Abstract
A simple, precise, and sensitive capillary electrophoresis technique coupled with a diode array detector has been developed for the separation and simultaneous determination of ezetimibe and atorvastatin in pharmaceutical formulations. Separation of both ezetimibe and atorvastatin was achieved utilizing fused silica capillary (58 cm × 75 μm ID) and background electrolyte solution that consisted of phosphate buffer (2.5 mM, pH 6.7): methanol (70:30 v/v). The proposed method was validated by testing its specificity, linearity, precision, accuracy, recovery, and detection limit/quantitation limit values. The method was linear over the range 2.5–50 μg/ml for ezetimibe (r = 0.9992) and 1–100 μg/ml for atorvastatin (r = 0.9999). Within-day and between-day RSD for ezetimibe and atorvastatin were ⩽5.6% and ⩽2.9%, respectively. The detection limit was 0.07 μg/ml for ezetimibe and 0.06 μg/ml for atorvastatin. The validated method was successfully employed for the determination of ezetimibe and atorvastatin in tablets with no interfering peaks from common pharmaceutical excipients. The percentage recoveries of the two drugs from their tablets were 99.80 ± 1.76 and 100.19 ± 1.83, respectively.
Keywords: Ezetimibe, Atorvastatin, Capillary electrophoresis, Diode array detector, Tablets
1. Introduction
Ezetimibe (1-(4-flurophenyl)-3(R)-[3(S)-(4-flurophenyl)-3-hydroxypropyl]-4(S) (4-hydroxyphenyl) azetidin-2-one), which belongs to a group of selective and very effective 2-azetidione cholesterol absorption inhibitors, acts at the level of cholesterol entry into enterocytes. It prevents transport of cholesterol through the intestinal wall by selectively blocking the absorption of cholesterol from dietary and biliary sources. This reduces the overall delivery of cholesterol to the liver, thereby promoting the synthesis of LDL receptors and a subsequent reduction in the serum LDL-C (Catapano, 2001; Heek and Davis, 2002; Bruckert et al., 2003).
Atorvastatin calcium, is [R-(R∗,R∗)]-2-(4-fluorophenyl)-β,δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino) carbonyl]-1H-pyrrole-1-heptanoic acid, a trihydrate of calcium salt (2:1). Atorvastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. This enzyme catalyzes the conversion of HMG-CoA to mevalonate, an early and rate-limiting step in cholesterol biosynthesis (Lea and McTavish, 1997; Beringer, 2005). The typical dose of atorvastatin calcium is 10–80 mg per day and it reduces 40–60% LDL (Page et al., 2002).
Combining the different mechanisms of action of these agents appears to provide substantial reductions in LDL-C, with additional favorable changes in total cholesterol, triglycerides, and HDL-C. Clinical studies have shown that co-administration of ezetimibe plus atorvastatin was significantly more effective at reducing LDL-C concentrations than ezetimibe or atorvastatin alone. Ezetimibe plus the starting dose of atorvastatin (10 mg) lowered direct LDL-C as effectively as the maximum dose of atorvastatin (80 mg) alone, reduced triglycerides by a similar amount, but provided significantly greater increase in HDL-C (9% versus 3%). Moreover, co-administration of ezetimibe with low dose atorvastatin was a well tolerated option to high dose atorvastatin monotherapy (Ballantyne et al., 2003, 2004; Conard et al., 2008a,b; Kaliappan et al., 2011).
There is an urgent need to develop an analytical method for the simultaneous analysis of ezetimibe and atorvastatin in pharmaceutical dosage forms due to the continuous increase in clinical use of these two agents as a combination.
A number of methods based on spectrophotometry (Sonawane et al., 2007; Baldha et al., 2009; Godse et al., 2009; Maher et al., 2011), densitometry (Dhaneshwar et al., 2007), and HPLC (Qutab et al., 2007; Seshachalam and Kothapally, 2008; Sama et al., 2010; Bhatt et al., 2010) were reported for the simultaneous determination of ezetimibe and atorvastatin in pharmaceutical preparations.
