Abstract
Suberoylanilide hydroxamic acid (SAHA, Vorinostat), the first FDA-approved histone deacetylase (HDAC) inhibitor, was modified at the C6 position to study the structural requirements for high potency and selectivity. Substituents on the C6 position only modestly influenced inhibitor potency, with poorer activity observed as substituent size increased. Interestingly, C6 substituents also modestly influenced selectivity compared to the parent compound, SAHA. This systematic study documenting the influence of substituents on the SAHA linker region will aid development of anti-cancer drugs targeting HDAC proteins.
Keywords: Histone Deacetylase, HDAC inhibitor, Isoform Selectivity, Vorinostat
Histone deacetylase (HDAC) proteins are transcription factors that influence gene expression by altering the acetylation status of lysine residues on nucleosomal histones. The HDAC protein family consists of 18 members and is divided into four classes based on size, cellular localization, number of catalytic active sites, and homology to yeast HDAC proteins.1 The eleven class I, II, and IV HDAC proteins are metal ion-dependant proteins and sensitive to the inhibitors discussed here.2
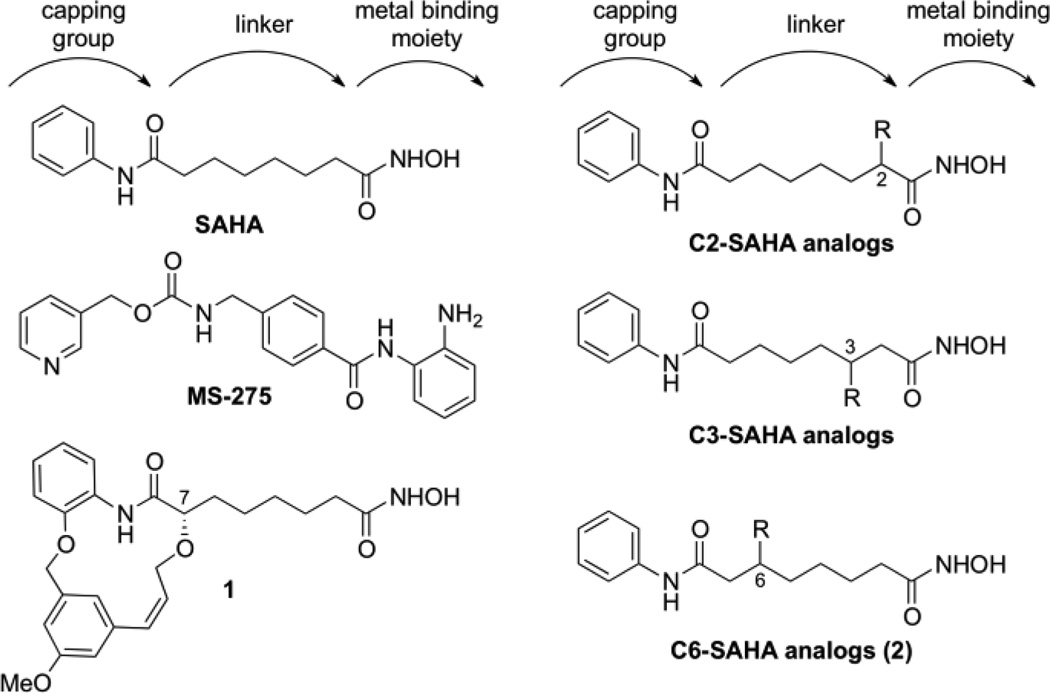
HDAC proteins are over-expressed in many cancers, making them attractive targets of anti-cancer drugs.3 In fact, two HDAC inhibitors, suberoylanilide hydroxamic acid (SAHA, Vorinostat, Figure 1) and Romidepsin, were approved for treatment of cutaneous T-cell lymphoma.4 In addition to lymphoma, HDAC activity has been associated with a variety of other cancers. Over-expression of class I HDAC proteins (HDAC1, 2, and 3) was observed in ovarian cancer.5 Altered HDAC2 activity in gastric cancers,6 mutations of HDAC1 and HDAC3 in lung cancers,7 and abnormal HDAC8 protein activity in acute myeloid leukemia tissues have been reported for the class I proteins.8 Overproduction of class II HDAC6 was observed in breast cancer tissues.9 Because individual HDAC isoforms play independent roles in specific cancers, development of isoform-selective inhibitors as anti-cancer drugs has been proposed. However, most HDAC inhibitors nonspecifically target all eleven metal ion-dependent HDAC proteins, including SAHA.10 It has been proposed that the non-selectivity of HDAC inhibitors may cause cancer patients in the clinic to suffer from the side effects, such as fatigue, anorexia, diarrhea, and cardiac arrhythmia.11 Unfortunately, the clinical toxicity of HDAC inhibitors is poorly characterized because few isoform-selective molecules have been reported. In addition, sequence similarity in the active site of the isoforms has challenged selective inhibitor design.12
Figure 1.
