Abstract
Objective:
To assess the properties of adrenal lesions with and without known primary cancer and investigate predictors for differential diagnosis between benign and malignant adrenal enlargement.
Methods:
This retrospective study used fluorine-18 fludeoxyglucose positron emission tomography (PET)/CT in 325 patients with adrenal lesions (229 with known primary cancer and 96 without primary cancer). Age, sex, the presence of right and left masses, nodules or hyperplasia, unenhanced attenuation, maximum standardised uptake value (SUVmax) ratio, and the presence of metastasis in other body parts and locations of the primary cancer were assessed. Univariate and multivariate analyses were used to assess variables associated with risk of adrenal metastasis.
Results:
Patients with adrenal metastasis vs those without had a higher frequency of primary lung cancer (52.3% vs 30.7%) but a lower frequency of gastrointestinal cancer (7.9% vs 16.6%). The frequency of other abnormalities, including adenoma and hyperplasia, was similar between patients with and without known primary cancer. A higher proportion of patients with adrenal metastasis regardless of primary cancer site were younger, had a nodule or a mass, had an unenhanced attenuation of >10 HU, had an SUVmax ratio of >2.5, and had metastasis in other body parts. Analysis found independent associations of age, unenhanced attenuation of >10 HU, SUVmax ratio of >2.5 and the presence of metastasis in other body parts with adrenal metastasis. The combination of the four variables was strongly associated with adrenal metastasis.
Conclusion:
PET/CT was useful in characterising adrenal lesions as benign or malignant and helpful in identifying adrenal metastasis and cancer severity.
Advances in knowledge:
PET/CT can help in the differential diagnosis between benign and malignant adrenal enlargement.
The adrenal gland is a common site of metastasis in patients with cancer. Up to 50% of adrenal lesions in patients with known primary non-adrenal cancers are malignant disease [1–4]. The most common malignant lesions that metastasise to the adrenal gland include lung, liver, colon, lymphoma, melanoma, breast, kidney, oesophagus, pancreas and stomach cancer [4–6]. However, diagnosis of an adrenal lesion as malignant or benign can be problematic. Characterisation of these adrenal lesions is therefore critical to stage the primary disease, direct therapy and predict prognosis. Although CT and MRI are typically used to characterise a lesion, a small but important number of adrenal lesions are found to be indeterminate on cross-sectional images [7–9].
Several reports have documented the effectiveness of stand-alone fluorine-18 fludeoxyglucose (18F-FDG) positron emission tomography (PET) to differentiate benign from malignant adrenal lesions [8–10]. Interest has focused on the ability of integrated in-line PET/CT to definitively characterise these lesions given that this technique combines the anatomical and densitometrical applications of CT and the functional and metabolic advantages of PET. Several studies have reported PET/CT's high sensitivity, specificity and accuracy for detecting adrenal metastatic lesions [7–10].
PET/CT can also be used as a non-invasive method to help assess the lesion, facilitating diagnosis and treatment decisions. The purpose of our study was to investigate whether PET/CT can reliably detect differences between malignant and benign lesions, tumour characteristics associated with the location of the primary cancer and predictors for adrenal metastasis.
METHODS AND MATERIALS
Our study was approved by Institutional Review Board of local general hospitals, and the requirement of informed consent was waived owing to the retrospective nature of our study.
Patient population
From January 2006 to July 2012, 325 consecutive patients had adrenal lesions as identified by an initial integrated in-line PET/CT that were diagnosed as adrenal metastasis (for those with known primary cancers) or adenoma and hyperplasia by examination 6–30 months after the initial PET/CT. The mean follow-up period was 18 months. During the follow-up period, we characterised the lesions as either benign or malignant.
Patients whose lesions were diagnosed as myelolipomas by the presence of macroscopic fat were excluded from our study, as were patients who had been treated for malignant or benign lesions in the adrenal gland, had diabetes or any other disorder affecting glucose metabolism.
PET/CT techniques
Patients fasted for at least 6 h before PET/CT. Blood glucose was measured 1 h before injection of 18F-FDG and was ideally less than 150 mg dL−1. The used 18F-FDG dose was 10–12 mCi (370–444 MBq), with 1 h uptake. Imaging was acquired with an integrated in-line PET/CT system (Discovery™ ST; GE Healthcare, Waukesha, WI). Unenhanced CT from the base of the skull to the upper thigh was performed for attenuation correction and diagnosis (300 mA; tube rotation time 0.5 s; 120 kVp; table speed 13.5 mm per rotation; beam collimation 8×1.25 mm). Axial CT images were reconstructed with a soft reconstruction kernel with a slice thickness of 3.75 mm and an interval of 3.27 mm to match the PET images.
The PET images were obtained in the two-dimensional mode for 3 min per bed position, and the images were reconstructed with standard vendor-provided reconstruction algorithms incorporating ordered subset expectation maximisation. Attenuation correction of PET images was performed with attenuation data from the CT component of the examination. The manufacturer's software was used to correct emission data for scatter, random events and dead-time losses.
