Abstract
Chromatographic fractionation of the alcoholic extract of the dried fronds of Adiantum capillus-veneris L. (Adiantaceae) yielded seven compounds: four triterpenoidal compounds belonging to adiantane and filicane groups were isolated from the hexane fraction and identified as isoadiantone (1); isoadiantol-B (2); 3-methoxy-4-hydroxyfilicane (3) and 3,4-dihydroxyfilicane (4) and three flavonoids were isolated from the ethyl acetate fraction and identified as: quercetin (5), quercetin-3-O-glucoside (6) and quercetin-3-O-rutinoside (rutin) (7). The identification of the isolated compounds has been established through their physical, chemical and spectroscopic methods including IR, 1H NMR, 13C NMR, HSQC, HMBC, NOESY and MS. Biological studies of the total alcoholic extract, hexane fraction and some of the isolated compounds showed an anti-inflammatory activity while the hypoglycemic study of the total alcoholic extract showed a significant activity.
Keywords: Adiantum capillus-veneris L., Adiantane, Filicane triterpenoids, Flavonoids, Anti-inflammatory, Hypoglycemic
1. Introduction
Adiantum capillus-veneris belonging to the Adiantaceae family is one of the most common and widely distributed species (Singh et al., 2008). Ethnomedicinally, the genus has been used as tonic and diuretic; in treatment of cold, fever, cough and bronchial disorders, as stimulant, emollient, purgative, demulcent, general tonic and hair tonic, in addition to skin diseases, tumors of spleen, liver and other viscera (Singh et al., 2008), in treatment of jaundice and hepatitis (Abbasi et al., 2009) and many other uses (Abbasi et al., 2010; Ahmad et al., 2008; Al-Qura’n, 2009; Camejo-Rodrigues et al., 2003; Dastagir, 2001; De Natale and Pollio, 2007; Ghorbani, 2005; Guarrera et al., 2008; Guarrera, 2005; Hamayun et al., 2006; Hammond et al., 1998; Inam et al., 2000; McGaw et al., 2008; Shinozaki et al., 2008; Shinwari and Khan, 2000; Uncini Manganelli et al., 2001). Concerning the phytoconstituents, the literature revealed the presence of flavonoids (Imperato, 1982a,b; Pourmorad et al., 2006), sulphate esters of hydroxycinnamic acid–sugars (Imperato, 1982c,d), different classes of triterpenoids (Abdel-Halim et al., 2002; Berti et al., 1963, 1969; Imperato, 1982c; Jankowski et al., 2004; Nakane et al., 1999, 2002; Shinozaki et al., 2008; Zaman et al., 1966), sterols (Guarrera et al., 2008; Marino et al., 1989), quinic and shikimic acids in addition to other constituents (Abbasi et al., 2010; Chiang and Lin, 1979; El-Tantawy et al., 1994; El-Tantawy, 1989; Imperato, 1982c; Mahran et al., 1994; McGaw et al., 2008; Victor et al., 2003). The anti-microbial (Abdel-Halim et al., 2002; Besharat et al., 2008; Guha et al., 2005; Hammond et al., 1998; Kumar and Aushik, 1999; Kumar et al., 2003; Mahmoud et al., 1989; Mahran et al., 1990; Singh et al., 2008; Victor et al., 2003), antioxidant, anti-implantation, antidiabetic in addition to other effects of this species (El-Sheimy et al., 1995; El-Tantawy et al., 1994; Gupta et al., 2010; Kumar et al., 2003; Kumar, 2009; Murthy et al., 1984; Pourmorad et al., 2006) were previously reported. The present study deals with the isolation and identification of the main constituents of the hexane and ethyl acetate fractions in addition to the study of the anti-inflammatory and hypoglycemic activities to verify these effects using experimental animals.
2. Materials and methods
2.1. Plant material
The dried fronds of A. capillus-veneris were collected from the local market of Assiut Governorate, Assiut, Egypt, April 2008. The identity of the plant was confirmed by Prof. Dr. Abd El-Aziz Fayed, Prof. of Taxonomy, Faculty of Science, Assiut University, Assiut, Egypt. A voucher sample (No. 20103) was kept in the Herbarium of Faculty of Pharmacy, Assiut University, Assiut, Egypt.
2.2. General experimental procedure
Melting points were uncorrected and determined using Stuart SMP3 digital melting point apparatus (UK). 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were measured using a Varian Unity INOVA spectrometer using TMS as an internal standard. UV spectra were measured using Evolution 300 UV–VIS spectrophotometer (England). IR spectra were recorded in KBr using Shimadzu IR-470 spectrometer (Japan). Mass spectra were carried out using JEOL JMS600 spectrometer. Column chromatography was performed on Kieselgel 60 (60–230 mesh, Merck) and TLC was carried out with silica gel 60 precoated plates F-254 (Merck) and Whatman sheets Nos. 1 and 3 (England) for paper chromatography. One touch apparatus and its strips (Lifescan, Johnson and Johnson Inc.) for blood glucose level determination.
2.3. Extraction and isolation
The dried powdered fronds of A. capillus-veneris (800 g) were extracted with alcohol (3 l × 3) by maceration and percolation. The combined alcoholic extract was concentrated under reduced pressure using rotary evaporator till dryness (220 g). Part of the dried residue (150 g) was mixed with distilled water (500 ml) and subjected to fractionation between hexane (700 ml × 4), chloroform (500 ml × 3), ethyl acetate (500 ml × 4) and finally n-butanol (500 ml × 3). Each fraction was dried under reduced pressure; the solvent-free residue in each case was weighed to give dry weight of hexane (70 g), chloroform (12 g), ethyl acetate (22 g) and n-butanol (30 g) extracts.
Twenty grams of the hexane fraction were subjected to silica gel CC. Elution was started with hexane then hexane–ethyl acetate in gradient elution manner; fractions (200 ml, each) were collected and monitored with silica gel TLC using hexane–ethyl acetate in different ratios, similar fractions were pooled together giving five groups (I–V). Group (I) eluted with hexane only contained gummy residue which showed many inseparable spots; group (II) which eluted with hexane–ethyl acetate (95:5) and group (III) which eluted with hexane–ethyl acetate (90:10) were subjected to repeated crystallization to afford compounds 1 (200 mg) and 2 (90 mg), respectively. Group (IV) eluted with hexane–ethyl acetate (80:20) was subjected to repeated column chromatography over silica gel column and eluted with benzene–ethyl acetate (85:15 to 80:20) that afforded two sub-groups (IV-a and IV-b) which upon re-crystallization afforded compounds 3 (50 mg) and 4 (63 mg), respectively. Group (V) now is still under investigation.
Ten grams of the dried ethyl acetate fraction was chromatographed over silica gel CC and eluted with chloroform–methanol gradiently where four fractions (F-1–F-4) were obtained. Fraction (F-2) eluted with chloroform–methanol (9:1) upon preparative silica gel TLC using chloroform–methanol (9:1), afforded compound 5 (32 mg). Fractions (F-3 and F-4) obtained by chloroform–methanol (85:15 to 70:30) were subjected to preparative paper chromatography using Whatman sheets No. 3 and butanol–acetic acid–water (4:1:5, v/v/v, upper layer) as a developer. They afforded compounds 6 (26 mg) and 7 (42 mg), respectively.
