Abstract
Free radical formation in heme proteins is recognized as a factor in mediating the toxicity of many drugs. Xenobiotics and drug therapy-related toxicity, due to oxidative modification of hemoglobin (Hb), has been attributed in part to the uncontrolled oxidative reactions. A variety of antioxidant strategies to ameliorate potential oxidative damage in vivo have been suggested. The present study was designed to evaluate the dose–response relationship of the free radical scavenging properties of silibinin dihemisuccinate (SDH) in nitrite-induced Hb oxidation in vitro and in vivo. Different concentrations of SDH were added, before and after different intervals of inducing Hb oxidation in erythrocytes lysate, and formation of methemoglobin (MetHb) was monitored spectrophotometrically; the same approach was utilized to evaluate the effect of the same doses of SDH on the integrity of erythrocytes after induction of hemolysis. Moreover, the most effective dose of SDH was administered in rats before challenge with toxic dose of sodium nitrite, and MetHb formation was monitored as mentioned before. The results showed that in both in vitro and in vivo models, SDH successfully attenuates Hb oxidation after challenge with sodium nitrite; this protective effect was not related to the stage of the catalytic stage of Hb oxidation, though the effect was more prominent when the compound was administered before nitrite. In conclusion, SDH can effectively, in concentration-dependent pattern, attenuate sodium nitrite-induced Hb oxidation and maintain integrity of red blood cells both in vitro and in vivo.
Keywords: Silibinin, Hemoglobin oxidation, Membrane fragility, Radical scavenging
1. Introduction
Increasing evidence suggests that free radical-induced oxidative damages lead to various pathological events including coronary heart disease, cancer and aging (Halliwell, 1996). In particular, lipid peroxidation in biological membranes has attracted much attention in relation to the deterioration of membrane structure and impairment of enzymatic functions (Mao and Poznansky, 1992; Koga et al., 1998). Reactive oxygen species have been implicated in damages of erythrocytes in patient with β-thalassemia, sickle cell anemia, glucose-6-phosphate dehydrogenase deficiency and other hemoglobinopathies (Hseu et al., 2002). The oxidation of erythrocyte membranes serves as a model for the oxidative damage of biomembranes (Einsele et al., 1985; Vissers et al., 1994). The oxidation of erythrocyte and its ghost membranes induced by free radicals have also been studied, and it has been found that free radicals generated in the aqueous phase attack the membrane to induce the chain oxidations of lipids and proteins and eventually cause hemolysis (Koga et al., 1998; Niki et al., 1988). Much interest exists in the possibility that antioxidants reduce the risk of degenerative diseases by inhibiting free radical induced oxidative damage (Halliwell, 1996). Therefore, several studies have examined both natural and synthetic antioxidants for the inhibition of lipid peroxidation in membrane systems. Flavonoids are naturally occurring substances that possess various pharmacological actions and therapeutic applications. Some due to their phenolic structures have antioxidant effect and inhibit free radical-mediated processes (Zhao, 2005; Mora et al., 1990). Silibinin, the major active flavonolignan in the seeds of milk thistle (Silybum marianum) is isolated from seeds. It is a free radical scavenger and a membrane stabilizer that prevents lipoperoxidation and its associated cell damage in some experimental models (Shalan et al., 2005). Silibinin is used clinically to treat chronic inflammatory liver disease and hepatic cirrhosis. Hepatoprotection can be attributed to its antioxidant properties by scavenging free radicals and increasing intracellular concentration of glutathione (DerMarderosian, 2001; Fleming, 2005). Silibinin received further attention due to its beneficial effects other than protection against tissue injury, effects partly bound to its radical-scavenging properties. These include mostly anticancer and chemopreventive actions (Gallo et al., 2003; Singh and Agarwal, 2006). Based on these precedents, it can be inferred that blocking of free radical propagation and lipid peroxidation would protect erythrocytes against the deleterious effect of oxidative stress due to exposure to chemicals like nitrites. In the present study, erythrocyte’s hemoglobin and plasma membrane were oxidized by sodium nitrite and the protective effect of different concentrations of silibinin dihemisuccinate on hemolysis and methemoglobin formation was investigated.
