Abstract
OBJECTIVES
Deep sternal wound infection (DSWI) is a devastating complication of cardiac surgery, with a historical incidence of 0.4–5%. Predicting which patients are at higher risk of infection may help instituting various preventive measures. Risk calculations for mortality have been used as surrogates to estimate the risk of deep sternal wound infection, with limited success. The Society of Thoracic Surgeons (STS) 2008 Risk Calculator modelled the risk of DSWI for cardiac surgical patients, but it has not been validated since its publication. We sought to assess the external validity of the STS-estimated risk of DSWI in a United Kingdom (UK) population.
METHODS
Using our prospectively captured database, we retrospectively calculated the risk of DSWI for 14 036 patients undergoing valve, coronary artery bypass grafts or combined procedures between February 2001 and March 2010. DSWI was identified according to the Centre for Disease Control and Prevention definition. The receiver operator characteristic (ROC) curve was employed to test the performance of the model using the area under the ROC curve (AUROC). The calibration of the model was interrogated using the Hosmer–Lemeshow test for Goodness of Fit.
RESULTS
A total of 135 (0.95%) patients developed DSWI. Although there was a statistically significant difference in the calculated risk of patients who contracted DSWI (0.44% ± 0.01) vs those who did not (0.28% ± 0.00, P < 0.0001), the AUROC of 0.699 (95% confidence interval: 0.6522–0.7414) denoted a modest discriminatory power, with the Hosmer–Lemeshow Goodness of Fit statistic (P < 0.001) suggesting poor calibration. A risk-adjusted modifier improved the calibration (P = 0.08).
CONCLUSIONS
The STS risk calculator lacks adequate discriminatory power for estimating the isolated risk of developing deep sternal wound infection in a UK population. The discrimination is similar to the tool's validation c-statistic and may have a place in an integrated calculator.
Keywords: Statistics, Risk analysis/modelling, Complications, Sternum, Infection
INTRODUCTION
Deep sternal wound infections (DSWIs) remain an uncommon but devastating complication of cardiac surgery, affecting 0.4–5% [1] of procedures performed via median sternotomy. They produce significant and prolonged morbidity and are associated with an increase in both short- and long-term mortalities [2]. Early retrospective studies identified a significant number of factors that might be involved in the aetiology of the condition. Preoperative risk factors included diabetes, renal failure, smoking, sex, age, reoperation, morbid obesity, breast size, steroid use and chronic obstructive pulmonary disease. Intraoperatively, length of surgery and the use of internal mammary arteries—both single and bilateral—were implicated. Postoperative complications contributing to DSWI included prolonged ventilation, inotropic support, reoperation for bleeding and the need for blood products [3–8].
The Society of Thoracic Surgeons (STS) risk score is, perhaps, the most well-established risk calculator for cardiac surgery in the world. It was developed from a multicentre database of over 100 000 index procedures in the USA and calculates the risk for coronary artery bypass grafting, valve procedures and combined cases [9–12]. Using 67 demographic and operative parameters, the STS risk tool calculates predicted mortality, as well as estimating a number of other comorbidities including DSWIs. In the UK, the EuroSCORE [13] has been validated as a reliable tool for predicting risk in the British population. However, while there are plans to develop a European model for estimating the risk of major comorbidities, at present EuroSCORE provides an estimated risk for mortality only. Many surgeons using the EuroSCORE to calculate mortality risk will therefore estimate the risk of DSWIs based on patient comorbidities.
A small number of studies [14–17] have tried to determine the validity of the STS or EuroSCORE calculators for calculating the risk of DSWI in their local populations, in some cases using the mortality risk as a surrogate for the risk of DSWI. The most recent study from the UK applied the 13 risk factors from the previous 2005 STS risk score to a population of 7602 patients undergoing coronary artery bypass grafting, with or without concomitant procedures, including those not stratified by the risk calculator. The authors found no statistical correlation between the expected and observed outcomes [17].
We sought to establish if there was any correlation between the newer 2008 STS risk score for DSWI in our population of patients undergoing cardiac surgery.