There is no reported method available in the literature for the simultaneous determination of ezetimibe and atorvastatin by capillary electrophoresis (CE).
CE has distinct advantages over other techniques in terms of fast analysis times, smaller quantities of solutions and minimal organic usage, and thus overall lower consumable expenses can be attained. More importantly, CE can produce much defined peaks with a high degree of resolution and the separation is achievable in a wide pH range.
Therefore, a simple, precise, and sensitive capillary electrophoresis method was developed in this study for the separation and simultaneous quantitation of the two drugs in tablets.
2. Experimental
2.1. Chemicals and reagents
Ezetimibe, atorvastatin calcium, and losartan, as internal standard (IS), were kindly obtained from the Drug Control Centre, Riyadh, Saudi Arabia. Ezetrol® tablets 10 mg (MSD–Schering-Plough) and Lipitor® tablets 10, 20, 40 mg (Parke-Davis) were purchased from the local market. HPLC grade methanol, analytical grade disodium hydrogen phosphate, and sodium dihydrogen phosphate were obtained from Merck (Germany). Deionized water was used throughout the experiments.
2.2. Electrophoretic instrumentation and conditions
The employed CE system consisted of an Agilent capillary electrophoresis instrument (Agilent Technologies, Germany) equipped with a diode array detector (DAD) and a data handling system comprised of an HP computer and Agilent Chem station software. Detection was performed at 210 nm. A deactivated fused silica capillary was obtained from Agilent Technologies and had the following dimensions 67 cm total length, 58 cm effective length, and 75 μm ID. The temperature of the capillary and the samples was maintained at 24 °C. The background electrolyte solution (BGE) consisted of phosphate buffer (2.5 mM, pH 6.7):methanol (70:30 v/v). Samples were injected into the capillary by pressure at the anodic side at 50 mbar for 10 s. The electrophoresis was carried out by applying 25 kV voltage to the capillary, with the cathode being at the detector end. The capillary was washed between run with deionized water (3 min), then equilibrated with the running buffer (5 min), to ensure reproducibility of the assay. The solutions were filtered through a Millipore membrane filter (0.2 μm) from Nihon, Millipore (Japan), before injection.
2.3. Preparation of stock and standard solutions
Stock solutions containing 1 mg/ml of ezetimibe and atorvastatin were prepared in methanol. The internal standard (IS) losartan was prepared in water to give a concentration of 1 mg/ml and was further diluted with water to get the working solution 0.2 mg/ml. Aliquots of the standard stock solutions of ezetimibe and atorvastatin were transferred into 10 ml volumetric flasks, 1 ml of IS working solution was added to each flask, then completed to the mark with the BGE to yield final concentrations of 2.5, 5, 10, 20, 40, and 50 μg/ml for ezetimibe, and 1, 10, 20, 50, 80, and 100 μg/ml for atorvastatin. Triplicate injections of each concentration were performed. The peak-area ratio of each concentration to the IS (As/Ast) against the corresponding standard concentration was plotted to construct the calibration curves, and the corresponding regression equations were derived.
2.4. Analysis of laboratory-made mixtures
Stock solutions of ezetimibe and atorvastatin (equivalent to 1 mg/ml) were prepared in methanol. Aliquots of the standard stock solutions of both drugs were transferred into 10 ml volumetric flasks, 1 ml of the IS working solution was added to each flask, then completed to the mark with the BGE to yield different concentrations ratios of ezetimibe:atorvastatin (1:1, 1:2, 1:4). Triplicate injections from each solution were made. The peak area ratio of each concentration to the IS was calculated. The concentration of each drug is obtained using the corresponding regression equations.
