Abstract
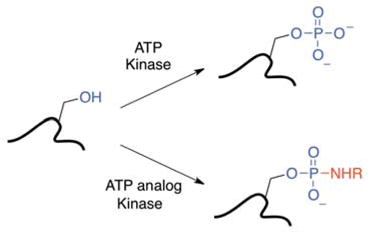
Kinase-catalyzed protein phosphorylation is an important biochemical process involved in cellular functions. We recently discovered that kinases promiscuously accept γ-modified ATP analogs as cosubstrates and used several ATP analogs as tools for studying protein phosphorylation. Herein, we explore the structural requirements of γ-modified ATP analogs for kinase compatibility. To understand the influence of linker length and composition, a series of ATP analogs was synthesized and the efficiency of kinase-catalyzed labeling was determined by quantitative mass spectrometry. This study on factors influencing kinase cosubstrate promiscuity will enable design of ATP analogs for a variety of kinase-catalyzed labeling reactions.
Introduction
Protein phosphorylation is mediated by kinase enzymes in a highly regulated manner to influence a variety of biological processes, including cell signaling, diseases, cancer, and immunosupression.(4) Over 500 different kinases containing a conserved catalytic domain have been characterized. Kinases phosphorylate using adenosine 5′-triphosphate (ATP) as a cosubstrate.(5) Based on crystal structures of various kinases, the adenine moiety of ATP binds in the hydrophobic pocket of the active site while the triphosphate chain of ATP protrudes out towards the solvent-exposed, substrate binding region. The proximity of the protein substrate and ATP facilitates transfer of the γ-phosphate of ATP to the hydroxyl of serine, threonine or tyrosine residues. When the neutral hydroxyl group is replaced with a negatively charged phosphate group, the activity of the protein may change, which influences cell biology. With a significant role in biochemical functions, it is important to identify and characterize phosphorylation events.
Techniques to monitor phosphorylation involve 32P radiolabeling, immobilized metal affinity chromatography, covalent modification of the phosphate, and gel-based visualization using specific antibodies or phosphate stains (for example, Pro-Q Diamond). (6–11) In addition to these methods, γ-phosphate modified ATP analogs have been developed for kinase-catalyzed labeling of phosphoproteins and phosphopeptides (Table 1). Specifically, ATP analogs containing biotin, dansyl, azide, and ferrocene groups at the γ-phosphate have highlighted the diversity of functional tags that can be enzymatically attached to phosphoprotein/peptide substrates.(1–3, 12, 13) However, a systematic study of the tolerance of kinases to γ-phosphate modified ATP analogs has yet to be reported.
Table 1.
Kinase-catalyzed labeling with previously reporteda
| |||
|---|---|---|---|
| ATP analog | PKA | CK2 | Abl |
|
ATP-dansyl |
91% | 81% | 87% |
|
ATP-biotin |
79% | 56% | 80% |
|
ATP-arylazide |
86% | 51% | 78% |
Quantitative mass spectrometric (QMS) analysis was performed with ATP analogs containing biotin, dansyl, or arylazide groups (Table 1).(1–3) In these studies, three different kinases (PKA, CK2 and Abl) showed the highest conversions with the ATP-dansyl derivative (81% – 91%) compared with the ATP-biotin or ATP-arylazide analogs (51% – 86%). Distinguishing features of the ATP-dansyl derivative that may account for its high conversions are the all carbon diamine linker and sulfonamide group. To understand the role of the linker and amide/sulfonamide groups in kinase-catalyzed labeling, we explored the relationship between ATP analog structure and kinase reaction efficiency. Herein, we report the synthesis and kinase compatibility of a variety of γ-phosphate modified ATP analogs related to ATP-dansyl, ATP-biotin, and ATP-arylazide.
Experimental Procedures
General protocol for synthesis of amines 5a–f, 8a–b, and 12
To an ice-cooled solution of diamine 4, 7, or 11 (1–5 eq) in dichloromethane (250 mL), a solution of acetyl, benzoyl, or benzenesulfonyl chloride (1 eq) in dichloromethane (100 mL) was added dropwise. The resultant mixture was allowed to stand in room temperature overnight under argon. The organic solution was concentrated in vacuo. The crude mixture was purified using flash chromatography on silica gel (ethanol, dichloromethane and ammonium hydroxide, 3:1:0.05) to afford the amine. Specific reagent amounts and spectra characterization for all amines are reported as supporting information.