Structures of HDAC inhibitors discussed in the text
Most HDAC inhibitors, including SAHA (Figure 1), have a similar construction consisting of a capping group that is solvent-exposed, a carbon linker that is surrounded by a hydrophobic tunnel, and a metal binding moiety that is buried in the protein active site.13 While the capping group and metal binding moiety have been modified extensively, few structure activity relationship studies focusing on the linker area of SAHA have been reported.12 However, MS275 (Figure 1) contains an intralinker aryl group and displays nanomolar class I selectivity,10, 14 while a recently reported nanomolar HDAC6-selective inhibitor (1, Figure 1) contains a cyclic substituent at the C7 position of SAHA.15 These examples highlight the possible role of the linker region in selectivity and the need for a systematic study of linker substituents.
To study the linker region, SAHA analogs containing hydrophobic substituents on the C2 and C3 positions were previously reported (Figure 1).16 Positioning substituents near the hydroxamic acid reduced inhibitor potency, with IC50 values in the µM range. However, the C3-ethyl SAHA analog displayed 12-fold selectivity for HDAC6 over HDAC3. Combining the data from compound 1 and the C2- and C3-SAHA analogs, we theorized that SAHA analogs with substituents positioned nearer to the solvent-exposed capping group might display potent inhibition with HDAC isoform selectivity.
We report here the synthesis and evaluation of SAHA analogs with substituents attached at the C6 position (Figure 1). To our knowledge, C6-modified SAHA analogs have not been reported despite the fact that SAHA analogs with substituents at the C7 position, such as 1, showed potency and selectivity. Due to the symmetrical relationship of the C6 and C3 positions