Abstract
For systemic drug delivery, the buccal region offers an attractive route of drug administration. Salbutamol sulfate is a short-acting β2-adrenergic receptor agonist used for the relief of bronchospasm in conditions such as asthma and chronic obstructive pulmonary disease. It’s oral bioavailability is ∼40% due to extensive first pass metabolism. Salbutamol sulfate patches were prepared using Eudragit L-100, HPMC, PVA and Carbopol 934 in various proportions and combinations using PEG-400/PG as plasticizers. Patches were laminated on one side with a water impermeable backing layer for unidirectional drug release. The thickness of medicated patches were ranged between 0.23 ± 0.008 and 0.59 ± 0.007 mm and mass varied between 65.23 ± 3.3 and 117.92 ± 4.2 mg. Patches showed an increase in mass and swelling index with PEG-400 when compared with PG. The surface-pH of patches ranged between 6 and 7. Formulations E7 (7.5 mL Eudragit L-100, 15 mL HPMC K4M, 7.5 mL PVA and 2 mL PEG-400), E12 (7.5 mL Eudragit L-100, 7.5 mL PVA, 15 mL Carbopol and 2 mL PEG-400), F7 (7.5 mL Eudragit L-100, 15 mL HPMC K4M, 7.5 mL PVA and 2 mL PG), and F12 (7.5 mL Eudragit L-100, 7.5 mL PVA, 15 mL Carbopol and 2 mL PG) showed high folding endurance. Residence time of the tested patches ranged between 101 and 110 min. The maximum in vitro release was found to be 99.93% over a period of 120 min for formulation F12. Data of in vitro release from patches were fitted to different kinetic models such as Higuchi and Korsmeyer–Peppas models to explain the release profile. Formulations E7 and F7 were best fitted to the non-Fickian, where as formulations E12 and F12 showed Fickian/anomalous drug release. Stability studies indicated that there was no change in the chemical and physical characteristics during the test period.
Keywords: Buccal drug delivery, Mucoadhesive patches, Salbutamol sulfate, 32 full factorial design
1. Introduction
Beta 2-adrenergic agonists represent an effective therapeutic treatment option for asthma, bronchospasm and conditions with reversible airways obstruction (including chronic obstructive pulmonary disease [COPD]). Salbutamol sulfate (SS), a selective beta 2-adrenergic agonist and bronchodilator, is one of the widely used drugs for the treatment of the most respiratory diseases arising due to airway obstruction (Kelly and Murphy, 1992). SS is a hydrophilic drug with a dissociation constant of (pKa) 9.2 and a log P value of 0.11. The drug undergoes extensive first-pass metabolism with a plasma half-life of 4–6 h (Ahrens and Smith, 1984).
SS is readily absorbed from the GI tract and has a site-specific absorption in the stomach and upper part of small intestine. SS undergoes extensive metabolism via intestinal sulfonation and first pass metabolism in the liver, it also undergoes degradation in the colon. Owing to these reasons, the oral bioavailability of SS is approximately 40% (Swarbrick and Boylon, 2002; Goldstein et al., 1987). Therefore, SS is now rarely delivered via the oral route. It is usually given by inhalation or slow intravenous infusions (Ehab and Mina, 2007).
SS is usually administered via inhaled route for direct effect on bronchial smooth muscle. This is usually achieved through metered dose inhalers (MDIs), nebulisers or other proprietary delivery devices (e.g. Rotahaler or Autohaler). All these drug delivery systems have reported to have many disadvantages like inaccuracy of dosing (require correct actuation and inhalation coordination to deliver accurate dose), patient compliance due to the presence of chloro fluoro carbon (CFC), cost of the preparation and frequency of administration. In order to overcome these disadvantages, in the present work, we aimed to formulate SS mucoadhesive buccal patches. Bioadhesion is the condition in which two materials, at least one of which is biological in nature, are held together for extended periods of time by interfacial forces. If the adhesive attachment is with a mucous or mucous membrane, the phenomenon is referred as mucoadhesion (John, 2005). The interest of mucoadhesion is to increase the intimate contact of the dosage form at the adhesion site and to improve the bioavailability of the drug (Chowdary and Srinivasa Rao, 2003). Over the past two decades mucoadhesion has become an interesting topic for pharmaceutical researchers due to its potential to optimize localized drug delivery, by retaining a dosage form at the site of action or systemic delivery. Owing to the ease of administration, the oral cavity is an attractive site for the mucosal delivery of drugs. Through this route it is possible to realize mucosal (local effect) and transmucosal (systemic effect) drug administration. Buccal drug delivery involves the administration of the desired drug through the buccal mucosal lining of the oral cavity (Silvia et al., 2005; Nazila et al., 2005). Other than the common advantages of novel drug delivery systems, buccal mucosa has several specific advantages like, faster and richer blood flow, lesser thickness of the buccal mucosa and increased permeability, low enzymatic activity in the buccal mucosa and versatility in designing multidirectional or unidirectional release systems for local or systemic action (Punitha and Girish, 2010).
