Abstract
OBJECTIVES
A prospective randomized multicentre trial was performed to analyse the efficacy of a vest (Posthorax support vest®) to prevent sternal wound infection after cardiac surgery, and to identify risk factors.
METHODS
From September 2007 to March 2010, 2539 patients undergoing cardiac surgery via median sternotomy were prospectively randomized into those who received a Posthorax® vest and those who did not. Patients were instructed to wear the vest postoperatively for 24 h a day for at least 6 weeks; the duration of follow-up was 90 days. Patients who did not use the vest within a period of 72 h postoperatively were regarded as study dropouts. Statistical calculations were based on an intention-to-treat (ITT) analysis. Further evaluations comprised all subgroups of patients.
RESULTS
Complete data were available for 2539 patients (age 67 ± 11years, 45% female). Of these, 1351 were randomized to receive a vest, while 1188 received no vest. No significant differences were observed between groups regarding age, gender, diabetes, body mass index, chronic obstructive pulmonary disease (COPD), renal failure, the logistic EuroSCORE and the indication for surgery. The frequency of deep wound complications (dWC: mediastinitis and sternal dehiscence) was significantly lower in vest (n = 14; 1.04%) vs non-vest (n = 27; 2.27%) patients (ITT, P < 0.01), but superficial complications did not differ between groups. Subanalysis of vest patients revealed that only 933 (Group A) wore the vest according to the protocol, while 202 (Group BR) refused to wear the vest (non-compliance) and 216 (Group BN) did not use the vest for other reasons. All dWC occurred in Groups BR (n = 7) and BN (n = 7), although these groups had the same preoperative risk profile as Group A. Postoperatively, Group BN had a prolonged intubation time, a longer stay in the intensive care unit, greater use of intra-aortic balloon pump, higher frequency of COPD and a larger percentage of patients who required prolonged surgery.
CONCLUSIONS
Consistent use of the Posthorax® vest prevented deep sternal wounds. The anticipated risk factors for wound complications did not prove to be relevant, whereas intra- and postoperative complications appear to be very significant.
Keywords: Sternum, Site infection, Prevention
INTRODUCTION
Postoperative wound complications after cardiac surgery are still one of the major causes of morbidity and mortality. Infections acquired during health care, such as sternal complications, including prolonged hospital stays, greater consumption of antibiotics, revision surgery and greater consumption of resources culminate in unpredictably high treatment costs [1–5].
Restrictions on the reimbursement of medical expenses, particularly in the last years, have magnified the importance of preventing post-surgical complications. Postoperative care must be focused on the patients' well-being and prudent use of resources.
Several risk factors have been identified in previous studies. However, the majority of these were based on retrospective analyses, and the results were contradictory; moreover, the studies were focused on risk factors and the outcome of mediastinitis [3–5]. We designed a prospective study based on the hypothesis that the most common cause of postoperative missing wound healing is early sternal instability.
A pilot study had been performed from 2006 to 2007 to test the concept of external stabilization of the sternum [6]. The present study was designed to evaluate the clinical efficacy of primary reinforcement of the sternum using the Posthorax® vest. High-risk patients were identified according to the Society of Thoracic Surgeons (STS) infection risk score published by Fowler et al. [7], which is based on an analysis in >300 000 patients.
The initial results confirmed the positive effects of external stabilization of the sternum with the vest. Patients with a low-risk score developed similar infection rates as those with high-risk score values, and therefore, all patients were included in the study. A prospective randomized multicentre study was performed to analyse the efficacy of the vest. The investigation comprised all patients and included a prospective risk factor analysis for wound-healing disorders.
MATERIALS AND METHODS
Posthorax vest
The Posthorax® sternum support vest (Epple, Inc., Vienna, Austria) is a recently developed and patented design (Fig. 1). Its outstanding feature is the fact that pressure and stabilization are specifically aimed towards the sternum. The Posthorax® vest provides anteroposterior stabilization while holding the two halves of the sternum in place. The two cushions are placed longitudinally on the left and right sides of the sternum and serve as shock absorbers when the patient coughs or breathes deeply, as well as supporting the sternum when the patient is turned in his/her bed (Fig. 2).
Figure 1:
Posthorax® sternum support vest.
Figure 2:

Shock absorbers on the lateral side of the sternum vest.
Multicentre study
Two thousand five hundred and thirty-nine patients were included in a prospective randomized multicentre trial conducted from September 2007 to March 2010. The centres that participated in the study were Hietzing Hospital, Vienna, Austria; University Heart Center, Hamburg, Germany and Heart Center of Nuremberg, Germany. The work was approved by the respective local ethics committees. Every patient provided his/her informed consent.
The majority of the operations were coronary artery bypass grafting in both groups (A: 53.3 vs B: 53%). Valve replacements in the aortic, mitral or tricuspid position were performed in 21.4% in Group A, and 22.7% in Group B. Combined procedures were defined as coronary artery bypass grafting with valve replacement or repair, which were equally distributed in both groups (A: 16.6% vs B: 15.8%). The frequency of replacement of the ascending aorta and off-pump coronary artery bypass grafting was also similar (aortic repair: A, 2.9% and B, 2.5%; off-pump: A, 5.7% and B, 5.9%). The distribution of all types of surgical procedures among the participating institutions is summarized in Table 1.