PATIENTS AND METHODS
Study design and population
We performed a retrospective study of patients undergoing cardiac surgery at the Liverpool Heart and Chest Hospital in the UK. Approval for the study was given by the hospital's Cardiothoracic Surgery Division and by the Data Quality Department. Data from our institutional database were gathered for all patients undergoing cardiac surgical procedures.
The study period was between February 2001 and March 2010, inclusive, during which a total of 15 499 cardiac operations were performed. Excluding those procedures that were not originally risk stratified by the STS risk score (e.g. aortovascular procedures, surgical ventricular remodelling, septal repairs, etc.), a total of 14 036 procedures remained. These included 9412 coronary artery bypass grafts; 2919 valve procedures and 1705 combined valve and graft operations.
Demographic information collected is summarized in Table 1 and included all risk factors described by the STS 2008 risk score for DSWIs, with the exception of ethnic origin, which was not routinely collected, and immunosuppressive treatment within 30 days of surgery, which was not available but had no effect on DSWI in the STS calculators as the coefficient was 0.00000. The algorithm for calculating risk was applied to these data, including those patients with missing data, to calculate a percentage risk score for each patient. We calculated the STS scores for risk of death in addition to risk of DSWI in order to characterize the overall validity of the risk stratification tool in a British population.
Table 1:
Demographic data for patients
| CABG and valve (N = 1705) | CABG only (N = 9412) | Valve only (N = 2919) | Total (N = 14 036) | Missing data [n (%)] | |
|---|---|---|---|---|---|
| Male [n (%)] | 1161 (68.0) | 7582 (80.6) | 1531 (52.4) | 10 274 (73.2) | 0 (0.0) |
| Age (years ± SD) | 71.3 ± 8.1 | 65.1 ± 9.2 | 64.6 ± 13.3 | 65.7 ± 10.3 | 0 (0.0) |
| BMI (kg/m2 ± SD) | 27.6 ± 4.6 | 28.6 ± 4.5 | 27.1 ± 5.2 | 28.2 ± 4.7 | 19 (0.1) |
| Preoperative creatinine (μmol/l ± SD) | 107.5 ± 56.3 | 100.0 ± 49.4 | 102.6 ± 65.6 | 101.5 ± 54.0 | 0 (0.0) |
| Renal failure [n (%)] | |||||
| ARI | 13 (0.8) | 29 (0.3) | 44 (1.5) | 86 (0.6) | 11 (0.1) |
| CRI | 137 (8.0) | 426 (4.5) | 154 (5.3) | 717 (5.1) | |
| CRF | 13 (0.8) | 46 (0.5) | 29 (1.0) | 88 (0.6) | |
| Ejection fraction [n (%)] | |||||
| Good | 1034 (60.7) | 5659 (60.1) | 2033 (69.8) | 8726 (62.2) | 7 (0.05) |
| Moderate | 473 (27.8) | 2882 (30.6) | 680 (23.3) | 4035 (28.8) | |
| Poor | 191 (11.2) | 829 (8.8) | 151 (5.2) | 1171 (8.3) | |
| Atrial fibrillation [n (%)] | 309 (18.1) | 325 (3.5) | 728 (25.0) | 1362 (9.7) | 18 (0.1) |
| NYHA class [n (%)] | |||||
| I | 173 (10.2) | 2868 (30.5) | 381 (13.1) | 3422 (24.4) | 4 (0.03) |
| II | 572 (33.6) | 4169 (44.3) | 813 (27.9) | 5554 (39.6) | |
| III | 838 (49.2) | 2246 (23.9) | 1427 (48.9) | 4511 (32.1) | |
| IV | 121 (7.1) | 127 (1.4) | 297 (10.2) | 545 (3.9) | |