PET/CT analysis
The PET/CT components were reviewed on a high-resolution workstation (Marosis; Infinity, Seoul, Republic of Korea). The PET and fused CT images were analysed in both axial and coronal planes. Two radiologists and one nuclear medicine physician reviewed the images, and decisions were reached by consensus.
The CT component measured the size of the adrenal lesion. The adrenal lesion unenhanced attenuations were measured from the unenhanced attenuation correction CT by taking the mean of two measurements from a region of interest (ROI). The ROI covered from one-half to two-thirds of the surface area of the lesion, avoiding adjacent retroperitoneal fat and inhomogeneous areas.
The PET and fused CT images were used to measure the average standardised uptake value (SUV) over an ROI placed on the liver and the adrenal lesion. The PET/CT images reconstructed in the coronal and axial planes were used to confirm accurate placement of the ROI on the adrenal gland. The ROI included at least two-thirds of the adrenal lesion. A similar-sized ROI was placed within the right hepatic lobe free from the detectable metastatic lesion. Care was taken to avoid the periphery of the lesion, thereby minimising the partial volume effect. Maximum SUV (SUVmax) and average SUV (SUVavg) were generated by the software with the equation SUV=Ctis/Dinj/body weight, in which SUV is normalised for body weight in kilograms, Ctis is tissue concentration in megabecquerels per milliliter and Dinj is the injected dose in megabecquerels. The adrenal SUVmax ratio was divided by the liver SUVavg to calculate a ratio (SUVratio) for each lesion.
Standard reference
The results of follow-up images were all used as reference standards for final characterisation of the adrenal lesion in our study. Lesions that remained stable in size for more than 6 months at follow up were considered benign. Criteria for malignancy included an interval increase in size or more than 20% decrease in size after appropriate therapy. The mean follow-up period for lesions was 18 months. As this study is a retrospective examination and analysis of the clinical data, there were a variety of follow-up methods, such as contrast-enhanced CT, MRI and PET/CT.
Statistical analysis
Age was shown as mean ± standard deviation and compared between groups by independent Student's t-test. Other categorical variables were shown as proportions, and the association between categorical variables was compared with Fisher's exact test. The variables associated with adrenal metastasis were shown by their odds ratios with the 95% confidence interval (CI) in the univariate and multivariate logistic regression models.
The cut-off point of unenhanced attenuation (10 HU) in the prediction of adrenal metastasis was determined by the commonly accepted value for distinguishing between benignancy and malignancy, and the cut-off point of SUVmax ratio (2.5) was determined by the Youden's index (the maximum of sensitivity and specificity-1) in the receiver operating characteristic curves analysis. The variables with p-values <0.05 were included in a multivariate logistic regression model, selected by a forward conditional method. Statistical analysis was performed using appropriate software (SPSS® v. 18.0 for Windows; SPSS, Chicago, IL). Null hypotheses of no difference were rejected if the p-value was <0.05 or, equivalently, if the 95% CI of odds ratio estimates excluded 1.
RESULTS
Patient demography analysis
PET/CT was performed on 10 750 patients, including 3562 patients with primary cancer and 7188 patients without primary cancer. Of these, 325 patients had adrenal lesions, 229 of whom had known primary cancer and 96 of whom did not have known primary cancer. Among the 325 patients with adrenal lesions, 28 were histologically diagnosed; of these, 21 were diagnosed as having malignant lesions and the remaining 7 were diagnosed as having benign lesions.
Patients' demographics and PET/CT characteristics are summarised in Table 1. The presence of lesions in both groups of patients was not associated with age or sex, and the majority of patients in both groups were males. Over half of the patients with primary cancer had a metastatic lesion. The types of primary cancers included lung (44.9%), gastrointestinal (13.5%), liver (10.9%) and others (30.7%).
Table 1.
Patients' demographics and PET/CT characteristics
| Parameters | Findings | With known primary cancer (n=229) | Without known primary cancer (n=96) | p-value |
| Age (years) | 59.5±14.2 | 54.3±16.5 | 0.250 | |
| Sex (male/female) | 146/67 | 88/34 | 0.380 | |
| Adrenal lesions | Metastasis | 163 (71.2%) | 0 (0.0%) | <0.001 |
| Benign | 66 (28.8%) | 96 (100.0%) | ||
| Primary cancer | Lung cancer | 103 (44.9%) | — | |
| Gastrointestinal cancer | 31 (13.5%) | — | ||
| Liver cancer | 25 (10.9%) | — | ||
| Others | 70 (30.7%) | — | ||
| PET/CT | ||||
| Left adrenal gland | Mass | 22 (9.6%) | 2 (2.0%) | 0.026 |
| Nodule | 78 (34.0%) | 44 (45.8%) | ||
| Hyperplasia | 62 (27.2%) | 32 (33.3%) | ||
| Normal | 67 (29.2%) | 18 (18.9%) | ||
| Right adrenal gland | Mass | 17 (7.45%) | 1 (1.0%) | 0.024 |
| Nodule | 43 (18.7%) | 17 (17.8%) | ||
| Hyperplasia | 27 (11.8%) | 15 (15.6%) | ||
| Normal | 142 (62.0%) | 63 (65.6%) | ||
| Unenhanced attenuation | >10 HU | 148 (64.6%) | 12 (8.4%) | <0.001 |
| ≤10 HU | 81 (36.4%) | 88 (91.6%) | ||
| SUVmax ratio | >2.5 | 52 (22.7%) | 3 (3.1%) | <0.001 |
| ≤2.5 | 109 (47.5%) | 25 (26.0%) | ||
| Absence of uptake | 68 (29.8%) | 68 (70.9%) | ||
| Unknown | — | 96% (100.0%) | ||
PET, positron emission tomography; SUVmax, maximum standardised uptake value.
