Abstract
An isocratic simple rapid assay has been developed and validated for the determination of carbamazepine (CBZ) in both solution form and rabbit plasma using propylparaben as an internal standard. The assay was performed using a μ-Bondapak C18 (150 mm × 4.6 mm i.d) with a mobile phase consisting of methanol and water (50:50), the flow rate was 1 ml/min and UV detection at 285 nm. The method was found to be specific for CBZ, no interfering peaks were observed with an overall analytical run time of 15 min. Accuracy reported as % recovery were found to be 98.37–100.45% and 97.53–103.58% for inter-day and intra-day accuracies, respectively. Inter-day precision (reproducibility) was found to be 0.53–2.75% RSD, while intra-day precision (repeatability) was found to be 1.06–3.7% RSD for the samples studied. The calibration curve was found to be linear with the equation y = 0.2847x + 0.0138, with a correlation coefficient of 0.9999 (R2) over a concentration range of 0.5–40 μg/ml. The limit of quantitation was the lowest concentration. The method is simple and rapid and does not require any preliminary treatment of the sample. The method was fully validated.
Keywords: Carbamazepine, Validation, Rabbit plasma, HPLC method
1. Introduction
Carbamazepine (CBZ), 5-H-dibenze[b,f]azepine-5-carboxamide (Fig. 1), is a tricyclic lipophilic compound that is a first-choice antiepileptic drug for the treatment of simple and complex partial seizures (Duche and Loiseau, 1995). It is almost completely metabolized in the body and only small traces are excreted unchanged in urine (Goodman et al., 2001). Therapeutic concentrations have been reported to be 6–12 μg/ml, although considerable variations may arise (Goodman et al., 2001). The use of an established therapeutic range for CBZ concentration is limited by the presence of carbamazepine-10,11-epoxide (CBZ-E), its active metabolite that significantly contributes to the efficacy and toxicity and also possesses pharmacological activity as an anticonvulsant (He et al., 1992), though it reaches lower concentrations than CBZ.
Figure 1.
Chemical structure of carbamazepine and propylparaben (IS).
Various methods have been reported in the literature for the determination of CBZ, in particular those using chromatography (Bhatti et al., 1998; Dasgupta et al., 1999; Ashy et al., 1986; Raggi et al., 2000; Franceschi and Furlanut, 2005; Chelberg et al., 1988; Hartley et al., 1986; Oh et al., 2006; Yoshida et al., 2006; Elizabeth et al., 2007; Budakova et al., 2008; Hemenway et al., 2010). High-performance liquid chromatography (HPLC) and fluorescence polarization immunoassay (FPIA) are usually employed as routine techniques for the determination of this and other anticonvulsants (Sanchez et al., 1999). Other techniques such as micellar electrokinetic capillary chromatography (MECC) (Lancas et al., 2003) and chemiluminescence (Lee et al., 2003) were used. Very recently, two approaches were presented in the literature. One of them uses spectrophotometry and multivariate calibration (Rezaei et al., 2005) for the simultaneous determination of CBZ and phenytoin. The other method exploits the unusual fluorescence of CBZ on a nylon membrane (Escandar et al., 2004).
Simultaneous determinations of CBZ and its metabolites in biological fluids and drug products (Burke and Thenot, 1985; Owen et al., 2001) have been published, including, solid–liquid extraction (Wad, 1984), liquid–liquid extraction (Rouan et al., 1992), column-switching (Juergens, 1984), deproteinization (Liu et al., 1993), spectrofluorimetry method (Huang et al., 2002), gas–liquid chromatography (Chen and Bashi, 1991), FT-Raman spectroscopy (Auer et al., 2003), planar chromatography (Mennickent et al., 2009), stir bar-sorptive extraction and high-performance liquid chromatography-UV detection (SBSE/HPLC-UV) (Queiroz et al., 2008) and high-performance thin layer chromatography (HPTLC) (Patel et al., 2011). Also, LC–mass spectrometry methods (Breton et al., 2005; Van Rooyen et al., 2002; Miao and Metcalfe, 2003; Zhu et al., 2005) have been reported for the detection of CBZ and its metabolites in aquatic environments and in plasma. Only one article that focused on forced degradation of CBZ used acid, base, oxidation, heat and photolytic conditions. Much degradation was not observed in CBZ samples under stress conditions like acid hydrolysis photolysis and thermal exposure. However, mild degradation was observed during alkaline hydrolysis and significant degradation was observed when the drug was exposed to oxidation by hydrogen peroxide (Srinivasa and Belorkar, 2010). There is no article concerning complete analysis and validation of CBZ in rabbit plasma, So, the aim of this study was to establish a method based on HPLC-UV that is capable of analyzing CBZ in both solution and rabbit plasma in order to facilitate nanotechnology studies of CBZ.