2. Experimental
2.1. Blood sample collection and preparation of lysate: in vitro study
Blood samples were obtained from healthy individuals by vein-puncture, and kept in ethylene diamine tetraacetic acid (EDTA) containing tubes; then centrifuged at 2500 rpm and 4 °C for 10 min to remove plasma and the buffy coat of white cells. The erythrocytes were washed thrice with phosphate buffer saline (PBS, pH 7.4) and lased by suspending in 20 volumes of 20 mM phosphate buffer (PB, pH 7.4) to yield the required hemolysate concentration of 1:20 (Doyle et al., 1982).
2.2. Preparation of silibinin dihemisuccinate sodium (SDHS) solution
Different concentrations of silibinin dihemisuccinate sodium (SDHS, Madaus, Germany) were prepared by dissolving the required quantity in phosphate buffer (pH 7.4) to prepare stock solution (10−3 mg/ml), from which serial dilutions were made to give concentrations of 10−15, 10−12, 10−9 and 10−6 mg/ml.
2.3. Effect of different SDHS concentrations on the time course of nitrite-induced oxidation of hemoglobin
In vitro model for oxidation of hemoglobin with sodium nitrite was utilized for production of methemoglobin (MetHb). To 1.5 ml freshly prepared hemolysate, 1.0 ml of different concentrations of sodium SDHS (10−6, 10−9, 10−12 and 10−15 mg/ml) were added each time concomitantly with 0.1 ml sodium nitrite (Analar BDH Ltd., Poole, England) (final concentration 6.0 mM), and the formation of MetHb was monitored spectrophotometrically at 631 nm for 50 min using computerized UV–visible spectrophotometer. Then to 1.5 ml freshly prepared hemolysate, 1.0 ml of the highly effective concentration of SDHS was added either 10 min before, or at 10 and 20 min after the addition of sodium nitrite to the hemolysate solution, and the formation of MetHb was monitored as previously mentioned.
2.4. Effect of SDH on the nitrite-induced osmotic fragility of red blood cells
Erythrocytes suspension was prepared by mixing a volume of fresh blood with 20 volumes of phosphate buffered saline (PBS, pH 7.4); aliquots (0.2 ml) of erythrocyte suspension (2.5% hematocrit) were added to 1.8 ml of buffered saline solutions of decreasing concentrations, pH 7.4 (NaCl concentration range of 9.0–1.0 g/l). Different concentrations of sodium SDHS (10−6, 10−9, 10−12 and 10−15 mg/ml) and 0.1 ml sodium nitrite (final concentration 6.0 mM) were incubated with the erythrocytes suspensions. The suspensions were allowed to stand for 30 min at room temperature, mixed again and then centrifuged for 5 min at 1200 rpm. The supernatants were obtained and the level of lysis was determined spectrophotometrically at 540 nm. The percentage of hemolysis was calculated from the ratios of the absorbance (Dacie et al., 2001).
2.5. In vivo study: experimental animals and treatment schedule
Seven to eight-weeks old female Wistar Albino rats, weighing 160–280 g were purchased from the animal house of the College of Pharmacy/Hawler Medical University. The animals were housed in the animal house of the College of Pharmacy/University of Sulaimani in well ventilated plastic cages at 24 ± 2 °C and 50 ± 10 relative humidity, and subjected to 12 h light/12 h dark cycle. They were acclimatized for 1 week before starting the experiments, during which they had free access to standard commercial diet purchased from (Iraqi Center for Cancer Research and Medical Genetics, Baghdad) and tap water ad libitum. After acclimatization of animals with the environment of the animal house, they were randomly allocated into three groups, each with six rats and treated as follows: first group, positive control group; in which 1.0 ml of normal saline (0.9% sodium chloride) was given orally; 1 h later, sodium nitrite (100 mg/kg) was given orally, after 45 min the animals were sacrificed by inhalation of high dose of anesthetic diethyl ether, and intra-cardiac blood samples were taken for measurement of MetHb level. Second and third groups: long- and short-term silibinin dihemisuccinate SDH (Tolbiac S.R., Argentina) exposure; in the former, SDH (100 mg/kg) once daily was given orally by gavage tube for 7 days; on the seventh day sodium nitrite (100 mg/kg) was given orally, 45 min later the animals were sacrificed by inhalation of high dose of anesthetic diethyl ether, and intra-cardiac blood samples were taken for measurement of MetHb level. In the third group, SDH (100 mg/kg) was given as a single oral dose; 1 h later, sodium nitrite (100 mg/kg) was given orally, after 45 min the animals were sacrificed by inhalation of high dose of anesthetic diethyl ether, and intra-cardiac blood samples were taken for measurement of MetHb level (Dacie et al., 2001).