on SAHA, the C6-SAHA analog synthesis had similarities to that of the C3-SAHA analogs, previously reported.16b The main difference between the two syntheses was the order of installation of the terminal amide groups. Similar to the C2 and C3-SAHA analogs, we selected hydrophobic substituents since the carbon linker is surrounded by a hydrophobic channel in the HDAC structure.13
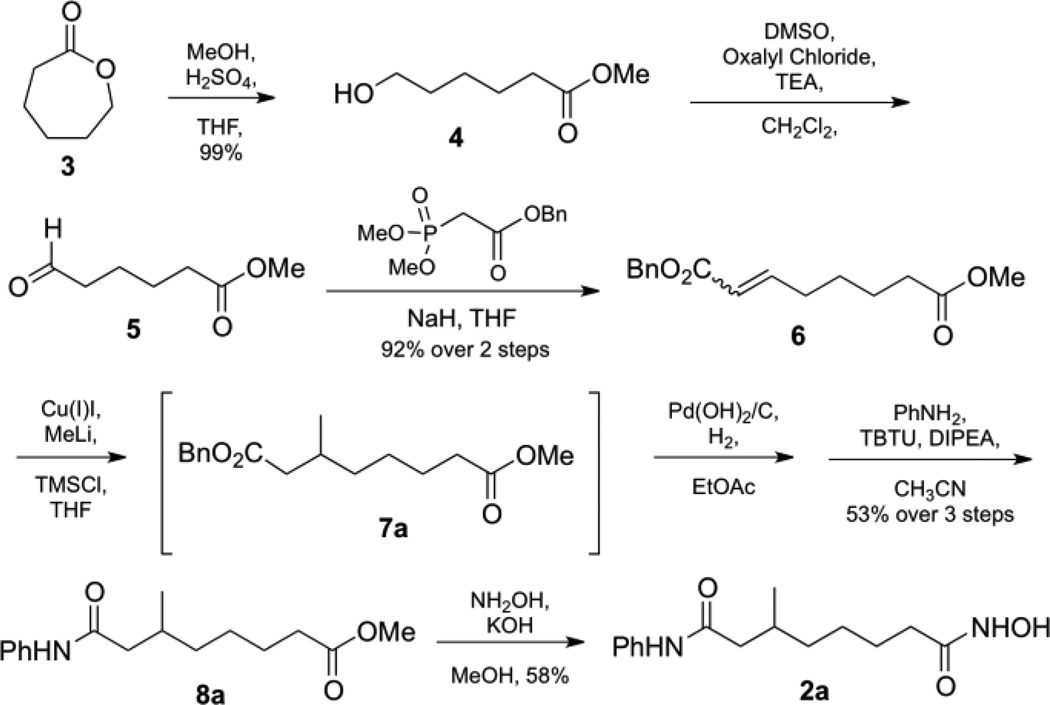
We initially synthesized the C6-methyl SAHA analog 2a, as outlined in Scheme 1. Under Fisher conditions, commercially available ε-caprolactone 3 was opened to give alcohol 4, which was subjected to Swern oxidation to give aldehyde 5. For the Horner-Wadsworth-Emmons reaction, benzyl dimethyl phosphono-acetate was added to crude compound 5 to give the corresponding α,β-unsturated benzyl ester 6. A mixture of (E) and (Z)-isomers of ester 6 was treated with a copper (I) iodide and methyl lithium to give the C6-methyl substituted benzyl ester 7a. Without purification, ester 7a was deprotected by hydrogenolysis and coupled with aniline to give anilide 8a, which was directly converted to the methyl hydroxamic acid final product 2a.
Scheme 1.
Initial synthesis of C6-SAHA methyl analog (2a).
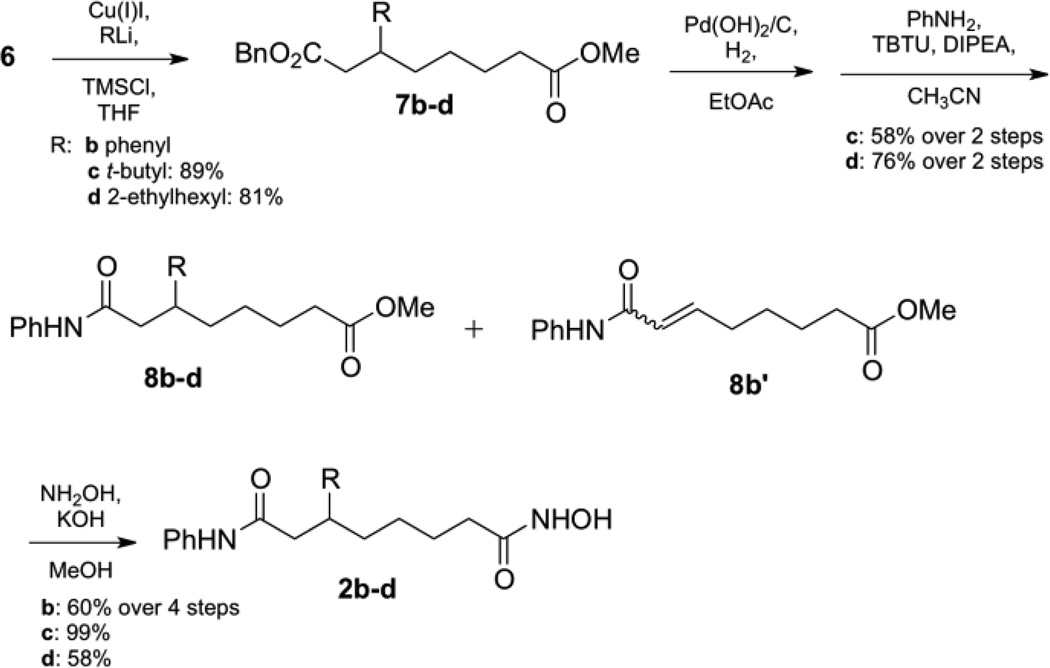
To create the remaining C6-SAHA analogs, purification by column chromatography by was required after 1,4-addition since the mixture of (E) and (Z) isomers 6 incompletely converted to ester 7 (Scheme 2). Using this additional purification step, the C3-t-butyl 2c and C3-2-ethylhexyl 2d SAHA analogs were synthesized as described for the methyl variant. Unfortunately, contaminating unsaturated ester 6 was present even after purification of phenyl ester 7b due to similar polarity. The mixture of unsaturated ester 6 and phenyl ester 7b were carried through the reaction series of hydrogenolysis and aniline coupling to give a mixture of anilide 8b and unsaturated anilide compound 8b´. Fortunately, after installation of the hydroxamic acid, final compound 2b was isolated.