2.4. Acid hydrolysis
Few milligrams of each of compounds 6 and 7 were separately dissolved in 5 ml MeOH to which an equal volume of 10% sulfuric acid was added. The mixture was refluxed on a boiling water bath for 3 h, then cooled. The hydrolyzate was shaken with ethyl acetate (3 × 50 ml). The combined extract was distilled off and the aglycone was subjected to TLC using CHCl3–MeOH (85:15) as solvent system. The acidic mother liquor containing the sugar moiety(s) was neutralized with barium carbonate, concentrated and separately spotted alongside authentic sugars on Whatman No. 1 sheets using n-butanol–acetic acid–water (4:1:2, v/v/v) as a solvent system.
2.5. Animals
Adult male mice (25–30 g) and rabbits (1.2–1.5 kg) were used. They were housed in standard environmental conditions in an experimental animal room and fed laboratory diet ad libitum with free access to water. Sixty-six mice were divided into 11 groups (six mice, each) and nine rabbits were used divided into 3 groups (three rabbits, each). The animals were kept for 7 days with 12 h light/dark cycle.
2.6. Anti-inflammatory activity
2.6.1. Formalin induced hind paw edema in mice
Fifty microliters of 3.5% formalin solution in 0.9% normal saline was injected into the right hind paw of the mice while the left paw was injected with the vehicle (2% PEG in 0.9% normal saline solution) as a control. One hour after formalin injection, the inflammation was measured by cutting both feet at the level of knee joint and comparing the weight of the right and left paws (Garrido et al., 2001). The increase in the paw weight was determined as follows:
where I% = increase in the paw weight; Wr = weight of the right hind paw that received formalin; Wl = weight of the left hind paw that received the vehicle.
Total alcoholic extract, hexane fraction (400 mg/kg, each) and sodium salicylate (200 mg/kg) were solubilized in 0.9% normal saline with the aid of 2% polyethylene glycol-400 (PEG-400) and administered orally once a day for four days via stomach tube. PEG-400 (2% in 0.9% normal saline solution) was used as a negative control. On the fourth day, the fractions under test and sodium salicylate were used 1 h before formalin injection.
2.6.2. Topical anti-inflammatory activity
2.6.2.1. Croton oil-induced edema
Croton oil inflammatory mixture was prepared by adding croton oil (250 mg) to a mixture of pyridine–ether–distilled water (4:5:1, v/v/v) and kept in the refrigerator till used.
2.6.2.2. Preparation of the tested fractions and pure compounds for topical application
The ointment base used consists of emulsifying wax (4.5 g), liquid paraffin (3.0 g) and white soft paraffin (7.5 g). The hexane extract was added to the ointment base by 10% replacement while the isolated compounds by 5% replacement. The ear thickness was measured before and after induction of inflammation by means of an Oditest caliper. The ear inflammation was induced according to (Tubaro et al., 1985), 10 μl of croton oil inflammatory mixture was applied by means of an Eppendorf pipette to the inner side of the left ear of each mouse while the right one was left untreated. The formulated extract (2.5 mg), pure compounds (1.25 mg) and positive control (niflumic acid, 1.25 mg) were applied to the respective mice groups and their effects were evaluated. Measurement of ear thickness was carried out at 2, 6, 24 and 30 h after treatment with total hexane fraction, pure compounds and the positive control. The edema was expressed as the difference between right and left ear thickness.
2.7. Hypoglycemic study
2.7.1. Assessment of hypoglycemic activity in normal healthy mice
Fasted mice (18 h) were divided into three groups of six animals each. Group I (control group) received vehicle (2% PEG-400), while groups II received the total alcoholic extract at dose 400 mg/kg body weight via stomach tube. Group III received glipizide at dose 8 mg/kg body weight as a reference standard drug. Blood samples were withdrawn from the cavernous sinus with a capillary tube and the blood glucose levels were estimated (mg/dl) at 0.0, 30, 60, 90, 120 and 150 min using one touch glucometer.
2.7.2. Assessment of anti-hyperglycemic activity using oral glucose tolerance test (OGTT)
The overnight fasted rabbits were divided into three groups of three animals each. Group I was given glucose solution (2.25 g/kg body weight) orally using stomach tube, the total alcoholic extract (600 mg/kg) was administered simultaneously (group II) and 30 min before glucose loading (group III). Blood samples were collected from the ear vein at zero time and after 30, 60, 120 and 240 min. after the glucose administration.
2.7.3. Determination of blood glucose level
The blood glucose level was determined by the glucose oxidase method (Rheney and Kirk, 2000).
2.8. Chemicals
Croton oil, formalin, glipizide and sodium salicylate were obtained from Sigma. Polyethylene glycol-400 (Merk, Germany), niflumic acid (Archimica GmbH, Germany).
2.9. Statistical analysis
All the results were expressed as mean ± SEM. The significance in results from control group was calculated using the Student’s t-test and a probability level lower than 0.05 was considered as statistically significant (Suleyman et al., 1999).
3. Results
Seven compounds were isolated from the fractionated alcoholic extract of A. capillus-veneris. The biological activity of the total alcoholic extract, hexane fraction and some of the isolated compounds were studied.
3.1. The physical and spectral data of the isolated compounds
3.1.1. Compound 1
Needle crystals (CHCl3–MeOH). m.p.: 236–238 °C. IR (KBr): 2933, 1691 cm−1, EI-MS m/z (% rel. int.) 412 [M]+ (27), 397 (6), 369 (5), 191 (100). 1H and 13C NMR data: Tables 1 and 2.
Table 1.
13C NMR spectral data of compounds 1–4 in CDCl3.
| C | Compound 1 | Compound 2 | Compound 3 | Compound 4 | C | Compound 1 | Compound 2 | Compound 3 | Compound 4 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 40.54 | 40.52 | 16.01 | 15.46 | 16 | 21.78 | 23.86 | 35.86 | 35.69 |
| 2 | 18.90 | 18.70 | 25.19 | 30.23 | 17 | 54.40 | 55.00 | 42.79 | 42.61 |
| 3 | 42.32a | 42.12 | 86.20 | 76.03 | 18 | 45.21 | 45.05 | 51.96 | 51.60 |
| 4 | 33.47 | 33.27 | 76.89 | 76.39 | 19 | 40.27 | 39.71 | 20.13 | 19.75 |
| 5 | 56.32 | 56.13 | 42.17 | 41.53 | 20 | 25.73 | 24.30 | 28.65 | 28.34 |
| 6 | 18.90 | 18.70 | 34.61 | 34.17 | 21 | 53.99 | 47.46 | 60.30 | 59.95 |
| 7 | 33.62 | 33.40 | 17.84 | 17.74 | 22 | 213.00 | 73.01 | 30.99 | 30.61 |
| 8 | 42.13 | 41.92 | 49.22 | 48.78 | 23 | 33.47 | 33.27 | 21.64 | 20.33 |
| 9 | 50.60 | 50.43 | 37.77 | 37.37 | 24 | 21.80 | 21.59 | 17.73 | 17.28 |
| 10 | 37.61 | 37.41 | 52.58 | 51.92 | 25 | 16.07 | 15.85 | 20.78 | 18.01 |
| 11 | 21.06 | 20.92 | 36.17 | 35.52 | 26 | 16.90 | 16.75 | 16.13 | 15.95 |
| 12 | 24.30 | 22.78 | 28.70 | 28.93 | 27 | 16.92 | 16.75 | 15.85 | 15.58 |
| 13 | 48.38 | 48.46 | 39.20 | 38.84 | 28 | 15.16 | 15.21 | 16.31 | 16.21 |
| 14 | 42.18a | 42.00 | 40.42 | 40.05 | 29 | 30.42 | 21.73 | 22.17 | 21.78 |
| 15 | 32.61 | 32.71 | 29.28 | 29.56 | 30 | 23.13 | 22.72 | ||
| OMe | 57.92 |
Data may be interchangeable.