The proportion of patients with a mass in both adrenal glands was small in both groups but was significantly higher for the patients with known primary cancer. The proportion of lesions that were nodule, hyperplasia or normal was similar in both groups. Unenhanced attenuation >10 HU and SUVmax ratios >2.5 were significantly higher in patients with known primary cancer than in those without. More patients without known primary cancer had evidence of no 18F-FDG uptake.
Analysis of characteristics in patients with known primary cancer
The characteristics of patients with known primary cancer and categorisation by primary cancer types are summarised in Table 2. Patients with adrenal metastasis were younger, more often had nodules or masses and more often both unenhanced attenuation >10 HU and an SUVmax ratio >2.5. As would be expected, more patients with adrenal metastasis had metastasised tumours in other parts of their body. Patients with adrenal metastasis most often had primary lung cancer and less often had primary tumours in the gastrointestinal tract and the liver. Patients without adrenal metastasis had a higher rate of gastrointestinal cancer. Those with adrenal metastasis and a gastrointestinal cancer primary were significantly younger than those without adrenal metastasis.
Table 2.
Characteristics in patients with known primary cancer and categorisation by primary cancer type
| Parameters | Findings | Adrenal metastasis | |||||||||||
| All patients with known primary cancer (n=229) | Patients with lung cancer (n=103) | Patients with gastrointestinal cancer (n=31) | Patients with liver cancer (n=25) | ||||||||||
| Yes (n=151) | No (n=78) | p-value | Yes (n=79) | No (n=24) | p-value | Yes (n=13) | No (n=18) | p-value | Yes (n=12) | No (n=13) | p-value | ||
| Age (years) | 54.8±12.5 | 65.5±11.7 | 0.028 | 59.2±13.0 | 62.4±11.7 | 0.090 | 56.1±12.7 | 64.8±10.4 | 0.010 | 60.4±9.9 | 57.3±11.2 | 0.160 | |
| Left adrenal | Nodule or mass | 76 (50.3%) | 24 (30.7%) | 0.003 | 36 (45.5%) | 8 (33.3%) | 0.125 | 6 (46.1%) | 7 (38.8%) | 0.381 | 5 (41.6%) | 7 (53.8%) | 0.580 |
| Right adrenal | Nodule or mass | 49 (32.4%) | 11 (14.1%) | 0.009 | 19 (24.0%) | 5 (20.8%) | 0.430 | 4 (30.7%) | 5 (27.7%) | 0.600 | 5 (41.6%) | 9 (69.2%) | 0.057 |
| Unenhanced | ≤10 HU | 11 (8.8%) | 31 (89.9%) | 0.001 | 5 (9.0%) | 10 (76.9%) | 0.009 | 2 (20.0%) | 11 (91.6%) | 0 | 5 | ||
| Attenuation | >10 HU | 114 (91.2%) | 4 (11.1%) | 0.001 | 50 (91.0%) | 3 (23.1%) | 0.010 | 8 (80.0%) | 1(8.4%) | 6 | 0 | ||
| SUVmax ratio | ≤2.5 | 68 (45.0%) | 41 (52.5%) | 29 (36.7%) | 5 (20.8%) | 8 (61.5%) | 6 (33.3%) | 0 (0.0%) | 0 (0.0%) | ||||
| >2.5 | 50 (33.1%) | 2 (2.1%) | 32 (40.5%) | 1 (4.1%) | 5 (38.4%) | 0 (0.0%) | 6 (50.0%) | 5 (38.4%) | |||||
| No 18F-FDG uptake | 9 (5.9%) | 59 (75.6%) | <0.001 | 3 (3.7%) | 15 (62.5%) | <0.001 | 1 (7.6%) | 11 (61.8%) | <0.001 | 1 (8.3%) | 7 (53.8%) | <0.001 | |
| Metastasis in other parts | Yes | 121 (73.5%) | 24 (29.4%) | <0.001 | 62 (74.6%) | 9 (29.1%) | <0.001 | 11 (84.6%) | 9 (50.0%) | 0.008 | 8 (58.3%) | 4 (23.0%) | 0.214 |
| No | 30 (13.2%) | 54 (66.6%) | 17 (18.9%) | 15 (54.1%) | 2 (15.3%) | 9 (50.0%) | 4 (0.25%) | 9 (61.5%) | |||||
| Primary | Lung cancer | 77 (49.0%) | 26 (37.1%) | 0.002 | |||||||||
| Gastrointestinal cancer | 15 (7.9%) | 16 (24.3%) | 0.014 | ||||||||||
| Liver cancer | 13 (7.2%) | 12 (17.9%) | 0.174 | ||||||||||
| Other | 46 (28.4%) | 24 (34.6%) | 0.156 | ||||||||||
18F-FDG, flourine-18 fludeoxyglucose; SUVmax, maximum standardised uptake value.