Table 1:
Distribution of operative procedures in the participating centres
| Vienna (%) | Hamburg (%) | Nuremberg (%) | Total (%) | |
|---|---|---|---|---|
| CABG | 52.4 | 41.5 | 57.1 | 50.40 |
| Use of bilateral mammary artery | 2.6 | 6.9 | 0 | 3.4 |
| AVR | 16.7 | 22.4 | 19.4 | 18.8 |
| MVR | 6.8 | 7.9 | 5.5 | 6.9 |
| AVR + CABG | 7.8 | 10.2 | 9.6 | 11.6 |
| MVR + CABG | 4.1 | 1.6 | 1.8 | 3 |
| AVR + MVR | 1.5 | 0.8 | 0.3 | 1.1 |
| All combined procedures | 18.5 | 17.9 | 16.7 | 17.8 |
| Ascending aorta replacement | 3 | 3.4 | 1.3 | 2.7 |
CABG: coronary artery bypass grafting; AVR: aortic valve replacement; MVR: mitral valve repair/replacement.
Randomization was performed immediately before the operation. The method of randomization was automatically computer-generated by chance. The sternum support vest was used 48 h after the operation, usually after the patient's stay in the intensive care unit.
Patients were advised to wear the vest for 6 weeks postoperatively. This was controlled by a specially trained nurse. Patients who refused to wear, or did not receive the vest were monitored as well according to the protocol. According to the therapy guidelines of the American Society of Hospital Pharmacists Commission on Therapeutics, 1 g of cefazoline was administered intravenously every 8 h or every 12 h for 48–72 h, or until the chest and mediastinal drainage tubes had been removed [8]. All patients received anti-inflammatory and analgesic agents per protocol: four times 1 g metamizole intravenously per day and up to four times 7500 mg piritramide subcutaneously until postoperative day 5.
Patients were shaved the evening prior to the operation and had a shower after shaving and again directly before the operation.
Patients were stratified on the basis of a modified STS risk score analysis for major infection after cardiac surgery in order to evaluate their potential infection risk at the surgical site [6, 7]. Exclusion criteria were age under 20 years due to the ethics committee recommendation, the presence of a congenital heart defect due to the complexity of procedure and the high rate of reoperations, cardiopulmonary reanimation, irradiation of the chest and heart transplantation to avoid any bias due to immunosuppressant patients or prior injured chest formation.
A power analysis was performed to determine the adequate sample size. A preliminary study with 455 patients revealed sternum wound complication rates of 0.6% in the vest and 5.4% in the non-vest group [9]. Based on a clinically relevant difference in cumulative complication rates, i.e. 4% in Group A and 2% in Group B, the projected sample size at a power of 0.8015 is 1000 per group. The actual study shows a test power of 0.7376.
Statistical calculations were based on an intention-to-treat (ITT) analysis. Further analysis involved all subgroups, including dropouts.
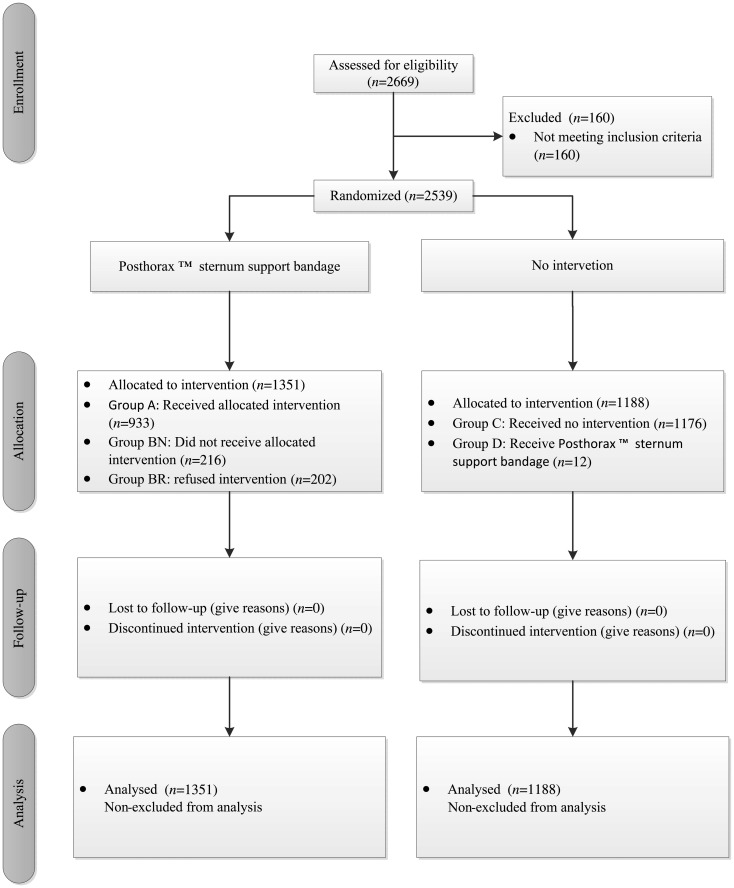
Reasons for not receiving the vest were prolonged stays at the intensive care unit, an open sternum or the patient being transferred to a different hospital within 48 h. Slippage and discomfort were the main reasons for declining to use the vest. A flow diagram of all allocated and analysed patients, in accordance with the Consolidated Standards of Reporting Trials (CONSORT) Statement, is provided in Fig. 3 [10].