2.5. Analysis of tablets
Ten tablets of each preparation (Ezetrol® 10 mg, and Lipitor® 10, 20, 40 mg) were weighed and powdered. Accurately weighed portions equivalent to one tablet content (10 mg ezetimibe, and 10, 20, 40 mg atorvastatin) were transferred into each of 10 ml volumetric flasks containing 5 ml methanol. The solutions were stirred and sonicated for 20 min, then made up to volume with methanol, mixed well, and centrifuged at 3000 rpm for 10 min. Aliquots of the tablets solutions were transferred to 10 ml volumetric flasks, 1 ml of the IS working solution was added to each flask, and completed to volume with the BGE, to yield different concentrations ratios of ezetimibe:atorvastatin (1:1, 1:2, 1:4). Triplicate injections from each solution were made. The peak area ratio of each concentration to the IS was calculated. The concentration of each drug is obtained using the corresponding regression equations.
3. Results and discussion
The electrophoretic parameters were preliminarily optimized to develop a capillary electrophoresis method for the simultaneous determination of ezetimibe and atorvastatin with short analysis time and acceptable resolution.
3.1. Method optimization
The choice of the electrolyte system (BGE) is still one of the key problems in the application of electrophoresis in analytical chemistry since its role is very complex. The main purposes of a BGE are to provide the transport of electric current and the separation of the analytes. But if an electric current passes through a BGE, some additional phenomena occur, such as the electroosmotic flow (EOF), which plays an important role in the electrophoretic processes. The BGE should primarily provide an appropriate migration of the analytes in a reasonable time with no peak broadening and migration interferences (Beckers and Bocek, 2003). Therefore, the most important parameters that affect the BGE were examined in order to reach the most suitable system for the optimum separation of the two drugs under study, and the results will be discussed as follow:
3.1.1. Effect of pH
The pH is of key importance for electromigration in systems with weak electrolytes (weak bases and weak acids). The pH of the BGE has to be regulated in order to keep the migration velocity of weak electrolyte and the velocity of the EOF constant. In that way, a stable and reproducible migration behavior of the analytes can be obtained. The effective mobility (the electromigration of a partially ionized substance) for weak anionic and cationic species is strongly dependent on their pK values related to the pH of the BGE. However, it should be emphasized, that even substances with zero effective mobility may move in the capillary due to the EOF, and this EOF is also strongly dependent on the pH of the BGE used (Beckers and Bocek, 2003).The pH in values from 5–10 were chosen for the study. The selected buffer was phosphate buffer. It is studied in three selected concentrations (1, 2.5, and 5 mM) under constant instrumentation conditions (voltage, injection time, temperature, pressure, and wave length). Phosphate buffer (2.5 mM, pH 6.7) has a good resolution and analysis time in comparison to the other electrolyte systems.
3.1.2. Effect of buffer concentration
Different BGEs have been tested. The best results considering selectivity, reproducibility, baseline separation, and current performance were obtained with sodium phosphate buffer (pH 6.7). Keeping other parameters constant (pH 6.7, 25 kV, 24 °C), the buffer concentration varied from 1 to 5 mM was studied. Increasing in migration times was observed when the buffer concentration increased. The resolution of ezetimibe and atorvastatin increased as the buffer concentration increased, but long migration times were observed at the buffer concentrations more than 2.5 mM. Therefore, 2.5 mM sodium phosphate buffer (pH 6.7) was selected as optimum BGE.
3.1.3. Effect of organic modifier concentration
The most obvious advantage from the addition of organic solvents into the run buffer is that it would extend the range of analytes that can be investigated, due to an enhancement in the solubility of various substances. Another major advantage is the potential increase in separation selectivity compared to the aqueous CE separations through changes in the physicochemical properties, such as changes in acid–base properties of the analytes, viscosity and dielectric constant of the separation medium, as well as interactions between analytes and solvent. Other advantages of organic solvents include the capability of significantly reducing the analysis time by employing relatively high applied voltages, since most organic solvents have lower dielectric constants compared to water and, thus, relatively high voltages can be applied without causing any significant band broadening due to Joule heating (Huie, 2003).
