Abstract
Emulgels have emerged as a promising drug delivery system for the delivery of hydrophobic drugs. The objective of the study was to prepare emulgel of mefenamic acid, a NSAID, using Carbapol 940 as a gelling agent. Mentha oil and clove oil were used as penetration enhancers. The emulsion was prepared and it was incorporated in gel base. The formulations were evaluated for rheological studies, spreading coefficient studies, bioadhesion strength, skin irritation studies, in vitro release, ex vivo release studies, anti-inflammatory activity and analgesic activity. Formulation F2 and F4 showed comparable analgesic and anti-inflammatory activity when they compared with marketed diclofenac sodium gel. So, it can be concluded that topical emulgel of mefenamic acid posses an effective anti-inflammatory and analgesic activity.
Keywords: Emulgel, Mefenamic acid, Topical drug delivery, NSAIDs
1. Introduction
Several analgesic preparations are available in the market as different topical preparations. Mefenamic acid, an effective NSAID has always been used as an anti-inflammatory and analgesic agent. Conventionally it is available in the form of tablets and suspensions. There is no marketed topical formulation of mefenamic acid available till date. Most of the topical preparations are used for the localized effects at the site of their application by virtue of drug penetration into the underlying layers of skin or mucous membranes (Lionberger and Brennan, 2010). Although some unintended drug absorption may occur, it is of sub therapeutic quantities and generally of minor concern. Gels are a relatively newer class of dosage form created by entrapment of large amounts of aqueous or hydroalcoholic liquid in a network of colloidal solid particles, which may consist of inorganic substances, such as aluminum salts or organic polymers of natural or synthetic origin (Kumar and Verma, 2010). They have a higher aqueous component that permits greater dissolution of drugs, and also permit easy migration of the drug through a vehicle that is essentially a liquid, compared with the ointment or cream base (Gennaro, 1995; Ansel et al., 1999). These are superior in terms of use and patient acceptability. In spite of many advantages of gels a major limitation is in the delivery of hydrophobic drugs. So to overcome this limitation, emulgels are prepared and used so that even a hydrophobic therapeutic moiety can enjoy the unique properties of gels (Topical Emulsion, 2004). When gels and emulsions are used in combined form the dosage forms are referred as EMULGELS (Mohamed, 2004). In recent years, there has been great interest in the use of novel polymers with complex functions as emulsifiers and thickeners because the gelling capacity of these compounds allows the formulation of stable emulsions and creams by decreasing surface and interfacial tension and at the same time increasing the viscosity of the aqueous phase (Gupta et al., 2010). Emulgels for dermatological use have several favorable properties such as being thixotropic, greaseless, easily spreadable, easily removable, emollient, nonstaining, long shelf life, bio-friendly, transparent and pleasing appearance (Stanos, 2007).
The aim of this work was to develop an emulgel formulation of mefenamic acid, a hydrophobic drug, using Carbopol 940 as gelling agent and two types of penetration enhancer, i.e., Clove oil and Mentha oil. The influence of gelling agent and penetration enhancers was investigated. The rheological studies, spreading coefficient studies, bioadhesion strength, skin irritation studies, in vitro release, ex vivo release studies, anti-inflammatory activity and analgesic activity of the prepared emulgels were also evaluated.
2. Materials and methods
2.1. Materials
Mefenamic acid was obtained as a gift sample from Lexicon Biotech Pvt. Ltd. Baddi (Himachal Pradesh), Carbopol 940 was obtained from Loba chemicals Mumbai. Dialysis membrane was procured from Hi media, Mumbai. All other chemicals used were of analytical grade and were used without any further chemical modification.
2.2. Preparation of emulgel
Different formulations were prepared using varying amount of gelling agent and penetration enhancers. The method only differed in process of making gel in different formulation. The preparation of emulsion was same in all the formulations. The gel phase in the formulations was prepared by dispersing Carbopol 940 in purified water with constant stirring at a moderate speed using mechanical shaker, then the pH was adjusted to 6–6.5 using tri ethanol amine (TEA). The oil phase of the emulsion was prepared by dissolving span 20 in light liquid paraffin while the aqueous phase was prepared by dissolving tween 20 in purified water. Methyl and propyl parabens were dissolved in propylene glycol whereas mefenamic acid was dissolved in ethanol, and both solutions were mixed with the aqueous phase. Clove oil and mentha oil were mixed in oil phase. Both the oily and aqueous phases were separately heated to 70–80 °C, then the oily phase was added to the aqueous phase with continuous stirring until it got cooled to room temperature. The obtained emulsion was mixed with the gel in 1:1 ratio with gentle stirring to obtain the emulgel (Jain et al., 2011). The composition of different formulations has been discussed in Table 1.