Figure 3:
Flow diagram of all allocated and analysed patients.
Follow-up
Patients were monitored regarding sternal dehiscence and wound infection for 90 days after cardiac surgery as reported by Jonkers et al. [11]. Sternal wound infections included superficial infections involving the skin and subcutaneous tissue of the incision, as well as deep infections. Infection data were obtained during hospitalization from the patients' medical records. During the 90-day follow-up period after discharge, patients were monitored at their visits to the out-patient clinic or by telephone interviews focusing on sternal wound problems.
Statistical methods
Data were analysed using the software programs Statistica, Version 10 MR1 (StatSoft, Inc., Tulsa, OK, USA), Hintze, J. (2012) and NCSS8, PASS 10, LLC. (Kaysville, UT, USA).
Statistically significant differences were determined according to the following scales: nominal scale–descriptive statistics was used for total sum and percent and inequality tests for two proportions with the Pearson's χ2 test.
Measures of central tendency median and dispersion parameter interquartile range (IQR) were described using ordinal scale–descriptive statistics. Statistically significant differences were determined using the Mann–Whitney U-test. Interval scale–descriptive statistics measured central tendency mean and dispersion parameter standard deviation (SD). Statistically significant differences were determined using the Mann–Whitney U-test.
Statistically significant differences were determined according to the following scales: median as well as IQR were described using ordinal scale–descriptive statistics. Calculations were performed using the Mann–Whitney U-test.
Tests for comparisons between groups were performed using the Mann–Whitney U-test, mean, SD using interval scale.
The P-value for risk ratios (RR) unity was determined according to Miettinen and Nurminen's test for ratio. The 95% confidence interval (CI) for RRs was calculated according to Miettinen and Nurminen's score. Factors determining the occurrence of wound infection before surgery and during surgery were analysed by logistic regression. P-values <0.05 were considered to indicate significance.
RESULTS
From September 2007 onward, patients who fulfilled the inclusion criteria were randomized into two groups, one of which received the sternum support vest for stabilization (n = 1351). Nine hundred and thirty-three patients actually received the vest (Group A); 418 were randomized to the vest group, but did not receive it within 48 h for various reasons (BN = 216), or refused the treatment (BR = 202). One thousand one hundred and seventy-six patients of the second group did not receive the Posthorax® support vest and were treated with an elastic chest brace, while 12 did receive the vest, although they were randomized into the non-vest group.
Baseline characteristics of the patients are presented in Table 2. No significant difference was registered between Groups A and B regarding age, gender, body mass index and potential risk factors such as diabetes, chronic renal failure, congestive heart failure, chronic obstructive pulmonary disease, peripheral artery disease and myocardial infarction. The linear and logistic EuroSCORE, as well as the modified STS infection risk score [6], and the New York Heart Association (NYHA) classification were equally distributed in both groups.
Table 2:
Baseline characteristics of participants receiving the Posthorax sternum support vest or not
| Received support vest (N = 1351) | Not received support vest (N = 1188) | P-value | |
|---|---|---|---|
| Age (years/mean ± SD) | 67.5 ± 10.54 | 67.5 ± 10.9 | 0.76 |
| BMI (total/mean ± SD) | 27.9 ± 5.8 | 28.9 ± 17.1 | 0.29 |
| Sex (female) | 956 (70.76%) | 821(69.22%) | 0.39 |
| Diabetes, n (%) | 433 (32.05%) | 351(29.55%) | 0.17 |
| IDDM, n (%) | 105 (7.77%) | 79 (6.65%) | 0.28 |
| NIDDM, n (%) | 242 (17.91%) | 200 (16.84%) | 0.47 |
| Chronic renal failure, n (%) | 146 (10.81%) | 123 (10.35%) | 0.71 |
| Congestive heart failure, n (%) | 84 (6.22%) | 62 (5.22%) | 0.28 |
| COPD, n (%) | 266 (19.69%) | 234 (19.70%) | 0.99 |
| Myocardial infarction/cardiogenic shock, n (%) | 347 (25.68%) | 268 (22.56%) | 0.07 |
| Peripheral vascular disease, n (%) | 160 (11.84%) | 132 (11.11%) | 0.56 |
| Modified STS infection risk score (median ± IRQ) | 10 ± 7.0 | 9 ± 7.0 | 0.47 |
| EuroSCORE (median ± IRQ) | 5 ± 4 | 5 ± 4 | 0.26 |
| EuroSCORE log (mean ± SD) | 6.3 ± 6.8 | 6.9 ± 8.3 | 0.56 |
| NYHA (median ± IRQ) | 3 ± 1 | 3 ± 1 | 0.07 |
BMI: body mass index; IDDM: insulin-dependent diabetes mellitus; NIDDM: not insulin-dependent diabetes mellitus needing therapy; COPD: chronic obstructive pulmonary disease; SD: standard deviation; IQR: interquartile range.