Univariate analysis of predictors of adrenal metastasis
The results of univariate analysis for predicting adrenal metastasis in patients with known primary cancer and categorisation by primary cancer are summarised in Table 3. Age, presence of left and right nodules or masses, unenhanced attenuation, SUVmax ratio, and metastasis in other parts and locations of the primary cancer are significantly associated with adrenal metastasis. However, only age, unenhanced CT attenuation, SUVmax ratio and the presence of metastasis in other parts were associated with adrenal metastasis after stepwise forward selection.
Table 3.
Univariate analysis for predicting adrenal metastasis in patients with known primary cancer and categorisation by primary cancer type
| Parameters | Findings | All patients with known primary tumour (n=229) | Patients with gastrointestinal cancer (n=103) | Patients with lung cancer (n=31) | Patients with liver cancer (n=25) | ||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Age (years) | 0.96 (0.94, 0.98) | 0.024 | 0.95 (0.94, 1.00) | 0.142 | 0.91 (0.86, 0.99) | 0.028 | 1.05 (0.97, 1.18) | 0.176 | |
| Left adrenal | Nodule or mass | 2.31 (1.34, 3.95) | 0.042 | 2.15 (0.88, 5.30) | 0.093 | 2.04 (0.51, 8.24) | 0.317 | 1.68 (0.31, 9.01) | 0.553 |
| Right adrenal | Nodule or mass | 2.38 (1.29, 4.21) | 0.038 | 1.35 (0.46, 3.58) | 0.631 | 1.45 (0.35, 7.29) | 0.638 | 9.07 (0.86, 97.53) | 0.068 |
| Unenhanced | ≤10 HU | 3.25 (1.54, 9.26) | <0.001 | 2.99 (1.34, 9.06) | <0.001 | 12.57 (1.42, 86.51) | 0.031 | NA | |
| Attenuation | >10 HU | 414.5 (34.1, 3712.1) | <0.001 | 321.5 (12.1, 2095.1) | <0.001 | 6.54 (1.01, 78.42) | 0.044 | NA | |
| SUVmax ratio | ≤2.5 | 15.4 (6.3, 38.2) | <0.001 | 39.4 (7.3, 210.8) | <0.001 | 17.5 (1.4, 171.5) | 0.015 | 8.30 (0.76, 93.22) | 0.085 |
| >2.5 | 509.8 (58.2, 4215.4) | <0.001 | 321.0 (27.2, 3712.8) | <0.001 | NA | NA | |||
| Metastasis in other parts | Yes | 5.35 (2.05, 8.99) | <0.001 | 5.05 (2.09, 12.51) | <0.001 | 14.2 (1.6, 129.2) | 0.019 | 3.68 (0.88, 23.23) | 0.141 |
| Primary | Lung cancer | 2.52 (1.32, 4.93) | 0.004 | ||||||
| Gastrointestinal cancer | 0.68 (0.32, 1.43) | 0.180 | |||||||
| Liver cancer | 0.71 (0.32, 1.81) | 0.006 | |||||||
| Other | — | ||||||||
NA, not applicable; OR, odds ratio; SUVmax, maximum standardised uptake value; 95% CI, confidence interval. Reference groups indicate that the corresponding variable had a significant influence on adrenal metastasis. NA indicates that the odds ratio was not performed owing to small or zero count.
The results of multivariate analysis for variables associated with adrenal metastasis are summarised in Table 4. After controlling for the unenhanced attenuation, SUVmax ratio and metastasis to other parts, the risk for developing adrenal metastasis decreased slightly by each year of increasing age. Controlling for age, unenhanced attenuation and SUVmax ratio, patients with metastasis to other parts had a significantly higher risk of adrenal metastasis than those with no adrenal metastasis. After controlling for the other two variables, unenhanced attenuation >10 HU and an SUVmax ratio >2.5 raised the risk of adrenal metastasis significantly. This relationship held consistently across the major sites of lung and gastrointestinal cancer, although numbers were too small for significance with liver cancer.
Table 4.