Table 1.
Composition of different formulation batches (%w/w).
| Ingredient | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Mefenamic acid | 1 | 1 | 1 | 1 |
| Carbapol 940 | 1 | 1 | 1 | 1 |
| Liquid paraffin | 7.5 | 7.5 | 7.5 | 7.5 |
| Tween 20 | 0.5 | 0.5 | 0.5 | 0.5 |
| Span 20 | 1 | 1 | 1 | 1 |
| Propylene glycol | 5 | 5 | 5 | 5 |
| Ethanol | 2.5 | 2.5 | 2.5 | 2.5 |
| Methyl parabene | 0.03 | 0.03 | 0.03 | 0.03 |
| Ethyl parabene | 0.01 | 0.01 | 0.01 | 0.01 |
| Clove oil | - | – | 8 | 10 |
| Mentha oil | 4 | 6 | – | – |
| Water | q.s | q.s | q.s | q.s |
2.3. Evaluation of emulgel
2.3.1. Physical examination
The prepared emulgel formulations were inspected visually for their color, appearance and consistency (Kasliwal et al., 2008).
2.4. Rheological study
The viscosity of the formulated batches was determined using a cone and plate viscometer with spindle 7 (Brookfield Engineering Laboratories). The assembly was connected to a thermostatically controlled circulating water bath maintained at 25 °C. The formulation whose viscosity was to be determined was added to a beaker covered with thermostatic jacket. Spindle was allowed to move freely into the emulgel and the reading was noted (Bonacucina et al., 2009).
2.5. Spreading coefficient
Spreading coefficient was determined by apparatus suggested by Mutimer. It consists of a wooden block, which is attached to a pulley at one end. Spreading coefficient was measured on the basis of ‘Slip’ and ‘Drag’ characteristics of emulgels. A ground glass slide was fixed on the wooden block. An excess of emulgel (about 2 g) under study was placed on this ground slide. The emulgel preparation was then sandwiched between this slide and second glass slide having same dimension as that of the fixed ground slide. The second glass slide is provided with the hook. Weight of 500 mg was placed on the top of the two slides for 5 min to expel air and to provide a uniform film of the emulgel between the two slides. Measured quantity of weight was placed in the pan attached to the pulley with the help of hook. The time (in s) required by the top slide to cover a distance of 5 cm was noted. A shorter interval indicates better spreading coefficient (Gupta and Gaud, 2005).
2.6. Skin irritation test (patch test)
A set of 8 rats was used in the study. The emulgel was applied on the properly shaven skin of rat. Undesirable skin changes, i.e., change in color, change in skin morphology were checked for a period of 24 h (Murty and Hiremath, 2001).
2.7. Bioadhesive strength measurement
The modified method was used for the measurement of bioadhesive strength. The apparatus consist of two arm balance. Both the ends are tied to glass plates using strings. One side contains two glass plates. Other side contains single glass plate for keeping weight. The right and left pans were balanced by adding extra weight on the left hand pan. The balance was kept in this position for 5 min.
Accurately weighed 1 g of emulgel was placed between these two slides containing hairless fresh rat skin pieces, and extra weight from the left pan was removed to sandwich the two pieces of glass and some pressure was applied to remove the presence of air. The balance was kept in this position for 5 min. Weight was added slowly at 200 mg/min to the left hand pan until the two glass slides got detached from each other. The weight (gram force) required to detach the emulgel from the glass surface gave the measure of bioadhesive strength (Choi et al., 2003). The bioadhesive strength is calculated by using following:
2.8. In vitro release studies
The in vitro drug release studies were carried out using a modified Franz diffusion (FD) cell. The formulation was applied on dialysis membrane which was placed between donor and receptor compartment of the FD cell. Phosphate buffer pH 7.4 was used as a dissolution media. The temperature of the cell was maintained at 37 °C by circulating water jacket. This whole assembly was kept on a magnetic stirrer and the solution was stirred continuously using a magnetic bead. A similar blank set was run simultaneously as a control. Sample (5 ml) was withdrawn at suitable time intervals and replaced with equal amounts of fresh dissolution media. Samples were analyzed spectrophotometrically at 285 nm and the cumulative % drug release was calculated. The difference between the readings of drug release and control was used as the actual reading in each case (Kakkar and Gupta, 1992).