2. Materials and methods
2.1. Materials
Carbamazepine was kindly supplied by Tabouk Pharmaceutical Company, Tabouk, Kingdom of Saudi Arabia. Methanol (HPLC grade) was purchased from BDH, Pool, England. Water for HPLC was purified from ELGA® PURELAB UHQ-II-MK3, HP143JH, UK. All other chemicals and solvents were of analytical reagent grade.
2.2. Equipment and chromatographic conditions
The study employed a high pressure liquid chromatography (Waters™ 1515 isocratic controller, Waters, USA) equipped with a Waters 2487 Dual λ Absorbance detector and an automatic sampling system (Waters™ 717). The mobile phase consisted of methanol and water (50:50), and the flow rate was 1 ml/min. Separation was achieved using a 150 mm × 4.6 mm (i.d.) C18, μ-Bondapak™, Waters, reversed phase column with an average particle size of 10 μm, and the column was kept at ambient temperature. The column effluent was monitored at 285 nm and the chromatographic data analysis was performed with the Empower™ Program (Waters, USA).
2.3. Stocks solutions and standards
Stock solutions of CBZ were prepared in triplicate by dissolving 50.0 mg CBZ in 50 ml methanol, resulting in a solution containing 1 mg/ml. This solution was diluted 20-fold by methanol to give working solution (50 μg/ml). A stock solution of the internal standard at a concentration of 1 mg/ml was prepared by dissolving 50.0 mg of propylparaben (Fig. 1) in 50 ml methanol. Working solutions of CBZ (50 μg/ml), and 250 μg/ml of propylparaben were prepared by dilution of the stock solutions in methanol.
Calibration curves were constructed in methanol by preparing a series of concentrations of the drug (0.5, 1, 2, 5, 10, 20 and 40 μg/ml) with the internal standard included at a fixed concentration of 10 μg/ml.
Calibration curves were also constructed in rabbit plasma. These involved replicate analysis of plasma samples spiked with varying concentrations of CBZ (0.5, 1, 2, 5, 10, 20 and 40 μg/ml) and a fixed concentration of the internal standard (10 μg/ml).
2.4. Treatment of plasma samples
The working solutions (100 μl) were separately transferred to clean dry centrifugation tubes. Rabbit plasma (500 μl) was added to each tube. These were vortex mixed for 2 min before adding 400 μl of methanol. These were vortex mixed before centrifugation at 5000 rpm for 5 min. The supernatant was separated and loaded into HPLC vials before injecting 50 μl into the HPLC system.
2.5. Method validation
The calibration curve of CBZ is a plot of the peak area ratio (PAR) of the drug to the internal standard as a function of the drug concentration (C). This gives the following equation: PAR = Slope × C + Intercept. The slope and the intercept are determined from the determined PAR and the nominal concentration of the drug. The unknown CBZ concentrations are determined from this equation.
The precision of the method based on intraday variability was determined by replicate analysis of the calibration standards in the same day. The reproducibility was taken as the interday variability and was determined by replicate analysis of the calibration standards in different days with one replicate being analyzed each day. The relative standard deviation values (RSD) were calculated from the ratios of the standard deviation (SD) to the mean and expressed as percentage.