Scheme 2.
Modified synthesis of C6-SAHA analog (1b–d).
HDAC inhibitory activities of the C6-SAHA analogs were measured using the Fluor de Lys® in vitro fluorescence activity assay kit (Enzo) using HeLa cell lysates as the source of HDAC activity (Table 1), as previously reported.16–17 The methyl and phenyl variants 2a and 2b were the most potent, displaying nanomolar IC50 values, which were only 4-fold reduced compared to SAHA. In addition, the 2-ethylhexyl variant 2d, which contained the longest substituent of the series, displayed potent inhibitory activity in the nanomolar range. The potent inhibition of these C6 analogs is in contrast to the C3-phenyl SAHA variant (Figure 1, IC50 of 73,000 nM), which displayed 811-fold reduced activity versus SAHA.16b The results indicate that the active site of HDAC proteins can accommodate a bulky substituent at the C6 position. Interestingly, the t-butyl variant 2c, which contains the bulkiest substituent with methyl groups on the α-carbon, displayed the weakest potency, which was 20-fold reduced compared to SAHA. In summary, the inhibition data show that most C6-SAHA analogs maintain nanomolar potency, but substitution at the α-carbon may decrease inhibitory activity.
Table 1.
HDAC inhibition by SAHA, MS-275, and the C6-SAHA analogs 2a–d using HeLa cell lysates
| Compounds | R | IC50, nMa |
|---|---|---|
| SAHA | 86 ± 4 | |
| MS-275 | 3160 ± 160 | |
| 2a | Methyl | 349 ± 28 |
| 2b | Phenyl | 344 ± 44 |
| 2c | t-Butyl | 1940 ± 300 |
| 2d | 2-Ethylhexyl | 456 ± 28 |
Values are the mean of at least three experiments with standard error given.
