Abstract
OBJECTIVES
While neoadjuvant chemoradiation followed by surgery has been shown to improve the survival of patients with locally advanced oesophageal cancer, it is not known whether neoadjuvant chemoradiation has a beneficial or harmful effect on the non-responders. We aimed to compare the outcomes among neoadjuvant chemoradiation responders, non-responders and patients receiving primary oesophagectomies for resectable locally advanced oesophageal squamous cell carcinoma.
METHODS
Eighty-four non-T1–2N0 oesophageal squamous cell carcinoma patients were included. Thirty-eight patients received primary resection and 46 patients received neoadjuvant chemoradiation. The overall survival of chemoradiation responders (<50% residual tumour), non-responders (>50% residual tumour and those who shifted to definitive chemoradiation instead of surgery due to tumour progression) and patients receiving primary resection were compared. Clinical parameters were also compared between responders and non-responders.
RESULTS
There was no overall difference in survival between neoadjuvant chemoradiation and primary resection groups (2-year overall survival rates: 45.6 vs 54.3%, P = 0.442). In patients receiving neoadjuvant chemoradiation followed by surgery, pathological responders had significantly higher 2-year overall survival rates than non-responders (64.5 vs 38.9%, P = 0.043). While the pathological responders had the highest survival rate, clinicopathological non-responders (pathological non-responders and patients with tumour progression during the neoadjuvant chemoradiation period) demonstrated significantly worse outcomes than those receiving primary resection (32.0 vs 54.3%, P = 0.036). However, none of the clinical parameters, including blood profiles, images and baseline tumour characteristics, predicted the response to chemoradiation before treatment.
CONCLUSIONS
Neoadjuvant chemoradiation non-responders demonstrated no benefit and an even worse outcome compared with those receiving primary resection for locally advanced oesophageal squamous cell carcinoma. However, no significant clinical parameters could be implemented in the clinics to predict the response to neoadjuvant chemoradiation before treatment.
Keywords: Oesophageal cancer, Neoadjuvant chemoradiation, Squamous cell carcinoma, Survival
INTRODUCTION
Oesophageal cancer is one of the most deadly cancers with a rapidly rising incidence. Even after resection with curative intent, the 5-year survival is rarely greater than 25% [1]. Furthermore, a large number of patients develop either distant metastases or locoregional recurrence within the first year after surgery, and the prognosis for these patients is dismal [2]. Given that surgery alone does not result in the complete cure of patients with advanced oesophageal cancer, multimodal therapy becomes necessary. Strategies of neoadjuvant chemoradiation have been applied in an attempt to improve survival in patients with locally advanced oesophageal cancer by downstaging the disease and thus increasing the curative resection rate in the subsequent surgery. However, the results of neoadjuvant chemoradiation for oesophageal cancer are variable. In the CALGB 9781 trial, which assigned T1–3Nx patients to either oesophagectomy alone or trimodality therapy consisting of cisplatin and fluorouracil concurrent with radiation therapy followed by oesophagectomy, the median survival was 4.48 vs 1.79 years in favour of trimodality therapy [3]. In a recent multicentre, randomized, controlled study comparing surgery alone and chemotherapy concurrent with radiotherapy followed by surgery for T1N1 or T2–3N0–1 oesophageal cancer, significant survival benefit was also noted in the preoperative chemoradiation group. The median overall survival was 49.4 months in the chemoradiation–surgery group vs 24 months in the surgery group [4]. However, such benefit could not be applied to all patients receiving preoperative chemoradiation. There have been randomized trials showing no survival difference between preoperative chemoradiation and surgery alone [5–7]. The potential explanation for this discrepancy is the heterogeneity of the patient populations, which implies that only patients with a good response to chemoradiation may enjoy a survival advantage. Indeed, many studies have identified pathological tumour response as a significant prognostic factor after preoperative chemoradiation and shown dramatic survival benefits in patients with complete or partial pathological responses to preoperative chemoradiation [8, 9]. However, only approximately 15–40% of oesophageal cancer patients who undergo neoadjuvant chemoradiation would achieve a pathological complete response and would benefit from this treatment protocol [3–9]. Given the potential morbidity, exposure to the risks of toxicity from the treatment, the delay to surgery and potentially higher rates of postoperative complications, neoadjuvant chemotherapy seems to be worse than useless for non-responders. Therefore, we hypothesized that the non-responders after neoadjuvant chemoradiation for locally advanced oesophageal cancer would have worse outcomes compared with patients receiving primary resection.
In this study, we aim to compare the outcomes between patients undergoing primary resection and neoadjuvant chemoradiation for resectable locally advanced oesophageal squamous cell carcinoma. We focus on the survival difference among neoadjuvant chemoradiation responders, non-responders and patients receiving primary oesophagectomies. In addition, whether any clinical parameter could predict neoadjuvant chemoradiation response, which is important for tailored treatment, is evaluated.