2.6. Statistical analysis
Data are expressed as the means ± SD. All the data were analyzed using Student’s test and Microsoft Excel software. P < 0.05 was considered statistically significant.
3. Results
3.1. Effect of SDHS on the time-course of nitrite-induced oxidation of Hb
Fig. 1 showed that SDHS, in concentration dependent pattern, attenuates the rate of Hb oxidation and MetHb formation. Linear relationship was reported between SDHS concentrations and percent inhibition of MetHb formation (82.4%, 79.5%, 72% and 64.3%, respectively). The time required to convert 50% of the available Hb to MetHb was 25 min in the absence of SDHS (control), while it was delayed to 70, 89.4, 122 and 150 min in the presence of 10−6, 10−9, 10−12 and 10−15 mg/ml SDHS, respectively (Table 1).
Figure 1.
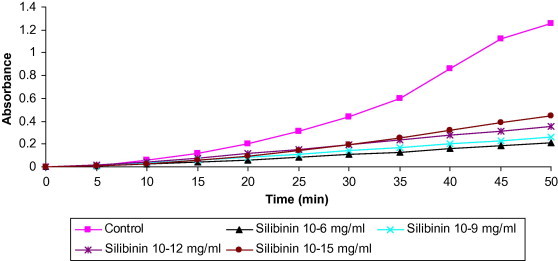
Effect of different concentrations of SDH (10−15, 10−12, 10−9 and 10−6 mg/ml) on the time-course of nitrite-induced oxidation of hemoglobin in erythrocyte lysate.
Table 1.
Effect of different concentrations of SDH (10−15, 10−12, 10−9 and 10−6 mg/ml) on the time-course of nitrite-induced oxidation of hemoglobin.
| Silibinin concentrations (mg/ml) | % Formation of MetHb | % Inhibition of MetHb | Time to form 50% MetHb (t1/2) (min) |
|---|---|---|---|
| Control | 100 | 0 | 25 |
| Silibinin 10−15 | 35.7 | 64.3 | 70 |
| Silibinin 10−12 | 28 | 72 | 89.4 |
| Silibinin 10−9 | 20.5 | 79.5 | 121.9 |
| Silibinin 10−6 | 17.6 | 82.4 | 150 |
Values represent mean of three experiments.
3.2. Effect of addition of SDHS at different time intervals
Addition of the highly effective concentration of SDHS (10−6 mg/ml) to the hemolysate at different time intervals (10 min before nitrite, 10 min and 20 min after nitrite addition, i.e., during autocatalytic phase) produced remarkable decrease in MetHb-related absorbance of light, attributed to significant inhibition of MetHb formation (83%, 77.4% and 75.3%, respectively, Fig. 2). The time required to convert 50% of the available Hb to MetHb was 25 min in the absence of SDHS (control), and delayed to 146, 110 and 101 min when SDHS (10−6 mg/ml) was added 10 min before nitrite, 10 min after and 20 min after nitrite addition, respectively (Table 2).
Figure 2.
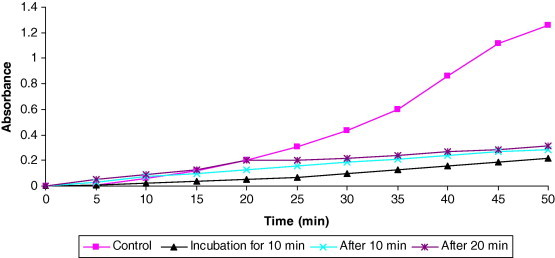
Effect of addition of SDH (10−6 mg/ml) at different time intervals (10 min before, 10 and 20 min after nitrite addition) on the time course of Hb oxidation erythrocyte lysate.
Table 2.
Effect of addition of SDH (10−6 mg/ml) at different time intervals (10 min before, 10 and 20 min after nitrite) on the time course of Hb oxidation in erythrocyte lysate.
| Time-course for addition of 10−6 mg/ml silibinin | % Formation of MetHb | % Inhibition of MetHb | Time to form 50% MetHb (t1/2) (min) |
|---|---|---|---|
| Control | 100 | 0 | 25 |
| Incubation before 10 min | 17 | 83 | 146 |
| Addition after 10 min | 22.6 | 77.4 | 110 |
| Addition after 20 min | 24.7 | 75.3 | 101 |
Values represent mean of three experiments.