Impact variables of adrenal metastasis by multivariate analysis
| Parameters | Findings | All patients with known primary tumoura (n=229) | Patients with lung cancerb (n=103) | Patients with gastrointestinal cancerc (n=31) | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Age (years) | 0.97 (0.94, 0.99) | 0.021 | NA | 0.87 (0.78, 1.00) | 0.070 | ||
| Unenhanced | >10 HU | 2.62 (1.23, 8.53) | <0.001 | 2.10 (1.24, 8.76) | <0.001 | 1.27 (0.29, 5.01) | 0.638 |
| Attenuation | ≤10 HU | 356.1 (29.8, 3213.2) | <0.001 | 278.3 (10.9, 1825.8) | <0.001 | 12.0 (1.3, 57.5) | 0.035 |
| SUVmax ratio | ≤2.5 | 13.9 (5.3, 36.8) | <0.001 | 35.8 (5.9, 194.6) | <0.001 | 21.7 (1.5, 280.5) | 0.020 |
| >2.5 | 471.5 (53.9, 4240.2) | <0.001 | 310.7 (25.4, 3750.7) | <0.001 | NA | ||
| Metastasis in other parts | Yes | 4.41 (1.74, 9.22) | < 0.001 | 4.07 (1.04, 14.23) | 0.042 | NA | |
NA, not applicable; OR, odds ratio, SUVmax, maximum standardised uptake value; 95% CI, confidence interval. Reference group indicates that the corresponding variable had a significant influence on adrenal metastasis. NA indicates that the odds ratio was not performed owing to small or zero count.
p=0.321 in Hosmer and Lemeshow test indicates that the multivariate model fit well.
p=0.686 in Hosmer and Lemeshow test indicates that the multivariate model fit well.
p=0.136 in Hosmer and Lemeshow test indicates that the multivariate model fit well.
Multivariate analysis of predictors of adrenal metastasis
The results of multivariate analysis for predicting adrenal metastasis in patients with known primary cancer and in patients with lung and gastrointestinal cancers are summarised in Table 5. For patients with known primary cancer, age, unenhanced attenuation, SUVmax ratio and metastasis to other parts were associated with adrenal metastasis. The highest accuracy for predicting adrenal metastasis was for the combination of “unenhanced attenuation >10 HU, SUVmax ratio >2.5, metastasis to other parts or age”, with 92.0%, 88.4% and 90.8% sensitivity, specificity and accuracy, respectively (Figure 1). The pairing of “unenhanced attenuation >10 HU or SUVmax ratio >2.5” had relative lower sensitivity, specificity and accuracy than the combination of the variables. These findings held for primary site-specific analysis as well as for both lung and gastrointestinal cancer.
Table 5.
The sensitivity, specificity, PPV and NPV of combining variables in the prediction for adrenal metastasis
| Parameters | Adrenal metastasis (n) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
| Yes | No | ||||||
| For known primary tumour | |||||||
| Unenhanced attenuation >10 HU | 128 | 20 | 84.7 | 74.3 | 86.4 | 71.6 | 81.2 |
| SUVmax ratio >2.5 | 125 | 22 | 82.7 | 70.5 | 85.0 | 67.9 | 78.6 |
| Unenhanced attenuation >10 HU and SUVmax ratio >2.5 | 132 | 13 | 87.4 | 83.3 | 91.0 | 77.3 | 81.6 |
| Unenhanced attenuation >10 HU and SUVmax ratio >2.5 and metastasis in other parts | 136 | 11 | 90.6 | 85.8 | 92.5 | 81.7 | 88.6 |
| Unenhanced attenuation >10 HU and SUVmax ratio >2.5 and metastasis in other parts and age <55 | 139 | 9 | 92.0 | 88.4 | 93.9 | 85.1 | 90.8 |
| For lung cancer | |||||||
| Unenhnaced attenuation >10 HU | 68 | 6 | 87.1 | 75.0 | 91.8 | 62.0 | 83.4 |
| SUVmax ratio >2.5 | 65 | 8 | 82.2 | 66.6 | 89.0 | 53.3 | 78.6 |
| Unenhanced attenuation >10 HU and SUVmax ratio >2.5 | 70 | 4 | 88.6 | 83.3 | 94.5 | 68.9 | 87.3 |
| Unenhanced attenuation >10 HU and SUVmax ratio >2.5 and metastasis in other parts | 72 | 3 | 91.1 | 87.5 | 96.0 | 75.0 | 90.2 |
| For gastrointestinal cancer | |||||||
| Unenhnaced attenuation >10 HU | 10 | 5 | 76.9 | 72.2 | 66.6 | 81.2 | 74.1 |
| SUVmax ratio >2.5 | 9 | 6 | 69.2 | 66.6 | 60.0 | 75.0 | 67.7 |
| Unenhanced attenuation >10 HU and SUVmax ratio >2.5 | 11 | 3 | 84.6 | 83.3 | 84.6 | 88.2 | 83.8 |
NPV, negative predictive value; PPV, positive predictive value; SUVmax, maximum standardised uptake value.