The accuracy of the method was determined by comparing practical amounts recovered from the control samples with actual values present in the samples (theoretical values). The selectivity of the method was determined by examining the interference from the endogenous materials in rabbit plasma or from the degradation products of the drug.
The limit of quantification LOQ was taken as the lowest concentration that can be accurately (relative error <20% for biological samples and <2% for the in vitro samples) and precisely determined (RSD <20% for biological samples and <2% for the in vitro samples). The LOD for CBZ was found to be 0.25 μg/ml and this result is in agreement with data reported in Elizabeth et al. (2007). The drug is stable in liquid preparation and the assay was not validated as stability indicated (Srinivasa and Belorkar, 2010).
3. Results and discussion
3.1. HPLC assay of CBZ
HPLC with UV detection was chosen as a simple, fast, and effective separation method for the determination of CBZ (Bhatti et al., 1998; Dasgupta et al., 1999; Ashy et al., 1986; Raggi et al., 2000; Franceschi and Furlanut, 2005; Chelberg et al., 1988; Hartley et al., 1986; Oh et al., 2006; Yoshida et al., 2006; Elizabeth et al., 2007; Budakova et al., 2008; Hemenway et al., 2010). In extensive preliminary experiments, a series of aqueous mobile phases with different pH values in combination with different organic modifiers were tested. Best results were obtained when using methanol and water (50:50) and adjusting the pH of the solution to 5, allowing adequate separation of the drug and the internal standard using a C18 μ-Bondapak™ column at a flow-rate of 1.0 ml/min. In addition, depending on the weak plasma protein binding of both drug and IS, methanol was used for protein precipitation (1:1) in order to obtain satisfactory values for recovery of CBZ and IS. The selected chromatographic conditions provided optimum resolution of CBZ and IS.
Fig. 2 shows representative chromatograms of low and high concentrations of CBZ in methanol. The CBZ and the IS were eluted after 7.75 and 11.36 min, respectively. Using the chromatographic conditions described above, the selectivity was further evidenced by the ability of the assay to separate the drug and the internal standard from plasma samples without interference from any endogenous material with a good separation and resolution of their peaks. This is clearly indicated from Fig. 3.
Figure 2.
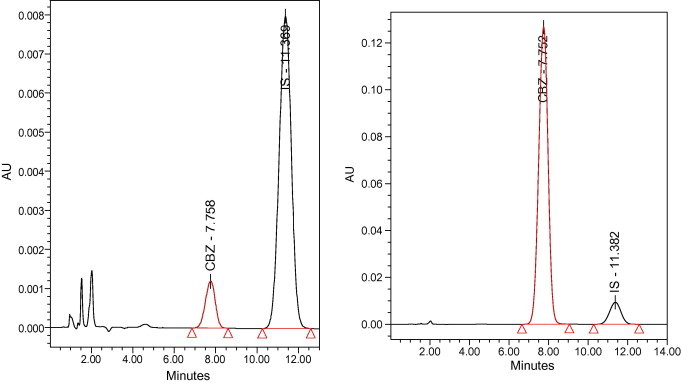
Representative chromatograms of carbamazepine showing 0.5 μg/ml as lower concentration (left) and 40 μg/ml as higher concentration (right). CBZ, carbamazepine; IS, internal standard.
Figure 3.
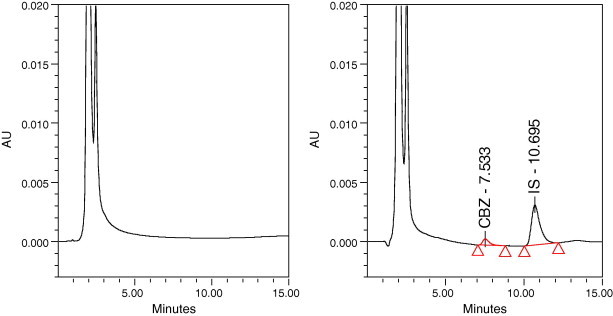
Representative chromatograms of blank plasma (left) and the plasma spiked with 0.5 μg/ml of carbamazepine and its internal standard (right).
