, although a beneficial effect of CMV replication with regard to relapse was observed in this study, the effect appeared strongest early after transplant and was later offset, if not outweighed, by a continued association of CMV reactivation with NRM. Thus, current strategies aimed at limiting CMV replication appear to be prudent.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants K23AI097234, K24HL093294, HL108307, CA18029, HL36444, CA78902, CA136551, AI053193, and P30CA015704-35S6. M.L.G. is supported by the Astellas IDSA/ERF postdoctoral fellowship in transplant infectious disease.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood commentary on this article in this issue.
Authorship
Contribution: M.L.G. participated in research design, performed data collection and analysis, and wrote the manuscript; H.X. performed statistical analyses under the direction of W.L., and both contributed to research design and review of the manuscript; R.B.W. contributed to data collection, research design, and writing and review of the manuscript; M.M., B.M.S, and S.R.R. contributed to research design and to the writing and review of the manuscript; M.B. conceived and designed the study and contributed to the writing and review of the manuscript.
Conflict-of-interest disclosure: M.B. has served as a consultant for Merck, Chimerix, Roche/Genentech, and Astellas and has received research support from Merck and Roche/Genentech.
Correspondence: Margaret L. Green, 1100 Fairview Ave North, Mailstop E4-100, Seattle, WA 98109; e-mail: mlgreen@fhcrc.org.
References
- 1.Lönnqvist B, Ringdèn O, Ljungman P, Wahren B, Gahrton G. Reduced risk of recurrent leukaemia in bone marrow transplant recipients after cytomegalovirus infection. Br J Haematol. 1986;63(4):671–679. doi: 10.1111/j.1365-2141.1986.tb07551.x. [DOI] [PubMed] [Google Scholar]
- 2.Elmaagacli AH, Steckel NK, Koldehoff M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118(5):1402–1412. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 3.Behrendt CE, Rosenthal J, Bolotin E, Nakamura R, Zaia J, Forman SJ. Donor and recipient CMV serostatus and outcome of pediatric allogeneic HSCT for acute leukemia in the era of CMV-preemptive therapy. Biol Blood Marrow Transplant. 2009;15(1):54–60. doi: 10.1016/j.bbmt.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nachbaur D, Clausen J, Kircher B. Donor cytomegalovirus seropositivity and the risk of leukemic relapse after reduced-intensity transplants. Eur J Haematol. 2006;76(5):414–419. doi: 10.1111/j.1600-0609.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 5.Remberger M, Ringdén O. Survival after bone-marrow transplantation. Lancet. 2002;359(9309):888. doi: 10.1016/S0140-6736(02)07926-6. [DOI] [PubMed] [Google Scholar]
- 6.Ljungman P, Brand R, Einsele H, Frassoni F, Niederwieser D, Cordonnier C. Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: an EBMT megafile analysis. Blood. 2003;102(13):4255–4260. doi: 10.1182/blood-2002-10-3263. [DOI] [PubMed] [Google Scholar]
- 7.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 8.Erard V, Guthrie KA, Riddell S, Boeckh M. Impact of HLA A2 and cytomegalovirus serostatus on outcomes in patients with leukemia following matched-sibling myeloablative allogeneic hematopoietic cell transplantation. Haematologica. 2006;91(10):1377–1383. [PubMed] [Google Scholar]
- 9.Farina L, Bruno B, Patriarca F, et al. The hematopoietic cell transplantation comorbidity index (HCT-CI) predicts clinical outcomes in lymphoma and myeloma patients after reduced-intensity or non-myeloablative allogeneic stem cell transplantation. Leukemia. 2009;23(6):1131–1138. doi: 10.1038/leu.2009.1. [DOI] [PubMed] [Google Scholar]
- 10.Ito S, Pophali P, Co W, et al. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transplant. 2013 doi: 10.1038/bmt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boeckh M, Gooley TA, Myerson D, et al. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88(10):4063. [PubMed]
- 12.Boeckh M, Bowden RA, Gooley T, Myerson D, Corey L. Successful modification of a pp65 antigenemia-based early treatment strategy for prevention of cytomegalovirus disease in allogeneic marrow transplant recipients. Blood. 1999;93(5):1781–1782. [PubMed] [Google Scholar]
- 13.Grimwade D, Hills RK, Moorman AV, et al. National Cancer Research Institute Adult Leukaemia Working Group. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 15.Venton G, Crocchiolo R, Fürst S, et al. Risk factors of Ganciclovir-related neutropenia after allogeneic stem cell transplantation: a retrospective monocentre study on 547 patients [published online ahead of print March 20, 2013]. Clin Microbiol Infect. [DOI] [PubMed]
- 16.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 17.Thomson KJ, Mackinnon S, Peggs KS. CMV-specific cellular therapy for acute myeloid leukemia? Blood. 2012;119(4):1088–1090, author reply 1090-1091. doi: 10.1182/blood-2011-10-383943. [DOI] [PubMed] [Google Scholar]
- 18.Foley B, Cooley S, Verneris MR, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119(11):2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charoudeh HN, Terszowski G, Czaja K, Gonzalez A, Schmitter K, Stern M. Modulation of the natural killer cell KIR repertoire by cytomegalovirus infection. Eur J Immunol. 2013;43(2):480–487. doi: 10.1002/eji.201242389. [DOI] [PubMed] [Google Scholar]
- 20.Béziat V, Liu LL, Malmberg J-A, et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013;121(14):2678–2688. doi: 10.1182/blood-2012-10-459545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Della Chiesa M, Falco M, Podestà M, Locatelli F, Moretta L, Frassoni F, Moretta A. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: a role for human cytomegalovirus? Blood. 2012;119(2):399–410. doi: 10.1182/blood-2011-08-372003. [DOI] [PubMed] [Google Scholar]
- 22.Gumá M, Budt M, Sáez A, Brckalo T, Hengel H, Angulo A, López-Botet M. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107(9):3624–3631. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- 23.Storek J, Dawson MA, Storer B, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood. 2001;97(11):3380–3389. doi: 10.1182/blood.v97.11.3380. [DOI] [PubMed] [Google Scholar]
- 24.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 25.Challa-Malladi M, Lieu YK, Califano O, et al. Combined genetic inactivation of β2-Microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell. 2011;20(6):728–740. doi: 10.1016/j.ccr.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George B, Pati N, Gilroy N, et al. Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transpl Infect Dis. 2010;12(4):322–329. doi: 10.1111/j.1399-3062.2010.00504.x. [DOI] [PubMed] [Google Scholar]
- 27.Green ML, Leisenring W, Stachel D, et al. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1687–1699. doi: 10.1016/j.bbmt.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiwarkar P, Gaspar HB, Gilmour K, et al. Impact of viral reactivations in the era of pre-emptive antiviral drug therapy following allogeneic haematopoietic SCT in paediatric recipients. Bone Marrow Transplant. 2013 doi: 10.1038/bmt.2012.221. 48(6):803-808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.