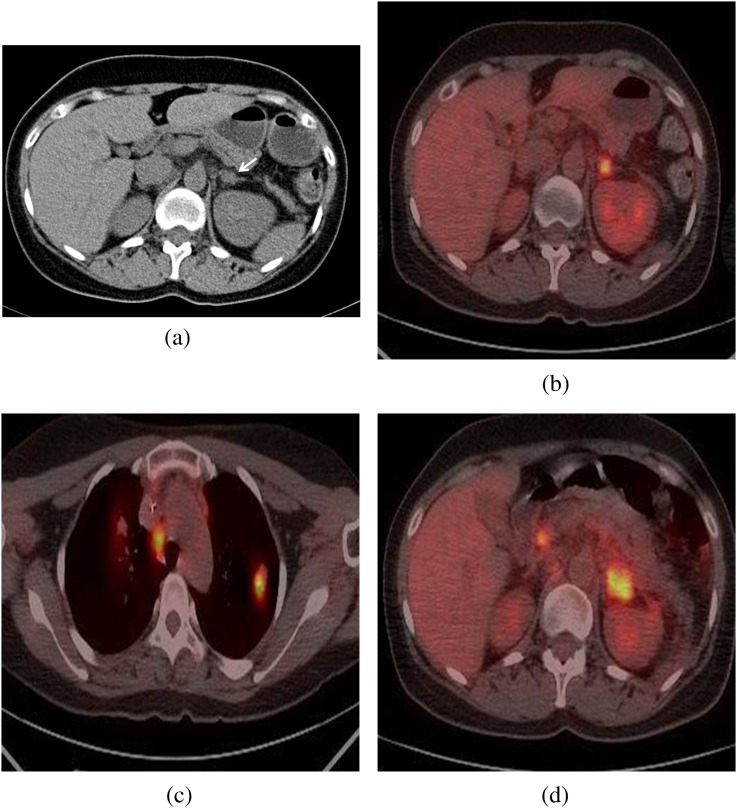
Figure 1.
A 39-year-old female with uterine cervical cancer. (a) The unenhanced CT scan shows a lesion measuring approximately 15 mm in the left adrenal nodule (arrow) with homogeneous attenuation (22 HU). (b) On integrated PET/CT scan, the lesion shows a high 18F-FDG uptake (SUVmax ratio 4.8). (c) A high 18F-FDG uptake lesion (SUVmax ratio 5.0) is shown in the left upper lung. (d) At follow-up 8 months later after appropriate chemotherapy, integrated PET/CT scan shows interval increases in size and aggravated 18F-FDG uptake (SUVmax ratio 12.1). The left adrenal lesion did prove to be metastasis. 18F-FDG, flourine-18 fludeoxyglucose; PET, positron emission tomography; SUVmax, maximum standardised uptake value.
DISCUSSION
Our retrospective study used PET/CT to assess the characteristics of adrenal lesions in patients with known primary cancer and without primary cancer. Once an adrenal lesion has been detected in patients with cancer, its characterisation is critical to stage the primary disease. The most common clinical conundrum is to differentiate benign non-functioning lesions from metastatic lesions. Patients with known primary cancer were more likely to have an adrenal metastatic lesion than patients with no primary tumour. The risk of adrenal metastases is very low in patients with no history of primary cancer. Song et al [11] reported that a study of 1049 consecutive incidental adrenal masses in patients with no history of cancer did not find a single malignancy.
Imaging techniques to differentiate these lesions must be as close to 100% specific as possible so as not to risk making a diagnosis of benignancy in error [12]. The unenhanced CT and contrast-enhanced CT washout study and chemical shift MRI meet these principles [13–17]. The use of an integrated PET/CT enables confident localisation and characterisation of adrenal lesions [18]. Unenhanced attenuation data from an integrated PET/CT can characterise most benign adenomas given that the 10-HU threshold to differentiate benign from indeterminate lesions has been firmly established in clinical practice [19–23]. The use of a 10-HU threshold brings the sensitivity up to 79% and decreases the specificity to 96% [13,16,23]. The integrated PET/CT is useful in cases in which the adrenal lesion has metabolic activity equal to or less than the background activity. Bagheri et al [24] found that 68% of normal adrenal glands had increased 18F-FDG activity compared with the background and that identification of adrenal glands was difficult with PET alone. Metser et al [20] suggested using an SUVmax of 3.1 for detecting malignant adrenal lesions.
The combination of unenhanced CT and SUVmax ratio is highly accurate in distinguishing between benign and malignant adrenal lesions [25]. In our study, applying the suggested unenhanced attenuation and SUVmax ratio cut-off values of 10 HU and 2.5, respectively, substantially increased the accuracy of the test and would have decreased the number of false-positive results. Our study found that PET/CT was efficient in differentiating between benign and malignant adrenal lesions. Our study found that approximately 40% of patients with primary cancer did not have a malignant adrenal lesion, similar to previous reports [26]. Moreover, adrenal lesions in patients without primary cancer were all benign or owing to hyperplasia, consistent with the low frequency (approximately 10%) of primary adrenal tumours [26]. Of the patients without known primary cancer, 8.4% had an unenhanced attenuation of >10 HU and 3.1% had an SUVmax ratio of >2.5. Of the patients with known primary cancer but no adrenal metastasis, 11.1% had unenhanced attenuation of >10 HU and 2.1% had an SUVmax ratio of >2.5.
Among patients with known primary cancer, metastatic tumours were predominantly found in the left adrenal gland (50.3% of patients). Generally, left adrenal lesions such as nodules or hyperplasia were overrepresented [27]. This may have been owing to developmental differences between the left and right adrenal glands, such as different anatomical positioning and formation of the asymmetrical mass. Also, liver SUVavg likely had no effect on our results, as the difference in the means of liver SUVavg was small and would have had minimal impact on a calculated SUV ratio.