Table 2.
1H NMR spectral data of compounds (1–4) and HMBC of compounds 3, 4 in CDCl3.
| H | 1 | 2 | 3 | HMBC | 4 | HMBC |
|---|---|---|---|---|---|---|
| 3 | 1.33, m 1.10, m |
1.35, m 1.10, m |
2.94, m | C-1, C-4, C-5, OMe | 3.56, dd, 7.0, J = 13.9 Hz | C-1, C-4, C-5 |
| 22 | – | 3.61, q | 1.42, m | – | 1.40, m | – |
| 23 | 0.85, s | 0.82, s | 1.16, s | C-3, C-5 | 1.15, s | C-3, C-5 |
| 24 | 0.77, s | 0.71, s | 0.99, s | C-4, C-6, C-10 | 1.06, s | C-4, C-6, C-10 |
| 25 | 0.82, s | 0.79, s | 0.87, s | C-8, C-10, C-11 | 0.82, s | C-8, C-10, C-11 |
| 26 | 0.93, s | 0.93, s | 0.87, s | C-8, C-15 | 0.81, s | C-8, C-15 |
| 27 | 0.94, s | 0.95, s | 0.92, s | C-12, C-14, C-18 | 0.99, s | C-12, C-14, C-18 |
| 28 | 0.67, s | 0.66, s | 0.75, s | C-16, C-18, C-21 | 0.71, s | C-16, C-18, C-21 |
| 29 | 2.12, s | 1.14, d, J = 6.2 Hz | 0.80, d, J = 6.7 Hz | C-21, C-30 | 0.74, d, J = 6.6 Hz | C-21, C-30 |
| 30 | – | – | 0.86, d, J = 6.7 Hz | C-21, C-29 | 0.79, d, J = 6.6 Hz | C-21, C-29 |
| OMe | – | – | 3.26, s | C-3 | – | – |
3.1.2. Compound 2
Needle crystals (Et2O–MeOH). m.p.: 213–215 °C. IR (KBr): 3390, 2935 cm−1, EI-MS m/z (% rel. int.) 414 [M]+ (24), 399 (14), 369 (11), 207 (16), 193 (49), 191 (100), 175 (36), 149 (20). 1H and 13C NMR data: Tables 1 and 2.
3.1.3. Compound 3
Needle crystals (CHCl3). m.p.: 237–239 °C. IR (KBr): 3410, 2920 cm−1, EI-MS m/z (% rel. int.) 458 [M]+ (2), 341 (81), 217 (9), 205 (35), 191 (21), 149 (17), 136 (29). 1H and 13C NMR data: Tables 1 and 2.
3.1.4. Compound 4
Needle crystals (MeOH). m.p.: 196–198 °C. IR (KBr): 3465, 2955, 1373, 1033, 875 cm−1, EI-MS m/z (% rel. int.) 444 [M]+ (34), 429 (66), 426 (15), 411 (17), 401 (4), 359 (14), 341 (19), 340 (56), 276 (13), 275 (24), 273 (36), 258 (16), 244 (12), 230 (14), 219 (29), 206 (19), 205 (61), 192 (23), 191 (68), 175 (22), 163 (32), 161 (26), 149 (38), 147 (21),137 (56), 136 (20), 107 (50). 1H and 13C NMR data: Tables 1 and 2.
3.1.5. Compound 5
Yellow needles (MeOH). m.p.: >300 °C, 1H NMR (400 MHz, DMSO-d6), δH 7.72 (1H, dd, J = 2.2 and 8.8 Hz, H-6′), 7.55 (1H, d, J = 2.2 Hz, H-2′), 6.85 (1H, d, J = 8.8 Hz, H-5′), 6.44 (1H, d, J = 2.1 Hz, H-8) and 6.27 (1H, d, J = 2.1 Hz, H-6). The UV spectral data with different ionizing and complexing reagents are cited in Table 3.
Table 3.
UV spectral data of flavonoidal compounds 5–7 with different ionizing and complexing reagents.
| Compounds | MeOH | NaOMe | AlCl3 | AlCl3 + HCl | NaOAc | NaOAc + H3BO3 |
|---|---|---|---|---|---|---|
| 5 | 256 266 295 370 |
270 297 415 |
254 276 299 445 |
267 277 299 404 |
264 280 297 411 |
259 300 392 |
| 6 | 253 305 357 |
271 327 410 |
273 301 330 433 |
273 297 353 404 |
274 322 383 |
259 379 |
| 7 | 259 266 299 359 |
272 327 410 |
275 303 433 |
271 300 364 402 |
271 325 393 |
262 298 389 |
3.1.6. Compound 6
Yellow fine needles (MeOH). m.p.: 207–210 °C, 1H NMR (400 MHz, DMSO-d6): δH 7.63 (1H, dd, J = 2.2 and 8.5 Hz, H-6′), 7.51 (1H, d, J = 2.2 Hz, H-2′), 6.81 (1H, d, J = 8.5 Hz, H-5′), 6.38 (1H, d, J = 2.0 Hz, H-8) and 6.22 (1H, d, J = 2.0 Hz, H-6), 5.22 (1H, d, J = 7.6 Hz, H-1″), 3.1–4.0 (6H, m, other-glu. protons). The UV spectral data with different ionizing and complexing reagents are cited in Table 3.
3.1.7. Compound 7
Fine yellowish needles (MeOH). m.p.: 197–199 °C, 1H NMR (400 MHz, DMSO-d6): δH 12.62 (1H, s, C-5-OH), 7.56 (1H, dd, J = 2.1 and 8.8 Hz, H-6′), 7.55 (1H, d, J = 2.1 Hz, H-2′), 6.87 (1H, d, J = 8.8 Hz, H-5′), 6.40 (1H, d, J = 2.0 Hz, H-8), 6.21 (1H, d, J = 2.0 Hz, H-6), 5.35 (1H, d, J = 7.4 Hz, H-1″-glu), 5.12 (1H, d, J = 1.9 Hz, H-1‴-Rham), 1.01 (3H, d, J = 6.1 Hz, CH3–Rh), 3.0–4.4 (10H, m, other-glu., rham. protons). The UV spectral data with different ionizing and complexing reagents are cited in Table 3.
3.2. Biological activity
3.2.1. Formalin induced inflammation
Both of the total alcoholic extract and its hexane fraction showed a significant anti-inflammatory activity in formalin induced edema. The total alcoholic extract showed a significant reduction (28%) compared with the positive control (sodium salicylate). The hexane fraction showed a reduction comparable with that of the positive control. The results were cited in Table 4.
Table 4.
Anti-inflammatory effects of total alcoholic extract and hexane fraction on formalin induced edema.
| Group | Swelling of paws (M ± SEM) | % of inhibition |
|---|---|---|
| Control 2% PEG (400) | 3.32 ± 0.33 | – |
| Total alcohol extract | 2.44 ± 0.26⁎ | 28 |
| Hexane fraction | 2.01 ± 0.21⁎ | 39 |
| Sodium salicylate | 1.89 ± 0.12⁎ | 41 |
SEM = Standard error of mean.