| CCS class [n (%)] | |||||
| I | 518 (30.4) | 636 (6.8) | 182 (6.2) | 960 (6.8) | 3 (0.03) |
| II | 142 (8.3) | 2668 (28.4) | 291 (10.0) | 3396 (24.2) | |
| III | 437 (25.6) | 3467 (36.8) | 131 (4.5) | 4035 (28.8) | |
| IV | 437 (25.6) | 1604 (17.0) | 31 (1.1) | 1766 (12.6) | |
| Unstable angina [n (%)] | 184 (10.8) | 1977 (21.0) | 39 (1.3) | 2200 (15.7) | 9 (0.06) |
| Chronic airways disease [n (%)] | 195 (11.4) | 705 (7.5) | 253 (8.7) | 1153 (8.2) | 0 (0.0) |
| PVD [n (%)] | 257 (15.1) | 1345 (14.3) | 173 (5.9) | 1775 (12.6) | 0 (0.0) |
| Diabetes mellitus [n (%)] | |||||
| Diet controlled | 89 (5.2) | 423 (4.5) | 89 (3.1) | 601 (4.3) | 3 (0.03) |
| Oral meds | 186 (10.9) | 1144 (12.2) | 194 (6.6) | 1524 (10.9) | |
| Insulin | 79 (4.6) | 614 (6.5) | 67 (2.3) | 760 (5.4) | |
| Coronary artery disease extent [n (%)] | |||||
| 1 vessel | 573 (33.8) | 317 (3.4) | 104 (21.8) | 994 (8.6) | 0 (0.0) |
| 2 vessels | 492 (29.0) | 1835 (19.5) | 9 (1.9) | 2336 (20.2) | |
| 3 vessels | 631 (37.2) | 7250 (77.1) | 38 (8.6) | 7919 (68.4) | |
| Preop IABP [n (%)] | 31 (1.8) | 170 (1.8) | 17 (0.8) | 218 (1.5) | 0 (0.0) |
| Preop shock [n (%)] | 14 (0.8) | 28 (0.3) | 29 (1.0) | 71 (0.5) | 0 (0.0) |
| Hypertension [n (%)] | 1040 (61.0) | 6103 (64.8) | 1233 (42.2) | 8376 (59.7) | 0 (0.0) |
| Left main stem disease [n (%)] | 192 (11.3) | 2244 (23.8) | 5 (0.2) | 2441 (17.4) | 0 (0.0) |
| Q-wave MI [n (%)] | 139 (8.2) | 1198 (12.7) | 19 (0.7) | 1356 (9.7) | 0 (0.0) |
| First operation [n (%)] | 1634 (95.8) | 9255 (98.3) | 2605 (89.2) | 13 494 (96.1) | 0 (0.0) |
| Status [n (%)] | |||||
| Urgent | 255 (14.9) | 1762 (18.7) | 320 (11.) | 2337 (16.7) | 1 (0.0) |
| Emergent/salvage | 15 (0.8) | 112 (1.1) | 57 (2.0) | 184 (1.3) | |
| Endocarditis [n (%)] | 14 (0.8) | 0 (0.0) | 121 (4.1) | 135 (1.0) | 0 (0.0) |
| Smoking [n (%)] | |||||
| Current | 164 (9.6) | 1287 (13.7) | 335 (11.5) | 1786 (12.7) | 10 (0.06) |
| Ex-smoker | 1013 (59.4) | 5434 (57.8) | 1289 (44.2) | 7736 (55.2) | |
| Cerebrovascular disease [n (%)] | 173 (10.1) | 702 (7.5) | 250 (8.6) | 1125 (8.0) | 0 (0.0) |
| Ventilation preop [n (%)] | 7 (0.4) | 12 (0.1) | 25 (0.9) | 44 (0.3) | 0 (0.0) |
| Inotropes preop [n (%)] | |||||
| 1 | 88 (5.9) | 283 (3.4) | 138 (5.3) | 509 (4.1) | 1622 (11.6) |
| 2 | 50 (3.4) | 103 (1.2) | 65 (2.4) | 218 (1.8) | |
| ≥3 | 25 (1.6) | 23 (0.3) | 25 (0.9) | 62 (0.4) | |
| FEV1, (% predicted ± SD) | 78.8 ± 27.2 | 82.6 ± 28.3 | 77.7 ± 27.1 | 86.3 ± 19.7 | 840 (6.0) |
| Reoperation [n (%)] | 17 (1.0) | 37 (0.4) | 6 (0.2) | 60 (0.4) | 0 (0.0) |
| DSWI [n (%)] | 31 (1.8) | 90 (1.0) | 14 (0.5) | 135 (1.0) | 0 (0.0) |
ARI: acute renal injury; BMI: body mass index; CCS: Canadian cardiovascular society; CRF: chronic renal failure; CRI: chronic renal injury; FEV1: forced expiratory volume in 1 second; IABP: intra-aortic balloon pump; MI: myocardial infarction; NYHA: New York Heart Association; PVD: peripheral vascular disease; SD: standard deviation.
