Abstract
Sildenafil citrate (SIL) is used in the treatment of erectile dysfunction and other chronic disorders. For the pharmacokinetic investigation of SIL we developed a simple and sensitive method for the estimation of SIL in rat plasma by reverse phase high-performance liquid chromatography (RP-HPLC). The drug samples were extracted by liquid–liquid extraction with 300 μl of acetonitrile and 5 ml of diethyl ether. Chromatographic separation was achieved on C18 column using methanol:water (85:15 v/v) as mobile phase at a flow rate of 1 ml/min and UV detection at 230 nm. The retention time of SIL was found to be 4.0 min having a separation time less than 5 min. The developed method was validated for accuracy, precision, linearity and recovery. Linearity studies were found to be acceptable over the range of 0.1–6 μg/ml. The method was successfully applied for the analysis of rat plasma sample for the application in pharmacokinetic study, drug interaction, bioavailability and bioequivalence.
Abbreviations: SIL, sildenafil citrate; DAD, diode-array detection; % RSD, % relative standard deviation
Keywords: Sildenafil citrate, RP-HPLC, Rat plasma
1. Introduction
Sildenafil citrate, 1-[4-ethoxy-3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)phenylsulfonyl]-4 methylpiperazine, is primarily indicated in the treatment of erectile dysfunction (Vardi and Nini, 2007). It acts by inhibiting cGMP-specific phosphodiesterase type 5, an enzyme that promotes degradation of cGMP, which regulates the blood flow in the penis. The chemical structure of SIL is shown in Fig. 1.
Figure 1.
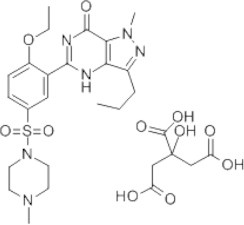
Chemical structure of SIL.
Literatures have been reported for the estimation of SIL in the human plasma and biological samples. Methods such as high-performance liquid chromatography (HPLC) have been reported for the determination of SIL separately in biological samples. Gas chromatography–mass spectrometry (GC/MS) (Saisho et al., 2001), micellar electrokinetic chromatography (Nevado et al., 2002), liquid chromatography–mass spectrometry (LC/MS) (Weinmann et al., 2001; Dumestre-Toulet et al., 2002) as well as liquid chromatography–tandem mass spectrometry (LC/MS/MS) (Eerkes et al., 2002; Kim et al., 2003; Wang et al., 2005) methods have also been reported. Liquid chromatography–mass spectrometry and liquid chromatography–tandem mass spectrometry (LC/MS/MS) are expensive and thus unavailable in many laboratories. High-performance liquid chromatographic methods with UV detection have been reported for the simultaneous determination of SIL and its active metabolite (Hyland et al., 2000; Cooper et al., 1997; Jeong et al., 2001; Liaw and Chang, 2001; Guermouchea and Bensalah, 2006).
2. Experimental
2.1. Instrumentation
A double beam UV–vis spectrophotometer, model UV-2401 PC (Japan) with 10 mm matched quartz cell was used.
The HPLC instrument consisted of thermo separation product quaternary gradient equipped with a pump spectra system P-4000 having an inline membrane degasser, detector was a UV–vis detector belonging to spectra system UV 1000 and Rheodyne 9725 injector with 20 μl loop. All the data were processed using Data Ace software. Separation was achieved using a Prontosil C18 stationary phase (150 × 4.6 mm i.d. 5 μm particle size) and the analytical column was protected by a Phenomenex C18 guard column (4 × 2.0 mm, i.d.).
2.2. Materials and reagents
Sildenafil citrate was donated by Ajanta Pharmaceuticals Pvt. Ltd. All the reagents and chemicals used were of AR analytical and HPLC grade. Methanol (Spectrochem) and water (Lobachem) used were of HPLC grade.
2.3. Chromatographic conditions
All determinations were carried out at room temperature. The isocratic separation of compounds was carried out by using mobile phase consisting of methanol:water (85:15 v/v). The flow rate was maintained at 1 ml min−1. The volume of injection was 20 μl. The mobile phase was filtered through 0.45 μm membrane filter and degassed by ultrasonification.
