Abstract
OBJECTIVES
Transcatheter aortic valve implantation (TAVI) has been proposed as a treatment alternative for patients with aortic valve stenosis (AS) at high or prohibitive risk for surgical aortic valve replacement (AVR). We aimed to assess real-world outcomes after treatment according to the decisions of the multidisciplinary heart team.
METHODS
At a tertiary centre, all high-risk patients referred between 1 March 2008 and 31 October 2011 for symptomatic AS were screened and planned to undergo AVR, TAVI or medical treatment. We report clinical outcomes as defined by the Valve Academic Research Consortium.
RESULTS
Of 163 high-risk patients, those selected for AVR had lower logistic EuroSCORE and STS scores when compared with TAVI or medical treatment (median [interquartile range] 18 [12–26]; 26 [17–36]; 21 [14–32]% (P = 0.015) and 6.5 [5.1–10.7]; 7.6 [5.8–10.5]; 7.6 [6.1–15.7]% (P = 0.056)). All-cause mortalities at 1 year in 35, 73 and 55 patients effectively undergoing AVR, TAVI and medical treatment were 20, 21 and 38%, respectively (P = 0.051). Cardiovascular death and major stroke occurred in 9, 8 and 33% (P < 0.001) and 6, 4 and 2% (P = 0.62), respectively. For patients undergoing valve implantation, device success was 91 and 92% for AVR and TAVI, respectively. The combined safety endpoint at 30 days was in favour of TAVI (29%) vs AVR (63%) (P = 0.001). In contrast, the combined efficacy endpoint at 1 year tended to be more favourable for AVR (10 vs 24% for TAVI, P = 0.12).
CONCLUSIONS
Patients who are less suitable for AVR can be treated safely and effectively with TAVI with similar outcomes when compared with patients with a lower-risk profile undergoing AVR. Patients with TAVI or AVR have better survival than those undergoing medical treatment only.
Keywords: Transcatheter aortic valve implantation, Aortic valve replacement, Valve academic research consortium
INTRODUCTION
Surgical aortic valve replacement (AVR) has long been the mainstay of therapy for severe aortic valve stenosis (AS). Recently, transcatheter aortic valve implantation (TAVI) has been proposed as the new standard of care for patients with symptomatic AS who are not candidates for open surgery [1, 2]. Arguably, TAVI may also be a preferred alternative to AVR in carefully selected high-risk, but still operable, patients in whom morbidity and mortality may be reduced [1, 3].
Treatment recommendations for TAVI advocate the use of multidisciplinary team discussions to define the most appropriate treatment strategies for individual patients with AS at higher risk for surgical AVR [4]. Published data, however, reflect outcomes in highly selected patients in large-scaled national [5–7] or device-related [8] registries of patients undergoing TAVI as a single strategy, or in relatively small-scaled randomized studies with strictly selected patients undergoing TAVI vs AVR [3], or vs medical treatment only [2]. Hence, these studies do not encompass the whole spectrum of high-risk patients with AS, and thus do not reflect real-world outcomes in all-comer high-risk patients with AS treated according to the heart team's best option. Finally, standardized endpoint definitions have only been used partially and in a minority of these studies [9].
With 10 years of clinical experience with TAVI and >50 000 implants in >40 countries, individual heart teams and multicentre initiatives should evaluate the position of TAVI in daily practice. As cost-benefit of AS intervention in a high-risk patient population remains a matter of debate [10], cardiovascular teams should assess whether patient and treatment selection translate into improved outcomes in their practice. To our knowledge, this is the first study reporting standardized clinical outcomes in all patients presenting to a single centre for treatment of AS at high risk for AVR and who were treated with AVR, TAVI or medical treatment only, according to the decision of the multidisciplinary heart team.
MATERIALS AND METHODS
This study is a prospective single-centre initiative. The local ethics committee approved the design of the registry and all enrolled patients provided written informed consent before enrolment.
Patient population
The study population consisted of all symptomatic adults with severe AS who were at high risk for surgical AVR because of coexisting illnesses and who presented or were referred for further management to a single centre after 29 February 2008. Severe AS was defined as an aortic valve area (AVA) of <1 cm2, a mean aortic valve gradient of ≥40 mmHg or a peak aortic jet velocity of ≥4.0 m/s. All patients had New York Heart Association (NYHA) class II, III or IV symptoms. Advanced age as an isolated risk criterion was not considered sufficient, and a minimum of one coexisting illness or technical condition significantly impacting on perioperative outcome was required to be included in the registry. These conditions included severe pulmonary dysfunction (requiring systemic immunosuppression or oxygen therapy), extensive calcification of the ascending aorta, previous chest wall radiation, former coronary artery bypass graft surgery (CABG) using mammary arterial grafts crossing the midline, anatomical variations precluding a conventional surgical approach or patient frailty. We prospectively evaluated risk factors for cardiovascular surgery using the Society of Thoracic Surgeons (STS) score [11] and the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) [12]. We equally retrospectively calculated the EuroSCORE II [13], a recently updated logistic regression model with improved calibration and powerful discrimination, reflecting the reduction in cardiac surgical mortality in the last 15 years despite the treatment of older and sicker patients.
Patient evaluation and treatment assignment
All patients underwent systematic workup including clinical evaluation, laboratory testing and detailed imaging. Functional status of the patients was assessed at baseline with an index of independence in activities of daily living, a mini-mental exam and a 6-min walking test. No systematic tests were performed for the evaluation of frailty, and patients were considered frail mainly on the basis of the criteria of the medical team evaluating them.
Patients were evaluated in a weekly meeting by a multidisciplinary team (the heart team) composed of interventional cardiologists (C.D., M.C. and T.A.), cardiac surgeons (P.H. and F.R.) and non-invasive imaging and clinical cardiologists (M.C.H. and K.G.). In specific cases, geriatricians' advice was taken into consideration. Finally, patients were proposed to undergo surgical AVR (with or without CABG), TAVI (with or without percutaneous coronary intervention (PCI)) or medical treatment (with or without PCI and/or percutaneous transluminal aortic valvuloplasty (PTAV)). Selection of AVR or TAVI was made based on patient's overall risk profile, anatomical criteria and his potential to improve after intervention, following evolving guidelines of international cardiovascular societies [4, 14]. Medical treatment was proposed for those patients at prohibitive risk for any intervention, in whom any intervention seemed futile, or in whom uncertainty prevailed regarding the haemodynamic effect of valve replacement. Some patients underwent first PTAV as an eventual bridge to TAVI or AVR.