P < 0.05.
3.2.2. Croton oil-induced inflammation
The hexane fraction obtained from the total alcoholic extract showed topical anti-inflammatory activity after 6 h and continued for 30 h. Compound 2 showed non-significant topical anti-inflammatory activity; whereas compounds 3 and 4 showed topical anti-inflammatory activity after 6 h and continued for 30 h. The results are listed in Tables 5 and 6.
Table 5.
Anti-inflammatory effect of the total hexane fraction using croton oil-induced inflammation.
| Group No. | M (μm) ± SEM |
|||
|---|---|---|---|---|
| After 2 h | After 6 h | After 24 h | After 30 h | |
| Group 1 (niflumic acid) | 33.3 ± 1.2 | 31.0 ± 2.4 | 29.3 ± 2.2 | 28.0 ± 0.8 |
| Group 2 (hexane fraction) | 51.4 ± 2.2 | 47.0 ± 1.0⁎ | 46.0 ± 1.8⁎ | 44.0 ± 1.1⁎ |
| Group 3 (negative control) | 85.6 ± 2.2 | 83.0 ± 1.3 | 59.0 ± 3.9 | 59.0 ± 4.1 |
SEM = Standard error of mean.
P < 0.05.
Table 6.
Anti-inflammatory effect of compounds 2–4 using croton oil-induced inflammation.
| M (μm) ± SEM |
||||
|---|---|---|---|---|
| After 2 h | After 6 h | After 24 h | After 30 h | |
| Compound 2 | 53 ± 2.9 | 46 ± 3.1 | 45 ± 1.8 | 48 ± 3.7 |
| Compound 3 | 51 ± 4.8 | 43 ± 2.7⁎ | 44 ± 1.9⁎ | 45 ± 3.7⁎ |
| Compound 4 | 49 ± 2.2 | 46 ± 1.2⁎ | 39 ± 1.4⁎ | 36 ± 6.4⁎ |
| Control | 55 ± 2.8 | 50 ± 1.7 | 48 ± 1.6 | 56 ± 4.7 |
Each group contains six mice.
P < 0.05.
3.2.3. Hypoglycemic activity
3.2.3.1. Effect on normal mice
The total alcoholic extract showed non-significant hyperglycemia. The results are listed in Table 7.
Table 7.
Hypoglycemic effect of total alcoholic extract on normal mice.
| 0 min | 30 min | 60 min | 90 min | 120 min | 150 min | |
|---|---|---|---|---|---|---|
| Gp. I | 65.5 ± 0.5 | 78.5 ± 9.6 | 79.0 ± 7.6 | 82.5 ± 11.6 | 82.0 ± 2.0 | 72.0 ± 3.0 |
| Gp. II | 65.3 ± 3.5 | 90.0 ± 7.4 | 89.8 ± 7.6 | 75.7 ± 8.2 | 73.6 ± 7.0 | 71.1 ± 7.7 |
| Gp. III | 59.4 ± 5.6 | 52.5 ± 3.2 | 52.4 ± 3.4 | 49.2 ± 3.3 |
Gp. I: negative control; Gp. II: dose 400 mg; Gp. III: positive control (glipizide). These data represent the blood glucose level (mg/dl).
3.2.3.2. Effect on glucose loaded rabbits
The OGTT showed the highest blood glucose level after 30 min. After concurrently administered alcoholic extract at dose 600 mg/kg, a significant hypoglycemic effect was obtained after 30 min for group II which continued for 4 h, while in group III the hypoglycemic effect was continued for 2 h. The results are listed in Table 8.
Table 8.
Hypoglycemic effect of the alcoholic extract administered simultaneously and 30 min before glucose loading.
| 0 min | 30 min | 60 min | 120 min | 240 min | |
|---|---|---|---|---|---|
| Gp. I | 120.7 ± 10.4 | 310.3 ± 12.0 | 221.5 ± 17.3 | 151.5 ± 11.2 | 146.3 ± 9.7 |
| Gp. II | 127.0 ± 13.7 | 165.0 ± 19.4⁎ | 145.5 ± 10.9⁎ | 123.7 ± 10.6⁎ | 117.5 ± 10.1⁎ |
| Gp. III | 123.0 ± 8.8 | 120.9 ± 10.4⁎ | 121.4 ± 9.8⁎ | 131.7 ± 10.8⁎ | 137 ± 10.4 |
Gp. I: glucose load only; Gp. II: dose 600 mg; Gp. III: 600 mg extract fed 30 min before glucose loading. These data represent the blood glucose level (mg/dl).
P < 0.05.
4. Discussion
4.1. Identification of the isolated compounds
The physical characters and spectral data (1H and 13C NMR) of compounds 1 and 2 (experimental part and Tables 1 and 2) were found to be identical to those reported for isoadiantone and isoadiantol-B, respectively (Shiojima and Ageta, 1994; Shiojima et al., 1993). Further confirmation was carried out by co-chromatography using authentic samples. These compounds were previously isolated from the same plant (Abdel-Halim et al., 2002) (Fig. 1).
Figure 1.
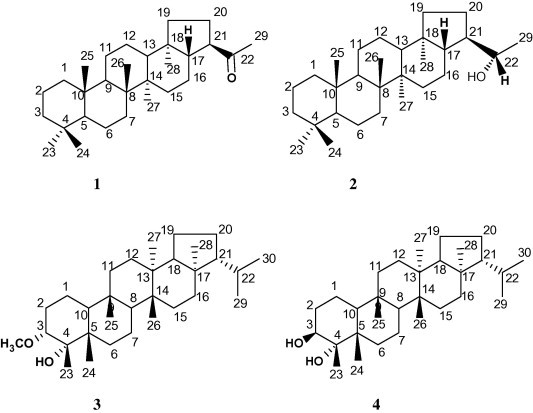
Structures of the isolated triterpenoids.