3.2. Method validation
The linearity of the assay method was evaluated by seven-point standard curves; with concentration range selection of (0.5–40 μg/ml) based on drug concentration. These standards were analyzed in replicates of the range mentioned above. Standard curves were constructed over a 6-week period to determine the variability of the slopes and intercepts. The results show little day-to-day variability in the slopes and intercepts. The mean (±standard deviation; SD) regression equation for replicated calibration curves constructed on different days was determined using the least-squares linear regression analysis method. Standard calibration curves reflected good linearity of the assay in the concentration range of 0.5–40 μg/ml. The linear regression of the calibration curve produced an equation of (y = 0.2847x + 0.0138), with a correlation coefficient of 0.9999 (R2).
The interday and intraday accuracies were expressed as the closeness to the true value and are calculated as the percent recovery related to the nominal values. Tables 1 and 2 present the percentage of drug recovered relative to the nominal values. The recovered values were close to the true value suggesting the accuracy of the assay. The % recoveries of CBZ was ranged from 98.37% to 100.45% and 97.53% to 103.58% for interday and intraday, respectively. These high values of the % drug recovered reflect the accuracy of the assay method.
Table 1.
Validation parameters calculation for interday (n = 3).
| Nominal value (μg/ml) | Recovered conc. (μg/ml) | SD (μg/ml) | RSD% | % Recovery |
|---|---|---|---|---|
| 0.5 | 0.493 | 0.012 | 2.5 | 98.59429 |
| 1 | 0.984 | 0.027 | 2.75 | 98.36924 |
| 2 | 2.00 | 0.054 | 2.69 | 100.0095 |
| 5 | 4.99 | 0.14 | 2.75 | 99.76992 |
| 10 | 9.97 | 0.24 | 2.43 | 99.72529 |
| 20 | 19.70 | 0.20 | 0.99 | 98.48914 |
| 40 | 40.2 | 0.21 | 0.53 | 100.4535 |
Table 2.
Validation parameters calculation for intraday (n = 3).
| Nominal value (μg/ml) | Recovered conc. (μg/ml) | SD (μg/ml) | RSD% | % Recovery |
|---|---|---|---|---|
| 0.5 | 0.49 | 0.018 | 3.7 | 97.87 |
| 1 | 1.0 | 0.11 | 3.4 | 100.23 |
| 2 | 2.07 | 0.025 | 1.21 | 103.58 |
| 5 | 5.04 | 0.092 | 1.82 | 100.85 |
| 10 | 9.8 | 0.29 | 2.94 | 97.53 |
| 20 | 19.9 | 0.21 | 1.06 | 99.28 |
| 40 | 39.95 | 1.06 | 2.65 | 99.9 |
The interday and intraday precisions were measured as the relative standard deviation (RSD) expressed as percentage over the concentration range of CBZ during the course of validation. This is presented in Tables 1 and 2 for the interday and intraday precisions. The results indicated an acceptable precision for all concentrations assayed for both intraday and interday samples. The RSD% of CBZ ranged from 0.53% to 2.75% and 1.06% to 3.7% for both interday and intraday precisions, respectively. The low values of RSD% reflect the precision of the assay method.
The LOQ for this method was found to be 0.5 μg/ml. This is indicated from the precision and accuracy of such concentration. Both accuracy and precision values throughout the concentration range (0.5–40 μg/ml) were acceptable (ICH Guideline Q2 (R1), 2005). The specificity of the method with regard to CBZ was ascertained by the absence of any co-eluted or chromatographic interference peaks (Figs. 2 and 3).
4. Conclusion
The HPLC method developed in this article is rapid, sensitive, and specific. The accuracy and precision of the method are within the acceptable range (ICH Guideline Q2 (R1), 2005). The simplicity of technique and the high sensitivity make this technique particularly attractive for the quantification of CBZ in both solution and rabbit plasma. The method can also be readily adapted to routine quality control analysis.