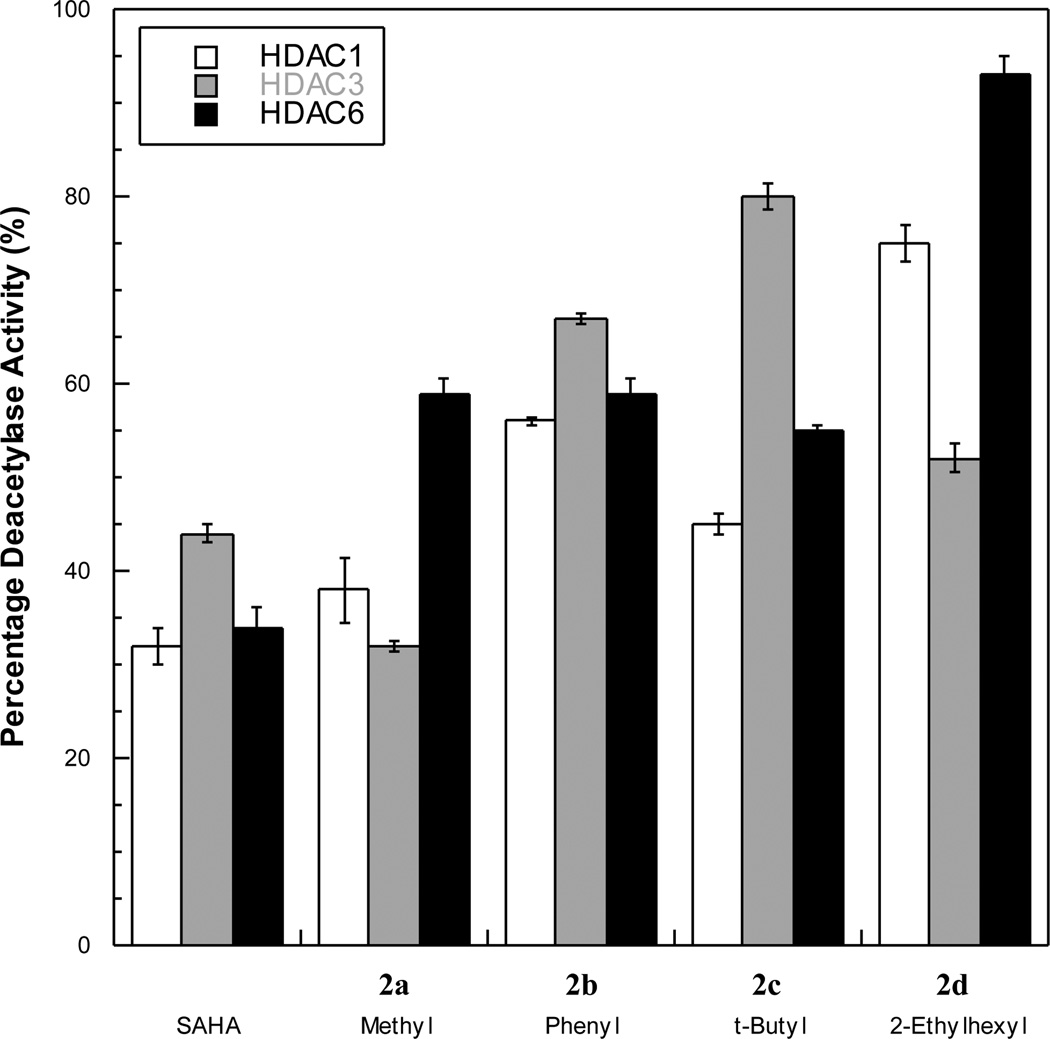
The C6-SAHA analogs were next evaluated for potency against individual HDAC isoforms- HDAC1 and HDAC3 representing class I and HDAC6 representing class II. All compounds were tested at a single concentration near their IC50 values using the Flour de Lys™ kit (Figure 2). Consistent with previous data,10, 16a SAHA exhibited roughly equal inhibition against HDAC1, HDAC3, and HDAC6. The phenyl variant 2b also similarly inhibited HDAC1, HDAC3, and HDAC6. In contrast, the methyl variant 2a showed modest dual-preference for HDAC1 and HDAC3 over HDAC6 at 500 nM. The 2-ethylhexyl variant 2d also showed preference for HDAC3 over HDAC1 and HDAC6. However, the bulkiest analog, the t-butyl variant 2c, displayed preference for HDAC1 and HDAC6 over HDAC3. The data indicate that the methyl, t-butyl, and 2-ethylhexyl variants (2a, 2c, and 2d) display modestly different preferences for each HDAC isoform while still maintaining nanomolar or low micromolar potency.