3.3. Effect of different concentrations of SDHS on osmotic fragility of red blood cells
The resistance of erythrocytes to lysis by diluted buffer saline solutions of decreasing concentrations (NaCl range of 9.0–1.0 g/l) was assayed. In Fig. 3 and 50% RBC hemolysis occurred with 0.49% NaCl buffer saline solution when RBCs were treated with sodium nitrite alone; whereas 0.47%, 0.45%, 0.43% and 0.36% buffer saline solution needed to cause 50% lysis of RBCs when different concentrations of SDHS (10−15, 10−12, 10−9 and 10−6 mg/ml, respectively) were added to the incubation mixture in addition to sodium nitrite. The figure shows the difference in susceptibility of human RBCs to osmotic lysis when subjected to increasing hypotonicity with and without addition of SDHS, which shift the curve toward that of control RBCs which are not challenged by sodium nitrite.
Figure 3.
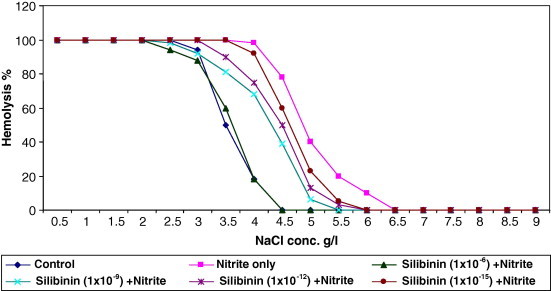
Effects of different concentrations of SDH on the osmotic fragility of red blood cells challenged with sodium nitrite in vitro.
3.4. Effect of SDH on nitrite-induced MetHb formation in rats
The results in Table 3 showed that MetHb% formed was significantly decreased (44.6%) in animals treated with SDH (100 mg/kg orally) once daily for 7 days (long term silibinin exposure) before induction of MetHb formation with orally administered sodium nitrite (100 mg/kg), compared with saline treated only animals (P < 0.05); whereas non-significant difference was observed when SDH (100 mg/kg) administered as single oral dose one hour before challenge with nitrite (short term silibinin exposure) compared to control group.
Table 3.
Effects of single and multiple doses of SDH (100 mg/kg) on nitrite-induced MetHb formation in rats.
| Type of treatment | n | % MetHb formation |
|---|---|---|
| Saline treated only | 6 | 55.6 ± 6.0 |
| Pre-treatment with SDH for 7 days | 6 | 30.8 ± 13.6⁎,a |
| Treatment with single dose SDH | 6 | 55.9 ± 9.3b |
Each value represents mean ± SD; n = number of animals.
Values with non-identical superscripts (a and b) were considered significantly different (P < 0.05).
Significantly different compared to saline treated group (P < 0.05).
4. Discussion
Sodium nitrite as a pro-oxidant induces a primary extensive MetHb formation as a result of generation of several free radical species like superoxide anion, hydroxyl, peroxynitrite and nitrogen oxide radicals which are implicated in promoting the autocatalytic stage of oxidation of Hb by nitrite (Tesorierre et al., 2001; Kumar et al., 2003); this reaction takes place with a peculiar S-shaped profile, and a noticeable increase in the rate of the process after a clear lag time (Lissi, 1998). In the present study, the formation of MetHb and the S-shaped curve obtained during kinetic behavior of Hb oxidation may provide an evidence for the formation of various free radicals during the process of hemoglobin oxidation, which seems compatible with previous findings reported by others (Lissi, 1998). Silibinin, the naturally occurring flavonolignan, acts mainly as an effective antioxidant (Wellington and Adis, 2001) and displays potential free radical scavenging property (Gharagozloo et al., 2008). Although the pharmacology of silibinin has been extensively studied, its molecular mechanisms of the antioxidative activity have not been systematically investigated and remain unclear (Gazak et al., 2009) and few studies have been devoted to the identification of silibinin active sites (Gyorgy et al., 1992). In the present study, the ability of different concentrations of SDHS to scavenge various types of free radicals was evaluated, and concentration–effect relationship was reported in the in vitro model of Hb oxidation in hemolysate. In accordance with the previous findings, several free radical species are generated during the course of nitrite-induced oxidation of Hb (Kumar et al., 2003). The present study has shown that SDHS can protect hemoglobin against oxidation by sodium nitrite in hemolysate. Structure–activity relationship (SAR) of flavonoids has been studied in details (Choi et al., 2002); however, only few data are known regarding SAR of flavonolignans. On the basis of these studies, the major determinants important for a high