Patients were subsequently informed; those refusing the assigned procedure were redirected to medical treatment only, and the resulting treatment groups constituted the intention-to-treat population. The as-treated population consisted of patients (i) effectively undergoing AVR at high risk; (ii) at prohibitive risk for AVR and effectively undergoing TAVI as a valuable alternative and (iii) at prohibitive risk for any intervention, who refused intervention, or who died awaiting intervention.
Procedures
The SAPIEN heart-valve system (Edwards Lifesciences, Irvine, CA, USA) and the transcatheter implantation procedure have been described previously [1]. Patients who were assigned to TAVI underwent either transfemoral or transapical placement of the aortic valve on the basis of whether peripheral arteries could accommodate the large sheaths required (22 French for the 23-mm valve and 24 French for the 26-mm valve). Transapical placement was performed through a small intercostal incision over the left ventricular apex with the use of a dedicated delivery catheter and the same Edwards SAPIEN valve. In patients treated after April 2010, the SAPIEN-XT valve was used, allowing for smaller femoral sheaths (18 and 19 French for the 23- and 26-mm valve, respectively). The 29-mm SAPIEN-XT valve was used through the apical access route as soon as it became available. All procedures were performed in a hybrid operating room under general anaesthesia with the use of transoesophageal echocardiographic monitoring. Patients requiring coronary revascularization underwent PCI with drug-eluting or bare metal stents at the operators' discretion, >1 month before TAVI.
Patients assigned to AVR underwent median sternotomy, extracorporeal circulation and implantation of an aortic valve bioprosthesis. In selected cases, a mitral or tricuspid annuloplasty could be performed, at the discretion of the surgeon. All patients requiring coronary revascularization underwent concomitant CABG.
Follow-up
A clinical follow-up was carried out in all patients in office visits at 1, 6 and 12 months after inclusion in the registry, and was continued yearly thereafter. A telephone contact replaced the outpatient clinic visit whenever the patient failed to attend. We assessed NYHA functional class and performed transthoracic echocardiography whenever possible.
Endpoints and definitions
We used standardized definitions and endpoints for clinical outcomes and prosthetic valve performance as proposed by the Valve Academic Research Consortium (VARC) [15]. We report procedural outcome and device performance for patients undergoing TAVI or AVR. Device success is defined as a composite of successful vascular access, delivery and deployment of the valve, retrieval of the delivery system, correct position of the device, intended performance (AVA >1.2 cm2, mean AV gradient <20 mmHg or peak velocity <3 m/s, without moderate or severe prosthetic aortic valve regurgitation (AR)) and use of a single prosthesis. We report periprocedural outcome at 30 days, including eventual in-hospital deaths occurring after 30 days, as defined by VARC. The combined safety endpoint at 30 days includes all-cause mortality, major stroke, life-threatening bleeding, Stage 3 acute kidney injury, periprocedural myocardial infarction, major vascular complication or repeat procedure for valve-related dysfunction. We report the combined efficacy endpoint at 1 year as a composite of all-cause mortality after discharge (excluding early deaths), rehospitalization for cardiovascular causes and prosthetic heart-valve dysfunction (see above).
Statistical analysis
Continuous data are presented as means ± standard deviation or medians [interquartile range] as appropriate. Normally distributed data are compared using analysis of variance; otherwise, a Kruskal–Wallis test was used. Categorical data and clinical outcomes at 30 days and 1 year are summarized as frequencies and compared by the χ2 test or Fisher's exact test, as appropriate. Adverse event analyses were carried out using survival analyses techniques whereby outcomes were compared with the log-rank test and event rates were estimated with the Kaplan–Meier methodology. All analyses were performed in the as-treated population; comparisons for clinical characteristics or endpoints and functional recovery were carried out between all three treatment groups, while comparisons for acute and periprocedural outcomes and haemodynamic/echocardiographic data are limited to the patient groups undergoing valve implantation. A similar endpoint analysis was equally performed in the intention-to-treat population. Finally, a supplementary analysis of the combined risk scores was performed according to the initial treatment assignment by the heart team.
For between-group comparisons of NYHA classification and degree of AR, baseline values were assessed using the Kruskal–Wallis test or Wilcoxon rank-sum test, respectively. To assess within-group changes from baseline to the follow-up visits, a Wilcoxon signed-rank test was performed. The follow-up measurements were also compared using a Kruskal–Wallis test. For the assessment of changes in functional performance, data at 1 and 6 months were missing for patients who died. Two different analyses were done to account for this: (i) ignoring/excluding patients with missing data; the results of this analysis should therefore be interpreted as being ‘conditional on the patient being alive’, keeping in mind that the probability of being alive is dependent on the group to which the patient belongs; (ii) imputing the missing data for patients who died with an artificial worst possible category of death for the endpoint of interest, e.g. considering death as an extra category that is worse than NYHA class IV.
All statistical tests were two-sided and assessed at a significance level of 5%. Due to the exploratory nature of the study, no adjustments were made to the significance level to account for multiple testing. All analyses were performed using the SAS software version 9.2 (SAS Institute, Inc., Cary, NC, USA).