The organic modifier used in this study is methanol. The amount added to the phosphate buffer ranges from 20% to 60%. It causes an increase in the separation selectivity and resolution factor (Rs) between the two drugs. Although the Rs factor is increased, late migration times were observed at a concentration more than 50%. Therefore, the 30% methanol was chosen as the optimum concentration.
3.1.4. Effect of applied voltage
The applied voltage can affect the efficiency of analysis since the resolution of compounds is directly proportional to it (McLaughlin et al., 1992). The applied voltage was gradually increased from 15 to 30 kV. The best resolution was observed when applying a voltage of 25 kV. At 15 kV, no signals were detected. Further increase in the applied voltage of more than 25 kV resulted in a decreased resolution.
Finally, the separation of ezetimibe and atorvastatin was carried out under the above optimized conditions and the migration times were 8.59 ± 0.03 and 13.21 ± 0.20 min for ezetimibe and atorvastatin, respectively (Fig. 1).
Figure 1.
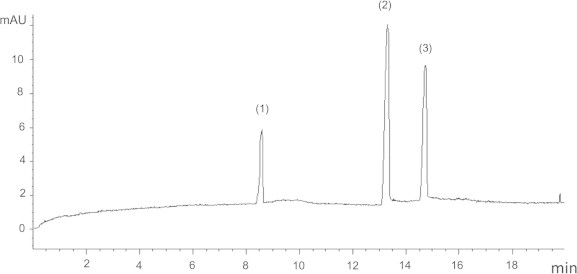
Electropherogram of 20 μg/ml ezetimibe (1), 50 μg/ml atorvastatin (2), and 20 μg/ml IS (3).
3.2. Method validation
The main objective of validation of an analytical procedure is to demonstrate that the procedure is suitable for its intended purpose. Therefore, the capillary electrophoresis method developed for the simultaneous determination of ezetimibe and atorvastatin in this study was validated according to the ICH guidelines for its specificity, linearity, range, accuracy, precision, detection limit and quantitation limit (ICH, 2005).
3.2.1. Specificity
Specificity is the ability to assess unequivocally the analyte in the presence of components which may be expected to be present. These might include impurities, degradation product, and excipients. The electropherogram in Fig. 1, demonstrates the specificity of the method. There are no peaks detected at the migration times of both drugs and internal standard from excipients that is commonly co-formulated with the studied drugs. Also, peak purity tests may be useful to show that the analyte peak is not attributable to more than one component. Peak purities of ezetimibe and atorvastatin were evaluated by the use of the DAD detector.
3.2.2. Linearity
The linearity of the method was evaluated by constructing six-point calibration curves. Excellent linearity was observed over the concentration range of 2.5–50 μg/ml for ezetimibe and 1.0–100 μg/ml for atorvastatin. The peak area ratios of the drugs to the IS (As/Ast) versus concentrations were plotted and a linear least-square regression analysis was conducted to determine the slope, intercept, and correlation coefficient (r) to demonstrate the linearity of the method. The correlation coefficient (r) was found to be 0.9992 and 0.9999 for ezetimibe and atorvastatin, respectively. The linear regression analysis data are summarized in Table 1.
Table 1.
Validation parameters for the determination of ezetimibe and atorvastatin by the proposed method.
| Parameter | Ezetimibe | Atorvastatin |
|---|---|---|
| Concentration range (μg/ml) | 2.5–50 | 1.0–100 |
| Intercept (b) | 0.005 | −0.012 |
| Slope (m) | 0.018 | 0.033 |
| Correlation coefficient (r) | 0.9992 | 0.9999 |
| Sb | 0.0099 | 0.0112 |
| Sm | 0.0003 | 0.0001 |
| Sx/y | 0.0157 | 0.0178 |
| DL (μg/ml) | 0.07 | 0.06 |
| QL (μg/ml) | 0.25 | 0.20 |
Sb = standard deviation of intercept.