Acknowledgment
This is work is fully supported by grant from National Plan for Science, Technology and Innovation (08NAN307-2).
References
- Ashy A.R., El Sayed Y.M., Islam S.I. Comparison of fluorescence polarization immunoassay and high performance liquid chromatography for the quantitative determination of phenytoin, phenobarbitone and carbamazepine in serum. J. Pharm. Pharmacol. 1986;38:572–577. doi: 10.1111/j.2042-7158.1986.tb03083.x. [DOI] [PubMed] [Google Scholar]
- Auer M.E., Griesser U.J., Sawatzki J. Qualitative and quantitative study of polymorphic forms in drug formulations by near infrared FT-Raman spectroscopy. J. Mol. Struct. 2003;661–662(1–3):307–317. [Google Scholar]
- Bhatti M.M., Hanson G.D., Schultz L. Simultaneous determination of phenytoin, carbamazepine, and 10,11-carbamazepine epoxide in human plasma by high-performance liquid chromatography with ultraviolet detection. J. Pharm. Biomed. Anal. 1998;16:1233–1240. doi: 10.1016/s0731-7085(97)00265-3. [DOI] [PubMed] [Google Scholar]
- Breton H., Cociglio M., Bressolle F., Peyriere H., Blayac J.P., Hillaire-Buys D. Liquid chromatography–electrospray mass spectrometry determination of carbamazepine, oxcarbazepine and eight of their metabolites in human plasma. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2005;828:80–90. doi: 10.1016/j.jchromb.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Budakova L., Brozmanova H., Grundmann M., Fischer J. Simultaneous determination of antiepileptic drugs and their two active metabolites by HPLC. J. Sep. Sci. 2008;31:1–8. doi: 10.1002/jssc.200700253. [DOI] [PubMed] [Google Scholar]
- Burke J.T., Thenot J.P. Determination of antiepileptic drugs. J. Chromatogr. 1985;340:199–241. doi: 10.1016/0378-4347(85)80198-5. [DOI] [PubMed] [Google Scholar]
- Chelberg R.D., Gunawan S., Treiman D.M. Simultaneous high-performance liquid-chromatographic determination of carbamazepine and its principal metabolites in human plasma and urine. Ther. Drug Monit. 1988;10:188–193. doi: 10.1097/00007691-198802000-00013. [DOI] [PubMed] [Google Scholar]
- Chen K., Bashi H.K. Comparative analysis of antiepileptic drugs by gas chromatography using capillary or packed columns and by fluorescence polarization immunoassay. J. Anal. Toxicol. 1991;15(2):82–85. doi: 10.1093/jat/15.2.82. [DOI] [PubMed] [Google Scholar]
- Dasgupta A., Vega A.E., Wells A., Datta P. Effect of heating human sera at a temperature necessary to deactivate human immunodeficiency virus on measurement of free phenytoin, free valproic acid, and free carbamazepine concentrations. Ther. Drug Monit. 1999;21:421–425. doi: 10.1097/00007691-199908000-00007. [DOI] [PubMed] [Google Scholar]
- Duche P., Loiseau B. In: Antiepileptic Drugs. fourth ed. Levy R.H., Mattson R.H., Meldrum B.S., Penry J.K., Dreyfuss F.E., editors. Raven; New York: 1995. p. 555. [Google Scholar]
- Elizabeth G.-S., Darla R.L., Mohamed A.V., Matthew D.K. Simultaneous determination of lamotrigine, zonisamide, and carbamazepine in human plasma by high-performance liquid chromatography. Biomed. Chromatogr. 2007;21(3):225–228. doi: 10.1002/bmc.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escandar G.M., González Gómez D., Espinosa Mansilla A., Muñoz de la Peña A., Goicoechea H.C. Determination of carbamazepine in serum and pharmaceutical preparations using immobilization on a nylon support and fluorescent detection. Anal. Chim. Acta. 2004;506:161–170. [Google Scholar]