RESULTS
Patient population
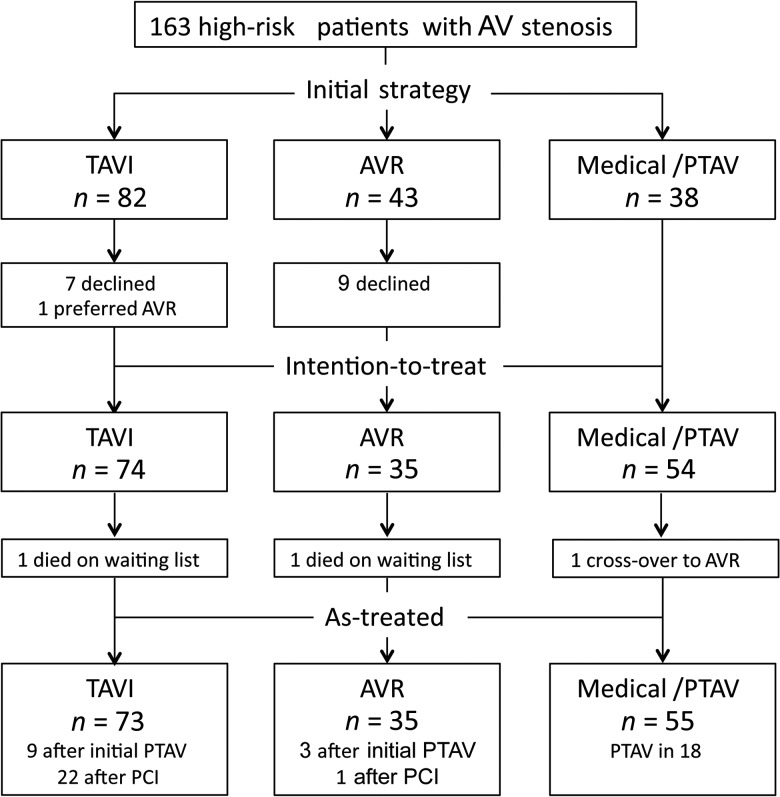
Between 1 March 2008 and 31 October 2011, 163 consecutive patients with symptomatic AS considered to be at high risk for surgical AVR were referred to a single centre for further management. After careful assessment by the heart team, patients were proposed to undergo TAVI (n = 82), surgical AVR (n = 43) or medical treatment (n = 38) (Fig. 1). Sixteen patients declined valve implantation and opted for a medical treatment only. Two patients died awaiting TAVI or AVR, and 1 ultimately underwent AVR, despite his initial assignment to the conservative treatment group. Finally, 73 patients underwent TAVI, 35 had AVR and 55 received medical treatment only, 18 of whom underwent PTAV. In the same period, 1211 other patients at lower risk underwent surgical AVR in the same centre.
Figure 1:
Patient flow according to heart team's assessment. AV: aortic valve; AVR: surgical aortic valve replacement; PCI: percutaneous coronary intervention; PTAV: percutaneous transluminal aortic valvuloplasty; TAVI: transcatheter aortic valve implantation.
Baseline characteristics and echocardiographic findings of the patients are presented in Table 1. Thirty-six percent of patients undergoing TAVI presented pulmonary dysfunction, when compared with 11% in patients undergoing AVR. Severe chronic obstructive pulmonary disease (COPD) was seen in 26 and 18% of TAVI and medically-treated patients, respectively, whereas none of the patients scheduled for AVR required oxygen therapy or systemic immunosuppression. Finally, patients assigned to TAVI or medical treatment more often presented cerebrovascular disease and underwent more previous cardiac interventions.
Table 1:
Baseline characteristics of the patients and echocardiographic findings
| Characteristic | TAVI (n = 73) | AVR (n = 35) | PTAV/medical (n = 55) | P-value |
|---|---|---|---|---|
| Age (year) | 82 ± 5 | 83 ± 5 | 83 ± 5 | 0.47 |
| Male sex [n (%)] | 36 (49) | 16 (46) | 20 (36) | 0.34 |
| STS score (%) | 7.3 [5.7–10.6] | 6.6 [5.3–10.8] | 7.8 [5.9–11.9] | 0.104 |
| Logistic EuroSCORE (%) | 25.0 [17.2–35.0] | 18.0 [13–26.5] | 21.0 [13.7–33.5] | 0.068 |
| EuroSCORE II (%) | 7.8 [4.8–12.9] | 7.7 [4.2–13.6] | 7.3 [4.2–14.4] | 0.51 |
| NYHA class [n (%)] | ||||
| I or II | 6 (8) | 10 (29) | 7 (13) | 0.016 |
| III or IV | 67 (92) | 25 (71) | 48 (87) | |
| Coronary artery disease [n (%)] | 47 (64) | 19 (54) | 30 (55) | 0.44 |
| Previous myocardial infarction [n (%)] | 13 (18) | 4 (11) | 8 (15) | 0.68 |
| Previous intervention [n (%)] | ||||
| CABG | 17 (23) | 2 (6) | 10 (18) | 0.069 |
| Valve surgery | 2 (3) | 2 (6) | 2 (4) | 0.76 |
| PCI | 36 (49) | 4 (11) | 9 (16) | <0.001 |
| PTAV | 16 (22) | 3 (9) | 0 | <0.001 |
| Cerebral vascular disease [n (%)] | 34 (47) | 7 (20) | 16 (29) | 0.013 |
| Previous stroke or TIA [n (%)] | 16 (22) | 2 (6) | 12 (22) | 0.075 |
| Peripheral vascular disease [n (%)] | 26 (36) | 8 (23) | 16 (29) | 0.39 |
| Arterial hypertension [n (%)] | 51 (70) | 26 (74) | 43 (78) | 0.57 |
| Diabetes mellitus [n (%)] | 22 (30) | 7 (20) | 12 (22) | 0.42 |
| Hyperlipidemia [n (%)] | 54 (74) | 24 (69) | 39 (71) | 0.83 |
| COPD [n (%)] | ||||
| Any | 26 (36) | 4 (11) | 20 (36) | 0.020 |
| Requiring systemic immunosuppression or oxygen therapy | 19 (26) | 0 | 10 (18) | <0.001 |
| Creatinine > 2 mg/dl [n (%)] | 8 (11) | 4 (11) | 9 (16) | 0.64 |
| Atrial fibrillation [n (%)] | 27 (37) | 14 (40) | 20 (36) | 0.94 |
| Permanent pacemaker [n (%)] | 8 (11) | 2 (6) | 2 (4) | 0.31 |
| Pulmonary hypertension [n (%)] | 51 (70) | 24 (69) | 40 (73) | 0.90 |
| Frailty [n (%)] | 15 (21) | 4 (11) | 13 (24) | 0.36 |
| Extensively calcified aorta [n (%)] | 17 (23) | 3 (9) | 5 (9) | 0.056 |
| Previous chest wall radiation [n (%)] | 5 (7) | 2 (6) | 2 (4) | 0.83 |
| Echocardiographic findings | ||||
| Aortic valve area (cm2) | 0.62 ± 0.17 | 0.65 ± 0.21 | 0.54 ± 0.15 | 0.010 |
| Mean aortic-valve gradient (mmHg) | 53 ± 17 | 48 ± 22 | 53 ± 23 | 0.42 |
| Mean LVEF (%) | 53 ± 13 | 50 ± 17 | 52 ± 14 | 0.57 |
AVR: surgical aortic valve replacement; CABG: coronary artery bypass graft surgery; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; NYHA class: New York Heart Association classification; PCI: percutaneous coronary intervention; PTAV: percutaneous transluminal aortic valvuloplasty; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation; TIA: transient ischaemic attack.