General protocol for synthesis of ATP-analogs 2a–b, 6a–f, 9a–b, and 13
ATP·2Na (0.05 mmol, 1eq) was dissolved into 3 mL of water and the pH was adjusted to 7.0 with 1 M sodium hydroxide, as assessed with a pH meter. EDCI (2 mmol, 40 eq) was added and the pH was adjusted to 5.6–5.8 followed by addition of 1 mL water. An aqueous solution of amine linker (1.5–3 mmol in 1 mL water, 30–60 eq) was added to the ATP mixture and the reaction was incubated for 2 hours under a controlled pH of 5.6–5.8. Progress of the reaction was monitored by TLC (6:3:1, isopropanol: NH4OH: H2O). The reaction mixture was brought to pH 8.5 using 1 M triethyl amine (TEA) and purified using an A-25 Sephadex anion exchange column with 0.1 M – 1 M triethyl ammonium bicarbonate (TEAB) buffer solution (pH 8.5) as eluent. The purified product was lyophilized to dryness to obtain the ATP analog as a white TEA salt. The product thus obtained was dissolved in methanol and stored at −20 °C for several months. Specific reagent amounts and spectra characterization for all ATP analogs are reported as supporting information.
Kinase reactions with peptides
Each reaction contained either PKA (26.6 U/μL) or CK2 (9 U/μL), along with either the PKA substrate peptide (LRRTSIIGT or LRRASLG, 33 μM) or CK2 substrate peptide (RRREEETEEE, 33.3 μM). A phosphopeptide was generated using ATP (1.3 mM), while a phosphoramidate peptides was created using the ATP-analog (1.3 mM). The ATP analog storage solvent methanol was evaporated using a ThermoSavant speedvac concentrator and the ATP analog was resuspended in a reaction buffer prior to reaction. For PKA reactions, the final concentration of buffer components was 39 mM Tris-HCl, 7.5 mM MgCl2, 3.7 mM NaCl, 3.7 mM KCl, 3.75% glycerol, 0.15 mM DTT, 0.25 mM EDTA, pH 7.5 @ 25 °C. For the CK2 reaction, the final concentration of buffer components was 11.3 mM Tris-HCl, 4.5 mM MgCl2, 15.75 mM NaCl, 22.5 mM KCl, 0.09 mM DTT, 0.09 mM EDTA, 0.005% Triton X-100, pH 7.5 @ 25 °C. The reaction mixtures were incubated at 30 °C for 2 hours without shaking. The final volume for the PKA reaction was 7.5μL, while that for the CK2 was 4.5 μL.
Quantitative mass spectrometric analysis
The phosphopeptides and phosphoramidate-peptides generated in kinase reactions were analyzed by QMS, as previously described.(14) To each of the peptides, 300 μL of anhydrous D0-MeOH or D4-MeOH was added, followed by 50 μL of acetyl chloride to generate 2N HCl in situ. The phosphopeptide was incubated with D4-MeOH while the phosphoramidate peptide was incubated with D0-MeOH. The reaction mixture was allowed to shake at 700 rpm at 16 °C for 3 hours to afford acid esterification. Since the conditions of esterification were acidic, the phosphoramidate bond linking the substituent to the peptide was cleaved, producing the two differentially-labeled phosphopeptides required for QMS analysis. The acidic methanol solution was evaporated in a ThermoSavant Speed vac. (~2 hrs). The peptide was further subjected to MALDI-TOF MS analysis after equal volumes of the two isotopically differentiated phosphopeptides were combined. The sample was prepared as follows: One of the differentially labeled peptide reactions was dissolved in a minimum amount of water (~2 μL) and combined with THE second vial. The first vial was washed (2 μL) and the resulting solution (4 μL) was mixed with 10 μL of a saturated solution of 4-hydroxy-α-cyanocinnamic acid in 1:1 acetonitrile: 0.1% TFA in water. The mixture (1 μL) was spotted into a MALDI plate (Standard 384 MTP, Bruker) and analyzed using a MALDI-TOF instrument.