Definitions
Where differences existed in the standard units of measurement between the USA and the UK (e.g. serum creatinine), appropriate conversions were applied for calculations, but we present the data here in British (SI) units. Quantitative measurements that referred to qualitative descriptors in the STS score (i.e. chronic lung disease, which is stratified as mild, moderate or severe in the STS risk variables but was measured at our centre as forced expiratory volume in 1 s) were graded according to published criteria [18]. Ejection fraction, which is quantified for the purposes of the calculator, was documented as good, moderate or poor in our database. For the conversion, we used 55% for good, 45% for moderate and 35% for poor ejection fractions.
We used the Centre for Disease Control and Prevention definition of DSWI as infection involving tissues or spaces beneath the sub-cutaneous tissue, fulfilling at least one of the following criteria: (i) an organism is isolated from a culture of mediastinal tissue or fluid; (ii) evidence of mediastinitis is seen during operation or by histopathological examination or (iii) one of the following, fever (>38°C), chest pain or sternal instability, is present and there is either purulent drainage from the mediastinum or an organism isolated from blood culture or culture of drainage of the mediastinal area [19].
Statistical analysis
Statistical tests were performed using JMP 9.0.2 for Mac (SAS Institute, Inc., Cary, NC, USA). The receiver operator characteristic (ROC) curve was employed to test the performance of each model using the area under the ROC (AUROC). The calibration of the model was interrogated using the Hosmer–Lemeshow test for Goodness of Fit.
RESULTS
Incidence of deep sternal wound infection
The total incidence of DSWI in our study group was 135 of 14 036 (0.96%). There were less sternal wound infections in patients undergoing isolated valve procedures alone than in those having coronary artery surgery or valve and graft procedures (0.48 vs 0.96 and 1.82% respectively, P < 0.0001 by Fisher's exact method).
Society of Thoracic Surgeon risk scores for mortality and deep sternal wound infection
The mean calculated risk of mortality in the study population was 2.40% ± 3.23 (range 0.20–72.78%). The AUROC for risk of perioperative death was 0.810, with a Hosmer–Lemeshow Goodness of Fit P < 0.0001.
The mean calculated risk of DSWI in our study population was 0.28% ± 0.20 (range 0.03–2.62%). There was a statistically significant difference in the mean risk score of patients who developed DSWI, compared with those who did not (0.44 ± 0.23 vs 0.28 ± 0.16%, P < 0.0001 by unpaired t-test).
The AUROC curve for all procedures was 0.699 (95% confidence interval: 0.6522–0.7414) (Fig. 1) with a Hosmer–Lemeshow test of P < 0.0001 (Table 2), indicating that the calibration of the tool was also poor.
Figure 1:
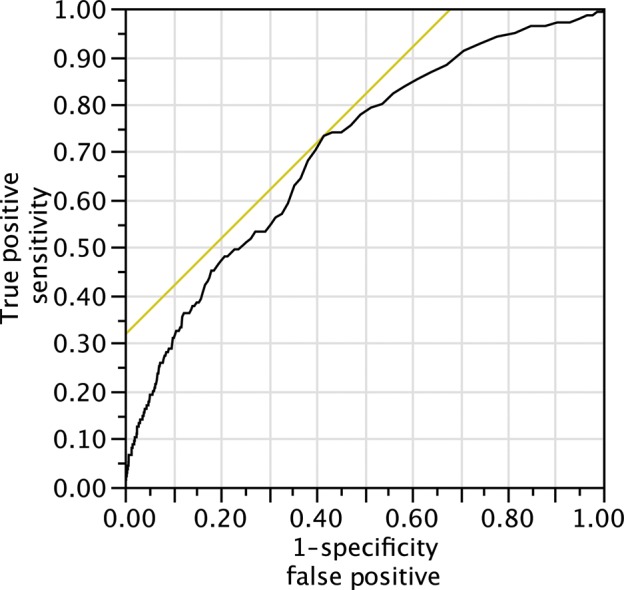
ROC curve for all procedures. AUROC 0.699.