Categorical data are presented as numbers and percentages, continuous data are presented as means ± standard deviations or medians [interquartile ranges], as appropriate.
Initial evaluation by the heart team assigned patients with distinct overall perioperative risk to TAVI, AVR and medical treatment (logistic EuroSCORE 26 [17–36]; 18 [12–26] and 21 [14–32] %, respectively (P = 0.015); STS score 7.6 [5.8–10.5]; 6.5 [5.1–10.7] and 7.6 [6.1–15.7] % (P = 0.056)). In the as-treated population, no significant differences were seen between groups, although a trend remained towards lower logistic EuroSCORE and STS score in patients undergoing AVR (Table 1). Of note, 28 of the 36 patients (78%) undergoing TAVI with a logistic EuroSCORE below the median of 25% presented at least one of the following characteristics, putting them at higher risk for AVR: extensive calcification of the ascending aorta (n = 13; 36%), severe COPD (n = 10; 28%), previous CABG with internal mammary arterial grafts crossing the midline (n = 3; 8%), former chest wall radiation (n = 2; 6%) or severe frailty (n = 6; 17%). Five others had logistic EuroSCOREs between 20 and 25% and presented severe pulmonary hypertension. Of the 3 TAVI patients with scores <20%, 2 suffered previous disabling stroke, and 1 was 93 years old. The group of patients with medical therapy included 10 patients (18%) with a logistic EuroSCORE of 14.9 [10–23.2] %, who were originally assigned to AVR but declined this treatment (n = 9) or died awaiting operation (n = 1). The remainder in this group had a logistic EuroSCORE of 22 [16–43] %, including 8 patients originally assigned to TAVI and 5 others who refused any invasive screening and treatment.
Procedures and acute outcomes
In the TAVI group, 9 patients (12%) underwent PTAV in the months before and as a bridge to valve implantation, and 22 (30%) had PCI. Thirty-six patients (49%) underwent transapical and 37-patients (51%) transfemoral valve implantation with 35 (48%) 23 mm, 35 (48%) 26-mm and 3 (4%) 29-mm valves. Five patients (7%) necessitated urgent cardiopulmonary bypass to correct haemodynamic compromise. Four transfemoral procedures (5%) were complicated with cardiac tamponade and 2 of these patients needed surgical revision and died in the postoperative period. One of these was urgently converted to AVR due to vascular access problems. In one transapical procedure, a surgical revision was needed for haemothorax. Overall, acute procedural vascular complications occurred in 2 patients (3%). A second SAPIEN valve was needed to secure a too ventricular position in 1, and as a consequence of distal valve embolization in another.
In the surgical group, 3 patients (9%) underwent PTAV as a bridge to valve replacement. Fifteen patients (43%) underwent concomitant CABG, 4 (11%) had mitral valve repair and 4 others underwent tricuspid valve annuloplasty. All patients received an aortic valve bioprosthesis. One patient needed surgical revision for haemothorax (3%). In another patient (3%), a sutureless valve had to be replaced by a conventional bioprosthesis due to severe paravalvular leakage. Two patients (6%) presented mean transvalvular pressure gradients >20 mmHg, most likely related to patient-prosthesis mismatch.
Overall, life-threatening or disabling bleeding was significantly more frequent in patients undergoing AVR (51%) when compared with TAVI (19%), mainly related to a significantly higher need for blood transfusion (P < 0.001). Device success was 92 and 91% for TAVI and AVR, respectively (P = 0.95). There were no intraprocedural deaths in either of the groups.
Periprocedural outcomes
Clinical outcomes at 30 days are presented in Table 2. No significant differences between groups were seen in all-cause mortality, cardiovascular mortality or stroke, although all-cause mortality and strokes/transient ischaemic attacks (TIA) were numerically higher in patients undergoing AVR. Of note, patients with AVR more frequently developed a first episode of atrial fibrillation after surgery (P = 0.001). Overall, the combined safety endpoint was reached significantly more often after AVR, mainly driven by a high transfusion rate (≥4 units packed cells). Duration of hospital stay was significantly longer after AVR when compared with TAVI (19 [14–26] vs 11 [7–18] days, P = 0.001), but patients spent a similar time in the intensive care unit (3 [2–7] vs 4 [3–6] days, P = 0.27).