2. Materials and methods
2.1. Materials
Salbutamol sulfate (SS) was obtained as a gift sample from Dr. Reddy’s laboratories, India. Polymers hydroxypropyl methyl cellulose (HPMC), polyvinyl alcohol (PVA), and Carbopol 934p (Cp), were procured from Sigma Chemicals. Eudragit L-100 (95% dispersion) was obtained as a gift sample from Rohm Pharma, Germany. Agar, methanol, sodium saccharinate, sodium hydroxide, potassium dihydrogen phosphate, polyethylene glycol 400 (PEG-400) and propylene glycol (PG), were purchased from Merck (India). Tween-80 was a gift sample from Sd Fine Chemicals, Bangalore, India. Biaxially-oriented polypropylene (BOPP) film was supplied by Pidilite, India. Pig buccal mucosa was obtained from a slaughter house. All other reagents used were of analytical grade.
2.2. Methods
2.2.1. Formulation of mucoadhesive buccal patches
Salbutamol sulfate (SS) buccal mucoadhesive patches were prepared by the solvent casting technique (Patel et al., 2007) using different polymer combinations of Eudragit L-100 (EU-L100), HPMC, PVA and Carbopol. The experiment was designed using a 32 full factorial design (Design Expert, Version 7, Stat-Ease Inc., Minneapolis, MN). Different concentrations of polymer solutions were mixed in specified ratios as shown in Table 1. Eudragit L-100 (95%) was dissolved in ethanol, HPMC in ethanol:acetone mixture (3:1 v/v) and, PVA in water. To 5 mL of Eudragit dispersion (95%), 5 mL of ethanol and 0.05% of tween 80 were added and mixed well on a magnetic stirrer. To the above solution known quantities of PVA (2% m/v) and HPMC (5% m/v) or Carbopol (1% m/v) were added and mixed thoroughly. To this mixture, 2 mL of PG or PEG was added and mixed well on a magnetic stirrer, at low rpm, for a period of 1 h to get a homogenous clear, bubble free solution. To this mixture, a drug solution corresponding to 230.4 mg was added and mixed thoroughly to obtain uniform distribution of the drug. This solution was then poured into a specially fabricated Teflon® coated circular dish (9.6 cm diameter). Patches were then dried at room temperature for 2 h and were further dried for 18 h at 40 °C in a hot air oven. Finally, the patches were vacuum dried for 4 h at room temperature in a vacuum desiccator. After careful examination, the dried patches were removed, checked for any imperfections or air bubbles and cut into 2 cm diameter patches using a specially fabricated circular stainless steel cutter. The patches were laminated on one side with a water impermeable backing layer (Pidilite BOPP film). The samples were packed in aluminum foil and stored in a glass container at room temperature.
Table 1.
Composition of various patch formulations.
| Formulation | Eudragit L-100 (10% m/v) (mL) | HPMC K4M (5% m/v) (mL) | PVA (2% m/v) (mL) | Carbopol 934P (1% m/v) (mL) | Salbutamol sulfate (mg) | |
|---|---|---|---|---|---|---|
| E1 | F1 | 10.0 | 10.0 | 10.0 | 10.0 | |
| E2 | F2 | 8.5 | 8.5 | 12.8 | 10.0 | |
| E3 | F3 | 7.5 | 7.5 | 15.0 | 10.0 | |
| E4 | F4 | 8.5 | 12.8 | 8.5 | 10.0 | |
| E5 | F5 | 7.5 | 11.2 | 11.2 | 10.0 | |
| E6 | F6 | 6.6 | 9.9 | 13.3 | 10.0 | |
| E7 | F7 | 7.5 | 15.0 | 7.5 | 10.0 | |
| E8 | F8 | 6.6 | 13.3 | 9.9 | 10.0 | |
| E9 | F9 | 6.0 | 12.0 | 12.0 | 10.0 | |
| E10 | F10 | 10.0 | 10.0 | 10.0 | 10.0 | |
| E11 | F11 | 8.5 | 8.5 | 12.8 | 10.0 | |
| E12 | F12 | 7.5 | 7.5 | 15.0 | 10.0 | |
| E13 | F13 | 8.7 | 12.8 | 8.7 | 10.0 | |
| E14 | F14 | 7.5 | 11.2 | 11.2 | 10.0 | |
| E15 | F15 | 6.6 | 9.9 | 13.3 | 10.0 | |
| E16 | F16 | 7.5 | 15.0 | 7.5 | 10.0 | |
| E17 | F17 | 6.6 | 13.3 | 9.9 | 10.0 | |
| E18 | F18 | 6.0 | 12.0 | 12.0 | 10.0 | |
E1–E18: plasticizer used is PEG-400; F1–F18: plasticizer used is PG.
Total volume of polymer solution added excluding plasticizer and drug solution was 30 mL.
2.2.2. Evaluation of patches
2.2.2.1. Mass uniformity, thickness and folding endurance
Mass of the patch, thickness and folding endurance were determined for the patches without the backing membrane. Mass uniformity was tested in three different, randomly selected, individual patches from each batch using an electronic balance. The thickness of each patch was determined using a standard screw gauge at three different positions of the patch and the average was calculated. Folding endurance of the patches was determined by repeatedly folding one patch at the same place till it broke or folded up to 300 times without breaking (Khanna et al., 1997).