Figure 2.
Screen of C6-SAHA analogs against HDAC1, HDAC3, and HDAC6 with 125 nM SAHA, 500 nM 2a, 2b, and 2d, and 2 µM 2c.
To more thoroughly assess the selectivity observed in the initial screen, we determined the IC50 values of the C6-t-butyl variant 2c against HDAC1, HDAC3, and HDAC6. We selected the t-butyl analog because it showed the most potential to create a dual HDAC1/HDAC6-selective inhibitor, which would be useful for the treatment and study of acute myeloid leukemia.18 As expected based on the initial screen, the C6-t-butyl analog 2c displayed modest preference for HDAC1 and HDAC6 compared to HDAC3 (6-fold and 2-fold, respectively, Table 2). As a control, SAHA showed no selectivity, as expected (Table 2).10 The analysis shows that substituents on the C6 position modestly influence inhibitor selectivity and may promote creation of dual selective inhibitors.
Table 2.
IC50 values of SAHA and the C6-SAHA t-butyl variant 2c for HDAC1, HDAC3, and HDAC6
| Compound | IC50/µM | ||
|---|---|---|---|
| HDAC1 | HDAC3 | HDAC6 | |
| SAHA | 0.096 ± 0.02 | 0.136 ± 0.01 | 0.074 ± 0.009 |
| 2c | 0.99 ± 0.06 | 5.4 ± 0.7 | 2.4 ± 0.5 |
In conclusion, SAHA analogs containing substituents on the C6 position in the linker region can display nanomolar IC50 values, indicating the subsitutents near the solvent-exposed capping group are accommodated in the HDAC active site. In addition, C6-substituents can also modestly influence selectivity for individual HDAC isoforms. Combined with earlier studies of SAHA analogs substituted on the C2 and C3 positions (Figure 1),16 the data suggest that the linker region of HDAC inhibitors, particularly near the capping group, is an interesting yet underexplored area of future drug design.
Supplementary Material
Acknowledgments
We thank the National Institute of Health (GM067657) and Wayne State University for funding, S.V.W. Weerasinghe, P. P. Das, B. B. Parida, and Z. Wu for technical assistance, and G. Padige and M. Wambua for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Synthetic procedures, characterization of all compounds, and HDAC assay data are provided as supplementary material.
References and notes
- 1.Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 2004;338(1):17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Grozinger CM, Schreiber SL. Deacetylase enzymes: biological functions and the use of small-molecule inhibitors. Chem Biol. 2002;9(1):3–16. doi: 10.1016/s1074-5521(02)00092-3. [DOI] [PubMed] [Google Scholar]
- 3.Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006;5(1):37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- 4.Grant S, Easley C, Kirkpatrick P. Vorinostat. Nat Rev Drug Discov. 2007;6(1):21–22. doi: 10.1038/nrd2227. [DOI] [PubMed] [Google Scholar]
- 5.Khabele D, Son DS, Parl AK, Goldberg GL, Augenlicht LH, Mariadason JM, Rice VM. Drug-induced inactivation or gene silencing of class I histone deacetylases suppresses ovarian cancer cell growth: implications for therapy. Cancer Biol Ther. 2007;6(5):795–801. doi: 10.4161/cbt.6.5.4007. [DOI] [PubMed] [Google Scholar]
- 6.Song J, Noh JH, Lee JH, Eun JW, Ahn YM, Kim SY, Lee SH, Park WS, Yoo NJ, Lee JY, Nam SW. Increased expression of histone deacetylase 2 is found in human gastric cancer. APMIS. 2005;113(4):264–268. doi: 10.1111/j.1600-0463.2005.apm_04.x. [DOI] [PubMed] [Google Scholar]