PATIENTS AND METHODS
Patients
We carried out a retrospective review of the prospectively collected database that was maintained by Taipei Veterans General Hospital Cancer Council. Between January 2010 and December 2011, there were 253 oesophageal cancer patients admitted to Taipei Veterans General Hospital. The treatment plan of each patient was determined by multidisciplinary tumour board discussions, and was in accordance with National Comprehensive Cancer Network (NCCN) guidelines [10]. In this study, we excluded the following patients: (i) non-squamous cell carcinoma histology; (ii) definitive chemoradiation treatment due to advanced stage of tumour or medically unfit for surgery; and (iii) clinical stage T1–2N0 tumours. Only operable patients with resectable, locally advanced non-T1–2N0 oesophageal squamous cell carcinoma were included.
Staging work-up
All patients were histologically confirmed before treatment. The staging work-up included a physical examination, laboratory tests, upper gastrointestinal tract endoscopy, flexible bronchoscopy (for upper-third and middle-third tumours), computed tomography (CT) scans from the neck to the upper abdomen, positron emission tomography/computed tomography (PET/CT) and cardiopulmonary function tests before the surgical intervention. The endoscopic ultrasound (EUS) was an optional procedure, but necessary for confirmation of cT1 lesion. Lymph nodes measuring >1 cm in diameter on image studies or with uptake on PET/CT were regarded as clinically involved.
Chemoradiation
In the neoadjuvant treatment group, two courses of chemotherapy were provided with an intervening interval of 4 weeks. The chemotherapy regimen included 80 mg/m2 of cisplatin intravenously on day 1 followed by 600 mg/m2/day of 5-fluorouracil and 90 mg/m2/day of leucovorin given by continuous intravenous infusion on days 1–4, concurrent with 45.0–50.4 Gy of external-beam radiation at 1.8–2.0 Gy per fraction. The clinical target volume was defined as 3–5 cm cephalic and at least 5 cm caudal margin beyond the gross target volume delineated by the CT scan and other diagnostic images.
Surgery
All operations were performed with curative intent and included removal of the primary tumour with its draining lymph nodes (McKeown oesophagectomy). The surgical resection methods during the thoracic stage included thoracotomy and video-assisted thoracoscopic approaches. The oesophagectomy and mediastinal lymph node dissection were performed in the thoracic stage. The extent of lymph node dissection encompassed paraoesophageal nodes, subcarinal nodes and paratracheal nodes. Oesophageal substitute mobilization and dissection of paracardial nodes and enlarged coeliac axis nodes were performed in the abdominal stage. Then the gastric tube was pulled to the cervical incision for anastomosis. Cervical lymph node sampling was also completed in the cervical stage.
Pathological examination
All resected tissue was labelled by the surgeon and sent for pathological examination, which was carried out according to the 7th edition AJCC TNM staging system [11]. The grading system for tumour response was according to the CAP (College of American Pathologists) Cancer Protocol for Esophageal Carcinoma [12], which is similar to the system developed by Wu et al. [13]. Tumour regression grade 0 (complete response) indicated no residual cancer cells. Tumour regression grade 1 (moderate response) was defined as 1–50% residual cancer; rare individual cancer cells or minute clusters of cancer cells. Tumour regression grades 2 (minimal response) and 3 (poor response) were defined as more than 50% residual cancer cells. We classified patients with tumour regression grade 0 or 1 as ‘pathological responders’ and patients with tumour regression grade 2 or 3 as ‘pathological non-responders'. We also defined ‘clinicopathological non-responders’, which included pathological non-responders and patients with clinical tumour progression that precluded surgical resection during the neoadjuvant chemoradiation period.
Follow-up
Patients were followed at our outpatient department every 3 months for the first 2 years, every 6 months for 2∼5 years and then annually. Routine follow-up examinations included chest radiography and CT scan from the neck to the upper abdomen. Endoscopy, radionuclide bone scans and PET/CT scans were carried out as indicated clinically. Overall survival, defined as the time from the date of diagnosis to death or last known follow-up, was used as a measure of prognosis.
Statistics
Pearson's χ2 test was used to compare categorical variables. Unpaired t-test and ANOVA were used for comparison of continuous variables. Survival curves were plotted by the Kaplan–Meier method and compared by the log-rank test. A P-value of <0.05 was considered significant. All calculations were performed using SPSS 17.0 software.