2.9. Ex vivo drug release study
The ex vivo drug release study of selected formulations (F2 and F4) was carried out in a modified Franz diffusion cell, using wistar male rat skin. A section of skin was cut and placed in the space between the donor and receptor compartment of the FD cell, keeping the dorsal side upward. Phosphate buffer pH 7.4 was used as dissolution media. The temperature of the cell was maintained constant at 32 °C by circulating water jacket. This whole assembly was kept on a magnetic stirrer and the solution was stirred continuously using a magnetic bead. A similar blank set was run simultaneously. The samples were withdrawn at suitable time intervals and replaced with equal amounts of fresh dissolution media (Schreier and Bouwstra, 1985). Samples were analyzed spectrophotometrically at 285 nm.
2.10. In vivo anti-inflammatory activity
2.10.1. Experimental design
Edema was induced on the left hind paw of the rats by subplantar injection of 1% (w/v) carrageenan. Formulations, i.e., F2, F4 and standard (diclofenac sodium gel) were applied 30 min before carrageenan administration. The paw volume was measured at intervals of 30, 60, 90, 120 min by mercury displacement method using plethysmometer (Crunkhorn and Mencock, 1971).
-
•
Group 1 (Control group): Carrageenan (1%) was administered in the plantar surface of rat.
-
•
Group 2 (Standard group): Topical marketed diclofenac gel + Carrageenan.
-
•
Group 3 (Test): Formulations F2 and F4 + Carrageenan.
The % inhibition of paw edema in drug treated group was compared with carrageenan control group and calculated according to the formula:
where Vc is the inflammatory increase in paw volume control group and Vt the inflammatory increase in paw volume in (drug + carrageenan) treated animals.
2.11. In vivo analgesic activity
The analgesic activity was carried out using hot plate method. Following groups were made and latency period in which rat responded to hot plate was calculated (Junping et al., 2005).
-
•
Group 1 (Control Group): No topical treatment was given and latency period was calculated.
-
•
Group 2 (Standard Group): The rats were treated with diclofenac gel and its latency period was calculated.
-
•
Group 3 (Test Group): The rats were treated with test formulations, i.e., F2 and F4 and latency period was calculated.
2.12. Stability studies
The prepared emulgels were packed in aluminum collapsible tubes (5 g) and subjected to stability studies at 5 °C, 25 °C/60% RH, 30 °C/65% RH, and 40 °C/75% RH for a period of 3 months. Samples were withdrawn at 15-day time intervals and evaluated for physical appearance, pH, rheological properties and drug content (Harmonized Tripartite Guidelines, 2003).
3. Results and discussion
3.1. Physical appearance
Emulgel formulations were yellowish white viscous creamy preparation with a smooth homogeneous texture and glossy appearance. Results have been discussed in Table 2.
Table 2.
Physical parameters of formulation batches.
| Formulation | Color | Homogeneity | Consistency | Phase separation |
|---|---|---|---|---|
| F1 | White | Excellent | Excellent | None |
| F2 | White | Excellent | Excellent | None |
| F3 | Pale yellow | Excellent | Excellent | None |
| F4 | Yellow | Excellent | Excellent | None |
3.2. .Spreading coefficient
The spreading coefficient of various emulgel formulations are given below in Fig. 1.
Figure 1.
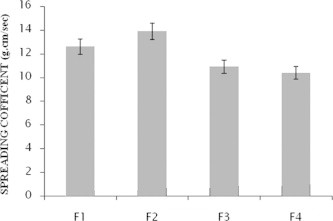
Spreading coefficient of the formulation F1–F4 (mean ± SD).
3.3. Rheological studies
The tests were performed at 100 rpm for 10 min. Results are given in Fig. 2.
Figure 2.
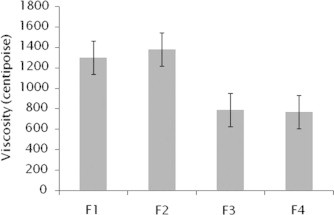
Viscosity of the formulations F1–F4 (mean ± SD).
3.4. Skin Irritation test
No allergic symptoms like inflammation, redness, irritation appeared on rats up to 24 h.