Sm = standard deviation of slope.
Sx/y = Standard deviation of the residual.
3.2.3. Range
The specified range is normally derived from the linearity studies and depends on the intended application of the procedure. The developed method provided an excellent linearity, accuracy, and precision when applied to the samples containing amounts of ezetimibe and atorvastatin within or at the extremes of the specified range of this method (2.5–50 μg/ml for ezetimibe and 1.0–100 μg/ml for atorvastatin).
3.2.4. Accuracy and precision
Accuracy and precision should be reported as percentage error (%E) and relative standard deviation (%RSD), respectively, and should be established across the range of the developed method. They were determined by the analysis of three different concentrations for each drug. The within-day accuracy and precision were assessed from the results of the analysis of the three concentrations on a single day. The between-day accuracy and precision were determined from the same three concentrations analyzed on three consecutive days (Table 2).
Table 2.
Accuracy and precision data for ezetimibe and atorvastatin.
| Analyte | Added conc. (μg/ml) | Found conc. (μg/ml) (mean ± SD) | RSD (%) | Error (%) |
|---|---|---|---|---|
| Within-day | ||||
| Ezetimibe | 8.50 | 8.54 ± 0.002 | 1.2 | 0.56 |
| 21.25 | 21.40 ± 0.007 | 1.7 | 0.70 | |
| 34.00 | 33.64 ± 0.009 | 1.5 | 1.04 | |
| Atorvastatin | 20.40 | 20.56 ± 0.014 | 2.0 | 0.18 |
| 51.00 | 50.75 ± 0.038 | 2.3 | 0.43 | |
| 81.60 | 80.78 ± 0.049 | 1.8 | 0.30 | |
| Between-day | ||||
| Ezetimibe | 8.50 | 8.49 ± 0.006 | 3.6 | 0.43 |
| 21.25 | 21.12 ± 0.015 | 3.7 | 0.59 | |
| 34.00 | 33.97 ± 0.035 | 5.6 | 0.07 | |
| Atorvastatin | 20.40 | 20.24 ± 0.019 | 2.9 | 1.44 |
| 51.00 | 50.45 ± 0.032 | 1.9 | 0.17 | |
| 81.60 | 80.87 ± 0.028 | 1.0 | 0.35 | |
3.2.5. Detection limit
The detection limit (DL) is the lowest amount of analyte in a sample which can be detected but not quantitated. Several approaches for determining the DL are present (visual evaluation, signal-to-noise, standard deviation of the intercept and the slope). The standard deviation of the intercept and the slope approach was used to determine the DL in this study. The detection limit was 0.07 μg/ml for ezetimibe and 0.06 μg/ml for atorvastatin (Table 1).
3.2.6. Quantitation limit
The quantitation limit (QL) is the lowest amount of analyte in a sample which can be quantitatively determined. The approach used in determining the DL, was also used for determining the QL. The quantitation limits of ezetimibe and atorvastatin were 0.25 and 0.20 μg/ml, respectively (Table 1).
3.2.7. Stability in solutions
Investigation of the stability was established for the 10 μg/ml solution of ezetimibe and atorvastatin kept at room temperature five days. Both drugs were stable under these conditions. No additional peaks were found in the electropherograms throughout the analysis time, indicating the stability of both drugs in the sample solution.
3.3. Application of the method in tablets
The proposed method was applied for the determination of ezetimibe and atorvastatin in their pharmaceutical formulations (Fig. 2) and the results are shown in Table 3.
Figure 2.