The use of red cell units, fresh-frozen plasma or thrombocyte transfusion during the operation and aortic cross-clamping time (61 ± 35.5 min vs 60 ± 37 min) were similar in the two groups. In both groups, 23% of patients required a longer perfusion time than 120 min.
An intra-aortic balloon pump was required in 44 patients (A: 1.9%, B: 1.5%; P = 0.43).
The mean duration of intubation was ∼9 h; the stay at the intensive care unit was 2 days, the total duration of the hospital stay was 13 days and the mean time from operation to discharge was 10 days in both groups.
Postoperative delirium as a synonym of organic brain syndrome occurred in >10% of patients in both groups. Surgical and postoperative data are summarized in Table 3.
Table 3:
Operative and postoperative variables
| Support vest (N = 1351) | No support vest (N = 1188) | P-value | |
|---|---|---|---|
| Coronary artery bypass grafting | 721(53.37%) | 638 (53.70%) | 0.87 |
| Valve replacement/repair | 295 (21.84%) | 296 (24.92%) | 0.07 |
| Combined coronary artery bypass grafting with valve replacement | 224 (16.58%) | 214 (18.01%) | 0.34 |
| Ascending aorta replacement | 39 (2.89%) | 30 (2.53%) | 0.58 |
| Off-pump coronary artery bypass grafting | 78 (5.77%) | 70 (5.89%) | 0.89 |
| Use of bilateral mammary artery | 35 (2.59%) | 47 (3.96%) | 0.67 |
| IABP (total %) | 26 (1.92%) | 18 (1.52%) | 0.43 |
| Postoperative hemodialysis | 41 (3.03%) | 25 (2.10%) | 0.14 |
| Use of FFP or thrombocyte transfusion | 188 (13.92%) | 186 (15.66%) | 0.22 |
| Revision for bleeding | 2 (0.15%) | 4 (0.34%) | 0.84 |
| Organic brain syndrome | 137 (10.14%) | 128 (10.77%) | 0.60 |
| Perfusion time >120 min | 322 (76.17%) | 284 (76.09%) | 0.97 |
| Aortic cross-clamp time (min, mean ± SD) | 67.2 ± 36.2 | 68.2 ± 32.2 | 0.47 |
| Use of red cell units (total ± SD) | 2 ± 2 | 2 ± 2 | 0.47 |
| Duration of intubation period (mean ± SD) | 27.9 ± 74.5 | 26.9 ± 82.0 | 0.23 |
| Intensive care unit stay (days, median ± IRQ) | 2 ± 2 | 2 ± 2 | 0.97 |
| Total length of hospital stay (days, median ± IRQ) | 13 ± 8 | 14 ± 8 | 0.75 |
| Days operation to discharge (median ± IRQ) | 10 ± 6 | 10 ± 6 | 0.62 |
IABP: intra-aortic balloon pump; FFP: fresh-frozen plasma; SD: standard deviation; IQR: interquartile range.
Factors influencing deep wound infection were investigated by logistic regression. Factors prior to surgery, baseline data (Table 2) and surgical variables including group membership (received the support vest and did not receive the support vest) were analysed. The following calculation steps were performed for both models. First, all variables were considered in the model, and a subset selection (hierarchical forward with switching) was performed. As the second step, those variables that called for quasi-complete separation were excluded from the analysis. After repeating the analysis, the numbers of variables were confined to those that demonstrated significance on the Wald test. Results are summarized in Table 4. As regards preoperative factors influencing deep wound infection, a higher risk was noted among non-vest patients (odds ratio, OR, 3.480) and diabetics (OR 2.059).
Table 4:
Logistic regression
| Parameter | Regression coefficient (β or Beta) | Standard error | Wald Z-value (β = 0) | Wald probability level | OR Exp(β) |
|---|---|---|---|---|---|
| Factors influencing deep sternal wound complications (preoperative) | |||||
| Intercept | −6.006 | 0.600 | −10.007 | 0.000 | 0.002 |
| ITT group = no vest | 1.247 | 0.453 | 2.754 | 0.006 | 3.480 |
| Myocardial infarction | −0.992 | 0.627 | −1.582 | 0.114 | 0.371 |
| Infection risk score | 0.084 | 0.029 | 2.856 | 0.004 | 1.087 |
| Diabetes | 0.722 | 0.423 | 1.709 | 0.087 | 2.059 |
| Factors influencing deep sternal wound complications (intraoperative) | |||||
| Intercept | −4.937 | 0.406 | −12.145 | 0.000 | 0.007 |
| Coronary artery bypass grafting | −1.733 | 0.663 | −2.613 | 0.009 | 0.177 |
| Organic brain syndrome | 0.898 | 0.393 | 2.283 | 0.022 | 2.454 |
| Perfusion time >100 min | 0.472 | 0.349 | 1.352 | 0.176 | 1.603 |
| Perfusion time >200 min | 1.209 | 0.579 | 2.086 | 0.037 | 3.350 |
| ITT group = no vest | 0.870 | 0.344 | 2.528 | 0.011 | 2.388 |
| Factors influencing deep sternal wound complications | |||||
| Intercept | −5.133 | 0.442 | −11.614 | 0 | 0.006 |
| Diabetes | 1.083 | 0.324 | 3.342 | 0.001 | 2.954 |
| Perfusion time >100 min | 0.677 | 0.343 | 1.97 | 0.049 | 1.967 |
| Perfusion time >200 min | 1.345 | 0.579 | 2.322 | 0.02 | 3.838 |
| ITT group = no vest | 0.878 | 0.343 | 2.556 | 0.011 | 2.406 |
| Coronary artery bypass grafting | −0.642 | 0.324 | −1.983 | 0.047 | 0.526 |
OR: odds ratio.