Autodocking analysis
The Autodock Vina program was designed by Dr. Oleg Trott of the Molecular Graphics Lab at The Scripps Research Institute (http://vina.scripps.edu/index.html). Crystal structures were downloaded from the RCSB Protein Data Bank (PKA: 1ATP and CK2: 1DAW). The PyMOL program was used to delete the ATP analog in the active site of the structures. The ATP dansyl structure was drawn in Chem 3D Pro and MM2 was used for energy minimization. The grid dimensions used for the autodock analysis and the output file with all binding modes are shown as supporting information.
Results
Synthesis of the ATP Analogs
ATP analogs with a variety linker lengths and terminal functional groups were prepared using a two-step synthetic strategy (Scheme 1), as previously described.(2) First, various amines were generated by reacting acetyl, benzoyl or benezenesulfonyl chlorides (3 or 10) with diamine linkers (4, 7, or 11). In some cases, the amine group was obtained from commercial sources (aniline 1a and benzylamine 1b). Syntheses of the ATP analogs were accomplished by activating the terminal phosphate of ATP using 1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydro chloride (EDCI), followed by coupling with the desired amines (Scheme 1).
Scheme 1.
Synthesis of ATP analogs 2a–b, 6a–f, 9a–b, and 13
The Acetyl Series of ATP Analogs
The initial series of ATP analogs was generated with an acetyl group to test whether an amide functionality would be tolerated. Three different types of linkers were employed. Ethylenediamine (4, n=2) and butylenediamine (4, n=4) were used because they are relatively short and hydrophobic, similar to the linker of ATP-dansyl. The third linker, 2,2′-ethylenedioxybis-(ethylamine) (7), was relatively long and hydrophilic, similar to the linker in ATP-biotin or ATP-arylazide.
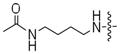
To perform a systematic study of kinase-catalyzed labeling, we chose CK2 as the model kinase due to its relatively variable conversion efficiencies with ATP-dansyl, ATP-biotin, and ATP-arylazide (Table 1). The efficiency of phosphorylation by CK2 and the ATP analogs was assessed by QMS analysis, as previously described.(14) The QMS data showed that the acetyl analogs were accepted by CK2, although with variable conversion efficiencies (Table 2). The analogs containing the all carbon linkers ethylenediamine (6a) and butylenediamine (6b) demonstrated 72% and 27% conversion, respectively (Table 2). Given the high conversion of ATP-dansyl with its all carbon linker (Table 1), these analogs suggest that the sulfonamide group of ATP-dansyl is important for its high efficiency. The analog containing a relatively long and hydrophilic polyethylene glycol linker 9a, similar to that in ATP-biotin or ATP-arylazide, displayed 62% conversion (Table 2). The slightly augmented conversion with a methyl group in place of the larger and more polar biotin or arylazide groups (compare CK2 conversions with 9a, 62%, to that with ATP-biotin and ATP-arylazide, 51–56%) suggests that the terminal group influences the efficiency. The QMS data from the acetyl series of ATP analogs provide our first evidence that the linker length and polarity play significant roles in dictating kinase compatibility.
Table 2.
Efficiency of phosphorylation with CK2 and the acetyl series of ATP analogs
| Analog | γ-phosphate group | Conversiona |
|---|---|---|
| 6a |
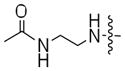
|
72% |
| 6b |
|
27% |
| 9a |
|
62% |
Percentage conversion was determined using QMS by comparing to ATP phosphorylation (set to 100 %). See Figures S45–S47 in supporting information
The Aryl Series of ATP Analogs
To further explore the role of the linker and terminal group on kinase-catalyzed labeling, an aryl analog series was created. Both the ATP-dansyl and ATP-arylazide derivatives (Table 1) contain aromatic terminal groups. In addition, aryl substituents are relatively hydrophobic, which will test the influence of polarity on the kinase reaction. To expand our study, the efficiency of phosphorylation with the aryl series of ATP analogs was determined with both CK2 and PKA using QMS. The percentage conversion of the aryl series ranged from <5% (the detection minimum of the MS technique) to 54% (Table 3). Given the high efficiencies observed with the acetyl series (up to 72%), the data initially suggest that the presence of an aryl group reduces conversion efficiency.
Table 3.