radical-scavenging capability were suggested, including the presence of catechol or pyrogallol group in ring B and the 3-OH group connected to a 2,3 double bond conjugated with the C-4 carbonyl group in the dehydrosilybin, a metabolite of silibinin (Gazak et al., 2009). Another factor which increases the potency of flavonoid to interact with radicals is the presence of a galloyl group in the molecule (Choi et al., 2002). Preventing the onset of the autocatalytic stage of nitrite-induced oxidation of Hb after addition of silibinin suggests that such protective effect might be due to its radical scavenging activity and not due to reduction of MetHb to Hb, because it fails to reverse the oxidized hemoglobin (i.e., the formed MetHb) after 10 and 20 min of addition of nitrite. Additionally, direct interaction between nitrite and silibinin, as a reason for protection, can be ruled out because the concentration of silibinin which protects the Hb is very low. SDH proved to be a powerful scavenger of OH• through different mechanisms, including addition reaction on the aromatic rings, abstraction of phenolic hydrogen and decarboxylation reaction (Khlebnikov et al., 2007). Furthermore, recent studies suggest that structural requirement for hydroxyl radical scavenging include the presence of the hydroxyl group in ring C, and probably 3′-methoxy-4′-hydroxylphenyl group in ring D. In spite of directly scavenging radicals (superoxide, hydrogen peroxide, nitric oxide and hydroxyl radicals) (Thomasset et al., 2007), the antioxidant effect of flavonoids may result from the interaction between flavonoids and metal ions, especially iron and copper, leading to chelate formation. It is believed that both iron chelation and free radical scavenging activities accounts for the antioxidative ability for flavonoids (Mira et al., 2002). However, few studies have investigated the molecular basis for these effects, where the polyphenol structure allows both the scavenging of free radicals, with concomitant formation of fairy stable aroxyl radicals, and chelation of transition metals including iron (El-Hajji et al., 2006). Silibinin, in addition to its antioxidant and free radical scavenging abilities, has the ability to act as iron chelator (Borsari et al., 2001); it possesses a hydroxyl group at C5 and C3 in addition to the carbonyl group at C4 and two carboxylate groups which may form chelates with divalent cation (Mora et al., 1990); the fact that hemoglobin Fe(II) can serve as a Fenton reagent catalyzing the conversion of H2O2 to OH• and OH−, and as long as is available to reduce the oxidized Fe(III), the catalytic cycle can continue to generate hydroxyl radicals (Kiefer and Snyder, 2000). Although the present study did not investigate the effect of silibinin as iron chelator, but utilizing hemoglobin oxidation system as a model cannot exclude this effect of silibinin. The protective effect of silibinin on hemolysate was found to be compatible with the reported protective effect of silymarin on erythrocyte hemolysate against benzo(a)pyren and exogenous reactive oxygen species (Kiruthiga et al., 2007). Oxidation of erythrocyte membrane induced by free radicals, which are generated in the aqueous phase, attack the membrane to induce a peroxidation of the unsaturated fatty acids and phospholipids that lead to hemolysis (Bensoltane et al., 2006). In the present study, erythrocytes were oxidized by sodium nitrite and the protective effect of silibinin on RBC hemolysis was investigated. Nitrite has a strong oxidant effect on RBC membrane and its hemoglobin; during the course of this reaction, reactive oxygen radicals are produced and start to induce a peroxidation of the unsaturated fatty acids of the phospholipid structure of the plasma membrane. Thus it appears like an osmotic brittleness of the erythrocyte membrane as well as a disturbance of membrane transport which leads to hemolysis (Bensoltane et al., 2006). The oxidative damage to hemoglobin molecule caused by inhaled nitrite can induce the dissociation of heme and globin chains, consequently forming polymerized globin aggregates known as Heinz bodies. These aggregates are attached to the membrane of the red blood cell, thereby altering its shape (Neuberger