Table 2:
Hosmer–Lemeshow groups
| Group | N | Probability interval | DSWI |
No DSWI |
||
|---|---|---|---|---|---|---|
| Observed | Expected | Observed | Expected | |||
| 1 | 1370 | 0.0003–0.0012 | 3 | 1.3 | 1367 | 1368.7 |
| 2 | 1371 | 0.0012–0.0014 | 4 | 1.8 | 1367 | 1369.2 |
| 3 | 1371 | 0.0014–0.0016 | 6 | 2.1 | 1365 | 1368.9 |
| 4 | 1371 | 0.0016–0.0019 | 3 | 2.4 | 1368 | 1368.6 |
| 5 | 1371 | 0.0019–0.0022 | 14 | 2.8 | 1357 | 1368.2 |
| 6 | 1371 | 0.0022–0.0025 | 8 | 3.2 | 1363 | 1367.8 |
| 7 | 1371 | 0.0025–0.0030 | 17 | 3.8 | 1354 | 1367.2 |
| 8 | 1371 | 0.0030–0.0037 | 13 | 4.5 | 1358 | 1366.5 |
| 9 | 1371 | 0.0037–0.0050 | 18 | 5.8 | 1353 | 1365.2 |
| 10 | 1372 | 0.0050–0.0275 | 46 | 10.1 | 1326 | 1361.9 |
Deep sternal wound infection and long-term mortality
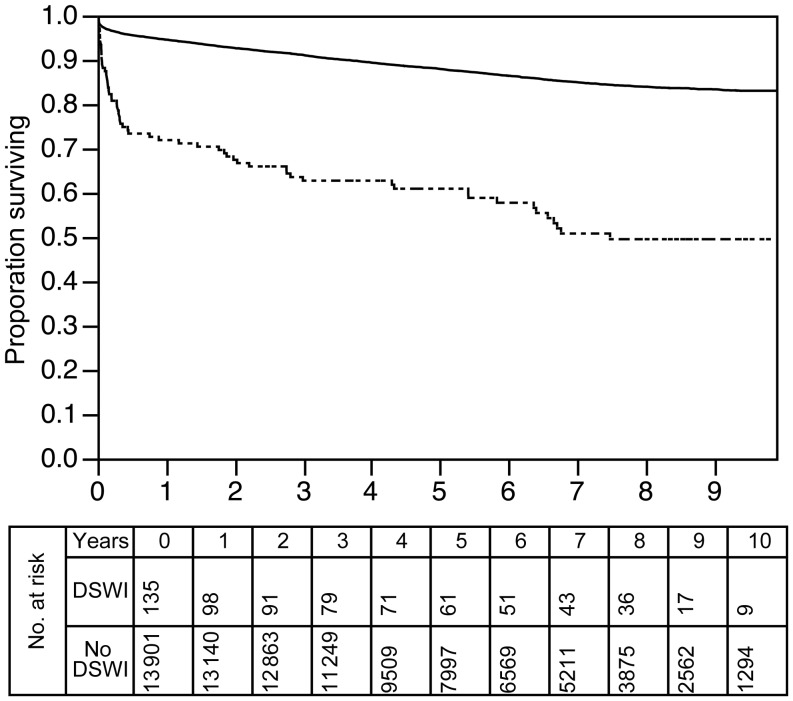
The 10-year survival following valve, graft or combined procedures at our institution was 83%. In patients who suffered from DSWI, 10-year survival was 50%, which was statistically significant by log-rank analysis (P < 0.0001). This is presented in Fig. 2.
Figure 2:
Kaplan–Meier survival curves for patients with and without DSWI. Solid line: no DSWI; dotted line: DSWI (P < 0.0001).
Comment
The overall incidence of DSWI in our study population (0.96%) was comparable with figures cited in other papers and towards the lower end of the range. The mean predicted STS risk score for DSWI was 0.28%, with a maximum predicted risk of 2.62%.