RESULTS
A total of 84 patients, who had technically resectable, locally advanced oesophageal squamous cell carcinoma (clinical non-T1–2N0 stage) and were medically fit for surgery, were eligible for analysis. Due to locoregional invasion, our multidisciplinary tumour board suggested neoadjuvant chemoradiation for these patients. After detailed explanation, 38 patients opted for primary resection and 46 underwent neoadjuvant chemoradiation. Among 46 patients in the neoadjuvant chemoradiation group, 34 completed oesophagectomies (neoadjuvant–surgery), whereas 12 patients were shifted to definitive chemoradiation (neoadjuvant–definitive chemoradiaton) due to tumour progression (progressive diasease or unresectable disease, e.g. invasion of the trachea, distant metastasis) during the chemoradiation period. The patient demographics of the neoadjuvant chemoradiation and primary resection groups are shown in Table 1. Patients in the primary resection group were older and had more lower-third tumours than those in the neoadjuvant chemoradiation groups. Otherwise, these groups were comparable in terms of the tumour length and pretreatment clinical stages. The final pathological stages are also listed in Table 1. The majority of patients receiving primary resection were of T3 and N0 or 1 stages. In patients receiving neoadjuvant chemoradiation followed by surgical resection, there were nine (26.4%) complete pathological responses (tumour regression grade 0) for the primary tumour. Pathological tumour regression grades 1, 2 and 3 were noted in 10, 10 and 5 patients, respectively.
Table 1:
Patient demographics of neoadjuvant chemoradiation and primary resection groups
| Treatment group |
|||
|---|---|---|---|
| Variables | Primary resection | Neoadjuvant chemoradiation | P-value |
| Number | 38 | 46 | |
| Age (years) | |||
| Mean ± SD | 62.0 ± 11.4 | 56.5 ± 8.4 | 0.01 |
| Range | 44–83 | 40–79 | |
| Sex | |||
| Male | 36 | 46 | 0.16 |
| Female | 2 | 0 | |
| Performance status | |||
| ECOG 0 | 36 | 45 | 0.54 |
| ECOG 1 | 1 | 1 | |
| ECOG 2 | 1 | 0 | |
| Location | |||
| Upper third | 4 | 8 | 0.04 |
| Middle third | 14 | 26 | |
| Lower third | 20 | 12 | |
| Endoscopic tumour length (cm) | |||
| Mean ± SD | 5.7 ± 2.4 | 6.2 ± 3.5 | 0.53 |
| Clinical TNM stage | |||
| T1N(+) | 1 | 4 | 0.07 |
| T2N(+) | 7 | 11 | |
| T3N(−) | 13 | 4 | |
| T3N(+) | 16 | 24 | |
| T4N(−) | 0 | 2 | |
| T4N(+) | 1 | 1 | |
| Total number of resected lymph nodesa | |||
| Mean ± SD | 25.3 ± 12.7 | 23.3 ± 19.0 | 0.59 |
| Range | 4–57 | 5–96 | |
| Pathological T stagea | |||
| T0 | 0 | 9 | 0.001 |
| T1 | 3 | 5 | |
| T2 | 5 | 8 | |
| T3 | 29 | 10 | |
| T4 | 1 | 2 | |
| Pathological N stagea | |||
| N0 | 16 | 18 | 0.71 |
| N1 | 13 | 11 | |
| N2 | 8 | 4 | |
| N3 | 1 | 1 | |
| Pathological stagea | |||
| Stage 0 | 0 | 7 | <0.001 |
| Stage I | 2 | 4 | |
| Stage II | 16 | 13 | |
| Stage III | 20 | 5 | |
| Stage IV | 0 | 5 | |
ECOG: Eastern Cooperative Oncology Group performance status.
aIn the neoadjuvant chemoradiation group, data were available for 34 patients who completed surgical resection.