et al., 2002). Additionally hyper-polarization of erythrocyte membranes and an increase in membrane rigidity have been observed as a result of RBC oxidation by sodium nitrite. The interaction of nitrite with cell membrane also drastically modified the erythrocyte membrane (Zavodnik et al., 1999), and nitrite-induced methemoglobinemia in RBC lead to an inhibition of red blood cell sodium/proton exchanger; the membrane potential changes after exposure of RBCs to nitrite which reflects the alteration of the activity of membrane ionic exchangers. The decrease in membrane fluidity may result from the changes in the membrane structural state due to the oxidative process within the membrane provoking hemolysis (Batina et al., 1990). The present study shows that SDHS protects human erythrocytes against nitrite-induced oxidative hemolysis in concentration-dependent manner. Free radicals attack erythrocyte membrane components such as proteins and lipids, and change the structure and function of this membrane which may result in hemolysis (Vissers et al., 1994). Various studies indicated that silibinin significantly increases glutathione levels, which serve as a free radical scavenger (Das and Vasudevan, 2006). Silibinin is relatively hydrophobic and its penetration into the cell is limited (Sainz-Pardo et al., 1994) and membrane structures are postulated to be one of the cellular targets for silibinin as it interacts with the surface of lipid bilayer (Wesolowska et al., 2007), its effect on erythrocytes may be explained by their incorporation into these lipid bilayers of the cell membrane leading to reducing hemolysis. Silibinin on the other hand, is able to increase the activity of both superoxide dismutase and glutathione peroxidase, which may explain the protective effect of the drug against free radicals and also stabilizing the effect on the red cell membrane (Altorjay et al., 1992). The possibility that silibinin exerts its cytoprotective effects at the membrane level, as a chain breaking antioxidant, should also be considered. In fact, Miki and Mino (1985) established that hemolysis happens when the erythrocyte membrane alpha tocopherol concentration has lowered to a critically low level; therefore silibinin may interact with lipoperoxyl radicals and spare alpha tocopherol molecules (Miki and Mino, 1985). In conclusion, silibinin dihemisuccinate protects hemoglobin and erythrocyte plasma membrane against the oxidative damage induced by sodium nitrite, which may be attributed to direct radical scavenging properties.
Acknowledgments
The authors gratefully thank the College of Pharmacy, University of Sulaimani and University of Baghdad for supporting the present work.
References
- Altorjay I., Dalmi L., Sari B., Imre S. The effect of silibinin (Legalon) on the free radical scavenger mechanisms of human erythrocytes in vitro. Acta Physiol. Hung. 1992;80(1–4):375–380. [PubMed] [Google Scholar]
- Batina P., Fritsch P., Saint Blanquat G., Mitiavila M.T. In vitro kinetics of the oxidative reactivity of nitrate and nitrite in the rat erythrocytes. Food Addit. Contam. 1990;1:145–149. doi: 10.1080/02652039009373868. [DOI] [PubMed] [Google Scholar]
- Bensoltane S., Messerer L., Youbi M., Houria B. Effects of acute and sub-chronic ammonium nitrate exposure on rat liver and blood tissues. Afr. J. Biotechnol. 2006;5(9):749–754. [Google Scholar]
- Borsari M., Gabbi C., Ghelfi F., Grandi R. Silybin, a new iron-chelating agent. J. Inorg. Biochem. 2001;85:123–129. doi: 10.1016/s0162-0134(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Choi J., Chung H.Y., Kang S.S., Jung M.J. The structure–activity relationship of flavonoids as scavengers of peroxynitrite. Phytotherapy. 2002;16:132–235. doi: 10.1002/ptr.828. [DOI] [PubMed] [Google Scholar]
- Dacie J.V., Lewis S.M., Bain B.J., Bates I. Churchill Livingstone; Edinburgh: 2001. Practical Haematology. pp. 210–215. [Google Scholar]
- Das S.K., Vasudevan D.M. Protective effects of silymarin, a milk thistle (Silybium marianum) derivative on ethanol-induced oxidative stress in liver. Indian J. Biochem. Biophys. 2006;43:306–311. [PubMed] [Google Scholar]
- DerMarderosian, A., 2001. The Review of Natural Product. Fact and Comparison, St. Louis, pp. 405–409.