2.2.2.2. Drug content
The medicated patch (without backing membrane) was dissolved in 10 mL of simulated saliva solution (pH 6.2) for 2–3 h under occasional shaking. The resultant solution was filtered through 0.45 μm membrane filter paper. After suitable dilution, the amount of SS present in the patch was determined spectrophotometrically at 278 nm (Shimadzu 1800, Japan).
2.2.2.3. Measurement of surface pH
A modified method actually reported by (Bottenberg et al., 1991) was used to determine the surface pH of the buccal patches (without backing membrane). Buccal patches were allowed to swell for 2 h on the surface of an agar plate (prepared by dissolving 2% (m/v) agar in warmed isotonic phosphate buffer (pH 6.8) under stirring and then pouring the solution into a Petri dish till it gelled at room temperature). The surface pH was measured by bringing a combined glass electrode in contact with the surface of the patch, allowing it to equilibrate for 1 min. The average of the three readings was recorded.
2.2.2.4. Swelling studies
A drug loaded patch of (without backing membrane), 2 cm diameter was allowed to swell on the surface of an agar plate (prepared as described under Section 2.2.2.3) kept in an incubator maintained at 37 °C. Measurement of the diameter of the swollen patch was carried out at predetermined time intervals for 90 min.
Swelling index was calculated from the following equation:
where SI (%) is the percent swelling index, Dt is the diameter of the swollen patch after time t and Do is the original patch diameter at time zero (Patel et al., 2007).
2.2.2.5. Residence time (ex vivo mucoadhesion time)
A locally modified USP (Erweka ZT72) disintegration apparatus was used to determine the ex vivo mucoadhesion (residence) time (Nakamura et al., 1996). The mucosal membrane (fresh pig buccal mucosa) was separated by removing the underlying fat and loose tissues. The membrane was washed with distilled water and then with simulated saliva (pH 6.2) at 37 °C. Pig cheek mucosa, 3 cm long, was glued to the surface of a glass slide. One side of the patch was wetted with one drop of simulated saliva (pH 6.2) and pasted to the pig buccal mucosa by applying a light force with fingertip for 20 s. The glass slide was vertically fixed to the apparatus and allowed to move up and down so that the patch was completely immersed in the buffer solution at the lowest point and was out at the highest point. The beaker was filled with 800 mL of simulated saliva at (pH 6.2) and was kept at 37 ± 1 °C. The time required for the patch to detach from the buccal mucosa was recorded as the mucoadhesion time (mean of three determinations).
2.2.2.6. In vitro drug dissolution
USP 23 Type-2 rotating paddle dissolution test apparatus (Electrolab, EDT-08Lx) was used to study the dissolution profile of SS buccal patches (Nafee et al., 2003). The dissolution medium used was 100 mL simulated saliva solution (pH 6.2) at 37 ± 0.5 °C which was stirred at 50 rpm. The patch of 2 cm diameter was fixed on the glass disk with the help of a cyanoacrylate adhesive. The disk was put at the bottom of the dissolution vessel so that the patch remained on the upper side of the disk. Samples (4 mL) were withdrawn at pre-determined time intervals (5, 10, 15, 30, 45, 60, 90, 120, 150 and 180 min) and replaced with equal volume of dissolution medium. The samples were filtered through 0.45 μm filter and appropriately diluted with simulated saliva solution (pH 6.2) and assayed spectrophotometrically at 278 nm. The mechanism of drug release from the buccal patches was determined by finding the best fit of the release data to Higuchi and Korsmeyer–Peppas plots (Mathew et al., 2009; Higuchi, 1961).
2.2.2.7. In vitro drug permeation
The in vitro buccal permeation of SS was studied through the pig buccal mucosa using Franz-diffusion cell. Freshly obtained buccal mucosa was mounted between the donor and receptor compartments so that the smooth surface of the mucosa faced the donor compartment. The patch was placed on the mucosa and the compartments clamped together. The donor compartment was slightly wetted with 1 mL of simulated saliva. The receptor compartment was filled with isotonic phosphate buffer pH 7.4. The diffusion cell was thermostated at 37 ± 0.2 °C and the receptor compartment was stirred at a rate of 100 rpm (Nafee et al., 2003). One milliliter sample was withdrawn at pre-determined time intervals using a butterfly canula and syringe. The buffer was immediately replaced using blank pre-warmed buffer. After filtration through 0.45 μm filter and appropriate dilution the samples were analyzed for the drug content spectrophotometrically at 278 nm.
2.2.2.8. Accelerated stability studies and stability in human saliva
Selected patches were subjected to accelerated stability testing by wrapping them in aluminum foil and packing them in glass vials. These patches were kept in an incubator maintained at 37 ± 0.5 °C and 75 ± 5% RH for 6 months. Changes in the appearance, residence time and drug content of the stored patches were investigated after 1, 2, 3, 5 and 6 months. The data presented were the mean of three determinations. Stability of the selected patches was assessed in natural human saliva collected from healthy human adult volunteers. Patches were placed in separate Petri dishes containing 5 mL of human saliva and kept in a temperature-controlled oven at 37 ± 0.2 °C for 4 h. The patches were examined for changes in color, shape and drug content.