As regards intraoperative factors, patients with an organic brain syndrome had an OR of 2.454. With regard to intraoperative parameters, the OR was 1.603 in a period of extracorporeal circulation >100 min, and 3.350 in patients with an extracorporeal perfusion time of >200 min. Patients without a vest had a high OR of 2.388.
All significant variables were then included in an overall model and calculation was performed again. The results are presented in Table 4. Based on the variables, the following significant factors were observed on overall assessment: diabetes (OR 2.954), extracorporeal perfusion time over 100 min (OR 1.967), extracorporeal perfusion time over 200 min (OR 3.838), the group that did not receive the support vest (OR 2.406) and coronary artery bypass grafting (OR 0.526).
Complications were divided into deep sternal complications and superficial wound infections. All complications required reoperation, including implantation of VAC® systems (KCI, Inc., San Antonio, TX, USA), surgical debridement, re-cerclage or closure of the chest with a pectoral muscle flap when appropriate. Complications during the hospital stay, re-admissions and re-operation rates within 90 days were evaluated according to the study protocol.
Rates of sternal site infections and dehiscence are summarized in Table 5.
Table 5:
Subgroups intention-to-treat group
| Group A (N = 933) | Group BN (N = 216) | P-value | RR | RR CI | P-value | RRR | |
|---|---|---|---|---|---|---|---|
| Posthorax® did not receive intervention: complication rates of the sternal wound | |||||||
| Deep sternal wound complications | 0 (0.0%) | 7 (3.24%) | 0.00 | 0.01 | 0.000–0.147 | 0.00 | 0.99 |
| During hospital stay | 0 (0.0%) | 5 (2.31%) | 0.00 | 0.01 | 0.001–0.209 | 0.00 | 0.99 |
| 90-day follow-up | 0 (0.0%) | 2 (0.93%) | 0.00 | 0.03 | 0.002–0.556 | 0.01 | 0.97 |
| Superficial wound complication | 8 (0.86%) | 5 (2.31%) | 0.06 | 0.37 | 0.129–1.070 | 0.07 | 0.63 |
| During hospital stay | 7 (0.75%) | 4 (1.85%) | 0.13 | 0.41 | 0.128–1.290 | 0.13 | 0.60 |
| 90-day follow-up | 1 (0.11%) | 1 (0.46%) | 0.26 | 0.23 | 0.024–2.241 | 0.26 | 0.77 |
| Group A (N = 933) | Group BR (N = 202) | P-value | RR | RR CI | P-value | RRR | |
| Posthorax® refused intervention: complication rates of the sternal wound | |||||||
| Deep sternal wound complications | 0 (0.0%) | 7 (3.46%) | 0.00 | 0.01 | 0.000–0.138 | 0.00 | 0.99 |
| During hospital stay | 0 (0.0%) | 4 (1.98%) | 0.00 | 0.01 | 0.001–0.247 | 0.00 | 0.99 |
| 90-day follow-up | 0 (0.0%) | 3 (1.49%) | 0.00 | 0.02 | 0.001–0.335 | 0.00 | 0.98 |
| Superficial wound complication | 8 (0.86%) | 8 (3.96%) | 0.00 | 0.22 | 0.085–0.553 | 0.00 | 0.78 |
| During hospital stay | 7 (0.75%) | 6 (2.97%) | 0.01 | 0.25 | 0.090–0.712 | 0.01 | 0.75 |
| 90-day follow-up | 1 (0.11%) | 2 (0.99%) | 0.03 | 0.11 | 0.014–0.825 | 0.03 | 0.89 |
BN: patients did not receive the support vest; BR: patients refused intervention; RR: relative risk; CI: confidence interval; RRR: relative risk reduction.
The rate of cumulative deep sternal wounds was 1.6%. On ITT analysis, it was found that, of those patients who received the Posthorax sternum support vest, this complication necessitated revision surgery in 9 (0.67%) during the hospital stay, and in 5 (0.37%) within 90 days. In the control group that did not receive the sternum vest, deep sternal infections occurred in 18 (1.52%) patients during the hospital stay and in about 9 (0.76%) during follow-up. The difference in overall rates of deep sternal wound infection between the two groups was statistically significant (P = 0.01714; Group A: 1.04 vs Group B: 2.27%; Pearson's χ2 test).
The relative risk of experiencing a complication was 0.46-fold lower (CI 0.24–0.86) in the patients who received the Posthorax® sternum support than in those who did not.