Efficiency of phosphorylation with PKA and CK2 and the aryl series of ATP analogs
| Analog | γ-phosphate group | Conversiona | |
|---|---|---|---|
| PKA | CK2 | ||
| 2a |
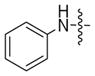
|
<5% | <5% |
| 2b |
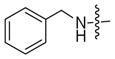
|
<5% | 6% |
| 6c |
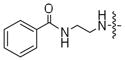
|
5% | 27% |
| 6d |
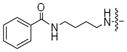
|
<5% | 33% |
| 6f |
|
22% | 38% |
| 9b |
|
54% | 54% |
Percentage conversion was determined using QMS by comparing to ATP phosphorylation (set to 100 %). See Figures S48–S59 in supporting information
The conversion efficiency with the aryl series was dependent on length and composition of the linker. Analogs lacking a linker, created with aniline (1a) or benzyl amine (1b) groups, showed undetectable or low levels of phosphorylation (Table 3, 2a and 2b), indicating that PKA and CK2 do not tolerate the presence of a bulky group directly attached to the terminal phosphate. The presence of an all carbon linker between the ATP and aryl substituent (6c, 6d, and 6f) resulted in low to moderate percentage conversions ranging from <5% to 38% (Table 3). Similarly, the acetyl analog containing an all carbon butylene diamine linker (Table 2, 6b) displayed 27% conversion. The low percentage conversion for analogs containing all carbon linkers suggests that hydrophobicity reduces kinase compatibility. However, as the linker length increased, there was an increase in the percent conversion with both PKA and CK2. Presumably, the increased linker length positions the hydrophobic aryl substituent away from kinase active site to augment conversion.
The most efficient enzymatic conversion among the aryl series of ATP analogs was observed with compound 9b, which contains a ethylene glycol linker (Table 3). Unlike the all carbon linkers, the hydrophilic ethylene glycol may counter balance the hydrophobic aryl substituent, while also positioning the aryl group away from the active site area. It is notable that compound 9a is identical to ATP-arylazide (Table 1), except for the absence of the terminal azide group. The relatively similar conversions of the two analogs with CK2 (54% versus 51%) suggest that the presence of the azide group does not significantly influence kinase compatibility. In contrast, the presence of the azide group significantly enhanced conversion with PKA (54% versus 86%), suggesting that polarity in the linker and terminal group are critical for high conversion with PKA. Overall, the data with the aryl series suggests that the composition and length of the linker are factors that influence cosubstrate tolerance.
The Role of the Sulfonamide Group
Earlier reports indicated that ATP-dansyl displays the highest kinase conversion efficiency of the analogs tested (Table 1). One of the distinguishing features of ATP-dansyl compared to ATP-biotin or ATP-arylazide is the presence of a sulfonamide group. To study the influence of the sulfonamide group on kinase-catalyzed labeling, ATP analogs containing a six-carbon linker, similar to ATP-dansyl, but displaying either a benzoyl (6e) or benzene sulfonyl (13) group were synthesized and subjected to kinase-catalyzed phosphorylation (Table 4). While the ATP-benzoyl analog 6e demonstrated 61% and 36% conversion, the ATP-benzenesulfonamide derivative 13 displayed 94% and 83% conversion (Table 4) with PKA and CK2, respectively. Given the similar percentage conversions observed with 13 and ATP-dansyl (91%/81% and 94%/83%, Tables 1 and 4), the data suggest that a sulfonamide group promotes high conversion.
Table 4.
Efficiency of phosphorylation with PKA and CK2 and the ATP-dansyl-like analogs
| Analog | γ-phosphate group | Conversiona | |
|---|---|---|---|
| PKA | CK2 | ||
| 6e |
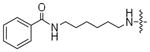
|
61% | 36% |
| 13 |
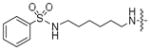
|
94% | 83% |
Percentage conversion was determined using quantitative MS by comparing to ATP phosphorylation (set to 100 %). See Figures S60–S63 in supporting information.
Docking Studies
To rationalize the high conversion with the dansyl analog, we docked ATP-dansyl into the active site of PKA (GenBank ID 6755076, pdb:1ATP)(15) using the Autodock Vina program (http://vina.scripps.edu/).(16) The most favorable binding mode (−9.5 kcal/mole) positioned the diamine linker and dansyl modification wrapped around the peptide inhibitor (Figure 1A). Although the dansyl modification protrudes from the active site, it is within 3.5–4.5 Å of several residues-G53 and S54 near the carbon chain and P203 and P244 near the dansyl group. G53 is well conserved among the kinases (conservation of 83%), while S54, P203, and P244 are not (conservation of 16%, 18%, and 9%, respectively) (http://sequoia.ucsf.edu/ksd/).(17)
Figure 1.