Table 2:
Clinical outcomes at 30 days and 1 year
| Outcome [n (%)] | TAVI (n = 73) | 30 days AVR (n = 35) | PTAV/medical (n = 55) | P-value | TAVI (n = 73) | 1 year AVR (n = 35) | PTAV/medical (n = 55) | P-value |
|---|---|---|---|---|---|---|---|---|
| Death | ||||||||
| From any cause | 7 (10) | 6 (17) | 7 (13) | 0.54 | 15 (21) | 7 (20) | 21 (38) | 0.051 |
| From cardiovascular cause | 5 (7) | 2 (6) | 7 (13) | 0.40 | 6 (8) | 3 (9) | 18 (33) | <0.001 |
| Repeat hospitalization for cardiovascular cause | 1 (1) | 0 | 0 | 0.54 | 9 (12) | 1 (3) | 10 (18) | 0.098 |
| Death or repeat hospitalization | 8 (11) | 6 (17) | 6 (11) | 0.62 | 21 (29) | 8 (23) | 24 (44) | 0.080 |
| Stroke or TIA | ||||||||
| All | 3 (4) | 3 (9) | 1 (2) | 0.31 | 9 (12) | 3 (9) | 3 (5) | 0.41 |
| TIA | 1 (1) | 0 | 0 | 0.54 | 3 (4) | 0 | 0 | 0.15 |
| Stroke | ||||||||
| Minor | 0 | 1 (3) | 0 | 0.16 | 3 (4) | 1 (3) | 2 (4) | 0.95 |
| Major | 2 (3) | 2 (6) | 1 (2) | 0.57 | 3 (4) | 2 (6) | 1 (2) | 0.62 |
| Death or major stroke | 9 (12) | 7 (20) | 6 (11) | 0.44 | 17 (23) | 8 (23) | 22 (40) | 0.081 |
| Major vascular complications | 3 (4) | 0 | 1 (2) | 0.41 | 4 (5) | 0 | 2 (4) | 0.37 |
| Life-threatening bleeding | 14 (19) | 18 (51) | NA | <0.001 | – | – | – | – |
| Acute kidney injury | ||||||||
| Modified RIFLE stage 2 or 3 | 11 (15) | 3 (9) | 9 (16) | 0.56 | – | – | – | – |
| Renal replacement therapy | 4 (5) | 2 (6) | 0 | 0.21 | – | – | – | – |
| Cardiac reintervention | ||||||||
| PTAV | 0 | 0 | 1 (2) | 0.38 | 0 | 0 | 2 (4) | 0.14 |
| AVR | 1 (1) | 0 | 0 | 0.54 | 2 (3) | 0 | 0 | 0.29 |
| Endocarditis | 0 | 0 | 0 | – | 1 (1) | 0 | 0 | 0.54 |
| New atrial fibrillation | 10 (14) | 12 (34) | 3 (5) | 0.001 | 10 (14) | 12 (34) | 3 (5) | 0.001 |
| New pacemaker | 7 (10) | 2 (6) | 2 (4) | 0.40 | 8 (11) | 2 (6) | 2 (4) | 0.27 |
| VARC device success | 67 (92) | 32 (91) | NA | 0.95 | – | – | – | – |
| VARC combined safety at 30 days | 21 (29) | 22 (63) | NA | 0.001 | – | – | – | – |
| VARC combined efficacy at 1 year | – | – | – | – | 16/66 (24) | 3/29 (10) | NA | 0.12 |
AVR: surgical aortic valve replacement; PTAV: percutaneous transluminal aortic valvuloplasty; TAVI: transcatheter aortic valve implantation; TIA: transient ischaemic attack; VARC combined safety and efficacy endpoints refer to composite endpoints unfavourably affecting safety or efficacy, respectively, and are defined by the VARC (see Methods and definitions).
Medium-term follow-up
The clinical follow-up was available at 25 [12–40], 38 [12–42] and 32 [18–41] months for patients undergoing TAVI, AVR and medical treatment, respectively. Clinical outcomes at 1 year are presented in Table 2. All-cause and cardiovascular mortalities were similar in patients who underwent TAVI or AVR, and significantly lower than in patients assigned to medical treatment (P = 0.051 and P < 0.001, respectively). Similar outcomes were seen when analysing all-cause and cardiovascular mortalities at 1 year in the intention-to-treat population (P = 0.09 and P = 0.001). Of note, 6 of 16 patients (38%) who were originally proposed to undergo TAVI or AVR, but refused the assigned treatment, died in the first year of the follow-up.
Stroke/TIA rate was numerically higher at 1 year after TAVI when compared with AVR or conservative treatment, but this difference was not statistically significant. Surprisingly, most neurological events in the TAVI group occurred after 30 days, while no new events were seen in patients after AVR. Finally, the combined efficacy endpoint after 1 year tended to be in favour of AVR (P = 0.12).
Of note, 1 patient underwent AVR >1 month after TAVI, because of severe AR secondary to premature leaflet degeneration. Another TAVI patient developed endocarditis of the bioprosthesis and could successfully be treated medically. Overall, more permanent pacemakers were implanted in patients after TAVI, but this difference was not statistically significant.
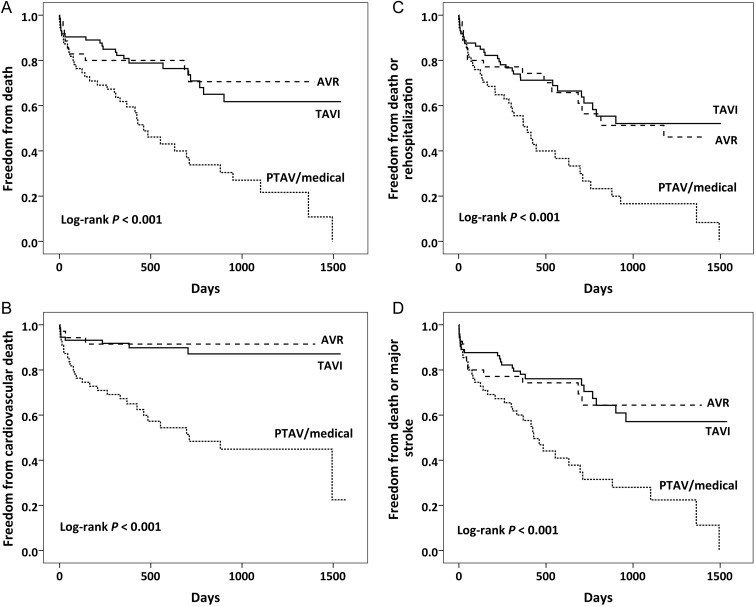
Event-free survival curves for all-cause death, cardiovascular death, death or rehospitalization and death or major stroke are presented in Fig. 2. For all outcomes, patients with medical treatment performed significantly worse than those undergoing TAVI or AVR.
Figure 2:
Event-free survival curves for major adverse outcomes. (A and B) All-cause and cardiovascular mortality, respectively. (C and D) All-cause death with rehospitalization for cardiovascular causes and major stroke, respectively. Event rates were calculated with the use of Kaplan–Meier methods and compared with the use of the log-rank test. AVR: surgical aortic valve replacement; PTAV: percutaneous transluminal aortic valvuloplasty; TAVI: transcatheter aortic valve implantation.
Functional recovery and haemodynamic outcome
We did not detect a difference in functional performance at baseline between patients undergoing TAVI, AVR or medical treatment (P = 0.16), but exercise tolerance improved significantly at 1 month in the TAVI group (P < 0.001), while no change was seen in the other groups (P = 0.65 and P = 0.96 for AVR and medical treatment, respectively) (Fig. 3). These changes differed significantly between groups (P < 0.001). This improvement in NYHA classification was maintained at 6 months after TAVI, both including and excluding patients who died. In contrast, a significant improvement in functional performance in the patients alive 6 months after AVR (P < 0.001) was no longer significant when taking into account the deaths in this group (P = 0.62). In patients with medical treatment, we noticed even a significant worsening of functional performance at 6 months (P = 0.011). Again, these changes differed significantly between groups, both when ignoring or considering the deaths in each group (P < 0.001 and P < 0.001).