3. Results and discussion
In the present study, buccal patches of SS were prepared with different polymer combinations of Eudragit L-100, HPMC, PVA, and Cp using solvent casting method. A total of 36 formulations were prepared in triplicate using a 32 factorial design. Factorial design was used only to design the experiments. PG or PEG-400 was used as the plasticizer.
One of our major aims during the formulation step was to avoid use of organic solvents to prevent any unwanted residual solvent complications when the patch is used in vivo. But, due to the presence of Eudragit L-100 dispersion, which is insoluble in water, we used ethanol as a solvent in the formulation. However, the ratio of Eudragit L-100 dispersion to ethanol was kept to the minimum (1:1) by using tween 80 as a surfactant. The high drying time of the patches is possibly related to the major proportion of water which was used to solubilize the polymers.
Impermeable backing membrane is an essential part of buccal mucoadhesive patch to obtain unidirectional drug flow. Backing membrane prevents the loss of drug at the required site and also minimizes the exposure of other tissues to the drug by preventing bidirectional flow. Many authors have used ethyl cellulose as the backing membrane but reports shows that it has some permeability. Also laminating the patches with ethyl cellulose film was not completely successful (Mukherjee et al., 2005; Rowe et al., 2009). Therefore, in the current study, we have used BOPP film as the backing membrane.
Physico-chemical characteristics of the medicated patches are shown in Tables 2 and 3. The prepared patches were smooth, uniform in thickness, mass and drug content. Patches showed no visible cracks or folds. The thickness of the medicated patches ranged between 0.23 ± 0.008 and 0.59 ± 0.007 mm and the mass varied between 65.23 ± 3.3 and 117.92 ± 4.2 mg, patches with PEG-400 as a plasticizer showed increased mass. This observation could be correlated to the high molecular weight of PEG-400 when compared to PG. The surface pH of the patches ranged between 6 and 7 and no mucosal irritation was expected. Patches showed favorable drug loading which varied between 9.0 ± 0.3 and 10.05 ± 0.82 mg/2 cm2 patches (i.e. drug loading efficacy of 90–100%). All the patches had satisfactory folding endurance of >240. Formulations E7, E12, F7 and F12 showed high folding endurance of over 300, and hence were selected for further evaluation and characterization.
Table 2.
Physico-chemical characteristics of the SS patches with PEG-400 as plastizer.
| Formulation code | Mass uniformity (mg ± SD)a | Thickness (mm ± SD)a | Folding endurance (times)a | Drug content (mg ± SD)a | Drug loading efficiency (%)a | Surface pHa |
|---|---|---|---|---|---|---|
| E1 | 82.23 ± 4.3 | 0.55 ± 0.002 | 250 | 9.6 ± 0.6 | 96 ± 0.6 | 7.1 ± 0.05 |
| E2 | 86.93 ± 3.1 | 0.41 ± 0.006 | 253 | 9.2 ± 0.4 | 92 ± 0.4 | 6.2 ± 0.08 |
| E3 | 91.42 ± 4.4 | 0.42 ± 0.002 | 255 | 9.1 ± 0.52 | 91 ± 0.52 | 6.1 ± 0.12 |
| E4 | 93.18 ± 2.6 | 0.43 ± 0.003 | 260 | 9.9 ± 0.12 | 99 ± 0.12 | 6.9 ± 0.08 |
| E5 | 101.58 ± 4.5 | 0.42 ± 0.004 | 268 | 9.6 ± 0.87 | 96 ± 0.87 | 6.8 ± 0.04 |
| E6 | 104.91 ± 6.2 | 0.41 ± 0.003 | 266 | 9.0 ± 0.3 | 90 ± 0.3 | 6.2 ± 0.17 |
| E7 | 109.52 ± 7.2 | 0.48 ± 0.002 | >300 | 9.8 ± 0.9 | 98 ± 0.9 | 6.9 ± 0.08 |
| E8 | 114.28 ± 5.6 | 0.56 ± 0.006 | 272 | 9.0 ± 0.7 | 90 ± 0.7 | 6.8 ± 0.01 |
| E9 | 117.92 ± 4.2 | 0.57 ± 0.007 | 275 | 9.4 ± 0.5 | 94 ± 0.5 | 6.1 ± 0.04 |
| E10 | 69.98 ± 4.2 | 0.59 ± 0.001 | 255 | 9.3 ± 0.8 | 93 ± 0.8 | 6.5 ± 0.03 |
| E11 | 77.58 ± 3.2 | 0.55 ± 0.002 | 259 | 9.8 ± 0.43 | 98 ± 0.43 | 6.9 ± 0.07 |
| E12 | 78.99 ± 6.9 | 0.48 ± 0.003 | >300 | 9.4 ± 0.92 | 94 ± 0.92 | 7.1 ± 0.01 |
| E13 | 77.32 ± 3.3 | 0.58 ± 0.008 | 262 | 9.1 ± 0.54 | 91 ± 0.54 | 6.9 ± 0.06 |
| E14 | 81.64 ± 2.0 | 0.52 ± 0.009 | 268 | 9.8 ± 0.86 | 98 ± 0.86 | 6.8 ± 0.02 |
| E15 | 85.24 ± 6.9 | 0.32 ± 0.006 | 273 | 9.1 ± 0.9 | 91 ± 0.9 | 7.0 ± 0.03 |
| E16 | 81.66 ± 1.1 | 0.42 ± 0.007 | 275 | 9.6 ± 0.5 | 96 ± 0.5 | 7.1 ± 0.04 |
| E17 | 83.01 ± 1.0 | 0.39 ± 0.008 | 274 | 9.4 ± 0.95 | 94 ± 0.95 | 6.8 ± 0.11 |
| E18 | 84.99 ± 8.1 | 0.59 ± 0.002 | 279 | 9.3 ± 0.32 | 93 ± 0.32 | 6.2 ± 0.05 |
Average of three determinations.