Risk reduction was calculated to determine the benefits of the Posthorax® sternum support. This was done by specifying the relative risk reduction. The relative risk reduction was 0.54 (CI 0.14–0.76). In other words, the risk of deep sternal wound infection was 54% lower when using the sternum support vest than it was without the vest. The absolute risk reduction was 1.23%.
In contrast, no benefit was observed in the rate of superficial wound complications; overall rates were 1.55% in the vest group (in hospital: 1.26% during 90-day follow-up 0.30%) and 1.09% in the non-vest group (in hospital 0.84%, during follow-up 0.25%) (P = 0.388).
Of all patients with sternal wound complications, 14 (34%) developed wound infection within 90 days after being discharged from the hospital. The mean duration of the hospital stay was extended to 49.4 days when a patient required repeat surgery due to infection. In cases of readmission during the follow-up period, the mean hospital stay was 41.2 days.
DISCUSSION
The Posthorax® support vest is a device for postoperative care after open heart surgery, and more specifically, an external stabilization device to aid unification of the longitudinally divided sternum after sternotomy. Complications after median sternotomy have been reported in several studies; these include breakage of wires, protrusion of wires through the skin of the sternum, no healing of the sternum, infection and an unstable sternum [12–16]. In addition to these physical problems, the patient has to face the mental burden of additional surgical procedures, a longer hospital stay and prolonged intake of antibiotics. These complications also signify a considerable financial burden for patients, families and the entire health-care system [17–19].
In the subgroup analysis, it was found that patients who did not receive, or refused to wear, the thorax support vest were at higher risk for deep sternal wounds. These data concern an important subset of the patient population, and stress the need for active intervention to prevent sternal site infection after cardiac surgery. The attitudes of physicians, nurses and patients regarding the prevention of these complications have not been well investigated. The main reasons why patients did not receive the chest support vest were organizational problems (such as patients being transferred to a different hospital) and doubts on the part of physicians and nurses regarding the effect of novel therapies. Patients' compliance rates improved after preoperative preparation and information. Protocols and patient teaching tools developed from research-driven standards of practice appear to be mandatory for implementing effective therapeutic strategies [20]. Future efforts should focus on creating a stronger culture of safety in the postoperative management of cardiovascular patients, increasing compliance with evidence-based infection control practices, improving communication and teamwork and developing partnership among all stakeholders to improve the design of tools and technologies [21, 22].
Particular attention should be given to those patients who refuse the sternum vest, as these had a significantly higher rate of sternal wound infection in the present study. The high rate of postoperative wound complications in non-compliant patients is one of the crucial points of this study. Patients lacking compliance do not undergo postoperative wound management and therefore experience a higher rate of deep sternal wound complications. A notably high rate of postoperative delirium, in other words organic brain syndrome, was noted in this group. Patients with a psychotic syndrome after cardiac surgery commonly do not realize the importance of appropriate postoperative behaviour or treatment. Prevention of sternal dehiscence and infection may be rendered very difficult in these patients, particularly when the organic psychological syndrome persists for several days. The rate of postoperative delirium was highly significant (OR = 2.45), and concurs with the data registered by Schimmer et al. [23] in a prospective randomized multicentre trial; the authors registered an even higher value (OR = 3.5, P = 0.01) regarding the influence of the organic brain syndrome.
LIMITATIONS
The focus of the study was to identify the potential reduction of risk for postoperative sternal infections after open heart surgery using the Posthorax sternum vest. For this purpose, we analysed all consecutive patients in a simple computed generated randomized prospective trial. Due to the ITT design of the study—which represents the standard for randomized controlled prospective studies—a remarkable amount of patients, who did not receive or refused the vest, were analysed and documented separately.
The multicentre study has some limitations in data recording. For example, the surgeon's experience and the closure of the sternum was made by surgeon's choice and not documented in this trial.
CONCLUSION
The additional stability provided by the supportive bandage prevents instability and infection of the sternum and also permits the patient to breathe more deeply because pain from friction at the edges of the sternum is minimized. The vest may be used after any operation based on a median sternotomy.
Routine use of the device reduces the relative risk of deep sternal complications by 54%.
The effect of the vest with its cushions results in the stabilization of the sternum. This study confirms the reduction of deep sternal infection using the Posthorax vest, but does not show any influence of superficial wound healing.
Funding
This work was supported by the Medical Scientific Fund of the Mayor of the City of Vienna (project number 09011).
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr W. Brinkman (Plano, TX, USA): This is a very interesting study of a difficult problem, sternal wound infections, for which there are multiple causes, and it is an expensive problem. The first question is, were there any guidelines for sternal closure amongst the various surgeons regarding ways in which they closed the sternum, number of wires, ways they closed the wires, and were adjuncts such as bone wax or platelet-rich plasma allowed, things such as that?
Dr Gorlitzer: We have a recommendation for the surgeons to close the sternum with one wire per 10 kg patient weight. So if you have a patient who is 80 kg, we usually use eight wires. But it is at least the choice of the surgeon how to close the sternum. Furthermore, we have analysed the use of bone wax, but there was no difference between the groups, so it does not have any influence on sternal infections.
Dr Brinkman: And the rates of bilateral internal mammary arteries were similar between the groups?