2.4. Preparation of standard solutions
2.4.1. Sildenafil citrate stock and working solutions
The stock solution of SIL was prepared by dissolving 10 mg in 100 ml of methanol and further dilutions were prepared in methanol to obtain the working solution of SIL in the range of 0.1–6 μg/ml.
2.5. Preparation of sample
Plasma samples were stored at −20 °C and allowed to thaw at room temperature before processing. In brief, to 100 μl of plasma, 100 μl aliquot of working standard solution of SIL was added in polypropylene centrifuge tubes and then were added 300 μl of acetonitrile and 5 ml of diethyl ether. Then tubes were centrifuged for 10 min at 3000 rpm. The clear supernatant layer was transferred into another conical glass tube and organic layer completely evaporated at room temperature. After evaporation the remaining things were dissolved in mobile phase. Resultant samples were injected in developed chromatographic conditions.
2.6. Application of the assay
The above method was successfully applied for the pharmacokinetic studies of SIL citrate in rats. Sprague–Dawley rats (200–250 g) were housed with free access to food and water. The rats were fasted overnight with free access to water before administration of drugs. After a single oral administration of 2.5 mg/kg of SIL, 0.5 ml of blood samples were collected from the retro orbital plexus sinus at 0.5, 1, 2, 4, 6, 12 and 24 h time-points. Plasma was separated by centrifugation and stored at −20 °C until analysis. Aliquots of 0.1 ml serum samples were processed and analyzed for SIL concentrations.
The pharmacokinetic parameters were calculated with a non-compartmental model using Kinetica TM Soft-ware (version 4.4.1 Thermo Electron Corporation, USA). Each value is expressed as mean ± SD.
3. Results and discussion
3.1. Method validation
3.1.1. Selectivity and specificity
Blank plasma was studied for endogenous interference. A representative chromatogram of the plasma blank is shown in Fig. 2a. No additional peaks of endogenous substances were observed. Fig. 2b shows the chromatograms of calibration standard containing 5 μg/ml of SIL in plasma.
Figure 2.
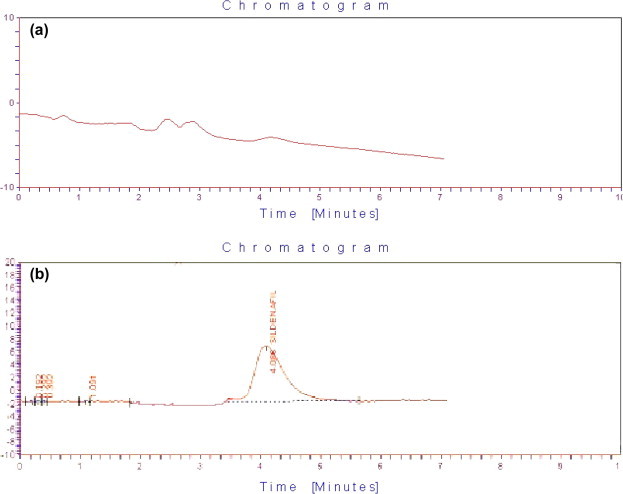
HPLC trace of SIL using ultraviolet detection at 230 nm. (a) Blank plasma sample; (b) quality control standard (5 μg/ml).
3.1.2. Linearity and limit of quantitation
Linear calibration curves with correlation coefficients greater than 0.9999 were obtained over the concentration range of 0.1–6 μg/ml for SIL in plasma. The co-efficient of regression i.e., r2 = 0.9996 for SIL. The results had shown that within the concentration range indicated there was an excellent correlation between peak area ratio and each concentration of SIL.
The limit of quantitation, defined as the lowest concentration was analyzed with an accuracy of ±15% and a co-efficient of variation of <15%, 0.16 μg/ml for SIL in plasma.
3.1.3. Accuracy
Accuracy studies were performed for SIL in terms of recovery studies. For this 0.2, 0.4 and 0.6 μg/ml of both drugs in plasma were injected and % recovery and % RSD were calculated. (See, Table 1).
Table 1.