Compound 3: Needle crystals that gave positive color with Liebermann–Burchard’s test suggested its triterpenoidal nature. The IR spectrum showed the presence of hydroxyl group (3410 cm−1) and aliphatic C–H stretching group (2920 cm−1). The molecular formula of compound 3 was deduced to be C31H54O2 on the basis of mass spectrum 1H and 13C NMR including DEPT experiment. The 1H NMR showed the presence of two doublet methyl signals at δH 0.80 and 0.86 (J = 6.7 Hz), six singlet methyl signals at δH 1.16, 0.99, 0.92, 0.87 (2CH3) and 0.75 in addition to a multiplet methine proton at δH 2.94 and singlet methoxy protons at δH 3.26. The 13C NMR spectrum and DEPT spectra showed the presence of 31 carbon signal for 31 carbons (CH3x8, –CH2x10, –CHx6, –Cx6, –OCH3x1) suggesting a triterpenoidal skeleton. The chemical shifts were found to be close to filicane triterpene nucleus (Tsuzuki et al., 2001), with oxygen function(s) in ring A. The doublet and singlet signals at δC 86.20 and 76.89, respectively, were assigned for two oxygen-carrying carbons. Comparing the chemical shifts of these two carbons and other neighboring carbons with those reported for the filicanes (Nakane et al., 2002; Tsuzuki et al., 2001), indicated that the oxygen-bearing carbons must be located at C-3 and C-4 (ring A). Also, by comparing the 13C NMR chemical shifts with those reported for 3α-hydroxy,4-methoxy filicane (Tsuzuki et al., 2001), it seems that the methoxy group is located at C-3 since it appears more downfield (δC 86.20) while the hydroxyl group is located at C-4 (δC 76.89) since it appears more upfield. Generally, the substitution of hydroxyl group causes an upfield shift of both C-2 and C-4 and downfield shift of C-3 (Brahmachari and Chatterjee, 2002; Mahato and Kundu, 1994; Ngouamegne et al., 2008). The upfield shift of C-2 (at δC 25.19) in compound 3 relative to that reported for 3α-hydroxy,4-methoxy filicane (Tsuzuki et al., 2001) (δC 30.54) confirmed the position of OCH3 to be at C-3. This suggestion was established by extensive study of 2D NMR experiments [1H–1H COSY, HSQC and HMBC]. The 1H–1H COSY showed the correlation of methine proton at δH 2.94 with the CH2 protons at δH 1.18 (CH2-2). The HSQC spectrum showed a correlation between the methine proton at δH 2.94 to the doublet carbon at δC 86.20, suggesting the position of the methoxy group at C-3 and the hydroxyl group at C-4. The HMBC spectrum revealed the correlations of the methoxy group protons at δH 3.26 with C-3 (δC 86.20) and the methine proton at δH 2.94 with C-4, C-5 and C-1, that established the OCH3 group at C-3 and the OH group to be at C-4, the other HMBC correlations were shown in Fig. 2. Finally the assignment of the structure was supported by the EI-MS, which exhibited beside the molecular ion peak at m/z 458 [M]+, other peaks at m/z 341, 217, 205 and 191 confirming that compound 3 is a filicane derivative with two oxygenated functional groups at ring A (Fig. 3). Based on these evidences, the structure of compound 3 was deduced as 3α-methoxy-4-hydroxyfilicane.
Figure 2.
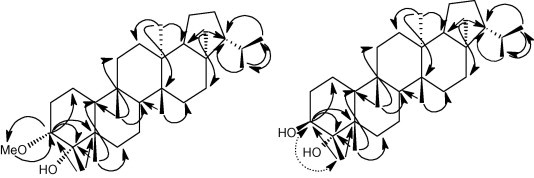
Some HMBC correlation (→) of compounds 3 and 4 and NOESY correlation (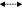 ) of compound 4.
) of compound 4.
Figure 3.
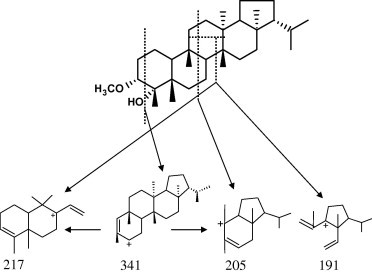
Some possible fragmentation pattern of compound 3.
Compound 4: Occured as fine needle crystals that gave positive color with Liebermann–Burchard’s test suggesting its triterpenoidal nature. EI-MS gave a molecular ion peak at m/z 444 [M]+ and its molecular formula was deduced from EI-MS, 1H, 13C NMR including DEPT experiment to be C30H52O2 indicating five degrees of unsaturation. IR spectrum showed absorption bands attributed to hydroxyl group at 3465 and 2955 cm−1 for C–H stretching. The 1H and 13C NMR data of compound 4 (Tables 1 and 2) showed close resemblance with those observed for compound 3 and 3α-hydroxy,4-methoxy filicane (Tsuzuki et al., 2001) except for the signals of ring A indicating that compound 4 is a filicane derivative. The 13C NMR spectrum showed 30 signals for 30 carbons (8 CH3, 10 CH2, 6 CH and 6 –C–), the signals at δC 76.03 and 76.30 were assigned for C-3 and C-4, respectively. The upfield shift of C-3 (δC 76.03) and the downfield shift of C-2 (δC 30.23) and the disappearance of the methoxy signal in both 1H and 13C NMR spectra comparing to those of compound 3 indicated the replacement of OCH3 group by OH group. The assignment of each carbon and its carrying proton was determined from the HSQC while the identification of each carbon and its neighboring protons was established from HMBC spectrum (Fig. 2, Table 2). From the previous data, compound 4 was deduced to be 3,4-dihydroxy filicane having an axial β-OH group and an equatorial α-H at C-3 from the large coupling constant of methine proton at δH 3.56 (dd, J = 7.0 and 13.9 Hz) (Li et al., 2009; Wei et al., 2008) and this was supported from the NOESY spectrum which showed NOE between the methyl group (C-23) and H-3 (Fig. 2). Due to the presence of CH3-23 in β-configuration in filicane triterpene, the OH group at C-4 must be present in α-configuration. The establishment of the structure was confirmed by EI-MS (m/z 444 [M]+) which is less than that of compound 3 by 14 mass units. The EI-MS spectrum exhibited besides the molecular ion peak at m/z 444 [M]+ other prominent peaks at m/z 429 [M-15], 426 [M-H2O], 411 (M−CH3−H2O), 401, 341, 273, 205 and 191. Naturally there is no evidence for the presence of 3,4-glycol, but it may be derived form the corresponding epoxide (adiantoxide) previously isolated from the plant (Berti et al., 1969; Nakane et al., 2002). Based on these evidences, the structure of compound 4 is 3-β,4-α-dihydroxy filicane (Fig. 1). This compound was identified previously as one of the hydrolytic products of adiantoxide (Berti et al., 1969); but this is the first report for its isolation from a natural source.
The physical characters and spectral data of compound 5 including 1H NMR and UV (experimental part and Table 3) were found to be identical to those reported for quercetin. Further confirmation was carried out by co-chromatography alongside authentic sample (Fig. 4).
Figure 4.
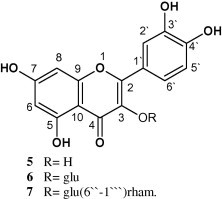
Structures of the isolated flavonoids.
Compound 6 gave positive tests for carbohydrates and/or glycosides and flavonoids (Mabry et al., 1970). The UV spectral data with different ionizing and complexing reagents (Table 3) indicated that this compound is a flavonoid. The 1H NMR (experimental part) showed the presence of five aromatic protons characteristic for quercetin pattern and an anomeric sugar proton that appears at δH 5.22 (1H, d, J = 7.6 Hz) indicating its β-nature, the other sugar protons appear between δH 3.1 and 4.0. The UV and 1H NMR of this compound are similar to those reported for quercetin-3-O-β-d-glucoside (Fathiazada et al., 2006). Mild acid hydrolysis showed that this compound was hydrolyzed in one step that indicated that it contains one sugar moiety. Complete acid hydrolysis afforded an aglycone identified as quercetin (m.p., m.m.p. and co-chromatography) and a sugar part identified as glucose (co-chromatography) using authentic sugars. So, compound 6 was identified as quercetin-3-O-β-d-glucoside (Fig. 4).
Compound 7 gives positive tests for carbohydrates and/or glycosides and flavonoids (Mabry et al., 1970). The UV spectral data with different ionizing and complexing reagents (Table 3) indicated that this compound is a flavonoid having free –OH groups at C-5, C-7, C-3′ and C-4′. The 1H NMR data (experimental part) showed a quercetin pattern and two anomeric sugar protons appear at δH 5.35 (1H, d, J = 7.4 Hz) for glucose indicating its β-nature and at δH 5.12 (1H, d, J = 1.9 Hz) together with the doublet methyl protons at δH 1.01 (3H, d, J = 6.1 Hz) for rhamnose indicating its α-nature. Complete acid hydrolysis of compound 7 afforded an aglycone identified as quercetin (m.p., m.m.p. and paper chromatography [PC] using authentic sample) and sugars identified as glucose and rhamnose [PC using butanol–acetic acid–water (4:1:5) solvent system using authentic sugars]. From the above-mentioned data compound 7 was identified as rutin (Fig. 4).