Dr Gorlitzer: Right. There was about a 4% use of bilateral mammary. It was a little bit more in the vest group, 4.3. But those offered significant differences.
Dr Brinkman: One other point is that the rates seem to be a little bit higher than reported in the STS database for your non-vest group (probably because of your better follow-up than your average American institution). So could you give me your explanation for that?
Dr Gorlitzer: The study protocol included a 90 day follow-up, in contrast to most US trials; the analysis of the STS database is limited to in-hospital morbidity or in-hospital complications and not longer. And as you are from the US, you will know that most patients are in hospital between seven and eight or nine days after cardiac surgery, and most US reports do not focus on patients discharged from the hospital. So that is maybe the reason for the sternal complications rate above 2% in the non-vest group.
Dr Brinkman: One other thing was that the patients who had trouble in your study were the ones who were non-compliant because they were confused or delirious or the MDs themselves were not compliant. How do you suggest doctor and patient compliance might be improved?
Dr Gorlitzer: We had two groups, one who refused and another group who did not receive the vest. The first group were mostly patients with organic brain syndrome. This is in fact a very big problem and we don't know how to solve this. But in regard to the second subgroup who refused it for other reasons, we changed our policy concerning the information given to our patients. Now we inform the patients preoperatively, and they also have to try this support vest before operation so they know what will be happening after the operation. This change in methods raised the compliance rate to nearly 90-100% because of this additional information preoperatively.
Dr Brinkman: I think this is an important tool to help our patients.
Dr B. Osswald (Bad Oeynhausen, Germany): You compared two groups which were very similar from the risk adjustment which you did, but I would recommend looking at the subgroups. It is a very low incidence overall, even if the numbers are perhaps a little bit higher than in the STS. But you are dealing with a very small number of patients. Maybe your group consists predominantly of these old ladies with osteoporosis where often these problems occur. And the recommendation to get such a vest for every patient I think will raise the costs to enormous amounts. I would be a little bit careful about it.
Dr Gorlitzer: Before this multicentre trial, we started a previous study involving very high-risk patients, e.g. obese patients, diabetic patients, with comparable results, and analysed that patients who do not have this high risk profile also have very common problems after sternotomy. So that is why we recommend using the support vest in all patients. So you lose one half of these sternal complications because you just treat this small group of high-risk patients.
Dr M. Moon (St. Louis, MO, USA): You noticed a difference in the patients who underwent coronary bypass versus valve procedures. In my experience, deep sternal infection is exceedingly rare in valve patients. Do you think maybe we would recommend it more for bypass patients only, or, as you said, all patients?
Dr Gorlitzer: We use it in all patients, but you are right, the logistic regression analysis shows that the coronary bypass graft patients had a higher risk for sternal wound complications.
Dr Moon: And did you do an analysis of sternal stability as well? Again, in my experience, the bilateral mammaries may not be associated with a higher incidence of infection necessarily in a healthier patient; however, they may have a little more instability with a little creaking or rocking of the chest.
Dr Gorlitzer: We don't know what initiates the sternal wound complication: is it the instability or is it an infection? Probably it is instability and then it progresses to the infection. We don't know now what comes first.
Dr M. Arbeus (Orebro, Sweden): The patients that were randomized to no vest, did they get any other support? I mean, if they belonged to one of the risk groups, because in our practice, we use some kind of stabilization, Heart Hugger and so on, for risk patients. So were they excluded from them?
Dr Gorlitzer: We analysed, in fact, the Posthorax sternum vest comparing it with patients who get an elastic bandage that we used formerly, but we saw that the elastic bandage did not offer any stabilization to the chest. A group from the Institute for Biophysics at the University of Vienna measured different points on the chest wall from outside, and there was no stabilization from the elastic bandage.