- Doyle M.P., Pickering R.A., Dykatra R.L. Nitrite-induced hemoglobin oxidation. Biochem. Biophys. Res. Commun. 1982;105:127–132. doi: 10.1016/s0006-291x(82)80020-x. [DOI] [PubMed] [Google Scholar]
- Einsele H., Clemens M.R., Remmer H. Effect of ascorbate on red blood cell lipid peroxidation. Free Radic. Res. Commun. 1985;1:63–67. doi: 10.3109/10715768509056537. [DOI] [PubMed] [Google Scholar]
- El-Hajji H., Nkhili E., Tomao V., Dangles O. Interactions of quercetin with iron and copper ions: complexation and autoxidation. Free Radic. Res. 2006;40:303–320. doi: 10.1080/10715760500484351. [DOI] [PubMed] [Google Scholar]
- Fleming T. Economic Company; New Jersey: 2005. PDR for Herbal Medicines Medical. pp. 566–567. [Google Scholar]
- Gallo D., Giacomelli S., Ferlini C., Raspaglio Antitumour activity of the silybin–phosphatidylcholine complex, IdB 1016, against human ovarian cancer. Eur. J. Cancer. 2003;39(16):2403–2410. doi: 10.1016/s0959-8049(03)00624-5. [DOI] [PubMed] [Google Scholar]
- Gazak R., Sedmera P., Vrbacky M., Vostalova J. Molecular mechanisms of silybin and 2,3-dehydrosilybin antiradical activity-role of individual hydroxyl groups. Free Radic. Biol. Med. 2009;46:745–758. doi: 10.1016/j.freeradbiomed.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Gharagozloo M., Khoshdel Z., Amirghofran Z. The effect of an iron (III) chelator, silybin, on the proliferation and cell cycle of Jurkat cells: a comparison with desferrioxamine. Eur. J. Pharmacol. 2008;589(1–3):1–7. doi: 10.1016/j.ejphar.2008.03.059. [DOI] [PubMed] [Google Scholar]
- Gyorgy I., Antus S., Foldiak G. Pulse radiolysis of silybin: one-electron oxidation of the flavonoid at neutral pH. Radiat. Phys. Chem. 1992;39:81–84. doi: 10.1080/09553009214551411. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic. Res. 1996;25:57–74. doi: 10.3109/10715769609145656. [DOI] [PubMed] [Google Scholar]
- Hseu Y.C., Chang W.C., Hseu Y.T., Lee C.Y. Protection of oxidative damage by aqueous extract from Antrodia camphorata mycelia in normal human erythrocytes. Life Sci. 2002;71:469–482. doi: 10.1016/s0024-3205(02)01686-7. [DOI] [PubMed] [Google Scholar]
- Khlebnikov A.I., Schepetkin I.A., Domina N.G., Kirpotina L.N. Improved quantitative structure–activity relationship models to predict antioxidant activity of flavonoids in chemical, enzymatic, and cellular systems. Bioorg. Med. Chem. 2007;15:1749–1770. doi: 10.1016/j.bmc.2006.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer C.R., Snyder M.L. Oxidation and erythrocyte senescence. Curr. Opin. Hematol. 2000;7(2):113–116. doi: 10.1097/00062752-200003000-00007. [DOI] [PubMed] [Google Scholar]
- Kiruthiga P.V., Shafreen R.B., Pandian S.K., Devi K.P. Silymarin protection against major reactive oxygen species released by environmental toxins: exogenous H2O2 exposure in erythrocytes. Basic Clin. Pharmacol. Toxicol. 2007;100(6):414–419. doi: 10.1111/j.1742-7843.2007.00069.x. [DOI] [PubMed] [Google Scholar]
- Koga T., Moro K., Terao J. Protective effect of a vitamin E analog, phosphatidylchromanol, against oxidative hemolysis of human erythrocytes. Lipids. 1998;33:589–595. doi: 10.1007/s11745-998-0244-4. [DOI] [PubMed] [Google Scholar]
- Kumar M.S., Unnikrishnan M.K., Patra S., Murthy K. Naringin and naringenin inhibit nitrite-induced methemoglobin formation. Pharmazie. 2003;58(8):564–566. [PubMed] [Google Scholar]
- Lissi E. Autocatalytic oxidation of hemoglobin by nitrite: a possible mechanism. Free Radic. Biol. Med. 1998;24(9):1535–1536. doi: 10.1016/s0891-5849(98)00004-5. [DOI] [PubMed] [Google Scholar]