- Franceschi L., Furlanut M. A simple method to monitor plasma concentration of oxcarbamazepine, carbamazepine, their main metabolites and lamotrignine in epileptic patients. Pharmacol. Res. 2005;51:297–302. doi: 10.1016/j.phrs.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Goodman A.G., Hardman J.G., Limbrid L.E. 10th ed. McGraw Hill; New York: 2001. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. [Google Scholar]
- Hartley R., Lucock M., Cookman J.R., Becker M., Forsythe W.I. High-performance liquid-chromatographic determination of carbamazepine and carbamazepine-10,11-epoxide in plasma and saliva following solidphase sample extraction. J. Chromatogr. 1986;380:347–356. doi: 10.1016/s0378-4347(00)83663-4. [DOI] [PubMed] [Google Scholar]
- He J., Shibukawa A., Nakagawa T. Direct injection analysis of carbamazepine and its active 10,11-epoxide metabolite in plasma by use of a semipermeable surface (SPS) silica column in LC. J. Pharm. Biomed. Anal. 1992;10(4):289–294. doi: 10.1016/0731-7085(92)80041-k. [DOI] [PubMed] [Google Scholar]
- Hemenway J.N., Jarho P., Henri J.T., Nair S.K., Velde D.V., Georg G.I., Stella V.J. Preparation and physicochemical characterization of a novel water-soluble prodrug of carbamazepine. J. Pharm. Sci. 2010;99(4):1810–1825. doi: 10.1002/jps.21952. [DOI] [PubMed] [Google Scholar]
- Huang C., He Q., Chen H. Flow injection photochemical spectrofluorimetry for the determination of carbamazepine in pharmaceutical preparations. J. Pharm. Biomed. Anal. 2002;30(1):59–65. doi: 10.1016/s0731-7085(02)00200-5. [DOI] [PubMed] [Google Scholar]
- ICH Guideline Q2 (R1), 2005. Validation of Analytical Procedures: Text and Methodology.
- Juergens U. Routine determination of eight common anti-epileptic drugs and metabolites by high-performance liquid chromatography using a column-switching system for direct injection of serum samples. J. Chromatogr. 1984;310:97–106. doi: 10.1016/0378-4347(84)80071-7. [DOI] [PubMed] [Google Scholar]
- Lancas F.M., Sozza M.A., Queiroz M.E.C. Simultaneous plasma lamotrignine analysis with carbamazepine, carbamazepine-10,11-epoxide, primidone, phenytoin, phenobarbital, and PEMA by micellar electrokinetic capillary chromatography (MECC) J. Anal. Toxicol. 2003;27:304–308. doi: 10.1093/jat/27.5.304. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Li M., Suh J.K. Determination of carbamazepine by chemiluminescence detection using chemically prepared tris(2,2V-bipyridine)ruthenium(III) as oxidant. Anal. Sci. 2003;19:903–906. doi: 10.2116/analsci.19.903. [DOI] [PubMed] [Google Scholar]
- Liu H., Delgado M., Forman L.J., Eggers C.M., Montoya J.L. Simultaneous determination of carbamazepine, phenytoin, phenobarbital, primidone and their principal metabolites by high-performance liquid chromatography with photodiode-array detection. J. Chromatogr. 1993;616:105–115. doi: 10.1016/0378-4347(93)80477-l. [DOI] [PubMed] [Google Scholar]
- Mennickent S., Fierro R., Vega M., de Diego M., Godoy C.G. Instrumental planar chromatographic method for determination of carbamazepine in human serum. J. Sep. Sci. 2009;32(9):1454–1458. doi: 10.1002/jssc.200800675. [DOI] [PubMed] [Google Scholar]
- Miao X.-S., Metcalfe C.D. Determination of carbamazepine and its metabolites in aqueous samples using liquid chromatography–electrospray tandem mass spectrometry. Anal. Chem. 2003;75:3731–3738. doi: 10.1021/ac030082k. [DOI] [PubMed] [Google Scholar]
- Oh E.K., Ban E., Woo J.S., Kim C.-K. Analysis of carbamazepine and its active metabolite, carbamazepine-10,11-epoxide, in human plasma using high-performance liquid chromatography. Anal. Bioanal. Chem. 2006;386:1931–1936. doi: 10.1007/s00216-006-0724-7. [DOI] [PubMed] [Google Scholar]