- 7.Bartling B, Hofmann HS, Boettger T, Hansen G, Burdach S, Silber RE, Simm A. Comparative application of antibody and gene array for expression profiling in human squamous cell lung carcinoma. Lung Cancer. 2005;49(2):145–154. doi: 10.1016/j.lungcan.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Krennhrubec K, Marshall BL, Hedglin M, Verdin E, Ulrich SM. Design and evaluation of 'Linkerless' hydroxamic acids as selective HDAC8 inhibitors. Bioorg Med Chem Lett. 2007;17(10):2874–2878. doi: 10.1016/j.bmcl.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 9.Saji S, Kawakami M, Hayashi S, Yoshida N, Hirose M, Horiguchi SI, Itoh A, Funata N, Schreiber SL, Yoshida M, Toi M. Significance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancer. Oncogene. 2005;24(28):4531–4539. doi: 10.1038/sj.onc.1208646. [DOI] [PubMed] [Google Scholar]
- 10.Khan N, Jeffers M, Kumar S, Hackett C, Boldog F, Khramtsov N, Qian X, Mills E, Berghs SC, Carey N, Finn PW, Collins LS, Tumber A, Ritchie JW, Jensen PB, Lichenstein HS, Sehested M. Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem J. 2008;409(2):581–589. doi: 10.1042/BJ20070779. [DOI] [PubMed] [Google Scholar]
- 11.O'Connor OA, Heaney ML, Schwartz L, Richardson S, Willim R, MacGregor-Cortelli B, Curly T, Moskowitz C, Portlock C, Horwitz S, Zelenetz AD, Frankel S, Richon V, Marks P, Kelly WK. Clinical experience with intravenous and oral formulations of the novel histone deacetylase inhibitor suberoylanilide hydroxamic acid in patients with advanced hematologic malignancies. J Clin Oncol. 2006;24(1):166–173. doi: 10.1200/JCO.2005.01.9679. [DOI] [PubMed] [Google Scholar]
- 12.Bieliauskas AV, Pflum MKH. Isoform-selective histone deacetylase inhibitors. Chem Soc Rev. 2008;37(7):1402–1413. doi: 10.1039/b703830p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, Pavletich NP. Structure of a histone deacetylase homologue bound to trichostatin A. Nature. 1999;401:188–193. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]; (b) Vannini A, Volpari C, Filocamo G, Casavola EC, Brunetti M, Renzoni D, Chakravarty P, Paolini C, Francesco RD, Gallinari P, Steinkuhler C, Marco SD. Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proc Natl Acad Sci U S A. 2004;101(42):15064–15069. doi: 10.1073/pnas.0404603101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Somoza JR, Skene RJ, Katz BA, Mol C, Ho JD, Jennings AJ, Luong C, Arvai A, Buggy JJ, Chi E, Tang J, Sang B-C, Verner E, Wynands R, Leahy EM, Dougan DR, Snell G, Navre M, Knuth MW, Swanson RV, McRee DE, Tari LW. Structural Snapshots of Human HDAC8 Provide Insights into the Class I Histone Deacetylases. Structure. 2004;12:1324–1334. doi: 10.1016/j.str.2004.04.012. [DOI] [PubMed] [Google Scholar]; (d) Bottomley MJ, Lo Surdo P, Di Giovine P, Cirillo A, Scarpelli R, Ferrigno F, Jones P, Neddermann P, De Francesco R, Steinkuhler C, Gallinari P, Carfi A. Structural and Functional Analysis of the Human HDAC4 Catalytic Domain Reveals a Regulatory Structural Zinc-binding Domain. The Journal of biological chemistry. 2008;283(39):26694–26704. doi: 10.1074/jbc.M803514200. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Schuetz A, Min J, Allali-Hassani A, Schapira M, Shuen M, Loppnau P, Mazitschek R, Kwiatkowski NP, Lewis TA, Maglathin RL, McLean TH, Bochkarev A, Plotnikov AN, Vedadi M, Arrowsmith CH. Human HDAC7 Harbors a Class IIa Histone Deacetylase-specific Zinc Binding Motif and Cryptic Deacetylase Activity. The Journal of biological chemistry. 2008;283(17):11355–11363. doi: 10.1074/jbc.M707362200. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Watson PJ, Fairall L, Santos GM, Schwabe JW. Structure of HDAC3 bound to co-repressor and inositol tetraphosphate. Nature. 2012;481(7381):335–340. doi: 10.1038/nature10728. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Bressi JC, Jennings AJ, Skene R, Wu Y, Melkus R, Jong RD, O'Connell S, Grimshaw CE, Navre M, Gangloff AR. Exploration of the HDAC2 foot pocket: Synthesis and SAR of substituted N-(2-aminophenyl)benzamides. Bioorg Med Chem Lett. 2010;20(10):3142–3145. doi: 10.1016/j.bmcl.2010.03.091. [DOI] [PubMed] [Google Scholar]
- 14.(a) Hu E, Dul E, Sung C-M, Chen Z, Kirkpatrick R, Zhang G-F, Johanson K, Liu R, Lago A, Hofmann G, Macarron R, De Los Frailes M, Perez P, Krawiec J, Winkler J, Jaye M. Identification of Novel Isoform-Selective Inhibitors within Class I Histone Deacetylases. J Pharmacol Exp Ther. 2003;307(2):720–728. doi: 10.1124/jpet.103.055541. [DOI] [PubMed] [Google Scholar]; (b) Beckers T, Burkhardt C, Wieland H, Gimmnich P, Ciossek T, Maier T, Sanders K. Distinct pharmacological properties of second generation HDAC inhibitors with the benzamide or hydroxamate head group. Int J Cancer. 2007;121(5):1138–1148. doi: 10.1002/ijc.22751. [DOI] [PubMed] [Google Scholar]
- 15.Auzzas L, Larsson A, Matera R, Baraldi A, Deschênes-Simard B, Giannini G, Cabri W, Battistuzzi G, Gallo G, Ciacci A, Vesci L, Pisano C, Hanessian S. Non-Natural Macrocyclic Inhibitors of Histone Deacetylases: Design, Synthesis, and Activity. J. Med. Chem. 2010;53:8387–8399. doi: 10.1021/jm101092u. [DOI] [PubMed] [Google Scholar]
- 16.(a) Bieliauskas A, Weerasinghe S, Pflum MH. Structural Requirements of HDAC Inhibitors: SAHA Analogs Functionalized Adjacent to the Hydroxamic Acid. Bioorg Med Chem Lett. 2007;17(8):2216–2219. doi: 10.1016/j.bmcl.2007.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Choi SE, Weerasinghe SV, Pflum MK. The structural requirements of histone deacetylase inhibitors: Suberoylanilide hydroxamic acid analogs modified at the C3 position display isoform selectivity. Bioorg Med Chem Lett. 2011;21(20):6139–6142. doi: 10.1016/j.bmcl.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Guan P, Sun Fe, Hou X, Wang F, Yi F, Xu W, Fang H. Design, synthesis and preliminary bioactivity studies of 1,3,4-thiadiazole hydroxamic acid derivatives as novel histone deacetylase inhibitors. Bioorg Med Chem. 2012;20(12):3865–3872. doi: 10.1016/j.bmc.2012.04.032. [DOI] [PubMed] [Google Scholar]; (b) Lee C, Choi E, Cho M, Lee B, Oh SJ, Park S-K, Lee K, Kim HM, Han G. Structure and property based design, synthesis and biological evaluation of γ -lactam based HDAC inhibitors: Part II. Bioorg Med Chem Lett. 2012;22(12):4189–4192. doi: 10.1016/j.bmcl.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 18.Xu X, Xie C, Edwards H, Zhou H, Buck SA, Ge Y. Inhibition of Histone Deacetylases 1 and 6 Enhances Cytarabine-Induced Apoptosis in Pediatric Acute Myeloid Leukemia Cells. PLoS ONE. 2011;6(2):e17138. doi: 10.1371/journal.pone.0017138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.