Figure 3:
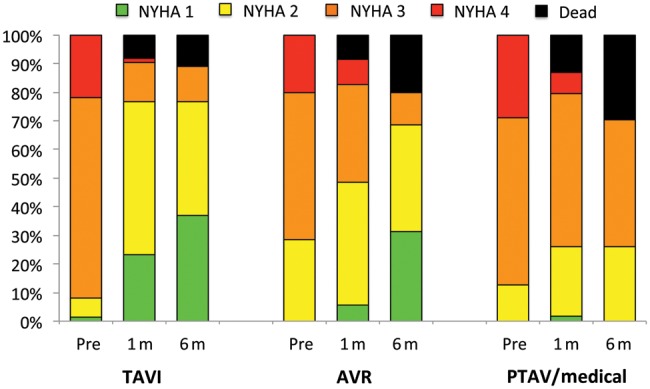
Functional assessment according to NYHA classification. Functional status was assessed at baseline and at 1 and 6 months after treatment. A significant improvement was seen in patients undergoing valve implantation, and this improvement occurred earlier in patients undergoing TAVI. AVR: surgical aortic valve replacement; PTAV: percutaneous transluminal aortic valvuloplasty; TAVI: transcatheter aortic valve implantation.
From a haemodynamic perspective, mean aortic valve gradient dropped from baseline to the 6-month follow-up from 54 ± 18 to 11 ± 4 mmHg and 51 ± 22 to 12 ± 6 mmHg after TAVI and AVR, respectively (change (Δ) of 43 ± 18 mmHg vs Δ39 ± 22 mmHg, P = 0.4). In the same time-span, ejection fraction improved from 53 ± 13 to 56 ± 10% and 52 ± 16 to 56 ± 10% with TAVI and AVR, respectively (Δ3 ± 10 vs Δ4 ± 12%, P = 0.8). This resulted in an improvement of the calculated AVA from 0.62 ± 0.18 to 1.72 ± 0.51 cm2 and 0.65 ± 0.22 to 1.51 ± 0.40 cm2 in the respective treatment groups (Δ1.09 ± 0.48 vs 0.86 ± 0.43 cm2, P = 0.048). Three patients presented moderate or severe AR 1 month after TAVI, and one of them needed surgical reintervention with AVR. No moderate to severe AR were seen in the surgical group. At 1 and 6 months, the surgical group counted 9 and 7 patients with mild AR, respectively, as opposed to 23 and 21 with no or only trivial regurgitation. Most mild AR at 1-month follow-up were valvular (5/9). Four patients had mild combined valvular and paravalvular AR, 3 of which received a Perceval™ sutureless bioprosthesis (Sorin, Milan, Italy). Overall, significantly more patients presented some degree of AR at 1 or 6 months after TAVI when compared with surgical AVR (P = 0.003) (Fig. 4). There were no differences in clinical outcome related to the degree of AR (data not shown).
Figure 4:
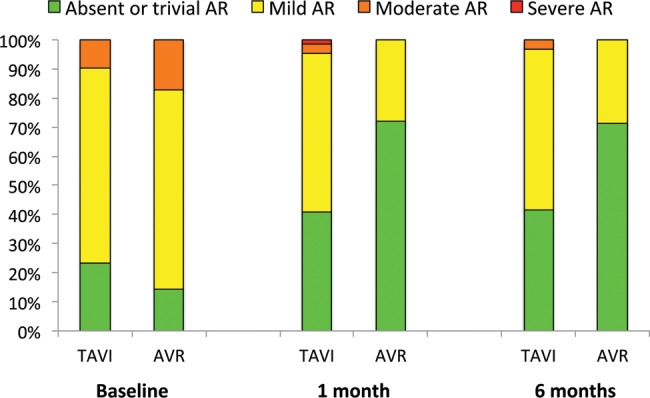
Prosthetic valve regurgitation at 1 and 6 months after TAVI and AVR. Patients with TAVI presented significantly more often some degree of AR after intervention when compared with patients undergoing AVR. AR: aortic valve regurgitation; AVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation.
DISCUSSION
The present study is the first to report standardized haemodynamic and medium-term clinical outcomes after TAVI, AVR or medical treatment in a sizable group of all-comer patients presenting to a single centre with severe AS at high risk for AVR. We observed the following main findings:
systematic and meticulous patient screening according to clinical and anatomic criteria allowed identifying patient subgroups with different overall risk profiles.
After multidisciplinary assessment, 4 in 5 patients were proposed to undergo a tailored valve implantation/replacement procedure with acceptable outcomes.
Patients who were less suitable for AVR could be treated safely and effectively with TAVI with similar outcomes when compared with patients with a lower risk phenotype undergoing AVR. Patients with TAVI or AVR had better survival than those undergoing medical treatment only.
(iv) Haemodynamic and functional improvement was similar after TAVI and AVR, but patients recovered earlier after TAVI. In contrast, patients with TAVI more presented often mild AR.
There are several methodological strengths in this study that reinforce the validity of the results obtained: all-comer nature, prospective enrolment, systematic and meticulous patient assessment, complete 1-year clinical follow-up, and the use of standardized definitions and endpoints for clinical outcomes and prosthetic valve performance.
Contemporary treatment recommendations advocate that the use of TAVI should be restricted to high- or prohibitive surgical-risk patients with severe AS [4, 14]. Many patients with high, but acceptable risk, however, can still be treated with AVR with good outcomes [16, 17]. Final treatment assignment thus implies a systematic and meticulous selection process, taking into account patient's overall risk profile as well as specific technical and anatomical criteria [18]. While available surgical risk algorithms do allow distinguishing high-risk surgical patients from those at low or intermediate risk, they are less suited to categorize patients within the high-risk spectrum. Moreover, high-risk patients often present anatomical or surgicotechnical characteristics, which are not properly reflected by these scoring systems. Despite these limitations, logistic EuroSCORE and STS score in the present study were higher in patients initially selected for TAVI, pointing towards an obvious difference in perioperative risk when compared with those scheduled for AVR. In the as-treated population, these differences were less prominent, since 16 patients were reassigned to the medical treatment group because of refusal of an intervention. However, patients with lower scores scheduled for TAVI presented by definition at least one coexisting illness, putting them at excessive risk for AVR.