Shahian and coworkers found that the AUROC (or c-index, as it is also known) for the validation subsets in the STS publications ranged from 0.580 to 0.714 [9–12]. Our findings of an AUROC of 0.699 are therefore not dissimilar, and suggest that the modest discrimination of the test is as expected. The use of the Hosmer–Lemeshow test for determining the calibration or ‘Goodness of Fit’ of risk stratification tools has been highly debated [20–22] and in particular, the use of the tool in large datasets has been cautioned by one of the original authors [23]. For these reasons, the test was not utilized by the authors of the STS 2008 risk score, but they instead demonstrated the calibration of the tools using graphical representation of observed vs expected outcomes. Producing the same illustration for DSWI in our population (Fig. 3) demonstrated a substantial, but consistent, underestimation of risk in our population. We considered the use of a risk-adjusted modifier, similar to the risk-adjusted mortality ratio used to recalibrate the EuroSCORE following its publication. As the mean predicted risk of 0.28% was approximately four times less than the mean observed risk, we employed a risk-adjusting modifier of 4 (i.e. multiplying the STS-predicted risk by a factor of four to account for underestimation). Repeating the Goodness of Fit calculations using this new predicted risk showed better calibration and a P-value of 0.08, implying better fit (Fig. 4). Whether or not this can be interpreted in the context of a modest AUROC, however, is debateable.
Figure 3:
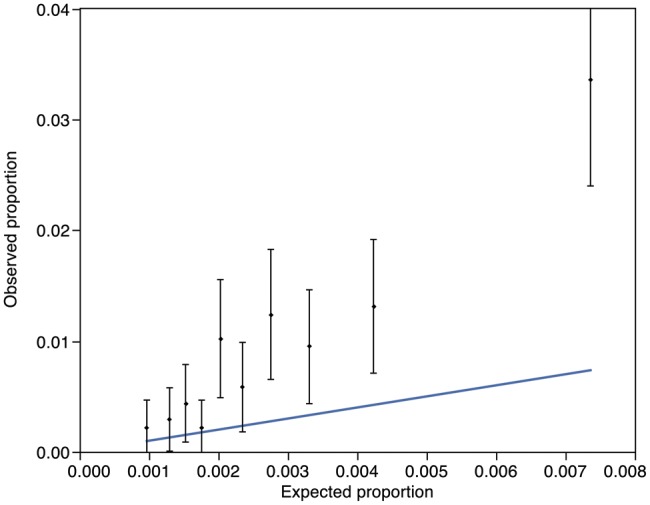
Observed vs expected mortality proportions derived from Hosmer–Lemeshow groups. Vertical bars and points represent mean and 95% confidence interval (CI) for observed mortality for each group. Oblique line is the expected mortality (Hosmer–Lemeshow Goodness of Fit statistic: P < 0.0001).
Figure 4:
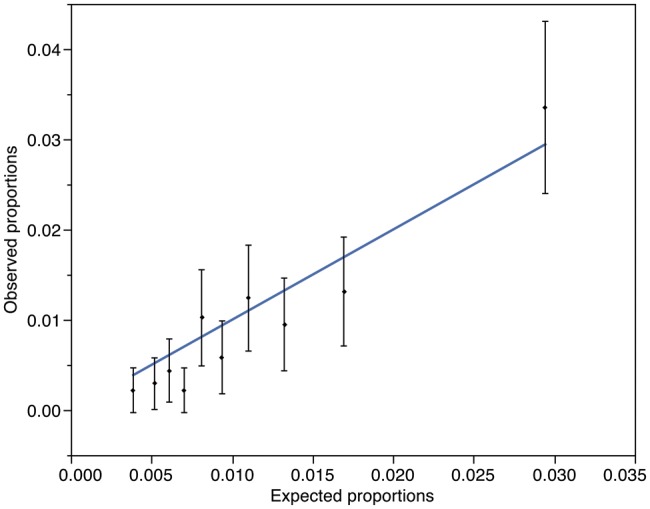
Observed vs expected mortality proportions with a risk-adjusted modifier applied, derived from Hosmer–Lemeshow groups. Vertical bars and points represent mean and 95% CI for observed mortality for each group. Oblique line is the expected mortality as calculated by the STS with a risk-modified of x4 applied (Hosmer–Lemeshow Goodness of Fit statistic: P = 0.08).