Table 3.
Physico-chemical characteristics of the SS patches with PG as plastizer.
| Formulation code | Mass uniformity (mg ± SD)a | Thickness (mm ± SD)a | Folding endurance (times)a | Drug content (mg ± SD)a | Drug loading efficiency (%)a | Surface pHa |
|---|---|---|---|---|---|---|
| F1 | 78.12 ± 8.2 | 0.57 ± 0.005 | 240 | 10.01 ± 0.54 | 100 ± 0.54 | 6.8 ± 0.03 |
| F2 | 83.28 ± 6.4 | 0.42 ± 0.004 | 245 | 9.8 ± 0.91 | 98 ± 0.91 | 7.0 ± 0.12 |
| F3 | 86.99 ± 5.9 | 0.47 ± 0.008 | 249 | 9.5 ± 0.42 | 95 ± 0.42 | 6.1 ± 0.08 |
| F4 | 89.78 ± 8.4 | 0.58 ± 0.002 | 252 | 9.4 ± 0.84 | 94 ± 0.84 | 6.9 ± 0.04 |
| F5 | 97.46 ± 2.9 | 0.52 ± 0.009 | 257 | 9.9 ± 0.61 | 99 ± 0.61 | 7.0 ± 0.03 |
| F6 | 99.52 ± 6.8 | 0.59 ± 0.007 | 254 | 9.2 ± 0.94 | 92 ± 0.94 | 6.9 ± 0.01 |
| F7 | 103.62 ± 4.1 | 0.58 ± 0.005 | >300 | 9.7 ± 0.34 | 97 ± 0.34 | 6.5 ± 0.06 |
| F8 | 109.19 ± 4.8 | 0.51 ± 0.004 | 259 | 9.8 ± 0.74 | 98 ± 0.74 | 6.9 ± 0.12 |
| F9 | 112.22 ± 2.2 | 0.23 ± 0.008 | 260 | 9.9 ± 0.81 | 99 ± 0.81 | 6.8 ± 0.04 |
| F10 | 65.23 ± 3.3 | 0.47 ± 0.009 | 245 | 9.8 ± 0.72 | 98 ± 0.72 | 6.1 ± 0.08 |
| F11 | 72.99 ± 4.8 | 0.56 ± 0.007 | 249 | 9.8 ± 0.83 | 98 ± 0.83 | 7.0 ± 0.04 |
| F12 | 74.63 ± 3.9 | 0.59 ± 0.005 | >300 | 9.1 ± 0.94 | 91 ± 0.94 | 6.8 ± 0.04 |
| F13 | 72.99 ± 8.7 | 0.59 ± 0.004 | 252 | 10.05 ± 0.82 | 100 ± 0.82 | 6.1 ± 0.02 |
| F14 | 76.99 ± 4.9 | 0.52 ± 0.009 | 258 | 9.2 ± 0.94 | 92 ± 0.94 | 7.0 ± 0.01 |
| F15 | 81.89 ± 6.7 | 0.49 ± 0.002 | 263 | 9.18 ± 0.91 | 91 ± 0.91 | 7.1 ± 0.09 |
| F16 | 77.38 ± 91 | 0.48 ± 0.006 | 259 | 9.01 ± 0.45 | 90 ± 0.45 | 6.2 ± 0.06 |
| F17 | 79.89 ± 4.2 | 0.53 ± 0.005 | 264 | 9.4 ± 0.84 | 94 ± 0.84 | 6.0 ± 0.02 |
| F18 | 80.99 ± 9.9 | 0.39 ± 0.008 | 265 | 9.08 ± 0.52 | 90 ± 0.52 | 7.1 ± 0.13 |
Average of three determinations.
Swelling behavior of selected SS patches as a function of time is illustrated in Fig. 1. The swelling indices of the prepared patches were found to be moderate and varied between the formulations, which could be due to the presence of the water insoluble polymer Eudragit. It was difficult to interpret the relation between hydrophilicity of polymers and swelling index from these results, since the patch was composed of both hydrophilic and hydrophobic polymers. At the same time, when we consider the fact that all tested patches contained one part of Eudragit polymer and there by assuming that the effect of Eudragit in swelling of the patches as common and can be neglected, then the differences in swelling of the tested hydrophilic polymers could be explained by the difference in resistance of the matrix network structure (hydrogen bond) to the movement of water molecules (Panomsuk et al., 1995). While analyzing these observations, we should also account for the presence of water-soluble SS which might have improved the surface wetting of the matrix. The swelling indices of the selected patches were in the following order E12 > E7 > F12 > F7. There was a noticeable reduction in the swelling index of patches prepared using PG as plasticizer. The swelling indices of patches prepared with PG as plasticizer was as low as 29 (F7) and 31 (F12) where as that of the patches prepared by PEG as plasticizer was 41 and 35 for formulations E12 and E7, respectively. This observation suggests that the presence of PEG-400 would have altered the water distribution within such systems. This would have modified the matrix structure (Lucy et al., 1995). Even though the swelling indices were moderate, the patches did not show any appreciable changes in their shape and form and maintained their integrity during the study period.