Results of accuracy studies.
| Sr. No. | Nominal concentration (μg/ml) SIL | Recovered amount (μg/ml) SIL | Accuracy (%) SIL |
|---|---|---|---|
| 01 | 0.2 | 0.195 | 97.5 |
| 02 | 0.4 | 0.390 | 97.5 |
| 03 | 0.6 | 0.598 | 99.6 |
3.1.4. Precision
Inter-day and intra-day precision studies were done by injecting three serial dilutions in developed chromatographic conditions (n = 6). For precision studies 0.2, 0.4 and 0.8 μg/ml were injected (n = 6). Peak areas were calculated for % RSD values, results for inter-day and intra-day precision are shown in Table 2.
Table 2.
Results of precision studies (inter-day and intra-day).
| Sr. No. | Concentration of drug solution (μg/ml) | Inter-day precision |
Intra-day precision |
||
|---|---|---|---|---|---|
| Peak area SIL | % RSD | Peak area SIL | % RSD | ||
| 1 | 0.2 | 29.3 ± 0.52 | 2.09 | 28.5 ± 0.45 | 2.01 |
| 2 | 0.4 | 56.54 ± 0.66 | 2.04 | 57.24 ± 0.66 | 2.03 |
| 3 | 0.8 | 205.43 ± 0.60 | 0.92 | 203.13 ± 0.45 | 0.98 |
3.1.5. Extraction recovery
Extraction recovery of SIL was determined by comparing peak areas obtained from extracted plasma samples with those found by extracting blank matrices through the extraction procedure and spiking with a known amount of SIL. The results showed that the mean extraction recoveries of SIL were >85% at concentrations of 1.0, 5.0, and 10.0 μg/ml, respectively (Table 3). Different organic extraction solvents were evaluated in the experiment, including methanol, acetonitrile, chloroform and diethyl ether. Diethyl ether and acetonitrile combination proved to be the most efficient in extracting SIL from plasma and had a small variation in extraction recoveries over the concentration range.
Table 3.
The percentage extraction recovery of measurement of SIL from plasma.
| Nominal concentration (μg/ml) | Mean % recovery |
|
|---|---|---|
| GLIM | SIL | |
| 1 | 91.2 | 89.3 |
| 5 | 86.4 | 88.5 |
| 10 | 89.7 | 89.4 |
Averaged for six measurements at each concentration level (n = 6);
3.2. Application of the analytical method in pharmacokinetic studies
The described method was applied to a pharmacokinetic study in rats. After a single oral administration of SIL (2.5 mg/kg) to rats, plasma concentrations were determined over a period of 24 h after administration. The mean serum concentration–time curve after an oral dose of SIL (2.5 mg/kg) is shown in Fig. 3 and the main pharmacokinetic parameters are summarized in Table 4. The Cmax of SIL detected in the rats was 2.19 μg/ml, and the Tmax was 4 h.
Figure 3.
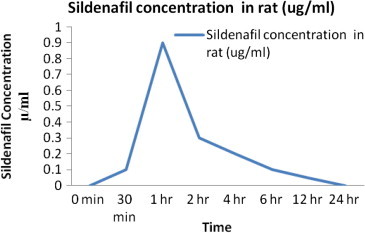
Mean serum concentration–time profile of SIL after oral administration of 2.5 mg/kg of SIL in rats.
Table 4.
The main pharmacokinetic parameters of mean drug serum concentration time curve (mean ± SD, n = 6) of SIL in rats after single oral administration of 2.5 mg/kg of SIL.
| Drugs | AUCo-t (μg h/ml) | AUC 0–∞ (μg h/ml) | Cmax (μg) | Tmax (h) | Kel | T1/2 (h) |
|---|---|---|---|---|---|---|
| SIL | 2.19 ± 0.052 | 0.5 ± 0.02 | 0.8 ± 0.05 | 1 h | 0.10 ± 0.01 | 6.93 |
4. Conclusion
In the present study a simple, accurate, and precise method was developed for the estimation of SIL by RP-HPLC. The developed method was simple employing water and not a buffer as component of mobile phase. The developed method was short with elution of SIL less than 5 min and specific with no interferences of blank matrix interfering with the quantification of SIL. The developed method was applied successfully for pharmacokinetic studies of SIL in rats. The applicability of method suggests its further application for bioequivalence, bioavailability and drug interaction studies.
Footnotes
Peer review under responsibility of King Saud University.