Many malignant lesions are capable of adrenal gland metastasis [10]. In our study, the most common primary cancer was lung (44.9%). Our study was performed with a heterogeneous group of patients with all types of malignant diseases. It may be that groups of patients with lung, gastrointestinal and liver cancer are more likely to have adrenal metastasis than are group of patients with different cancers, potentially introducing interpreter bias. The majority of patients with adrenal metastasis also had metastatic cancer in other body parts (73.5%), especially patients with primary gastrointestinal cancer (84.6%). These results were consistent with the fact that patients in our study who had adrenal metastasis had advanced cancer.
Univariate analysis revealed that age, unenhanced attenuation, SUVmax ratio and the presence of metastasis in other parts were independent predictors for adrenal metastasis in patients with known primary cancer. The risk of adrenal metastasis from known primary cancer decreased with every increasing year of age. The same variables, except age, were associated with adrenal metastasis in patients with lung and gastrointestinal cancer. Multivariate analysis revealed that in patients with primary cancer, unenhanced attenuation >10 HU, an SUVmax ratio >2.5 and the presence of metastasis in other parts yielded high sensitivity, specificity and accuracy for predicting adrenal metastasis. For patients with gastrointestinal cancer, unenhanced attenuation >10 HU and an SUVmax ratio >2.5 were the best combination of sensitivity, specificity and accuracy for predicting adrenal metastasis.
Our study had a few important limitations. First, like many similar studies, it was conducted retrospectively. Second, we used follow-up data to determine the nature of the adrenal lesions because we had pathological proof for only a few patients. However, this situation is reflective of current practice. Most adrenal nodules are characterised with either follow-up data or imaging, and biopsy is reserved for a few selected indeterminate lesions. Third, our analysis was quantitative. It can be argued as to whether quantitative or qualitative methods are more effective in distinguishing between malignant and benign adrenal lesions. Finally, the population of the study was small (n=325), and patients with lung cancer accounted for roughly 50% of our population. Therefore, the findings of the total population with known primary cancers may reflect the characteristics of a lung cancer population. Larger scaled studies including sizable populations representing multiple primary cancer types are needed to more fully assess the variables that predict adrenal metastasis.
In conclusion, on the basis of all our findings, we conclude that PET/CT is useful for characterising adrenal lesions present with or without known primary cancers. We identified variables that may be useful in assessing the risk of developing adrenal metastasis. PET/CT may help the clinician in diagnosis and in determining the optimal treatment strategies in patients with cancer.
REFERENCES
- 1.Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med 2000;41:1369–79 [PubMed] [Google Scholar]
- 2.Reinartz P, Wieres FJ, Schneider W, Schur A, Buell U. Side-by-side reading of PET and CT scans in oncology: which patients might profit from integrated PET/CT? Eur J Nucl Med Mol Imaging 2004;31:1456–61 10.1007/s00259-004-1593-y [DOI] [PubMed] [Google Scholar]
- 3.Metser U, Golan O, Levine CD, Even-Sapir E. Tumor lesion detection: when is integrated positron emission tomography/computed tomography more accurate than side-by-side interpretation of positron emission tomography and computed tomography? J Comput Assist Tomogr 2005;29:554–9 [DOI] [PubMed] [Google Scholar]
- 4.Yun M, Kim W, Alnafisi N, Lacorte L, Jang S, Alavi A. 18F-FDG PET in characterizing adrenal lesions detected on CT or MRI. J Nucl Med 2001;42:1795–9 [PubMed] [Google Scholar]
- 5.Maurea S, Mainolfi C, Bazzicalupo L, Panico MR, Imparato C, Alfano B, et al. Imaging of adrenal tumors using FDG PET: comparison of benign and malignant lesions. AJR Am J Roentgenol 1999;173:25–9 10.2214/ajr.173.1.10397094 [DOI] [PubMed] [Google Scholar]
- 6.Namimoto T, Yamashita Y, Mitsuzaki K, Nakayama Y, Makita O, Kadota M, et al. Adrenal masses: quantification of fat content with double-echo chemical shift in-phase and opposed-phase FLASH MR images for differentiation of adrenal adenomas. Radiology 2001;218:642–6 [DOI] [PubMed] [Google Scholar]