- Owen A., Tettey J.N., Morgan P., Pirmonhamed M., Park B.K. LC determination of carbamazepine in murine brain. J. Pharm. Biomed. Anal. 2001;26(4):573–577. doi: 10.1016/s0731-7085(01)00477-0. [DOI] [PubMed] [Google Scholar]
- Patel R.B., Patel M.R., Bhatt K.K., Patel B.G. Development and validation of HPTLC method for estimation of carbamazepine in formulations and its in vitro release study. Chromatogr. Res. Int. 2011 8pp. [Google Scholar]
- Queiroz R.H.C., Bertucci C., Malfará W.R., Dreossi S.A.C., Chaves A.R., Valério D.A.R., Queiroz M.E.C. Quantification of carbamazepine, carbamazepine-10,11-epoxide, phenytoin and phenobarbital in plasma samples by stir bar-sorptive extraction and liquid chromatography. J. Pharm. Biomed. Anal. 2008;48:428–434. doi: 10.1016/j.jpba.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Raggi M.A., Casamenti G., Mandrioli R., Sabbioni C., Volterra V. A rapid LC method for the identification and determination of CNS drugs in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2000;23:161–167. doi: 10.1016/s0731-7085(00)00265-x. [DOI] [PubMed] [Google Scholar]
- Rezaei Z., Hemmateenejad B., Khabnadideh S., Gorgin M. Simultaneous spectrophotometric determination of carbamazepine and phenytoin in serum by PLS regression and comparison Ruth HPLC. Talanta. 2005;65:21–28. doi: 10.1016/j.talanta.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Rouan M.C., Campestrini J., Le Clanche V., Lecaillon J.B., Godbillon J. Automated microanalysis of carbamazepine and its epoxide and trans-diol metabolites in plasma by column liquid chromatography. J. Chromatogr. 1992;573(1):65–68. doi: 10.1016/0378-4347(92)80475-6. [DOI] [PubMed] [Google Scholar]
- Sanchez A., Garcia R., Abadin J.A., Duran J.A. Determination of free serum carbamazepine by protein precipitation with sulfosalicylic acid. Pharm. Pharmacol. Commun. 1999;5:435–438. [Google Scholar]
- Srinivasa R.K., Belorkar N. Development and validation of a specific stability indicating liquid chromatographic method for carbamazepine in bulk and pharmaceutical dosage forms. J. Adv. Pharm. Res. 2010;1:36–47. [Google Scholar]
- Van Rooyen G.F., Badenhorst D., Swart K.J., Hundt H.K., Scanes T., Hundt A.F. Determination of carbamazepine and carbamazepine-10,11-epoxide in human plasma by tandem liquid chromatography–mass spectrometry with electrospray ionisation. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2002;769(1):1–7. doi: 10.1016/s1570-0232(01)00590-6. [DOI] [PubMed] [Google Scholar]
- Wad N.J. Simultaneous determination of eleven antiepileptic compounds in serum by high-performance liquid chromatography. J. Chromatogr. 1984;305:127–133. doi: 10.1016/s0378-4347(00)83320-4. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Imai K., Motohashi S., Hamano S., Sato M. Simultaneous determination of zonisamide, carbamazepine, carbamazepine-10,11-epoxide in infant serum by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2006;41:1386–1390. doi: 10.1016/j.jpba.2006.02.044. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Chiang H., Wulster-Radcliffe M., Hilt R., Wong P., Kissinger C.B., Kissinger P.T. Liquid chromatography/tandem mass spectrometry for the determination of carbamazepine and its main metabolite in rat plasma utilizing an automated blood sampling system. J. Pharm. Biomed. Anal. 2005;38(1):119–125. doi: 10.1016/j.jpba.2004.11.058. [DOI] [PubMed] [Google Scholar]