- Mao G.D., Poznansky M.J. Electron-spin resonance study on the permeability of superoxide radicals in lipid bilyers and biological membranes. FEBS Lett. 1992;305:233–236. doi: 10.1016/0014-5793(92)80675-7. [DOI] [PubMed] [Google Scholar]
- Miki M., Mino M. Changes in RBC membrane lipids affected by two radical-producing systems and effect of alpha-tocopherol as a radical scavenger. J. Osaka Med. Cell. 1985;44:60–63. [Google Scholar]
- Mira L., Fernandez M.T., Santos M., Rocha R. Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic. Res. 2002;36:1199–1208. doi: 10.1080/1071576021000016463. [DOI] [PubMed] [Google Scholar]
- Mora A., Paya M., Rios J., Alcaraz M. Structure–activity relationships of polymetoxyflavones and other flavonoids as inhibitors of non-enzymatic lipid peroxidation. Biochem. Pharmacol. 1990;40:793–794. doi: 10.1016/0006-2952(90)90317-e. [DOI] [PubMed] [Google Scholar]
- Neuberger A., Fishman S., Golik A. Hemolytic anemia in G6PD-deficient man after inhalation of amyl nitrite (Poppers) IMAJ. 2002;4:1085–1086. [PubMed] [Google Scholar]
- Niki E., Komur E., Takahashi M., Urano S. Oxidative hemolysis of erythrocytes and its inhibition by free radical scavengers. J. Biol. Chem. 1988;263:19809–19814. [PubMed] [Google Scholar]
- Sainz-Pardo L.A., Anundit I., Miguez M.P., Lindors K.O. The effects of silymarin on the attachment and viability of primary cultures of rat hepatocytes. Toxicology. 1994;8:841–847. doi: 10.1016/0887-2333(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Shalan M.G., Mostafa M.S., Hassouna M.M., EI-Nabi S.E., EI-Refaie A. Amelioration of lead toxicity on rat liver with vitamin C and silymarin supplements. Toxicology. 2005;206:1–15. doi: 10.1016/j.tox.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Singh R.P., Agarwal R. Prostate cancer chemoprevention by silibinin: bench to bedside. Mol. Carcinogen. 2006;45(6):436–442. doi: 10.1002/mc.20223. [DOI] [PubMed] [Google Scholar]
- Tesorierre L., Allegra M., DiArpa D. Reaction of melatonin with hemoglobin-derived oxoferryl radicals and inhibition of the hydroperoxide-induced hemoglobin denaturation in red blood cells. J. Pineal Res. 2001;31:114–119. doi: 10.1034/j.1600-079x.2001.310204.x. [DOI] [PubMed] [Google Scholar]
- Thomasset S.C., Berry D.P., Garcea G., Marczylo T. Dietary polyphenolic phytochemicals: promising cancer chemopreventive agents in humans? A review of their clinical properties. Int. J. Cancer. 2007;120:451–458. doi: 10.1002/ijc.22419. [DOI] [PubMed] [Google Scholar]
- Vissers M.C., Stern A., Kuypers F., Van denBerg J. Membrane changes associated with lysis of red blood cells by hypochlorous acid. Free Radic. Biol. Med. 1994;16:703–712. doi: 10.1016/0891-5849(94)90185-6. [DOI] [PubMed] [Google Scholar]
- Wellington K., Adis B.J. Silymarin: a review of its clinical properties in the management of hepatic disorders. BioDrugs. 2001;15:465–489. doi: 10.2165/00063030-200115070-00005. [DOI] [PubMed] [Google Scholar]
- Wesolowska O., Lania-Pietrzak B., Kuzdzal M., Stanczak K. Influence of silybin on biophysical properties of phospholipids bilayers. Acta Pharmacol. Sin. 2007;28(2):296–306. doi: 10.1111/j.1745-7254.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- Zavodnik I.B., Lapshina E.A., Rekawiecka K., Zavodnik L.B. Membrane effects of nitrite-induced oxidation of human red blood cells. Biochim. Biophys. Acta. 1999;1421(2):306–316. doi: 10.1016/s0005-2736(99)00136-4. [DOI] [PubMed] [Google Scholar]
- Zhao B. Natural antioxidants for neurodegenerative diseases. Mol. Neurobiol. 2005;31:283–294. doi: 10.1385/MN:31:1-3:283. [DOI] [PubMed] [Google Scholar]