Figure 1.
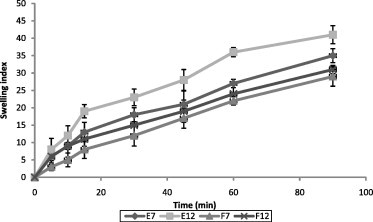
Swelling behavior of selected SS patches. Values presented as mean ± SD, n = 3.
The ex vivo mucoadhesion time is presented in Fig. 2. The residence time of the tested patches ranged between 101 and 110 min. Previous studies reported, no relation between mucoadhesion strength and mucoadhesion time (Nafee et al., 2003). However, none of the patches were detached from the mucosal membrane over the study period, which indicated that the bioadhesion of all patches was satisfactory to retain the patch on the buccal mucosa.
Figure 2.
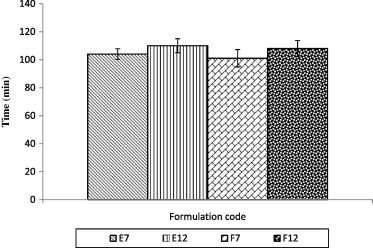
Ex vivo mucoadhesion time. Values presented as mean ± SD, n = 3.
In vitro release of SS from different patches is shown in Fig. 3. All the tested formulations released >80% of the drug within 90 min. Formulation F12 showed the maximum release of 99.93% at the end of 120 min. Formulation E7 showed slower drug release, and showed maximum drug release of 98.99% after 150 min. We could not detect any relationship between the drug release profile and polymer composition. The drug release profile of selected patches showed an erratic drug release, which is not ideal for controlled drug delivery systems. Controlled drug delivery systems release the drug through a variety of complex mechanisms, and are not yet completely defined. Many of these mechanisms are classified as either purely diffusion controlled or purely erosion controlled; many others can only be interpreted as being governed by both. The release data of the tested patches were analyzed on the basis of Korsmeyer–Peppas equation and Higuchi kinetics. The release rates ‘k’ and ‘n’ of each model were calculated by linear regression analysis using Microsoft Excel 2003 software. Coefficients of correlation (R2) were used to evaluate the accuracy of the fit (Mathew et al., 2009; Higuchi, 1961; Korsmeyer et al., 1983; Peppas, 1985). The R2, ‘k’ and ‘n’ values are given in Table 4.
Figure 3.
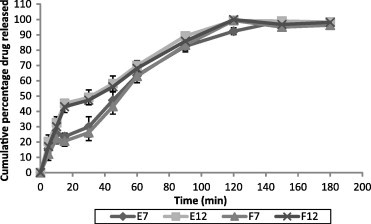
In vitro release of SS from different patch formulations. Values presented as mean ± SD, n = 3.
Table 4.
Values of R2, k and n for selected formulations.
| Formulation | Higuchi |
Korsmeyer–Peppas |
Mechanism of drug release | ||||
|---|---|---|---|---|---|---|---|
| R2 | y | k | R2 | y | n | ||
| E7 | 0.9484 | 0.8461x − 0.5352 | 0.8461 | 0.9601 | 0.552x − 1.2179 | 0.552 | Non-Fickian |
| E12 | 0.9532 | 0.7335x + 1.0874 | 0.7335 | 0.9675 | 0.4341x − 0.9342 | 0.4341 | Fickian |
| F7 | 0.9421 | 0.875x − 0.982 | 0.875 | 0.9548 | 0.6221x − 1.3639 | 0.6221 | Non-Fickian |
| F12 | 0.9547 | 0.7522x + 0.7581 | 0.7522 | 0.9613 | 0.4714x − 1.0149 | 0.4714 | Fickian |
The R2 values for Higuchi and Peppas kinetic models were calculated and compared. All the tested formulations gave good fit to the Korsmeyer–Peppas model. Formulations E12 and F12 exhibited Fickian release. Formulations E7 and F7 showed non-Fickian drug release. In non-Fickian model, the drug release varies with time (t) according to the power law. When swelling is predominant, drug diffusion probably occurs through the solvent-filled pathways of the swollen patch. Erosion of the matrix can also influence the drug release from this polymer matrix. A relative contribution of erosion and diffusion to the overall release mechanism is suggested. Since all the tested patches had water soluble and water insoluble polymers we could not correlate the difference in mechanism of drug release with the polymer properties.