3.5. Biodhesive strength measurement
The bioadhesive strength of various emulgel formulations have been shown below in Fig. 3.
Figure 3.
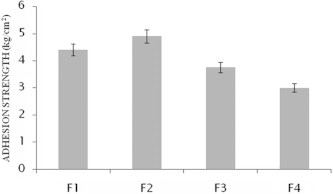
Bioadhesive strength of formulations F1–F4 (mean ± SD).
3.6. In vitro release study
The study showed the release of the drugs from its emulsified gel formulation can be ranked in the following descending order: F4 > F1 > F2 > F3 where the amounts of the drug release of the drug released after 240 min were 56.01%, 53.48%, 52.23%, 51.21%, respectively (Fig. 4, Table 3).
Figure 4.
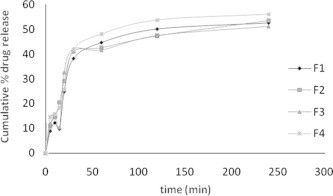
In vitro cumulative % drug release of formulation F1–F4.
Table 3.
Data for in vitro cumulative % drug release data of formulations F1–F4.
| Time (min) | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| 0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 5 | 08.90 ± 0.010 | 11.02 ± 8.65 | 11.82 ± 6.83 | 14.55 ± 30.44 |
| 10 | 12.20 ± 0.05 | 14.92 ± 9.64 | 14.54 ± 13.30 | 15.76 ± 10.20 |
| 15 | 09.78 ± 1.30 | 20.43 ± 6.83 | 10.44 ± 10.2 | 18.24 ± 15.70 |
| 20 | 24.70 ± 2.25 | 29.04 ± 10.2 | 32.74 ± 0.03 | 25.75 ± 0.020 |
| 30 | 38.10 ± 31.70 | 40.95 ± 24.9 | 42.14 ± 0.38 | 41.84 ± 0.03 |
| 60 | 44.60 ± 31.70 | 42.56 ± 3.10 | 41.64 ± 7.55 | 48.04 ± 3.10 |
| 120 | 50.00 ± 7.55 | 47.37 ± 2.90 | 47.44 ± 3.10 | 53.74 ± 13.30 |
| 240 | 52.23 ± 2.85 | 53.48 ± 2.31 | 51.25 ± 0.49 | 56.01 ± 2.30 |
3.7. Ex vivo release study
This study was carried out only on two best optimized formulations. The study showed the release of the drugs from its emulsified gel formulation F2 and F4 were 55.47% and 56.60%, respectively in 240 min. The results are show in Fig. 5.
Figure 5.
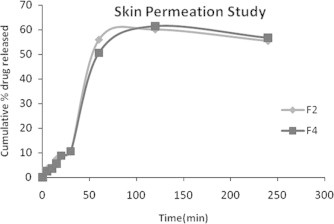
Ex vivo cumulative % release of formulations F2 and F4.
3.8. Anti-inflammatory activity
The anti-inflammatory action of formulation F2 and F4 was calculated and it was compared with diclofenac sodium (marketed preparation). The % inhibition of diclofenac sodium, F2 and F4 were found to be 65.71%, 54.28% and 55.72%, respectively. This showed that the formulations were as effective as marketed formulation.
3.9. Analgesic activity
The formulations showed hike in lapse time. They were compared with diclofenac sodium gel (marketed preparation). The lapse times of diclofenac sodium gel, F2 and F4 were found to be 6.8 s, 5 s and 5.1 s.
3.10. Stability study
All the prepared emulgel formulations were found to be stable upon storage for 3 months, no change was observed in their physical appearance, pH, rheological properties and drug content.
4. Conclusion
In the coming years, topical drug delivery will be used extensively to impart better patient compliance. Since emulgel is helpful in enhancing spreadability, adhesion, viscosity and extrusion, this novel drug delivery become popular. Moreover, they will become a solution for loading hydrophobic drugs in water soluble gel bases for the long term stability.
Similarly in the study, topical emulgels of mefenamic acid were formulated and subjected to physicochemical studies i.e. rheological studies, spreading coefficient studies and bioadhesion strength, in vitro release studies and ex vivo release studies through rat skin. In vitro release of the tests formulations were performed to determine drug release from emulgel rate and duration of drug release. From the in vitro studies, formulation F4 showed maximum release of 56.23% in 240 min. Ex vivo drug release was also performed in which formulation F4 showed best release of 56% in 240 min. Carrageenan induced paw edema and hot plate tests revealed anti-inflammatory and analgesic activity. The formulations F2 and F4 were comparable with marketed diclofenac topical gel.