References
- Cooper J.D.H., Muirhead D.C., Taylor J.E., Baker R.P. Development of an assay for the simultaneous determination of sildenafil (viagra) and its metabolite (UK-103,320) using automated sequential trace enrichment of dialysates and high-performance liquid chromatography. J. Chromatogr. B. 1997;701:87–95. doi: 10.1016/s0378-4347(97)00339-3. [DOI] [PubMed] [Google Scholar]
- Dumestre-Toulet V., Cirimele V., Gromb S., Belooussoff T., Lavault D., Ludes B., Kintz P. Last performance with VIAGRA®: post-mortem identification of sildenafil and its metabolites in biological specimens including hair sample. Forensic Sci. Int. 2002;126:71–76. doi: 10.1016/s0379-0738(02)00012-9. [DOI] [PubMed] [Google Scholar]
- Eerkes A., Addison T., Naidong W. Simultaneous assay of sildenafil and desmethylsildenafil in human plasma using liquid chromatography–tandem mass spectrometry on silica column with aqueous–organic mobile phase. J. Chromatogr. B. 2002;768:277–284. doi: 10.1016/s1570-0232(01)00602-x. [DOI] [PubMed] [Google Scholar]
- Guermouchea M.H., Bensalah K. Solid phase extraction and liquid chromatographic determination of sildenafil and N-demethylsildenafil in rat serum with basic mobile phase. J. Pharm. Biomed. Anal. 2006;40:952–957. doi: 10.1016/j.jpba.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Hyland R., Roe E.G.H., Jones B.C., Smith D.A. Effect of P-glycoprotein modulation on the clinical pharmacokinetics and adverse effects of morphine. Br. J. Clin. Pharmacol. 2000;51:239–248. doi: 10.1046/j.1365-2125.2000.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong C.K., Lee H.Y., Jang M.S., Kim W.B., Lee H.S. Narrow bore high-performance liquid chromatography for the simultaneous determination of sildenafil and its metabolite UK-103,320 in human plasma using column switching. J. Chromatogr. B. 2001;752:141–147. doi: 10.1016/s0378-4347(00)00536-3. [DOI] [PubMed] [Google Scholar]
- Kim J., Ji H., Kim S., Lee H., Lee S., Kim D., Yoo M., Kim W., Lee H. Simultaneous determination of sildenafil and its active metabolite UK-103,320 in human plasma using liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2003;32:317–322. doi: 10.1016/s0731-7085(03)00088-8. [DOI] [PubMed] [Google Scholar]
- Liaw J., Chang T.W. Determination of transdermal sildenafil in nude mouse skin by reversed-phase high-performance liquid chromatography. J. Chromatogr. B. 2001;765:161–166. doi: 10.1016/s0378-4347(01)00421-2. [DOI] [PubMed] [Google Scholar]
- Nevado J.J.B., Flores J.R., Penalvo G.C., Farinas N.R. Determination of sildenafil citrate and its main metabolite by sample stacking with polarity switching using micellar electrokinetic chromatography. J. Chromatogr. A. 2002;953:279–286. doi: 10.1016/s0021-9673(02)00131-0. [DOI] [PubMed] [Google Scholar]
- Saisho K., Scott K.S., Morimoto S., Nakahara Y. Extraction and determination of sildenafil (viagra) and its N-desmethyl metabolite in rat and human hair by GC–MS. Biol. Pharm. Bull. 2001;24:1384–1388. doi: 10.1248/bpb.24.1384. [DOI] [PubMed] [Google Scholar]
- Vardi M., Nini A. Phosphodiesterase inhibitors for erectile dysfunction in patients with diabetes mellitus. Cochrane Database Syst. Rev. 1. 2007:CD002187. doi: 10.1002/14651858.CD002187.pub3. (PMID 17253475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang J., Cui Y., Fawcett J., Gua J. Liquid chromatographic–tandem mass spectrometric method for the quantitation of sildenafil in human plasma. J. Chromatogr. B. 2005;828:118–121. doi: 10.1016/j.jchromb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Weinmann W., Bohnert M., Wiedemann A., Renz M., Lehmann N., Pollak S. Post-mortem detection and identification of sildenafil (viagra) and its metabolites by LC/MS and LC/MS/MS. Int. J. Legal Med. 2001;114:252–258. doi: 10.1007/s004140000178. [DOI] [PubMed] [Google Scholar]