The isolation of above-mentioned compounds from A. capillus-veneris (Adiantaceae) is important from the chemotaxonomic point of view, since the isolated compounds belong to different natural groups previously isolated from other fern families (Soeder, 1985).
4.2. Biological activity
4.2.1. Formalin induced inflammation
Using formalin induced inflammation, both of the total alcoholic extract and its hexane fraction showed a significant anti-inflammatory activity. Usually, the pain and edema produced by formalin are mediated by substance P and bradykinin in the early phase, followed by tissue mediated response induced by histamine, 5HT, prostaglandins and bradykinins (Wheeler-Aceto and Cowan, 1991). Thus, the anti-inflammatory activity of the total extract and its hexane fraction may be attributed to inhibition of one or some of these inflammatory mediators (Nakazato and Takeo, 1998) where the total extract contains flavonoidal compounds that have anti-inflammatory activity (Bai and Zhu, 2010; Bai et al., 2010; Guardia et al., 2001; Kazłowska et al., 2010; Rogerio et al., 2010) and in case of the n-hexane fraction that may be attributed to the presence of sterols and triterpenes (Ding et al., 2010; Marino et al., 1989; Micallef and Garg, 2009; Ríos et al., 2000).
4.2.2. Croton oil-induced inflammation
The hexane fraction and compounds 3, 4 showed topical anti-inflammatory activity after 6 h and continued for 30 h. The delayed topical anti-inflammatory activity may be due to the poor absorption of these compounds from topical application or may be due to the unsuitability of the formulation or the used compounds may have mild effect to exert a clear action (De Souza et al., 2009).
4.2.3. Hypoglycemic activity
4.2.3.1. Effect on normal mice
The alcoholic extract showed a non-significant effect using mice model. The started non-significant hyperglycemic effect and the non-significant hypoglycemic effects after 1.5 h may be due to the presence of sugars or hyperglycemic agents, or may be the used dose is small to exert a clear action.
4.2.3.2. Effect on glucose loaded rabbits
The OGTT using rabbit model showed a significant hypoglycemic effect started after 30 min for group II and continued for 4 hours; this effect may be due to non-insulin mechanism. When the alcoholic extract was given 30 min before glucose loading, a significant decrease in blood glucose level was observed after 30 min of glucose loading which may be due to enhancing insulin secretion from β-cells. The hypoglycemic effects of the alcoholic extract may be due to the presence of flavonoids which are known for their hypoglycemic and antioxidant effects (Kessler et al., 2003; van Acker et al., 1996; Zhou et al., 2009). Further investigations are warranted to identify the hypoglycemic mechanism of the active principles.
References
- Abbasi A.M., Khan M.A., Ahmad M., Zafar M., Jahan S., Sultana S. Ethnopharmacological application of medicinal plants to cure skin diseases and in folk cosmetics among the tribal communities of North-West Frontier Province, Pakistan. J. Ethnopharmacol. 2010;128:322–335. doi: 10.1016/j.jep.2010.01.052. [DOI] [PubMed] [Google Scholar]
- Abbasi M., Khan M.A., Ahmad M., Zafar M., Khan H., Muhammad N., Sultana S. Medicinal plants used for the treatment of jaundice and hepatitis based on socio-economic documentation. Afr. J. Biotechnol. 2009;8:1643–1650. [Google Scholar]
- Abdel-Halim O.B., Ibraheim Z.Z., Shiojima K. Oleanane triterpenes from Adiantum capillus-veneris growing in Egypt. Alex. J. Pharm. Sci. 2002;16:87–92. [Google Scholar]
- Ahmad I., Hussain M., Ahmad M.S.A., Ashraf M.Y., Ahmad R., Ali A. Spatio-temporal variations in physiochemical attributes of Adiantum capillus-veneris from Soone valley of salt range (Pakistan) Pak. J. Bot. 2008;40:1387–1398. [Google Scholar]
- Al-Qura’n S. Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 2009;123:45–50. doi: 10.1016/j.jep.2009.02.031. [DOI] [PubMed] [Google Scholar]
- Bai H.W., Zhu B.T. Myricetin and quercetin are naturally occurring co-substrates of cyclooxygenases in vivo. Prostag. Leukot. Essent. Fatty Acids. 2010;82:45–50. doi: 10.1016/j.plefa.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai N., He K., Zhou Z., Lai C.S., Zhang L., Quan Z., Shao X., Pan M.H., Ho C.T. Flavonoids from Rabdosia rubescens exert anti-inflammatory and growth inhibitory effect against human leukemia HL-60 cells. Food Chem. 2010;122:831–835. [Google Scholar]
- Berti G., Bottari F., Marsili A., Lehn J.M., Witz P., Ourisson G. Structure de l’adiantone un nor-triterpene naturel. Tetrahedron Lett. 1963;4:1283–1287. [Google Scholar]
- Berti G., Bottiari F., Marsili A. Structure and stereochemistry of a triterpenoid epoxide from Adiantum capillus-veneris. Tetrahedron. 1969;25:2939–2947. doi: 10.1016/s0040-4020(01)82826-x. [DOI] [PubMed] [Google Scholar]
- Besharat M., Rahimian M., Besharat S., Ghaemi E. Antibacterial effects of Adiantum capillus-veneris ethanolic extract on three pathogenic bacteria in vitro. J. Clin. Diagn. Res. 2008;2:1242–1243. [Google Scholar]
- Brahmachari G., Chatterjee D. Triterpenes from Adiantum lunulactum. Fitoterapia. 2002;73:363–368. doi: 10.1016/s0367-326x(02)00119-3. [DOI] [PubMed] [Google Scholar]
- Camejo-Rodrigues J., Ascensão L., Bonet M.À., Vallès J. An ethnobotanical study of medicinal and aromatic plants in the Natural Park of Serra de São Mamede (Portugal) J. Ethnopharmacol. 2003;89:199–209. doi: 10.1016/s0378-8741(03)00270-8. [DOI] [PubMed] [Google Scholar]
- Chiang S.T., Lin B.L. Insoluble carbohydrates in the shoot apical meristem of Adiantum capillus-veneris L. Taiwania. 1979;24:1–10. [Google Scholar]
- Dastagir G. Medicinal plants of Mai Dhani hill Muzaffarabad (AJK), Pakistan. Hamdard Med. 2001;44:29–35. [Google Scholar]
- De Natale A., Pollio A. Plants species in the folk medicine of Montecorvino Rovella (inland Campania, Italy) J. Ethnopharmacol. 2007;109:295–303. doi: 10.1016/j.jep.2006.07.038. [DOI] [PubMed] [Google Scholar]
- De Souza M.M., Pereira M.A., Ardenghi J.V., Mora T.C., Bresciani L.F., Yunes R.A., Monache F.D., Cechinel-Filho V. Filicene obtained from Adiantum cuneatum interacts with the cholinergic, dopaminergic, glutamatergic, GABAergic, and tachykinergic systems to exert antinociceptive effect in mice. Pharmacol. Biochem. Behav. 2009;93:40–46. doi: 10.1016/j.pbb.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Ding Y., Liang C., Kim J.H., Lee Y.M., Hyun J.H., Kang H.K., Kim J.A., Min B.S., Kim Y.H. Triterpene compounds isolated from Acer mandshuricum and their anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2010;20:1528–1531. doi: 10.1016/j.bmcl.2010.01.096. [DOI] [PubMed] [Google Scholar]
- El-Sheimy, I.R., Kamal, I.H., Khalifa, M.M., Hassan, A.B., El-Makhzngy, M.N., El-Alfy, T.S., 1995. Biochemical study and the biological effects of the petroleum ether extract and the isolates of Adiantum capillus-veneris L. In: A.S.P. 36th Annual Meeting, July, Oxford, MS, USA.