The introduction of TAVI in clinical practice has opened new treatment perspectives for 32–60% of patients in the general population with severe symptomatic single valvular heart disease who formerly did not undergo valve intervention, most frequently because of comorbidities [19, 20]. In our experience, 77% of these higher-risk patients with comorbidities were assigned to TAVI or AVR, and 66% ultimately underwent valve implantation (45% TAVI; 21% AVR), while 34% received medical treatment only. These figures compare favourably with reports in similar patients referred for multidisciplinary evaluation of high-risk AS [21, 22]. In these registries, ∼35% of patients received a valve prosthesis (20% TAVI; 15% AVR), while up to 65% of patients were treated medically. The study by Jahangiri et al. [23], however, reports higher bioprosthesis implantation rates (36% TAVI; 42% AVR), but this analysis cannot be considered as a true all-comers study, since it was restricted to patients specifically referred for TAVI. Indeed, a number of patients will even not be considered for TAVI, when they are at prohibitive risk for any intervention, or when the intervention is considered to be futile. Moreover, a significant number of patients in our series refused AVR or TAVI, while others died awaiting intervention. With increasing experience and widespread use of TAVI, these refusals may become more rare, and earlier intervention may prevent deaths on the waiting list. Of note, most refusals occurred in the group selected for AVR (21%).
The clinical outcomes in our study justify the choice of TAVI in those patients considered to be at excessive risk for AVR: indeed, in these patients, event-free survival curves for all-cause and cardiovascular mortalities (Fig. 2, A and B), and all-cause mortality combined with rehospitalization or major stroke (C and D), were virtually overlapping during the course of the study with the patients undergoing AVR, except for an early death hazard in the AVR group. This early hazard was limited to death from non-cardiac causes, underscoring the impact of comorbid conditions on outcome of cardiovascular surgery. We can, however, not completely exclude that our TAVI patients would have fared equally well with AVR, but given the specific risk spectrum in this group and the early death hazard associated with higher risk, this scenario is very unlikely. In contrast, patients who did not undergo valve intervention performed significantly worse at the follow-up, and TAVI or AVR could have saved lives, at least in those who refused the intervention or could not be treated early enough. Indeed, 38% of patients who refused TAVI or AVR died within the first year, identical to the 1-year mortality rate in the global medically treated group.
The results in our TAVI patients are in line with the large meta-analysis by Généreux et al. [9]. In this meta-analysis, the pooled estimate rates of major clinical outcomes according to VARC definitions were almost identical to our figures, including 1-year all-cause and cardiovascular mortalities, stroke, life-threatening bleeding, acute kidney injury and the composite safety endpoint at 30 days. Overall, our results can thus be considered as representative for contemporary TAVI practice. We experienced, however, a lower incidence of major vascular complications (4 vs 12%), possibly related to our restrictive selection of patients with diseased aortofemoral routes for the transfemoral approach. Indeed, in our series, half of the patients underwent transapical TAVI, mainly due to the very large vessel size required to accommodate the introducer sheath for the femoral approach in the first 2 years of our experience. The combined VARC efficacy endpoint at 1 year was strikingly lower in our series when compared with the results reported by Généreux et al. (24 vs 71%). These high rates in the meta-analysis were explained by the inclusion of recurrent heart failure requiring admission as a component of this outcome; in our study, however, rehospitalization for cardiovascular causes was limited to 12% at 1 year.
A number of interesting outcome differences between TAVI and AVR came to our attention. First, patients undergoing TAVI needed significantly less frequent blood transfusions, pointing towards an obvious difference in the invasiveness of the procedure. Secondly, patients with TAVI developed significantly less atrial fibrillation when compared with AVR with sternotomy and extracorporeal circulation. Thirdly, the less invasive procedure translated into a significantly shorter hospital stay for patients undergoing TAVI. This shorter recovery was equally reflected in an improved functional status at 1 month after intervention, a functional level that AVR patients obtained later (Fig. 3). Fourthly, the combined safety endpoint at 30 days was significantly in favour of TAVI, driven by the early death hazard and life-threatening bleeding with AVR, but this was in part compensated by a trend towards more favourable combined efficacy at 1 year with AVR. Indeed, TAVI patients tended to be rehospitalized more frequently during the first year of the follow-up, and 3 of them had moderate or severe prosthetic AR. In contrast to the results reported by Makkar et al. [2] and Kodali et al. [3], however, in our experience, mild, moderate or severe prosthetic AR after TAVI did not implicate a less favourable late survival.
Overall, the results after TAVI in our patient population at high risk for AVR can be considered satisfactory and promising. Indeed, only 8% presented cardiovascular complications that ultimately resulted in cardiovascular death at 1 year. Retrospectively, half of these complications could eventually have been avoided, and should be considered as inherent to the learning curve of performing TAVI. Moreover, prosthesis size selection and implantation technique should be considered as a continuously evolving field, in which multimodality imaging has recently been playing an increasing role, hereby optimizing prosthesis positioning and limiting AR [18, 24, 25]. Finally, various transcatheter valves are being developed, specifically striving to reduce cardiovascular complications. In general, newer devices will incorporate features that reduce delivery catheter diameter, improve ease of positioning and sealing, or facilitate repositioning or removal [1].
Our study has several limitations. It concerns a prospective single-centre experience of all patients evaluated and treated for AS at high risk for AVR, and carries all the shortcomings of its non-randomized and monocentric design. This limitation represents strength since it replicates a real-world situation in which all-comer nature and randomization can never be reconciled. We realize that the numbers in the different treatment groups are relatively small, though still allowing the discernment of important differences in baseline characteristics and hard clinical endpoints. In contrast to other much larger TAVI registries [5–8], we did not perform uni- or multivariate analyses to identify predictors for worse outcome in our patient population, or report subgroup analyses between transfemoral and transapical TAVI or between tertiles of operative risk scores. We did, however, meticulously assign endpoints according to VARC definitions, to allow comparisons with similar future experiences.