The in vitro permeation of SS from different patches is shown in Fig. 4. The drug permeation was fast and showed the similar profile as that of the drug release. From formulation F12 showed a maximum drug permeation of 97.91% over a period of 180 min followed by E12 (97.52% at 120 min), F7 (96.98 at 120 min) and E7 (96.41 at 150 min). The results of drug permeation from buccal patches of SS through the porcine buccal mucosa reveal that SS was released from the formulations and permeated through the porcine buccal membrane and hence could possibly permeate through the human buccal membrane. There was a good correlation between the in vitro drug release and in vitro drug permeation study results. The correlation between in vitro release and in vitro permeation is shown in Fig. 5. The correlation coefficient (R2) between the drug permeation and drug release was as follows. Formulation E7 was 0.9996, E12 was 0.9997, F7 was 0.9997 and F12 was 0.9991.
Figure 4.
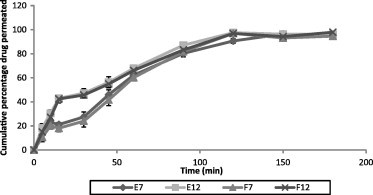
In vitro permeation of SS from different patch formulations. Values are presented as mean ± SD, n = 3.
Figure 5.
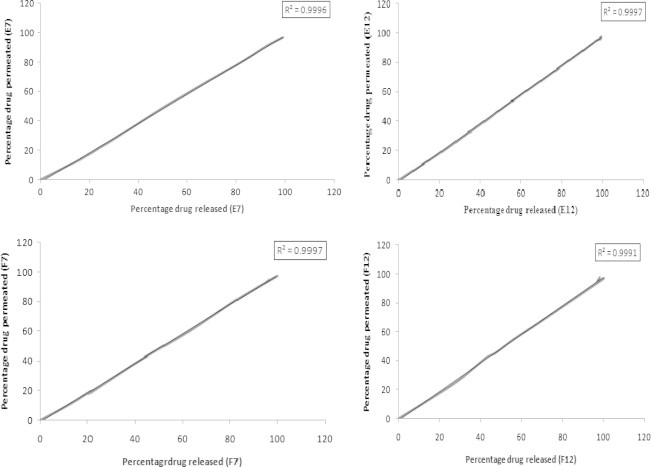
Correlation between in vitro release and in vitro permeation of selected SS patches.
Accelerated stability study data of the medicated patches are shown in Table 5. During and at the end of the accelerated stability study, the tested patches showed similar drug content as observed at the beginning of the study. They also showed satisfactory flexibility and elastic properties during and at the end of the accelerated study period. We have conducted the stability studies in normal human saliva since would appropriately mimic the stability of drug and device in the oral cavity in vivo. No color changes or unexpected change in the texture was observed. The drug content of the tested patches was in the range of 9.0 ± 0.1 and 9.7 ± 0.8 mg. The results of stability studies indicated that there was no influence on the chemical and physical stability of the formulations during the test period.
Table 5.
Physical stability characteristics of selected SS patches.
| Evaluation parameter | Formulation code | 1st month | 2nd month | 3rd month | 5th month | 6th month |
|---|---|---|---|---|---|---|
| Drug content (%)⁎ | E7 | 9.7 ± 0.6 | 9.7 ± 0.8 | 9.6 ± 0.6 | 9.6 ± 0.5 | 9.6 ± 0.6 |
| E12 | 9.3 ± 0.9 | 9.3 ± 0.8 | 9.2 ± 0.9 | 9.0 ± 0.2 | 9.0 ± 0.1 | |
| F7 | 9.6 ± 0.24 | 9.6 ± 0.34 | 9.5 ± 0.3 | 9.5 ± 0.24 | 9.5 ± 0.4 | |
| F12 | 9.1 ± 0.3 | 9.0 ± 0.62 | 9.0 ± 0.12 | 9.0 ± 0.9 | 9.0 ± 0.4 | |
| Residence time⁎ | E7 | 103 ± 2.9 | 103 ± 2.4 | 103 ± 3.2 | 101 ± 3.1 | 100 ± 2.5 |
| E12 | 110 ± 3.2 | 109 ± 4.1 | 109 ± 4.6 | 107 ± 3.5 | 107 ± 3.9 | |
| F7 | 100 ± 5.5 | 109 ± 5.1 | 109 ± 6.1 | 109 ± 5.1 | 107 ± 4.9 | |
| F12 | 106 ± 5.1 | 106 ± 5.7 | 105 ± 4.2 | 105 ± 4.3 | 105 ± 5.2 | |
| Appearance | E7 | No change | No change | No change | No change | No change |
| E12 | No change | No change | No change | No change | No change | |
| F7 | No change | No change | No change | No change | No change | |
| F12 | No change | No change | No change | No change | No change | |
Average of three determinations.
4. Conclusions
Novel mucoadhesive buccal patches of SS with unidirectional drug delivery were developed to overcome the first-pass metabolism and subsequent low bioavailability of the drug. The in vitro studies have shown that this is a potential drug delivery system for SS with considerably good stability and release profile. But, future in vivo studies are warranted to confirm these results in vivo.