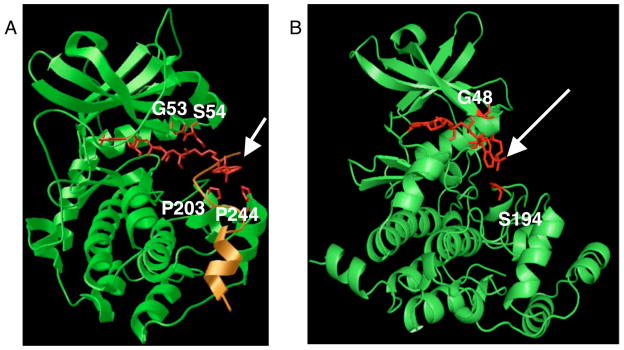
Docking of ATP-dansyl (red) into the active sites of PKA (A, green) in complex with peptide substrate inhibitor (yellow) or CK2 (B, green). The dansyl group protrudes from both active site (arrows), but within close proximity to G53, S54, P203, and P244 of PKA (A, red) and G48 and S194 of CK2 (B, red).
Similarly, docking of ATP-dansyl into the active site of CK2 (GenBank ID 7766821, pdb:1DAW)(18) resulted in a favorable binding mode (−8.9 kcal/mole) where the phosphate groups are positioned close to G48 and the dansyl group is near S194 (Figure 1B). Comparison of the favorable binding modes of ATP-dansyl with CK2 and PKA show that both contain a GXGX sequence (X being a hydrogen bond donor or acceptor) near the ATP binding site (Table S7). These docking results suggest that the conserved glycine creates an open active site to accommodate the enlarged ATP analog. Non-conserved S54 and S194 may create a polar environment where the phosphate diesters and ethylene glycol or sulfonamide linker bind favorably. In addition, the preference of PKA for an eight atom linker (Table 3, compounds 6f and 9b, and Table 4) may be due to optimal positioning of the aromatic group near a binding pocket comprised of non-conserved P203 and P244.
To understand the influence of the linker and terminal groups on binding interactions, we also docked analogs 2b and 13 into the active sites of CKII and PKA. With 2b, higher energy binding energies were observed with CKII (−6.3 kcal/mol) and PKA (−6.7 kcal/mol) compared to ATP-dansyl, which suggests that the shorter benzyl group of 2b does not maintain the favorable binding interactions of the dansyl group (Tables S3–S4 and Figures S64–S65). The high energy binding is consistent with the low conversions observed in kinase reactions (Table 3, <5% and 6%). In contrast, comparable binding energies were observed with ATP-dansyl and compound 13 with CKII (−8.6 kcal/mol) and PKA (−8.4 kcal/mol) (Tables S5–S6 and Figures S66–S67), which in consistent with the high conversion data (Tables 1 and 4, 81–94%) and strongly suggests that the sulfonamide group leads to favorable interactions. In total, the docking analysis confirms the need for long linkers and polar groups to enhance binding interactions between the ATP analogs and kinases.
Discussion
The data reported here provide guiding principles for the design of new ATP analogs for kinase-catalyzed labeling. First, the data suggests that the functional tag attached at the γ-phosphate of ATP should be positioned sufficiently distant from the kinase active site to allow high conversions. With the aryl series of ATP analogs, compounds with an eight atom diamine linker demonstrated the highest conversion efficiencies (Table 3, compounds 6f and 9b). Docking studies were consistent with the conversion data, showing that compounds with eight-atom linkers (ATP-dansyl and compound 13) showed more favorable binding energies than a compound lacking a linker (compound 2b). In addition, a previous report using ATP-ferrocene derivatives indicated that an eight to twelve-atom all carbon linker gave optimal electrochemical signal.(19) Interestingly, when the terminal group is relatively small, like with the acetyl analog series, a four-atom diamine linker was sufficient to observe high conversions (Table 2, compound 6a). The linker distance required to obtain high kinase-catalyzed labeling is dependent on the size of the functional tag.