- El-Tantawy M., El-Sakhawy F., El-Deeb K., Fathy M., Hassan A. A phytochemical and pharmacological study of Adiantum capillus-veneris L. growing in Egypt. Zagazig J. Pharm. Sci. 1994;3:97–103. [Google Scholar]
- El-Tantawy, M., 1989. A pharmacognostical study of Adiantum capillus-veneris L. growing in Egypt. Ph.D. Thesis, Cairo University.
- Fathiazada F., Delazar A., Amiri R., Sarker S.D. Extraction of flavonoids and quantification of rutin from waste tobacco leaves. Iran J. Pharm. Res. 2006;3:222–227. [Google Scholar]
- Garrido G., Gonzalez D., Delporte C., Backhouse N., Quintero G., Nunez-Selles A.J., Morales M.A. Antinociceptive and anti-inflammatory effects of Mangifera indica L. extracts (Vimang) Phytother. Res. 2001;15:18–21. doi: 10.1002/1099-1573(200102)15:1<18::aid-ptr676>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Ghorbani A. Studies on pharmaceutical ethnobotany in the region of Turkmen Sahra, North of Iran. J. Ethnopharmacol. 2005;102:58–68. doi: 10.1016/j.jep.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Guardia T., Rotelli A.E., Juarez A.O., Pelzer L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56:683–687. doi: 10.1016/s0014-827x(01)01111-9. [DOI] [PubMed] [Google Scholar]
- Guarrera P.M. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium) Fitoterapia. 2005;76:1–25. doi: 10.1016/j.fitote.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Guarrera P.M., Lucchese F., Medori S. Ethnophytotherapeutical research in the high Molise region (Central-Southern Italy) J. Ethnobiol. Ethnomed. 2008;4:7. doi: 10.1186/1746-4269-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha P., Mukhopadhyay R., Gupta K. Antifungal activity of the crude extracts and extracted phenols from gametophytes and sporophytes of two species of Adiantum. Taiwania. 2005;50:272–283. [Google Scholar]
- Gupta V., Bansal P., Kumar P., Kaur G. Anti-inflammatory and anti-nociceptive activity of Adiantum capillus. Res. J. Pharm. Tech. 2010;3:432–434. [Google Scholar]
- Hamayun M., Khan S.A., Sohn E.Y., Lee I.J. Folk medicinal knowledge and conservation status of some economically valued medicinal plants of District Swat, Pakistan. Lyonia. 2006;11:101–113. [Google Scholar]
- Hammond G.B., Fernandez I.D., Villegas L.F., Vaisberg A.J. A survey of traditional medicinal plants from the Callejo´n de Huaylas, Department of Ancash, Peru. J. Ethnopharmacol. 1998;61:17–30. doi: 10.1016/s0378-8741(98)00009-9. [DOI] [PubMed] [Google Scholar]
- Imperato F. Kaempferol 3-sulphate in the fern Adiantum capillus-veneris. Phytochemistry. 1982;21:2158–2159. [Google Scholar]
- Imperato F. A new acylated flavonol glycoside from the fern Adiantum capillus-veneris L. Chem. Ind. 1982;16:604. [Google Scholar]
- Imperato F. Sulphate esters of hydroxycinnamic acid-sugar derivatives from Adiantum capillus-veneris. Phytochemistry. 1982;21:2717–2718. [Google Scholar]
- Imperato F. New phenolic glycosides in the fern Adiantum capillus-veneris L. Chem. Ind. 1982:957–958. [Google Scholar]
- Inam B., Sultana K., Qureshi R.A., Malik S. A checklist of plants of Bhogarmang, Siran Valley, N.W.F.P., Pakistan. Hamdard Med. 2000;43:62–75. [Google Scholar]
- Jankowski C.K., Aumelas A., Thuéry P., Reyes-Chilpa R., Jimenez-Estrada M., Barrios H., Diaz E. X-ray, 1H/13C 2D and 3D NMR studies of the structures of davallene and adipedatol, two triterpenes isolated from american Adiantum capillus-veneris. Polish J. Chem. 2004;78:389–408. [Google Scholar]
- Kazłowska K., Hsu T., Hou C.C., Yang W.C., Tsai G.J. Anti-inflammatory properties of phenolic compounds and crude extract from Porphyra dentata. J. Ethnopharmacol. 2010;128:123–130. doi: 10.1016/j.jep.2009.12.037. [DOI] [PubMed] [Google Scholar]
- Kessler M., Ubeaud G., Jung L. Anti- and pro-oxidant activity of rutin and quercetin derivatives. J. Pharm. Pharmacol. 2003;55:131–142. doi: 10.1211/002235702559. [DOI] [PubMed] [Google Scholar]
- Kumar A. Antioxidant effect of Adiantum capillus-veneris Linn. on human lymphocyte: an in vitro study. J. Cell Tissue Res. 2009;9:1899–1902. [Google Scholar]
- Kumar A., Aushik P. Antibacterial effect of Adiantum capillus-veneris Linn. Ind. Fern. J. 1999;16:72–74. [Google Scholar]
- Kumar M., Ramesh M., Sequiera S. Medicinal pteridophytes of Kerala, South India. Ind. Fern. J. 2003;20:1–28. [Google Scholar]
- Li G.Y., Zeng Y.M., Meng H., Li X., Wang J.H. A new triterpenoid saponin from the leaves and stems of Panax quinquefolium L. Chin. Chem. Lett. 2009;20:1207–1210. [Google Scholar]
- Mabry T.J., Markham K.R., Thomas M.B. Springer-Verlag; New York/Heidelberg/Berlin: 1970. The Systematic Identification of Flavonoids. [Google Scholar]
- Mahato S.B., Kundu A.P. 13C NMR spectra of pentacyclic triterpenoids – a compilation and some salient features. Review article. Phytochemistry. 1994;37:1517–1575. [Google Scholar]
- Mahmoud M.J., Jawad A.L., Hussain A.M., Al-Omari M., Al-Naib A. In vitro antimicrobial activity of Salsola rosmarinus and Adiantum capillus-veneris. Int. J. Crude Drug Res. 1989;27:14–16. [Google Scholar]
- Mahran G.H., El-Alfy T.S., El-Tantawy M.M., El-Sakhaw Y.F. A Contribution to the study of chemical constituents of Adiantum capillus-veneris L. growing in Egypt. Az. J. Pharm. Sci. 1994;13:1–14. [Google Scholar]
- Mahran G.H., El-Alfy T.S., Taha K.F., El-Tantawy M.M. Chemical composition and antimicrobial activity of the volatile oil and extracts of fronds of Adiantum capillus-veneris L. Bull. Fac. Agric. Cairo Univers. 1990;41:555. [Google Scholar]
- Marino A., Elberti M.G., Cataldo A. Phytochemical investigation of Adiantum capillus-veneris. Boll. Soc. Ital. Biol. Sper. 1989;65:461–463. [PubMed] [Google Scholar]