References
- Ahrens R.C., Smith G.D. Albuterol: an adrenergic agent for use in the treatment of asthma pharmacology, pharmacokinetics and clinical use. Pharmacotherapy. 1984;4:105–121. doi: 10.1002/j.1875-9114.1984.tb03330.x. [DOI] [PubMed] [Google Scholar]
- Bottenberg P., Cleymaet R., De Muynck C., Remon J.P., Coomans D., Michotte Y., Slop D. Development and testing of bioadhesive, fluoride containing slow-release tablets for oral use. J. Pharm. Pharmacol. 1991;43:457–464. doi: 10.1111/j.2042-7158.1991.tb03514.x. [DOI] [PubMed] [Google Scholar]
- Chowdary K.P.R., Srinivasa Rao Y. Preparation and evaluation of mucoadhesive microcapsules of indomethacin. Saudi Pharm. J. 2003;11:97–103. [Google Scholar]
- Ehab R.B., Mina I.T. Enhanced transdermal delivery of salbutamol sulfate via ethosomes. AAPS PharmSciTech. 2007;8:E1–E8. doi: 10.1208/pt0804107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D.A., Tan Y.K., Soldin S.J. Pharmacokinetics and absolute bioavailability of salbutamol in healthy adult volunteers. Eur. J. Clin. Pharmacol. 1987;32:631–634. doi: 10.1007/BF02456001. [DOI] [PubMed] [Google Scholar]
- Higuchi T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961;50:874–875. doi: 10.1002/jps.2600501018. [DOI] [PubMed] [Google Scholar]
- John D.S. The basic and underlying mechanism of mucoadhesion. Adv. Drug Deliv. Rev. 2005;57:1556–1568. doi: 10.1016/j.addr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kelly H.W., Murphy S. Beta-adrenergic agonists for acute severe asthma. Ann. Pharmacother. 1992;26:81–91. doi: 10.1177/106002809202600115. [DOI] [PubMed] [Google Scholar]
- Khanna R., Agarwal S.P., Ahuja A. Preparation and evaluation of mucoadhesive buccal films of clotrimazole for oral Candida infections. Indian J. Pharm. Sci. 1997;59:299–305. [Google Scholar]
- Korsmeyer R.W., Gurny R., Doelker E., Buri P., Peppas N.A. Mechanism of potassium chloride release from compressed, hydrophilic, polymeric matrices: effect of entrapped air. J. Pharm. Sci. 1983;72:1189–1191. doi: 10.1002/jps.2600721021. [DOI] [PubMed] [Google Scholar]
- Lucy S.C.W., Paul W.S.H., Lee F.W. Matrix swelling: a simple model describing the extent of swelling of HPMC matrices. Int. J. Pharm. 1995;116:159–168. [Google Scholar]
- Mathew S.T., Devi S.G., Prasanth V.V., Vinod B. Formulation and in vitro–in vivo evaluation of ketoprofen-loaded albumin microspheres for intramuscular administration. J. Microencapsul. 2009;26:456–469. doi: 10.1080/02652040802420367. [DOI] [PubMed] [Google Scholar]
- Mukherjee B., Mahapatra S., Gupta R., Patra B., Tiwari A., Arora P. A comparison between povidone-ethylcellulose and povidone-eudragit transdermal dexamethasone matrix patches based on in vitro skin permeation. Eur. J. Pharm. Biopharm. 2005;59:475–483. doi: 10.1016/j.ejpb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Nafee N.A., Boraie M.A., Ismail F.A., Mortada L.M. Design and characterization of mucoadhesive buccal patches containing cetylpyridinium chloride. Acta Pharm. 2003;53:199–212. [PubMed] [Google Scholar]
- Nakamura F., Ohta R., Machida Y., Nagai T. In vitro and in vivo nasal mucoadhesion of some water-soluble polymers. Int. J. Pharm. 1996;134:173–181. [Google Scholar]
- Nazila S.M., Montakarn C., Thomas P.J. The use of mucoadhesive polymers in buccal drug delivery. Adv. Drug Deliv. Rev. 2005;57:1666–1691. doi: 10.1016/j.addr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Panomsuk S.P., Hatanaka T., Aiba T., Katayama K., Koizumi T. A study of the hydrophilic cellulose matrix: effect of endomethacine and a water soluble additive on swelling properties. Int. J. Pharm. 1995;126:147–153. [Google Scholar]
- Patel V.M., Prajapati B.G., Patel M.M. Design and characterization of chitosan-containing mucoadhesive buccal patches of propranolol hydrochloride. Acta Pharm. 2007;57:61–72. doi: 10.2478/v10007-007-0005-9. [DOI] [PubMed] [Google Scholar]
- Peppas N.A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985;60:110–111. [PubMed] [Google Scholar]
- Punitha S., Girish Y. Polymers in mucoadhesive buccal drug delivery system – a review. Int. J. Res. Pharm. Sci. 2010;1:170–186. [Google Scholar]
- Rowe Raymond C., Sheskey Paul J., Owen Sian C. sixth ed. Pharmaceutical Press; Washington, USA: 2009. Hand Book of Pharmaceutical Excipients. [Google Scholar]
- Silvia R., Giuseppina S., Caramella M.C. Buccal drug delivery: a challenge already won. Drug Discov. Today: Technol. 2005;2:59–65. doi: 10.1016/j.ddtec.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Swarbrick J., Boylon J.C. second ed. Marcel Dekker Inc.; New York, USA: 2002. Encyclopedia of Pharmaceutical Technology. [Google Scholar]