The substantial differences in the STS-predicted against the actual or observed risks of DSWI were a cause for consternation. However, we noted that our average incidence of DSWI of <1% is comparable with others in the literature, and that the mean predicted risk of 0.28% is below that described in even the best series. Indeed, even where a hypothetical ‘worst-case scenario’ patient is risk-stratified using the 2008 STS calculator, selecting all possible comorbidities (Table 3), the calculated risk of DSWI is still only 8.3%. This is likely to represent the probability that such a patient—if ever accepted for surgery—would be unlikely to survive long enough to develop mediastinitis, but nonetheless highlights the limitations of the tools.
Table 3:
Hypothetical worst-case scenario patient
|
BMI: body mass index; COPD: chronic obstructive pulmonary disease; NYHA: New York Heart Association.
It is of note that while the risk of DSWI is frequently cited as up to 5%, the references for these figures are largely historical, representing the incidence over two or three decades ago. The majority of recent publications describe an incidence of DSWI with contemporaneous data of <2%. The UK National Database figures cite the number of reoperations for DSWI as 0.5% in 2008 [24]. The national incidence of mediastinal infections including those not treated surgically is not known, but our institutional reoperation rate for DSWI of 0.4% would suggest that we have comparable results. Nonetheless, we accept the limitations of a single-centre retrospective study in which all the variables required for the calculator under scrutiny were not available.
The STS risk score for mortality and morbidity has not been widely adopted in the British setting due to the popularity of the parsimonious dataset associated with the EuroSCORE. With an AUROC of 0.699, this element of the STS calculator lacks sufficient discriminatory power to estimate the risk of DSWI in our population, despite an adequate risk-adjusted calibration. However, as part of an integrated mortality and morbidity calculator, the modest discrimination might be overlooked on account of the overall ability to quantify risk for patients.
ACKNOWLEDGEMENTS
Many thanks to Khalid Haneef for help with statistical manipulations.
Conflict of interest: none declared.
REFERENCES
- 1.Ottino G, De Paulis R, Pansini S, Rocca G, Tallone MV, Comoglio C, et al. Major sternal wound infection after open-heart surgery: a multivariate analysis of risk factors in 2,579 consecutive operative procedures. Ann Thorac Surg. 1987;44:173–9. doi: 10.1016/s0003-4975(10)62035-8. doi:10.1016/S0003-4975(10)62035-8. [DOI] [PubMed] [Google Scholar]
- 2.Braxton JH, Marrin CAS, McGrath PD, Morton JR, Norotsky M, Charlesworth DC, et al. 10-year follow-up of patients with and without mediastinitis. Semin Thorac Cardiovasc Surg. 2004;16:70–6. doi: 10.1053/j.semtcvs.2004.01.006. doi:10.1053/j.semtcvs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 3.He G-W, Ryan WH, Acuff TE, Bowman RT, Douthit MB, Yang C-Q, et al. Risk factors for operative mortality and sternal wound infection in bilateral internal mammary artery grafting. J Thorac Cardiovasc Surg. 1994;107:196–202. [PubMed] [Google Scholar]
- 4.Kouchoukos NT, Wareing TH, Murphy SF, Pelate C, Marshall WG. Risks of bilateral internal mammary artery bypass grafting. Ann Thorac Surg. 1990;49:210–9. doi: 10.1016/0003-4975(90)90140-2. doi:10.1016/0003-4975(90)90140-2. [DOI] [PubMed] [Google Scholar]
- 5.The Parisian Mediastinitis Study Group. Risk factors for deep sternal wound infection after sternotomy: a prospective, multicenter study. J Thorac Cardiovasc Surg. 1996;111:1200–7. doi: 10.1016/s0022-5223(96)70222-2. doi:10.1016/S0022-5223(96)70222-2. [DOI] [PubMed] [Google Scholar]
- 6.Gummert JF, Barten MJ, Hans C, Kluge M, Doll N, Walther T, et al. Mediastinitis and cardiac surgery—an updated risk factor analysis in 10,373 consecutive adult patients. Thorac Cardiovasc Surg. 2002;50:87–91. doi: 10.1055/s-2002-26691. doi:10.1055/s-2002-26691. [DOI] [PubMed] [Google Scholar]