In addition to linker length, the data also suggest that the composition of the linker is important for kinase compatibility. With both the acetyl and aryl series of ATP analogs, the percentage conversion increased with analogs containing an ethylene glycol linker compared to a similar all carbon linker (Table 2, compounds 6b and 9a and Table 3, compounds 6f and 9b). The data suggest that a polar linker promotes high conversions. Interestingly, the ATP-benzenesulfonamide analog 13 displayed excellent conversion despite containing an all carbon linker (Table 4). Perhaps the sulfonyl group provides sufficient polarity to promote high efficiency labeling. In total, the data indicate that the linker connecting the γ-phosphate of ATP to the functional tag should contain polar groups, whether within the diamine linker itself or the presence of a sulfonamide group.
Prior work with ATP-ferrocene analogs and surface-mobilized substrates reported higher electrochemical signal with an all carbon linker compared to polyethylene glycol linkers.(20) However, when the substrate was in solution, polyethylene glycol and all carbon linkers performed similarly. Combined with the work reported here showing preferrence for polar linkers, the optimal linker composition and polarity may depend on the environment of the substrate.
To characterize the efficiency of ATP-dansyl as a kinase cosubstrate, kinetics measurements were previously reported using an enzyme coupled or FRET-based assay (21). With PKA and Abl, ATP-dansyl showed similar KM values but significantly reduced kcat values compared to the natural ATP.(3) Therefore, prior data suggest that the reduced conversion with the ATP analogs compared to ATP is primarily due to a change in Vmax. A more complete kinetics analysis is currently ongoing and will be published in due course. We note however, that dansylation with ATP-dansyl displayed similar catalytic efficiency (kcat/KM) to thiophosphorylation with ATP-γS,(3) which is well documented for phosphoprotein labeling.(22) Hence, ATP-dansyl and the other ATP anlaogs maintain appropriate catalytic efficiency for a variety of applications.
In conclusion, efficient kinase-catalzyed labeling requires ATP analogs with a polar linker containing at least eight atoms. However, the size of the functional tag can influence linker length, while the substrate environment can influence the linker composition. These linker guidelines will aid in development of additional analogs for phosphopeptide and phosphoprotein labeling. Given the role of kinases and their phosphoprotein products in cell biology, new biochemical tools based on kinase-catalyzed labeling have the potential to augment cell signaling research.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM079529. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank T. Anthony and A. Fouda from comments on the manuscript.
Footnotes
The authors declare no competing financial interests.
Associated Content
Supporting Information Available: Information on materials and instrumentation, compound synthesis and characterization, ATP analog spectral data, QMS analysis, docking studies, and sequence alignment.
References
- 1.Green KD, Pflum MH. Kinase-Catalyzed Biotinylation for Phosphoprotein Detection. J Am Chem Soc. 2007;129:10–11. doi: 10.1021/ja066828o. [DOI] [PubMed] [Google Scholar]
- 2.Suwal S, Pflum MH. Phosphorylation-dependent kinase-substrate cross-linking. Angew Chem Int Ed Engl. 2010;49:1627–30. doi: 10.1002/anie.200905244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green KD, Pflum MH. Exploring Kinase Cosubstrate Promiscuity: Monitoring Kinase Activity through dansylation. ChemBioChem. 2009;10:234–237. doi: 10.1002/cbic.200800393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter T. Signaling-2000 and beyond. Cell. 2000;100:113–127. doi: 10.1016/s0092-8674(00)81688-8. [DOI] [PubMed] [Google Scholar]
- 5.Adams JA. Kinetic and Catalytic Mechanisms of Protein Kinases. Chem Rev. 2001;101:2271–2290. doi: 10.1021/cr000230w. [DOI] [PubMed] [Google Scholar]