REFERENCES
- 1.Loop FD, Lytle BW, Cosgrove DM, Mahfood S, McHenry MC, Goormastic M, et al. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity and cost of care. Ann Surg. 1990;49:179–87. doi: 10.1016/0003-4975(90)90136-t. [DOI] [PubMed] [Google Scholar]
- 2.Zacharias A, Habib RH. Factors predisposing to median sternotomy complications: deep vs superficial infection. Chest. 1996;110:1173–8. doi: 10.1378/chest.110.5.1173. [DOI] [PubMed] [Google Scholar]
- 3.Ridderstolpe L, Gill H, Granfeldt H, Ahlfeldt H, Rutberg H. Superficial and deep sternal wound complications: incidence, risk factors and mortality. Eur J Cardiothorac Surg. 2001;20:1168–75. doi: 10.1016/s1010-7940(01)00991-5. [DOI] [PubMed] [Google Scholar]
- 4.Stahle E, Tammelin A, Bergstrom R, Hambreus A, Nystrom SO, Hansson HE. Sternal wound complications—incidence, microbiology and risk factors. Eur J Cardiothorac Surg. 1997;11:1146–53. doi: 10.1016/s1010-7940(97)01210-4. [DOI] [PubMed] [Google Scholar]
- 5.El Oakley RM, Wright JE. Postoperative mediastinitis: classification and management. Ann Thorac Surg. 1996;61:1030–6. doi: 10.1016/0003-4975(95)01035-1. [DOI] [PubMed] [Google Scholar]
- 6.Gorlitzer M, Folkmann S, Meinhart J, Poslussny P, Thalmann M, Weiss G, et al. A newly designed thorax support vest prevents sternum instability after median sternotomy. Eur J Cardiothorac Surg. 2009;36:335–9. doi: 10.1016/j.ejcts.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 7.Fowler VG, Jr, O'Brien SM, Muhlbaier LH, Corey GR, Ferguson TB, Peterson ED. Clinical predictors of major infections after cardiac surgery. Circulation. 2005;112:I358–65. doi: 10.1161/CIRCULATIONAHA.104.525790. [DOI] [PubMed] [Google Scholar]
- 8.ASHP Commission on Therapeutics. ASHP therapeutic guidelines on antimicrobial prophylaxis in surgery. Clin Pharm. 1992;11:483–513. [PubMed] [Google Scholar]
- 9.Gorlitzer M, Wagner F, Pfeiffer S, Folkmann S, Meinhart J, Fischlein T, et al. A prospective randomized multicenter trial shows improvement of sternum related complications in cardiac surgery with the Posthorax support vest. Interact CardioVasc Thorac Surg. 2010;10:714–8. doi: 10.1510/icvts.2009.223305. [DOI] [PubMed] [Google Scholar]
- 10.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:1–8. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 11.Jonkers D, Elenbaas T, Terporten P, Nieman F, Stobberingh E. Prevalence of 90-days postoperative wound infections after cardiac surgery. Eur J Cardiothorac Surg. 2003;23:97–102. doi: 10.1016/s1010-7940(02)00662-0. [DOI] [PubMed] [Google Scholar]
- 12.Shih CC, Shih CM, Su YY, Lin SJ. Potential risk of sternal wires. Eur J Cardiothorac Surg. 2004;25:812–8. doi: 10.1016/j.ejcts.2003.11.043. [DOI] [PubMed] [Google Scholar]
- 13.Cohen DJ, Griffin LV. A biomechanical comparison of three sternotomy closure techniques. Ann Thorac Surg. 2002;73:563–8. doi: 10.1016/s0003-4975(01)03389-6. [DOI] [PubMed] [Google Scholar]
- 14.Zeitani J, Penta de Peppo A, Moscarelli M, Guerrieri Wolf L, Scafuri A, Nardi P, et al. Influence of sternal size and inadvertent paramedian sternotomy on stability of the closure site: a clinical and mechanical study. J Thorac Cardiovasc Surg. 2006;132:38–42. doi: 10.1016/j.jtcvs.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Casha AR, Yang L, Kay PH, Saleh M, Cooper GJ. A biomechanical study of median sternotomy closure techniques. Eur J Cardiothorac Surg. 1999;15:365–9. doi: 10.1016/s1010-7940(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 16.Losanoff JE, Collier AD, Wagner-Mann CC, Richman BW, Huff H, Hsieh F, et al. Biomechanical comparison of median sternotomy closures. Ann Thorac Surg. 2004;77:203–9. doi: 10.1016/s0003-4975(03)01468-1. [DOI] [PubMed] [Google Scholar]
- 17.Speir AM, Kasirajan V, Barnett SD, Fonner E. Additive costs of postoperative complications for isolated coronary artery bypass grafting patients in Virginia. Ann Thorac Surg. 2009;88:40–5. doi: 10.1016/j.athoracsur.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 18.Kurki T, Häkkinnen U, Lauharanta J, Rämö J, Leijala M. Evaluation of the relationship between preoperative risk scores, postoperative and total length of stays and hospital costs in coronary bypass surgery. Eur J Cardiothorac Surg. 2001;20:1183–7. doi: 10.1016/s1010-7940(01)00988-5. [DOI] [PubMed] [Google Scholar]
- 19.Jenney AW, Harrington GA, Russo PL, Spelman DW. Cost of surgical site infections following coronary artery bypass surgery. ANZ J Surg. 2001;71:662–4. doi: 10.1046/j.1445-1433.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 20.McBride T, Beamer J. Pre-operative patient preparation in the prevention of surgical site infections. Can Oper Room Nurs J. 2007;25:26–7. 29–32, 34. [PubMed] [Google Scholar]
- 21.Gurses AP, Kim G, Martinez EA, Marsteller J, Bauer L, Lubomski LH, et al. Identifying and categorising patient safety hazards in cardiovascular operating rooms using an interdisciplinary approach: a multisite study. BMJ Qual Saf. 2012;21:810–8. doi: 10.1136/bmjqs-2011-000625. [DOI] [PubMed] [Google Scholar]
- 22.Gillespie BM, Chaboyer W, Longbottom P, Wallis M. The impact of organisational and individual factors on team communication in surgery: a qualitative study. Int J Nurs Stud. 2010;47:732–41. doi: 10.1016/j.ijnurstu.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Schimmer C, Reents W, Berneder S, Eigel P, Sezer O, Scheld H, et al. Prevention of sternal dehiscence and infection in high-risk patients: a prospective randomized multicenter trial. Ann Thorac Surg. 2008;86:1897–904. doi: 10.1016/j.athoracsur.2008.08.071. [DOI] [PubMed] [Google Scholar]