- 7.Lu JCY, Grayson AD, Jha P, Srinivasan AK, Fabri BM. Risk factors for sternal wound infection and mid-term survival following coronary artery bypass surgery. Eur J Cardiothorac Surg. 2003;23:943–9. doi: 10.1016/s1010-7940(03)00137-4. doi:10.1016/S1010-7940(03)00137-4. [DOI] [PubMed] [Google Scholar]
- 8.Copeland M, Senkowski C, Ulcickas M, Mendelson M, Griepp RB. Breast size as a risk factor for sternal wound complications following cardiac surgery. Arch Surg. 1994;129:757–9. doi: 10.1001/archsurg.1994.01420310089016. doi:10.1001/archsurg.1994.01420310089016. [DOI] [PubMed] [Google Scholar]
- 9.Shahian DM, Edwards FH. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: introduction. Ann Thorac Surg. 2009;88:S1. doi: 10.1016/j.athoracsur.2009.05.054. doi:10.1016/j.athoracsur.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 10.Shahian DM, O'Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1—coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S2–22. doi: 10.1016/j.athoracsur.2009.05.053. doi:10.1016/j.athoracsur.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2—isolated valve surgery. Ann Thorac Surg. 2009;88:S23–42. doi: 10.1016/j.athoracsur.2009.05.056. doi:10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 12.Shahian DM, O'Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 3—valve plus coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S43–62. doi: 10.1016/j.athoracsur.2009.05.055. doi:10.1016/j.athoracsur.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 13.Nashef SAM, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R the Euro SCORE study group. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/s1010-7940(99)00134-7. doi:10.1016/S1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 14.Paul M, Raz A, Leibovici L, Madar H, Holinger R, Rubinovitch B. Sternal wound infection after coronary artery bypass graft surgery: validation of existing risk scores. J Thorac Cardiovasc Surg. 2007;133:397–403. doi: 10.1016/j.jtcvs.2006.10.012. doi:10.1016/j.jtcvs.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg. 2008;135:180–7. doi: 10.1016/j.jtcvs.2007.09.011. doi:10.1016/j.jtcvs.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Farsky PS, Graner H, Duccini P, Zandonadi Eda C, Amato VL, Anger J, et al. Risk factors for sternal wound infections and application of the STS score in coronary artery bypass graft surgery. Rev Bras Cir Cardiovasc. 2011;26:624–9. doi: 10.5935/1678-9741.20110054. doi:10.5935/1678-9741.20110054. [DOI] [PubMed] [Google Scholar]
- 17.Ariyaratnam P, Bland M, Loubani M. Risk factors and mortality associated with deep sternal wound infections following coronary bypass surgery with or without concomitant procedures in a UK population: a basis for a new risk model? Interact CardioVasc Thorac Surg. 2010;11:543–6. doi: 10.1510/icvts.2010.237883. doi:10.1510/icvts.2010.237883. [DOI] [PubMed] [Google Scholar]
- 18.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. doi:10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 19.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–40. doi: 10.1016/0196-6553(88)90053-3. doi:10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 20.Nezic D, Borzanovic M, Spasic T, Vukovic P. Calibration of the EuroSCORE II risk stratification model: is the Hosmer–Lemeshow test acceptable any more? Eur J Cardiothorac Surg. 2013;43:206. doi: 10.1093/ejcts/ezs346. [DOI] [PubMed] [Google Scholar]
- 21.Hickey GL, Bridgewater B. How well calibrated is EuroSCORE II? Eur J Cardiothorac Surg. 2013;43:208. doi: 10.1093/ejcts/ezs349. [DOI] [PubMed] [Google Scholar]
- 22.Sergeant P, Meuris B, Pettinari M. EuroSCORE II, illum qui est gravitates magni observe*. Eur J Cardiothorac Surg. 2012;41:729–31. doi: 10.1093/ejcts/ezs057. doi:10.1093/ejcts/ezs057. [DOI] [PubMed] [Google Scholar]
- 23.Paul P, Pennell ML, Lemeshow S. Standardizing the power of the Hosmer–Lemeshow goodness of fit test in large data sets. Stat Med. 2013;32:67–80. doi: 10.1002/sim.5525. [DOI] [PubMed] [Google Scholar]
- 24.Society of Cardiothoracic Surgeons. National adult cardiac surgical database report. 1999.