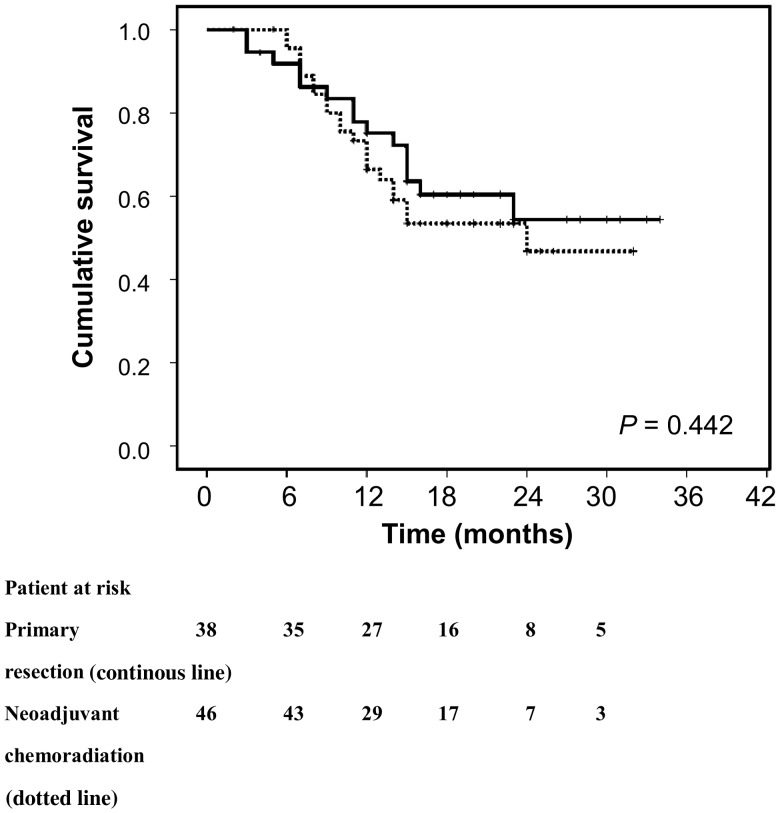
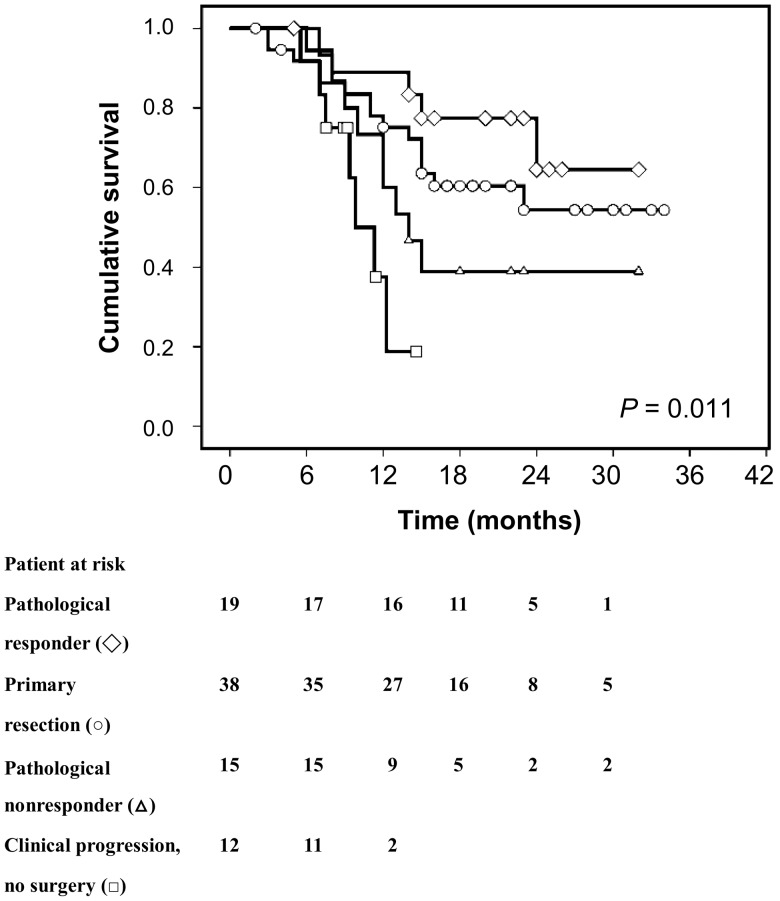
Survival curves of patients receiving primary resection and neoadjuvant chemoradiation are shown in Fig. 1. The 1-year/2-year overall survival rates of patients in the neoadjuvant chemoradiation and primary resection groups were 65.1/45.6 and 75.1/54.3%, respectively. The survival curves did not show any difference between the neoadjuvant chemoradiation and primary resection groups (P = 0.442). In patients receiving neoadjuvant chemoradiation followed by surgery, the 2-year survival rates were 64.5 and 38.9% for pathological responders and non-responders, respectively. The survival curve analysis showed significantly better outcomes for pathological responders than pathological non-responders (P = 0.043; Fig. 2). For those with tumour progression during the chemoradiation period, the 1- and 2-year survival rates were 19.7 and 0.0%, respectively. There were significant differences among ‘chemoradiation responder–surgery’, ‘primary oesophagectomy’, ‘chemoradiation non-responder–surgery’ and ‘chemoradiation non-responder–definitive chemoradiation’ (P = 0.011; Fig. 2). While the pathological responders had the highest survival rates, clinicopathological non-responders (pathological non-responders and patients with tumour progression during neoadjuvant chemoradiation period that precluded surgical resection) demonstrated significant worse outcomes than those receiving primary resection (32.0 vs 54.3%, P = 0.036).
Figure 1:
There was no survival difference between primary resection (continuous line) and neoadjuvant chemoradiation groups (dotted line; P = 0.442).