- 6.Kalume DE, Molina H, Pandey A. Tackling the phosphoproteome: tools and strategies. Current Opinion in Chemical Biology. 2003;7:64–69. doi: 10.1016/s1367-5931(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 7.Garcia BA, Shabanowitz J, Hunt DF. Analysis of protein phosphorylation by mass spectrometry. Methods. 2005;35:256–64. doi: 10.1016/j.ymeth.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg TH, Agnew BJ, Gee KR, Leung WY, Goodman T, Schulenberg B, Hendrickson J, Beechem JM, Haugland RP, Patton WF. Global quantitative phosphoprotein analysis using multiplexed proteomics technology. Proteomics. 2003;3:1128–1144. doi: 10.1002/pmic.200300434. [DOI] [PubMed] [Google Scholar]
- 9.Mann M, Ong SE, Gronborg M, Steen H, Jensen ON, Pandey A. Analysis of protein phosphorylation using mass spectrometry: deciphering the phosphoproteome. Trends Biotechnol. 2002;20:261–8. doi: 10.1016/s0167-7799(02)01944-3. [DOI] [PubMed] [Google Scholar]
- 10.Posewitz MC, Tempst P. Immobilized gallium(III) affinity chromatography of phosphopeptides. Anal Chem. 1999;71:2883–92. doi: 10.1021/ac981409y. [DOI] [PubMed] [Google Scholar]
- 11.Sun T, Campbell M, Gordon W, Arlinghaus RB. Preparation and application of antibodies to phosphoamino acid sequences. Biopolymers. 2001;60:61–75. doi: 10.1002/1097-0282(2001)60:1<61::AID-BIP1004>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Song H, Kerman K, Kraatz HB. Electrochemical detection of kinase-catalyzed phosphorylation using ferrocene-conjugated ATP. Chem Commun (Camb) 2008:502–4. doi: 10.1039/b714383d. [DOI] [PubMed] [Google Scholar]
- 13.Lee SE, Elphick LM, Anderson AA, Bonnac L, Child ES, Mann DJ, Gouverneur V. Synthesis and reactivity of novel gamma-phosphate modified ATP analogues. Bioorg Med Chem Lett. 2009;19:3804–7. doi: 10.1016/j.bmcl.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Senevirathne C, Green KD, Pflum MKH. Current Protocols in Chemical Biology. John Wiley & Sons, Inc; 2009. Kinase-Catalyzed Biotinylation of Peptides, Proteins, and Lysates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng J, Knighton DR, Ten Eyck LF, Karlsson R, Xuong N, Taylor SS, Sowadski JM. Crystal structure of the catalytic subunit of cAMP-dependent protein kinase complexed with magnesium-ATP and peptide inhibitor. Biochemistry. 1993;32:2154–2161. doi: 10.1021/bi00060a005. [DOI] [PubMed] [Google Scholar]
- 16.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–61. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buzko O, Shokat KM. A kinase sequence database: sequence alignments and family assignment. Bioinformatics. 2002;18:1274–1275. doi: 10.1093/bioinformatics/18.9.1274. [DOI] [PubMed] [Google Scholar]
- 18.Niefind K, Putter M, Guerra B, Issinger OG, Schomburg D. GTP plus water mimic ATP in the active site of protein kinase CK2. Nat Struct Mol Biol. 1999;6:1100–1103. doi: 10.1038/70033. [DOI] [PubMed] [Google Scholar]
- 19.Martić S, Labib M, Freeman D, Kraatz HB. Probing the Role of the Linker in Ferrocene–ATP Conjugates: Monitoring Protein Kinase Catalyzed Phosphorylations Electrochemically. Chemistry – A European Journal. 2011;17:6744–6752. doi: 10.1002/chem.201003535. [DOI] [PubMed] [Google Scholar]
- 20.Martić S, Rains MK, Freeman D, Kraatz HB. Use of 5′-γ-Ferrocenyl Adenosine Triphosphate (Fc-ATP) Bioconjugates Having Poly(ethylene glycol) Spacers in Kinase-Catalyzed Phosphorylations. Bioconjugate Chemistry. 2011;22:1663–1672. doi: 10.1021/bc200229y. [DOI] [PubMed] [Google Scholar]
- 21.Cook PF, Neville ME, Jr, Vrana KE, Hartl FT, Roskoski R., Jr Adenosine cyclic 3′,5′-monophosphate dependent protein kinase: kinetic mechanism for the bovine skeletal muscle catalytic subunit. Biochemistry. 1982;21:5794–9. doi: 10.1021/bi00266a011. [DOI] [PubMed] [Google Scholar]
- 22.Kwon SW, Kim SC, Jaunbergs J, Falck JR, Zhao Y. Selective enrichment of thiophosphorylated polypeptides as a tool for the analysis of protein phosphorylation. Molecular & cellular proteomics: MCP. 2003;2:242–7. doi: 10.1074/mcp.M300039-MCP200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.