In summary, meticulous patient assessment by a multidisciplinary heart team ultimately leads to aortic valve implantation in most patients with AS considered to be at high risk for AVR. Patients who are less suitable for AVR can be treated safely and effectively with TAVI with similar outcomes when compared with patients with a lower-risk phenotype undergoing AVR. Patients with TAVI or AVR had better survival when compared with those undergoing medical treatment only. Continuous improvements in patient evaluation, prosthesis design and selection and implantation technique will increasingly support the role of TAVI in this complex patient subset.
Acknowledgements
We thank Marina Claes for the help she provided in acquiring the data.
Conflict of interest: none declared.
REFERENCES
- 1.Webb JG, Wood DA. Current status of transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;60:483–92. doi: 10.1016/j.jacc.2012.01.071. [DOI] [PubMed] [Google Scholar]
- 2.Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Eng J Med. 2012;366:1696–704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 3.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Eng J Med. 2012;366:1686–95. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 4.Vahanian A, Alfieri O, Al-Attar N, Antunes M, Bax J, Cormier B, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2008;29:1463–70. doi: 10.1093/eurheartj/ehn183. [DOI] [PubMed] [Google Scholar]
- 5.Gilard M, Eltchaninoff H, Iung B, Donzeau-Gouge P, Chevreul K, Fajadet J, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Eng J Med. 2012;366:1705–15. doi: 10.1056/NEJMoa1114705. [DOI] [PubMed] [Google Scholar]
- 6.Moat NE, Ludman P, de Belder MA, Bridgewater B, Cunningham AD, Young CP, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol. 2011;58:2130–8. doi: 10.1016/j.jacc.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 7.Rodes-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–90. doi: 10.1016/j.jacc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Thomas M, Schymik G, Walther T, Himbert D, Lefevre T, Treede H, et al. One-year outcomes of cohort 1 in the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry: the European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2011;124:425–33. doi: 10.1161/CIRCULATIONAHA.110.001545. [DOI] [PubMed] [Google Scholar]
- 9.Généreux P, Head SJ, Van Mieghem NM, Kodali S, Kirtane AJ, Xu K, et al. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol. 2012;59:2317–26. doi: 10.1016/j.jacc.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Neyt M, Van Brabandt H, Devriese S, Van De Sande S. A cost-utility analysis of transcatheter aortic valve implantation in Belgium: focusing on a well-defined and identifiable population. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson TB, Jr, Dziuban SW, Jr, Edwards FH, Eiken MC, Shroyer AL, Pairolero PC, et al. The STS National Database: current changes and challenges for the new millennium. Committee to Establish a National Database in Cardiothoracic Surgery, the Society of Thoracic Surgeons. Ann Thorac Surg. 2000;69:680–91. doi: 10.1016/s0003-4975(99)01538-6. [DOI] [PubMed] [Google Scholar]
- 12.Roques F, Nashef SA, Michel P, Gauducheau E, de Vincentiis C, Baudet E, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. J Eur Cardiothorac Surg. 1999;15:816–22. doi: 10.1016/s1010-7940(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 13.Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, et al. EuroSCORE II. J Eur Cardiothorac Surg. 2012;41:734–44. doi: 10.1093/ejcts/ezs043. [DOI] [PubMed] [Google Scholar]
- 14.Holmes DR, Jr, Mack MJ. Transcatheter valve therapy a professional society overview from the american college of cardiology foundation and the society of thoracic surgeons. J Am Coll Cardiol. 2011;58:445–55. doi: 10.1016/j.jacc.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J. 2011;32:205–17. doi: 10.1093/eurheartj/ehq406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Eusanio M, Fortuna D, Cristell D, Pugliese P, Nicolini F, Pacini D, et al. Contemporary outcomes of conventional aortic valve replacement in 638 octogenarians: insights from an Italian Regional Cardiac Surgery Registry (RERIC) J Eur Cardiothorac Surg. 2012;41:1247–52. doi: 10.1093/ejcts/ezr204. [DOI] [PubMed] [Google Scholar]
- 17.Molstad P, Veel T, Rynning S. Long-term survival after aortic valve replacement in octogenarians and high-risk subgroups. J Eur Cardiothorac Surg. 2012;42:934–40. doi: 10.1093/ejcts/ezs190. [DOI] [PubMed] [Google Scholar]
- 18.Piazza N, Lange R, Martucci G, Serruys PW. Patient selection for transcatheter aortic valve implantation: patient risk profile and anatomical selection criteria. Arch Cardiovasc Dis. 2012;105:165–73. doi: 10.1016/j.acvd.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Charlson E, Legedza AT, Hamel MB. Decision-making and outcomes in severe symptomatic aortic stenosis. J Heart Valve Dis. 2006;15:312–21. [PubMed] [Google Scholar]
- 20.Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–43. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Dor I, Dvir D, Barbash IM, Okubagzi P, Torguson R, Xue Z, et al. Outcomes of patients with severe aortic stenosis at high surgical risk evaluated in a trial of transcatheter aortic valve implantation. Am J Cardiol. 2012;110:1008–14. doi: 10.1016/j.amjcard.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 22.Dewey TM, Brown DL, Das TS, Ryan WH, Fowler JE, Hoffman SD, et al. High-risk patients referred for transcatheter aortic valve implantation: management and outcomes. Ann Thorac Surg. 2008;86:1450–6. doi: 10.1016/j.athoracsur.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 23.Jahangiri M, Laborde JC, Roy D, Williams F, Abdulkareem N, Brecker S. Outcome of patients with aortic stenosis referred to a multidisciplinary meeting for transcatheter valve. Ann Thorac Surg. 2011;91:411–5. doi: 10.1016/j.athoracsur.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Binder RK, Leipsic J, Wood D, Moore T, Toggweiler S, Willson A, et al. Prediction of optimal deployment projection for transcatheter aortic valve replacement: angiographic 3-dimensional reconstruction of the aortic root versus multidetector computed tomography. Circ Cardiovasc Interv. 2012;5:247–52. doi: 10.1161/CIRCINTERVENTIONS.111.966531. [DOI] [PubMed] [Google Scholar]
- 25.Willson AB, Webb JG, Labounty TM, Achenbach S, Moss R, Wheeler M, et al. 3-dimensional aortic annular assessment by multidetector computed tomography predicts moderate or severe paravalvular regurgitation after transcatheter aortic valve replacement: a multicenter retrospective analysis. J Am Coll Cardiol. 2012;59:1287–94. doi: 10.1016/j.jacc.2011.12.015. [DOI] [PubMed] [Google Scholar]