Figure 2:
Differences in survival were noted among ‘chemoradiation responder followed by surgery’ (open diamonds), ‘primary resection’ (open circles), ‘chemoradiation non-responder followed by surgery’ (open triangles) and ‘chemoradiation non-responder followed by definitive chemoradiation’ (open square; all P = 0.011; open diamonds vs open triangles, P = 0.043; open diamonds vs open triangles + open squares, P = 0.008; open circles vs open triangles + open squares, P = 0.036).
To identify predictors for the response to chemoradiation, we compared clinical parameters, including blood profiles, functional imaging results and clinical tumour characteristics of 19 pathological responders with 12 pathological non-responders and 27 clinicopathological non-responders (Table 2). The non-responders had higher serum levels of the tumour marker, squamous cell carcinoma antigen (SCC) and lower maximal standard uptake value (SUVmax) on PET scan than responders, but without statistical significance. Otherwise, there was no factor that could differentiate between responders and non-responders. None of the clinical factors accurately predicted the response to chemoradiation before treatment.
Table 2:
Comparison between chemoradiation responders and non-responders
| Chemoradiation response |
|||||
|---|---|---|---|---|---|
| Variables | Pathological responder | Pathological non-responder | P-value | Clinicopathological non-responder | P-valuea |
| Number | 19 | 15 | 27 | ||
| Age (years) | |||||
| Mean ± SD | 56.0 ± 8.6 | 56.1 ± 7.2 | 0.98 | 56.8 ± 8.4 | 0.76 |
| Range | 40–69 | 47–70 | 43–79 | ||
| Sex | |||||
| Male | 19 | 15 | – | 27 | – |
| Female | 0 | 0 | 0 | ||
| Performance status | |||||
| ECOG 0 | 19 | 14 | 0.25 | 26 | 0.40 |
| ECOG 1 | 0 | 1 | 1 | ||
| Smoking index | |||||
| ≤30 | 10 | 7 | 0.73 | 11 | 0.43 |
| >30 | 9 | 8 | 16 | ||
| Alcohol | |||||
| No | 0 | 2 | 0.26 | 2 | 0.45 |
| Social | 3 | 2 | 3 | ||
| Heavy | 16 | 11 | 22 | ||
| Betal nut | |||||
| No | 9 | 4 | 0.33 | 8 | 0.44 |
| Social | 2 | 4 | 5 | ||
| Heavy | 8 | 7 | 14 | ||
| White blood cells (/mm3) | |||||
| Mean ± SD | 8017 ± 2528 | 8109 ± 3062 | 0.93 | 8142 ± 3060 | 0.89 |
| Haemoglobin (g/dl) | |||||
| Mean ± SD | 13.0 ± 1.4 | 13.5 ± 1.5 | 0.35 | 13.1 ± 1.4 | 0.25 |
| Platelets (×103/mm3) | |||||
| Mean ± SD | 287 ± 98 | 268 ± 126 | 0.65 | 287 ± 98 | 0.33 |
| Albumin level (g/dl) | |||||
| Mean ± SD | 4.0 ± 0.4 | 4.0 ± 0.3 | 0.91 | 3.9 ± 0.4 | 0.80 |
| SCC (ng/ml) | |||||
| Mean ± SD | 1.9 ± 2.0 | 3.7 ± 2.9 | 0.11 | 3.4 ± 3.6 | 0.19 |
| PET SUVmax | |||||
| Mean ± SD | 13.6 ± 2.9 | 10.6 ± 3.7 | 0.06 | 11.4 ± 5.4 | 0.06 |
| Clinical T stage | |||||
| T1/2 | 6 | 5 | 0.91 | 9 | 0.90 |
| T3/4 | 13 | 10 | 18 | ||
| Clinical N stage | |||||
| N(−) | 2 | 2 | 0.80 | 4 | 0.67 |
| N(+) | 17 | 13 | 23 | ||
| Clinical TNM stage | |||||
| T1N(+) | 1 | 2 | 0.71 | 3 | 0.79 |
| T2N(+) | 5 | 3 | 6 | ||
| T3N(−) | 1 | 2 | 3 | ||
| T3N(+) | 10 | 8 | 14 | ||
| T4N(−) | 1 | 0 | 1 | ||
| T4N(+) | 1 | 0 | 0 | ||
| Endoscopic tumour length (cm) | |||||
| Mean ± SD | 5.7 ± 3.0 | 4.7 ± 2.6 | 0.28 | 6.6 ± 3.9 | 0.26 |
| Range | 1–11 | 2–10 | 2–15 | ||
| Location | |||||
| Upper third | 3 | 3 | 0.21 | 5 | 0.34 |
| Middle third | 13 | 6 | 13 | ||
| Lower third | 3 | 6 | 9 | ||
ECOG: Eastern Cooperative Oncology Group; SCC: squamous cell carcinoma antigen; Smoking index = (packs smoked per day) × (years as a smoker); SUVmax: maximal standard uptake value.
aPathological responder vs clinicopathological non-responder.