- McGaw L.J., Lall N., Meyer J.J.M., Eloff J.N. The potential of South African plants against Mycobacterium infections. J. Ethnopharmacol. 2008;119:482–500. doi: 10.1016/j.jep.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Micallef M.A., Garg M.L. Anti-inflammatory and cardioprotective effects of n-3 polyunsaturated fatty acids and plant sterols in hyperlipidemic individuals. Atherosclerosis. 2009;204:476–482. doi: 10.1016/j.atherosclerosis.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Murthy R.Sr., Basu D.K., Murti V.Vs. Anti-implantation activity of isoadiantone. Indian Drugs. 1984;21:141–144. [Google Scholar]
- Nakane T., Arai Y., Masuda K., Ishizaki Y., Agate H., Shiojima K. Fern constituents: six new triterpenoid alcohols from Adiantum capillus-veneris. Chem. Pharm. Bull. 1999;47:543–547. doi: 10.1248/cpb.50.1273. [DOI] [PubMed] [Google Scholar]
- Nakane T., Maeda Y., Ebihara H., Arai Y., Masuda K., Takano A., Ageta H., Shiojima K., Cai S., Abdel-Halim O.B. Fern constituents: triterpenoids from Adiantum capillus-veneris. Chem. Pharm. Bull. 2002;50:1273–1275. doi: 10.1248/cpb.50.1273. [DOI] [PubMed] [Google Scholar]
- Nakazato K., Takeo T. Anti-inflammatory effect of oolong tea polyphenols. Nippon-Nogeikagaku-Kaishi. 1998;72:51–54. [Google Scholar]
- Ngouamegne E.T., Fongang R.S., Ngouela S., Boyom F.F., Rohmer M., Tsamo E., Gut J., Rosenthal P.J. Endodesmiadiol, a friedelane triterpenoid and other antiplasmodial compounds from Endodesmia calophylloides. Chem. Pharm. Bull. 2008;56:374–377. doi: 10.1248/cpb.56.374. [DOI] [PubMed] [Google Scholar]
- Pourmorad F., Hosseinimehr S.J., Shahablmajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 2006;5:1142–1145. [Google Scholar]
- Rheney C.C., Kirk J.K. Performance of three blood glucose meters. Ann. Pharmacother. 2000;34:317–321. doi: 10.1345/aph.19187. [DOI] [PubMed] [Google Scholar]
- Ríos J.L., Recio M.C., Máñez S., Giner R.M. Natural triterpenoids as anti-inflammatory agents. In: Atta-Ur-Rahman, editor. Studies in Natural Products Chemistry. vol. 22, part C. Elsevier Science Publishers; Amsterdam, Netherlands: 2000. pp. 93–143. (Bioactive Natural Products). [Google Scholar]
- Rogerio A.P., Dora C.L., Andrade E.L., Chaves J.S., Silva L.F., Lemos-Senna E., Calixto J.B. Anti-inflammatory effect of quercetin-loaded microemulsion in the airways allergic inflammatory model in mice. Pharmacol. Res. 2010;61:288–297. doi: 10.1016/j.phrs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Shinozaki J., Shibuya M., Masuda K., Ebizuka Y. Squalene cyclase and oxidosqualene cyclase from a fern. FEBS Lett. 2008;582:310–318. doi: 10.1016/j.febslet.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Shinwari M.I., Khan M.A. Folk use of medicinal herbs of Margalla Hills National Park, Islamabad. J. Ethnopharmacol. 2000;69:45–56. doi: 10.1016/s0378-8741(99)00135-x. [DOI] [PubMed] [Google Scholar]
- Shiojima K., Ageta H. Fern constituents: triterpenoids isolated from the leaves of Adiantum edgeworthii. Structures of 19α-hydroxyadiantone and fern-9(11)-en-25-oic acid. Chem. Pharm. Bull. 1994;42:45–47. [Google Scholar]
- Shiojima K., Arai Y., Kasama T., Ageta H. Fern constituents: triterpenoids isolated from the leaves of Adiantum monochlamys. Filicenol A, Filicenol B, Isoadiantol B, Hakonanediol and Epihakonanediol. Chem. Pharm. Bull. 1993;41:262–267. [Google Scholar]
- Singh M., Singh N., Khare P.B., Rawat A.K.S. Antimicrobial activity of some important Adiantum species used traditionally in indigenous systems of medicine. J. Ethnopharmacol. 2008;115:327–329. doi: 10.1016/j.jep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Soeder R.W. Fern constituents: including occurrence, chemotaxonomy and physiological activity. Bot. Rev. 1985;51:442–536. [Google Scholar]
- Suleyman H., Demirezer L.O., Kuruuzum A., Banoglu Z.N., Gocer F., Ozbakir G., Gepdiremen A. Antiinflammatory effect of the aqueous extract from Rumex patientia L. roots. J. Ethnopharmacol. 1999;65:141–148. doi: 10.1016/s0378-8741(98)00175-5. [DOI] [PubMed] [Google Scholar]
- Tsuzuki K., Ôhashi A., Arai Y., Masuda K., Takano A., Shiojima K., Ageta H., Cai S.Q. Triterpenoids from Adiantum caudatum. Phytochemistry. 2001;58:363–367. doi: 10.1016/s0031-9422(01)00198-4. [DOI] [PubMed] [Google Scholar]
- Tubaro A., Dri P., Delbello G., Zilli C., Della Loggia R. The croton oil ear test revisited. Agents Actions. 1985;17:347–349. doi: 10.1007/BF01982641. [DOI] [PubMed] [Google Scholar]
- Uncini Manganelli R.E., Camangi F., Tomei P.E. Curing animals with plants: traditional usage in Tuscany (Italy) J. Ethnopharmacol. 2001;78:171–191. doi: 10.1016/s0378-8741(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Van Acker S.A.B.E., Van Den Berg D.J., Tromp M.N.J.L., Griffioen D.H., Van Bennekom W.P., Van Der Vijgh W.J.F., Bast A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996;20:331–342. doi: 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]
- Victor B., Maridass M., Ramesh U., Prabhu J.M.A. Antibacterial activity of essential oils from the leaves of Adiantum capillus-veneris Linn. Malaysian J. Sci. 2003;22:65–66. [Google Scholar]
- Wei Y., Ma C.M., Chen D., Hattori M. Anti-HIV-1 protease triterpenoids from Stauntonia obovatifoliola Hayata subsp. intermedia. Phytochemistry. 2008;69:1875–1879. doi: 10.1016/j.phytochem.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Wheeler-Aceto H., Cowan A. Neurogenic and tissue-mediated components of formalin-induced edema: evidence for supraspinal regulation. Agents Actions. 1991;34:264–269. doi: 10.1007/BF01993299. [DOI] [PubMed] [Google Scholar]
- Zaman A., Prakash A., Berti G., Bottari F., Macchia B., Marsili A., Morelli L. A new nortriterpenoid ketol from two Adiantum species. Tetrahedron Lett. 1966:3943–3947. [Google Scholar]
- Zhou T., Luo D., Li X., Luo Y. Hypoglycemic and hypolipidemic effects of flavonoids from lotus (Nelumbo nuficera Gaertn) leaf in diabetic mice. J. Med. Plants Res. 2009;3:290–293. [Google Scholar]