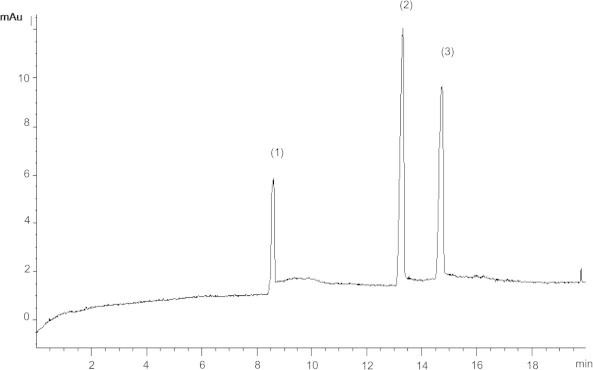
Electropherogram of tablet solution of 17 μg/ml ezetimibe (1), 48 μg/ml atorvastatin (2), and 20 μg/ml IS (3).
Table 3.
Analysis of ezetimibe and atorvastatin in tablets by the proposed method.
| Mixturea Ezetimibe:Atorvastatin | Added conc. (μg/ml) | Ezetimibe |
Atorvastatin |
||
|---|---|---|---|---|---|
| Found conc. (μg/ml) (mean ± SD) | Recovery (%) | Found conc. (μg/ml) (mean ± SD) | Recovery (%) | ||
| 1:1 | 17:24 | 16.62 ± 0.28 | 97.76 | 23.60 ± 0.16 | 98.33 |
| 1:2 | 17:48 | 17.14 ± 0.72 | 100.82 | 48.96 ± 0.40 | 102.00 |
| 1:4 | 17:96 | 17.14 ± 0.50 | 100.82 | 96.24 ± 1.16 | 100.25 |
| Average | 99.80 ± 1.76 | 100.19 ± 1.83 | |||
Laboratory-made mixture.
4. Conclusion
The developed method is considered as the first method for simultaneous separation and quantitation of ezetimibe and atorvastatin by capillary electrophoresis. It is a simple, rapid, and sensitive procedure and was successively applied for the quantitation of the two drugs in tablets. The various validation characteristics were applied and determined to assure the suitability of the method. It was found that the method is valid and suitable for its intended purpose which is the simultaneous determination of the studied drugs in tablets.
Acknowledgment
The author extends her appreciation to the Deanship of the Scientific Research at the King Saud University for funding the work through the research group Project No. RGP-VPP-065.
References
- Baldha R.G. Simultaneous spectrophotometric determination of atorvastatin calcium and ezetimibe in tablet dosage form. International Journal of PharmTech Research. 2009;1(2):233–236. [Google Scholar]
- Ballantyne C.M. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107:2409–2415. doi: 10.1161/01.CIR.0000068312.21969.C8. [DOI] [PubMed] [Google Scholar]
- Ballantyne C.M. Long-term safety and tolerability profile of ezetimibe and atorvastatin coadministration therapy in patients with primary hypercholesterolaemia. International Journal of Clinical Practice. 2004;58:653–658. doi: 10.1111/j.1368-5031.2004.00278.x. [DOI] [PubMed] [Google Scholar]
- Beckers J.L., Bocek P. The preparation of background electrolytes in capillary zone electrophoresis: golden rules and pitfalls, a review. Electrophoresis. 2003;24:518–535. doi: 10.1002/elps.200390060. [DOI] [PubMed] [Google Scholar]
- Beringer P. 21st ed. vol. 2. Mack Publishing; Easton, PA: 2005. (Remingtons – The Science and Practice of Pharmacy). [Google Scholar]
- Bhatt K.K. Simultaneous estimation of atorvastatin calcium and ezetimibe in tablet by RP-HPLC method. International Journal of Pharmaceutical and Applied Sciences. 2010;1(1):114–117. [Google Scholar]
- Bruckert E. Perspectives in Cholesterol-Lowering Therapy. The Role of Ezetimibe, a New Selective Inhibitor of Intestinal Cholesterol Absorption. Circulation. 2003;107:3124. doi: 10.1161/01.CIR.0000072345.98581.24. [DOI] [PubMed] [Google Scholar]
- Catapano A.L. Ezetimibe: a selective inhibitor of cholesterol absorption. European Heart Journal Supplements. 2001;3(Suppl. E):E6–E10. [Google Scholar]
- Conard S.E. Efficacy and safety of ezetimibe added on to atorvastatin (20 mg) versus uptitration of atorvastatin (to 40 mg) in hypercholesterolemic patients at moderately high risk for coronary heart disease. The American Journal of Cardiology. 2008;102:1489–1494. doi: 10.1016/j.amjcard.2008.09.075. [DOI] [PubMed] [Google Scholar]