DISCUSSION
Current NCCN guidelines suggest the use of chemoradiation for all patients with locally advanced, stage T1b or greater or node-positive, oesophageal cancer [10]. However, the results of neoadjuvant chemordiation for oesophageal cancer are variable. Although the CALGB 9781 trial and the recent multicentre, randomized, controlled study by van Hagen et al. did show survival benefit in the preoperative chemoradiation group [3, 4], there have also been randomized controlled trials showing that preoperative chemoradiation does not improve patient outcome. For example, Burmeister et al. randomly assigned 128 patients with resectable oesophageal cancer (T1–3N0–1 disease) to surgery alone and 128 patients to surgery after cisplatin and fluorouracil with concurrent radiotherapy. The results demonstrated that neither progression-free survival nor overall survival differed between groups [5]. The FFCD 9901 randomized controlled trial also showed that preoperative chemoradiation (radiotherapy with concomitant chemotherapy with 5-fluorouracil and cisplatin) did not improve overall survival for localized stage I or II oesophageal cancer compared with surgery alone [6]. Nonetheless, nearly all studies have observed the tumour response to neoadjuvant chemoradiation to be a significant factor for survival. While the responders are likely to have longer survival than patients receiving surgery alone, it is unknown whether neoadjuvant treatments provide beneficial or harmful effects to non-responders. Few reports have focused on the survival differences between non-responders and patients undergoing primary oesophagectomies. Dittrick et al. compared the survival of patients with a poor response to neoadjuvant chemoradiation with that of patients who underwent a primary oesophagectomy [14]. The disease-free survival and overall survival were significantly decreased in the non-responders group compared with the primary oesophagectomy group (10 vs 50 and 13 vs 50 months, respectively). However, the patient characteristics were not comparable between the two the groups in their study. Most importantly, there were significantly more stage III tumours in the non-responders. In our study, the tumour characteristics, including endoscopic tumour length as well as pretreatment clinical stages, were comparable among the groups. While the chemoradiation responders had the highest survival rates, our results demonstrated that the clinicopathological non-responders had significantly worse outcomes than those receiving primary oesophagectomies (P = 0.036). Our observation highlights the dilemma in current oesophageal cancer treatment. How to identify correctly the oesophageal cancer patients who will benefit from chemoradiation and avoid unnecessary chemoradiation in patients with chemoradiation-resistant oesophageal cancer is a major task in oesophageal cancer treatment planning. If we could predict the therapeutic effects, i.e. pathological tumour response, then perhaps we could develop individualized treatment approaches according to prediction results.
Various methods for predicting the pathological response to chemoradiation have already been proposed. For example, McLoughlin et al. compared post-chemoradiation PET with final pathological findings. The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of PET to predict pathological response were only 61.8, 43.8, 70, 35 and 56%, respectively [15]. Others have utilized endoscopic ultrasonography in assessing the pathological response after neoadjuvant therapy, but the results were controversial [16]. As for the endoscopic biopsy, Sarkaria and colleagues found that while a positive post-treatment biopsy was predictive of residual disease, a negative biopsy was a poor predictor of pathological response, with 69% having local disease at oesophagectomy [17]. Analysing 322 oesophageal cancer patients who underwent preoperative chemoradiation, Ajani et al. constructed a nomogram consisting of five variables (female sex, well or moderately differentiated histology, the absence of cancer cells on post-chemoradiation biopsy specimens, lower post-chemoradiation SUVmax and baseline T category) to predict pathological complete response after chemoradiation for oesophageal cancer [18]. However, the most influential factors were lower post-chemoradiation SUVmax and the absence of cancer cells on post-chemoradiation biopsy, and these variables cannot be obtained before treatment starts. To sum up, nearly all the above methods have yielded less-than-satisfying results. Furthermore, most were based on post-chemoradiation, preresection clinical studies. None could provide prediction before treatment.
Besides clinicopathological markers, some studies have used gene expression changes to predict the preoperative chemoradiation response. The expression of DNA repair markers, such as ERCC1, MLH1 and MRP, in pretreatment biopsy tissue have been reported to correlate with pathological tumour response [19–21]. Correlation between tumour response and the activity of 5-fluorouracil metabolism-associated genes, such as the gene for thymidylate synthase (TS1), which is the target enzyme for 5-fluorouracil, and the gene for methylenetetrahydrofolate reductase (MTHFR), which is an inhibitor of thymidylate synthase, have also been reported [22]. Furthermore, activation of the nuclear factor-κB pathway in pretreatment tissue was shown to be significantly associated with tumour aggressiveness and chemoradiation resistance in oesophageal cancer [23].