So mefenamic acid emulgel can be used as an anti-inflammatory analgesic agent for topical drug delivery.
References
- Ansel H.C., Allen L.V., Jr, Popovich N.G. 7th ed. Lippincott Williams and Wilkins; New York: 1999. Pharmaceutical Dosage Forms and Drug Delivery Systems. [Google Scholar]
- Bonacucina G., Cespi M., Palmieri G.F. Characterization and stability of emulsion gels based on acrylamide/sodium acryloyldimethyl taurate copolymer. AAPS PharmSciTech. 2009;2:10. doi: 10.1208/s12249-009-9218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.G., Yong C.S., Sah H., Jahng Y., Chang H.W., Son J.K., Lee S.H., Jeong T.C., Rhee J.D., Baek S.H. Physiochemical characterization of diclofenac sodium loaded poloxamer gels as a rectal delivery system with fast absorption. Drug Dev. Ind. Pharm. 2003;29:545–553. doi: 10.1081/ddc-120018643. [DOI] [PubMed] [Google Scholar]
- Crunkhorn P., Mencock S.C. Mediators of inflammation induced in rat paw carregeenan. Br. J. Pharmacol. 1971;42:371–402. doi: 10.1111/j.1476-5381.1971.tb07124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro AR., editor. Remington: the Science and Practice of Pharmacy. 19th ed. Mack Publishing Company; Easton: 1995. [Google Scholar]
- Gupta G.D., Gaud R.S. Release rate of tenoxicam from acrypol gels. Ind. Pharm. 2005:69–76. [Google Scholar]
- Gupta A., Mishra A.K., Singh A.K., Gupta V., Bansal P. Formulation and evaluation of topical gel of diclofenac sodium using different polymers. Drug Invention Today. 2010;2:250–253. [Google Scholar]
- ICH Harmonized Tripartite Guidelines, Stability Testing of New Drug Substances and Products. ICH Committee 2003; 8.
- Jain A., Deveda P., Vyas N., Chauhan J. Development of antifungal emulsion based gel for topical fungal infection(s) IJPRD. 2011;2(03) [Google Scholar]
- Junping K., Yun N., Wang N., Liang L., Zhi-Hong H. Analgesic and anti-inflammatory activities of total extract and individual fractions of Chinese medicinal plants Polyrhachis lamellidens. Bio. Pharm. Bull. 2005;23:176–180. doi: 10.1248/bpb.28.176. [DOI] [PubMed] [Google Scholar]
- Kakkar A.P., Gupta A. Gelatin based transdermal therapeutic system. Ind. Drugs. 1992;29:308–312. [Google Scholar]
- Kasliwal N., Derle D., Negi J., Gohil J. Effect of permeation enhancers on the release and permeationkinetics of meloxicam gel formulations through rat skin. Asian J. Pharm. Sci. 2008;3(5):193–199. [Google Scholar]
- Kumar L., Verma R. In vitro evaluation of topical gel prepared using natural polymer. International Journal of Drug Delivery. 2010;2:58–63. [Google Scholar]
- Lionberger D.R., Brennan M.J. Topical nonsteroidal anti-inflammatory drugs for the treatment of pain due to soft tissue injury: diclofenac epolamine topical patch. J Pain Research. 2010;3:223–233. doi: 10.2147/JPR.S13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M.I. Optimization of chlorphenesin emulgel formulation. AAPS J. 2004;6(3) doi: 10.1208/aapsj060326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty S.N., Hiremath S.R.R. Physical and chemical enhancers in transdermal delivery of terbutaline sulphate. AAPS Pharm. Sci. Tech. 2001;2:1–5. doi: 10.1208/pt0201_tn1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier H., Bouwstra J.A. Liposomes and Niosomes as topical drug carriers: Dermal and Transdermal delivery. J. Contr. Rel. 1985;30:863–868. [Google Scholar]
- Stanos S.P. Topical agents for the management of musculoskeletal pain. J. Pain Sympt. Manage. 2007:33. doi: 10.1016/j.jpainsymman.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Topical Emulsion, Gel Composition Comprising Diclofenac Sodium. Patent no. WO/2004/017998).