- 7.Kumar R, Xiu Y, Yu JQ, Takalkar A, El-Haddad G, Potenta S, et al. 18F-FDG PET in evaluation of adrenal lesions in patients with lung cancer. J Nucl Med 2004;45:2058–62 [PubMed] [Google Scholar]
- 8.Sung YM, Lee KS, Kim BT, Choi JY, Chung MJ, Shim YM, et al. (18)F-FDG PET versus (18)F-FDG PET/CT for adrenal gland lesion characterization: a comparison of diagnostic efficacy in lung cancer patients. Korean J Radiol 2008;9:19–28 10.3348/kjr.2008.9.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta NC, Graeber GM, Tamim WJ, Rogers JS, Irisari L, Bishop HA. Clinical utility of PET-FDG imaging in differentiation of benign from malignant adrenal masses in lung cancer. Clin Lung Cancer 2001;3:59–64 [DOI] [PubMed] [Google Scholar]
- 10.Vikram R, Yeung HD, Macapinlac HA, Iyer RB. Utility of PET/CT in differentiating benign from malignant adrenal nodules in patients with cancer. AJR Am J Roentgenol 2008;191:1545–51 10.2214/AJR.07.3447 [DOI] [PubMed] [Google Scholar]
- 11.Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am J Roentgenol 2008;190:1163–8 10.2214/AJR.07.2799 [DOI] [PubMed] [Google Scholar]
- 12.Boland GW, Blake MA, Hahn PF, Mayo-Smith WW. Incidental adrenal lesions: principles, techniques, and algorithms for imaging characterization. Radiology 2008;249:756–75 10.1148/radiol.2493070976 [DOI] [PubMed] [Google Scholar]
- 13.Lee MJ, Hahn PF, Papanicolaou N, Egglin TK, Saini S, Mueller PR, et al. Benign and malignant adrenal masses: CT distinction with attenuation coefficients, size, and observer analysis. Radiology 1991;179:415–18 [DOI] [PubMed] [Google Scholar]
- 14.Korobkin M, Brodeur FJ, Yutzy GG, Francis IR, Quint LE, Dunnick NR, et al. Differentiation of adrenal adenomas from nonadenomas using CT attenuation values. AJR Am J Roentgenol 1996;166:531–6 10.2214/ajr.166.3.8623622 [DOI] [PubMed] [Google Scholar]
- 15.Korobkin M. CT characterization of adrenal masses: the time has come. Radiology 2000;217:629–32 [DOI] [PubMed] [Google Scholar]
- 16.Caoili EM, Korobkin M, Francis IR, Cohan RH, Platt JF, Dunnick NR, et al. Adrenal masses: characterization with combined unenhanced and delayed enhanced CT. Radiology 2002;222:629–33 [DOI] [PubMed] [Google Scholar]
- 17.Mitchell DG, Crovello M, Matteucci T, Petersen RO, Miettinen MM. Benign adrenocortical masses: diagnosis with chemical shift MR imaging. Radiology 1992;185:345–51 [DOI] [PubMed] [Google Scholar]
- 18.Boland GW, Goldberg MA, Lee MJ, Mayo-Smith WW, Dixon J, McNicholas MM, et al. Indeterminate adrenal mass in patients with cancer: evaluation at PET with 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology 1995;194:131–4 [DOI] [PubMed] [Google Scholar]
- 19.Blake MA, Slattery JM, Kalra MK, Halpern EF, Fischman AJ, Mueller PR, et al. Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy—initial experience. Radiology 2006;238:970–7 10.1148/radiol.2383042164 [DOI] [PubMed] [Google Scholar]
- 20.Metser U, Miller E, Lerman H, Lievshitz G, Avital S, Even-Sapir E. 18F-FDG PET/CT in the evaluation of adrenal masses. J Nucl Med 2006;47:32–7 [PubMed] [Google Scholar]
- 21.Park BK, Kim CK, Kim B, Choi JY. Comparison of delayed enhanced CT and 18F-FDG PET/CT in the evaluation of adrenal masses in oncology patients. J Comput Assist Tomogr 2007;31:550–6 10.1097/rct.0b013e31802fa8e1 [DOI] [PubMed] [Google Scholar]
- 22.Caoili EM, Korobkin M, Brown RK, Mackie G, Shulkin BL. Differentiating adrenal adenomas from nonadenomas using (18)F-FDG PET/CT: quantitative and qualitative evaluation. Acad Radiol 2007;14:468–75 10.1016/j.acra.2007.01.009 [DOI] [PubMed] [Google Scholar]
- 23.Boland GW, Lee MJ, Gazelle GS, Halpern EF, McNicholas MM, Mueller PR. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol 1998;171:201–4 10.2214/ajr.171.1.9648789 [DOI] [PubMed] [Google Scholar]
- 24.Bagheri B, Maurer AH, Cone L, Doss M, Adler L. Characterization of the normal adrenal gland with 18F-FDG PET/CT. J Nucl Med 2004;45:1340–3 [PubMed] [Google Scholar]
- 25.Perri M, Erba P, Volterrani D, Guidoccio F, Lazzeri E, Caramella D, et al. Adrenal masses in patients with cancer: PET/CT characterization with combined CT histogram and standardized uptake value PET analysis. AJR Am J Roentgenol 2011;197:209–16 10.2214/AJR.10.5342 [DOI] [PubMed] [Google Scholar]
- 26.McLean K, Lilienfeld H, Caracciolo JT, Hoffe S, Tourtelot JB, Carter WB. Management of isolated Adrenal Lesions in Cancer Patients. Cancer Control 2011;18:113–26 [DOI] [PubMed] [Google Scholar]
- 27.Jana S, Zhang T, Milstein DM, Isasi CR, Blaufox MD. FDG-PET and CT characterization of adrenal lesions in cancer patients. Eur J Nucl Med Mol Imaging 2006;33:29–35 10.1007/s00259-005-1915-8 [DOI] [PubMed] [Google Scholar]