With the advent of high-throughput technology, gene classifiers have also been constructed to differentiate chemoradiation responders and non-responders [24, 25]. Luthra et al. even suggested the apoptotic pathway to be one of the key functions related to chemoradiation resistance [24]. However, there were few overlapping genes among these gene classifiers from different cohorts and none of these high-throughput-derived data have ever been validated in clinics. Whether these microarray analyses could help in individual treatment planning needs further investigation.
Our study is limited by its retrospective nature and the relatively small number of patients. In addition, not all patients received EUS for clinical staging. As most patients came to us with intolerable dysphagia and near-total obstruction on endoscopy, EUS was sometimes technically impossible. Therefore, EUS was an optional procedure, but necessary for confirmation of a cT1 lesion, which might be a candidate for endoscopic mucosal resection. However, the clinical staging and treatment plan of each patient was determined by multidisciplinary tumour board discussions and recorded by Taipei Veterans General Hospital Cancer Council. All patient data were based on the prospectively collected electronic database, which might minimize the possible bias. Despite the small number of patients, the survival curves did clearly demonstrate differences in survival. However, a longer follow-up time is needed to elucidate the survival differences further.
In conclusion, our results showed that for locally advanced oesophageal squamous cell carcinoma, there was no difference in survival between neoadjuvant chemoradiation and primary resection groups. Non-responders demonstrated no benefit and even worse survival compared with patients receiving primary resection. Unfortunately, there is no significant clinical marker that could be implemented in the clinics to predict preoperative chemoradiation response. Further investigation into biomarkers based on gene expression patterns may help in prediction of reponse to neoadjuvant chemoradiation, which could save non-responders from unnecessary treatment and allow the tailoring of such therapy to the individual patient with oesophageal cancer.
Conflict of interest: none declared.
REFERENCES
- 1.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–52. doi: 10.1056/NEJMra035010. doi:10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 2.Hsu PK, Wang BY, Huang CS, Wu YC, Hsu WH. Prognostic factors for post-recurrence survival in esophageal squamous cell carcinoma patients with recurrence after resection. J Gastrointest Surg. 2011;15:558–65. doi: 10.1007/s11605-011-1458-1. doi:10.1007/s11605-011-1458-1. [DOI] [PubMed] [Google Scholar]
- 3.Tepper J, Krasna MJ, Niedzwiecki N, Hollis D, Reed CE, Goldberg R, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–92. doi: 10.1200/JCO.2007.12.9593. doi:10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. doi: 10.1056/NEJMoa1112088. doi:10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 5.Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–68. doi: 10.1016/S1470-2045(05)70288-6. doi:10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 6.Mariette C, Seitz JF, Maillard E, Mornex F, Thomas PA, Raoul J, et al. Surgery alone versus chemoradiatherapy followed by surgery for localized esophageal cancer: analysis of a randomized controlled phase III FFCD 9901. J Clin Oncol. 2010;28(Suppl 15) doi: 10.1200/JCO.2013.53.6532. Abstract 4005. [DOI] [PubMed] [Google Scholar]
- 7.Urba SG, Orringer MB, Turrisi A, Lannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–13. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 8.Meredith KL, Weber JM, Turaga KK, Siegel EM, McLoughlin J, Hoffe S, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol. 2010;17:1159–67. doi: 10.1245/s10434-009-0862-1. doi:10.1245/s10434-009-0862-1. [DOI] [PubMed] [Google Scholar]
- 9.Donahue JM, Nichols FC, Li Z, Schomas DA, Allen MS, Cassivi SD, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg. 2009;87:392–8. doi: 10.1016/j.athoracsur.2008.11.001. doi:10.1016/j.athoracsur.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. Esophageal Cancer Clinical Practice Guidelines in Oncology. Available at www.nccn.org. (22 May, 2013, date last accessed). [Google Scholar]
- 11.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, et al. American Joint Committee on Cancer (AJCC) cancer staging manual. 7th edn. Chicago: Springer, Inc.; 2010. [Google Scholar]