- Conard S.E. Efficacy and safety of ezetimibe added on to atorvastatin (40 mg) compared with uptitration of atorvastatin (to 80 mg) in hypercholesterolemic patients at high risk of coronary heart disease. The American Journal of Cardiology. 2008;102:1495–1500. doi: 10.1016/j.amjcard.2008.09.076. [DOI] [PubMed] [Google Scholar]
- Dhaneshwar S.S. Development and validation of a method for simultaneous densitometric estimation of atorvastatin calcium and ezetimibe as the bulk drug and in tablet dosage forms. Acta Chromatographica. 2007;19:141–148. [Google Scholar]
- Godse V.P. Simultaneous spectrophotometric estimation of ezetimibe and atorvastatin in pharmaceutical dosage form. Asian Journal of Research in Chemistry. 2009;2(1):86–89. [Google Scholar]
- Heek M.V., Davis H. Pharmacology of ezetimibe. European Heart Journal Supplements. 2002;4(Suppl. J):J5–J8. [Google Scholar]
- Huie C.W. Effects of organic solvents on sample pretreatment and separation performances in capillary electrophoresis, a review. Electrophoresis. 2003;24:1508–1529. doi: 10.1002/elps.200305396. [DOI] [PubMed] [Google Scholar]
- ICH, 2005. Q2 (R1) Validation of analytical procedures: text and Methodology. In: Proceeding of the international conference on harmonization, Geneva.
- Kaliappan V.B. A comparative study of the novel cholesterol absorption inhibitor ezetimibe with atorvastatin and atorvastatin alone in type II diabetes mellitus patients with primary hypercholesterolemia. International Journal of Basic Medical Science. 2011;2(2) [Google Scholar]
- Lea A.P., McTavish D. Atorvastatin. A review of its pharmacology and therapeutic potential in the management of hyperlipidaemias. Drugs. 1997;53(5):828–847. doi: 10.2165/00003495-199753050-00011. [DOI] [PubMed] [Google Scholar]
- Maher H.M. Enhanced spectrophotometric determination of tow antihyperlipidemic mixtures containing ezetimibe in pharmaceutical preparations. Drug Testing and Analysis. 2011;3(2):97–105. doi: 10.1002/dta.165. [DOI] [PubMed] [Google Scholar]
- McLaughlin M. Pharmaceutical drug separations by HPCE, practical guidelines. Journal of Liquid Chromatography. 1992;15:961–973. [Google Scholar]
- Page, Curtis, Sutter . 2nd ed. Mosby International Ltd.; 2002. Walkar and Hoffmann Integrated Pharmacology. p.303. [Google Scholar]
- Qutab S.S. Simultaneous determination of atorvastatin calcium and ezetimibe in pharmaceutical formulations by liquid chromatography. Journal of Food and Drug Analysis. 2007;15(2):139–144. [Google Scholar]
- Sama J.R. Simultaneous estimation of atorvastatin and ezetimibe in pharmaceutical formulations by RP-HPLC method. Der Pharmacia Lettre. 2010;2(1):427–436. [Google Scholar]
- Seshachalam U., Kothapally C.B. HPLC analysis for simultaneous determination of atorvastatin and ezetimibe in pharmaceutical formulations. Journal of Liquid Chromatography and Related Technologies. 2008;31(5):714–721. [Google Scholar]
- Sonawane S.S. Simultaneous spectrophotometric estimation of atorvastatin calcium and ezetimibe in tablets. Indian Journal of Pharmaceutical Science. 2007;69(5):683–684. [Google Scholar]