- 12.Washington K, Berlin J, Branton P, Burgart LJ, Carter DK, Fitzgibbons P, et al. Protocol for the examination of specimens from patients with carcinoma of the esophagus. College of American Pathologists Cancer Protocols; 2009. pp. 1–16. Available at http://www.cap.org. (22 May, 2013, date last accessed). [Google Scholar]
- 13.Wu TT, Chirieac LR, Abraham SC, Krasinskas AM, Wang H, Rashid A, et al. Excellent interobserver agreement on grading the extent of residual carcinoma after preoperative chemoradiation in esophageal and esophagogastric junction carcinoma: a reliable predictor for patient outcome. Am J Surg Pathol. 2007;31:58–64. doi: 10.1097/01.pas.0000213312.36306.cc. doi:10.1097/01.pas.0000213312.36306.cc. [DOI] [PubMed] [Google Scholar]
- 14.Dittrick GW, Weber JM, Shridhar R, Hoffe S, Melis M, Almhanna K, et al. Pathologic nonresponders after neoadjuvant chemoradiation for esophageal cancer demonstrated no survival benefit compared with patients treated with primary esophagectomy. Ann Surg Oncol. 2012;19:1678–84. doi: 10.1245/s10434-011-2078-4. doi:10.1245/s10434-011-2078-4. [DOI] [PubMed] [Google Scholar]
- 15.McLoughlin JM, Melis M, Siegel EM, Dean EM, Weber JM, Chern J, et al. Are patients with esophageal cancer who become PET negative after neoadjuvant chemoradiation free of cancer? J Am Coll Surg. 2008;206:879–86. doi: 10.1016/j.jamcollsurg.2007.12.027. doi:10.1016/j.jamcollsurg.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Misra S, Choi M, Livingstone AS, Franceschi D. The role of endoscopic ultrasound in assessing tumor response and staging after neoadjuvant chemotherapy for esophageal cancer. Surg Endosc. 2012;26:518–22. doi: 10.1007/s00464-011-1911-y. doi:10.1007/s00464-011-1911-y. [DOI] [PubMed] [Google Scholar]
- 17.Sarkaria IS, Rizk NP, Bains MS, Tang LH, Ilson DH, Minsky BI, et al. Post-treatment endoscopic biopsy is a poor-predictor of pathologic response in patients undergoing chemoradiation therapy for esophageal cancer. Ann Surg. 2009;249:764–7. doi: 10.1097/SLA.0b013e3181a38e9e. doi:10.1097/SLA.0b013e3181a38e9e. [DOI] [PubMed] [Google Scholar]
- 18.Ajani JA, Correa AM, Hofstetter WL, Rice DC, Blum MA, Suzuki A, et al. Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol. 2012;23:2638–42. doi: 10.1093/annonc/mds210. doi:10.1093/annonc/mds210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim MK, Cho KJ, Kwon GY, Park SI, Kim YH, Kim JH, et al. Patients with ERCC1-negative locally advanced esophageal cancers may benefit from preoperative chemoradiotherapy. Clin Cancer Res. 2008;14:4225–31. doi: 10.1158/1078-0432.CCR-07-4848. doi:10.1158/1078-0432.CCR-07-4848. [DOI] [PubMed] [Google Scholar]
- 20.Alexander BM, Wang XZ, Niemierko A, Weaver DT, Mak RH, Roof KS, et al. DNA repair biomarkers predict response to neoadjuvant chemoradiotherapy in esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;83:164–71. doi: 10.1016/j.ijrobp.2011.05.033. doi:10.1016/j.ijrobp.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 21.Yamasaki M, Makino T, Masuzawa T, Kurokawa Y, Miyata H, Takiguchi S, et al. Role of multidrug resistance protein 2 (MRP2) in chemoresistance and clinical outcome of oesphageal squamous cell carcinoma. Br J Cancer. 2011;104:707–13. doi: 10.1038/sj.bjc.6606071. doi:10.1038/sj.bjc.6606071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langer R, Specht K, Becker K, Ewald P, Bekesch M, Sarbia M, et al. Association of pretherapeutic expression of chemotherapy-related genes with response to neoadjuvant chemotherapy in Barrett carcinoma. Clin Cancer Res. 2005;11:7462–9. doi: 10.1158/1078-0432.CCR-05-0042. doi:10.1158/1078-0432.CCR-05-0042. [DOI] [PubMed] [Google Scholar]
- 23.Izzo JG, Malhotra U, Wu TT, Ensor J, Luthra R, Lee JH, et al. Association of activated transcription factor nuclear factor κB with chemoradiation resistance and poor outcome in esophageal carcinoma. J Clin Oncol. 2006;24:748–54. doi: 10.1200/JCO.2005.03.8810. doi:10.1200/JCO.2005.03.8810. [DOI] [PubMed] [Google Scholar]
- 24.Luthra R, Wu TT, Luthra MG, Izzo J, Lopez-Alvarez E, Zhang L, et al. Gene expression profiling of localized esophageal carcinomas: association with pathologic response to preoperative chemoradiation. J Clin Oncol. 2006;24:259–67. doi: 10.1200/JCO.2005.03.3688. doi:10.1200/JCO.2005.03.3688. [DOI] [PubMed] [Google Scholar]
- 25.Maher SG, Gillham CM, Duggan SP, Smyth PC, Miller N, Muldoon C, et al. Gene expression analysis of diagnostic biopsies predicts pathological response to neoadjuvant chemoradiotherapy of esophageal cancer. Ann Surg. 2009;250:729–37. doi: 10.1097/SLA.0b013e3181bce7e1. doi:10.1097/SLA.0b013e3181bce7e1. [DOI] [PubMed] [Google Scholar]