Abstract
OBJECTIVES
Post-cardiac surgery vasoplegia is a common complication of cardiac surgery, characterized by profound loss of systemic vascular resistance. This results in severe hypotension, high cardiac output and metabolic acidosis reflecting inadequate tissue perfusion. The pathophysiological mechanisms underlying this syndrome remain unknown. We hypothesized that this vasoplegia reflects endothelial dysfunction, either as pre-existing condition or as a consequence of the surgical procedure.
METHODS
To examine these mechanisms, six established and distinct markers of endothelial cell activation were measured pre- and perioperatively in patients undergoing mitral valve surgery. Arterial (radial artery) and myocardial venous blood samples (coronary sinus) were collected simultaneously over the reperfused heart at various time points during the first hour after reperfusion. Additional samples were collected at baseline (brachial vein) and 1 day post-reperfusion (radial artery). Post-cardiac surgery vasoplegia was defined as a mean arterial blood pressure of <60 mmHg, with a cardiac index of ≥2.2 l/min/m2 treated with continuous intravenous administration of norepinephrine.
RESULTS
No myocardial release of endothelial cell activation markers was observed upon reperfusion in patients with vasoplegia (n = 15; mean age 71 years, 73% male). In contrast, in patients without vasoplegia (n = 24; mean age 64 years, 54% male), reperfusion was characterized by a myocardial release of three endothelial cell activation markers. Myocardial von Willebrand Factor propeptide, osteoprotegerin and interleukin-8 were increased 107% (P < 0.001), 106% (P = 0.02) and 116% (P = 0.009), respectively, compared with arterial levels upon reperfusion. Similar systemic levels of all markers were found upon reperfusion in both groups, except for 120% increased soluble P-selectin (sP-selectin) levels in vasoplegia patients (P = 0.03). Remarkably, postoperative vasoplegia was identified with baseline von Willebrand Factor propeptide levels with a cut-off value of 11.9 nM as well as with baseline sP-selectin levels with a cut-off value of 64.4 ng/ml.
CONCLUSIONS
Pre-existing endothelial cell activation, reflected by higher baseline von Willebrand Factor propeptide and sP-selectin levels, is a predisposing factor for post-cardiac surgery vasoplegia. The pre-existing endothelial cell activation may have resulted in desensibilization of endothelium in patients who develop vasoplegic syndrome, resulting in no myocardial release of endothelial cell activation markers upon reperfusion.
Keywords: Endothelial cell activation, Surgery, Vasoplegia
INTRODUCTION
Vasoplegic syndrome occurs in 9–44% of the patients after cardiac surgery [1, 2]. This syndrome is characterized by profound loss of systemic vascular resistance, resulting in severe hypotension, high cardiac output and metabolic acidosis reflecting inadequate tissue perfusion [2]. High levels of vasopressive agents are required to maintain adequate blood pressure postoperatively and, in a substantial proportion of the patients, vasoplegia appears refractory to these agents [3]. It is hypothesized that the surgical trauma and use of a cardiopulmonary bypass (CPB) machine activate vasoactive inflammatory mediators, neurohumoral factors and the coagulation system. This activation results in a disbalance in the regulation of the vascular tone [1]. Prolonged hypotension, defined as >24 h, and the accompanying hypoperfusion subsequently lead to end-organ dysfunction. Consequently, this syndrome is associated with increased morbidity and mortality [1, 4].
Yet, the pathophysiological basis of post-cardiac surgery vasoplegia is unknown. During on-pump cardiac surgery, endothelial cells are exposed to multiple disturbances, leading to significant vasomotor tone and vascular systemic resistance modifications. Exocytosis of Weibel-Palade bodies is one of the earliest responses to vascular damage [5] and plays a pivotal role in inflammation. Weibel-Palade bodies are endothelial granules that store vascular modulators, such as von Willebrand Factor (vWF), vWF propeptide (vWFpp), soluble P-selectin (sP-selectin), osteoprotegerin (OPG) and angiopoeitin-2. Weibel-Palade body release into the circulation is induced by physical injuries (that is, hypoxia, ischaemia and trauma), endogenous chemicals (that is, epinephrine and histamine), proteins (that is, complement and thrombin) and lipid messengers (that is, oxidized low-density lipoprotein), resulting in vasoactive and inflammatory responses [5, 6].
Preventive or treatment strategies are for unmet medical needs, however, the understanding of the pathophysiology is therefore essential. We hypothesized that the development of post-cardiac surgery syndrome could be caused by each or a combination of the following factors: pre-existing endothelial dysfunction, endothelial dysfunction caused by myocardial ischaemia/reperfusion that occurs during surgery and endothelial dysfunction caused by surgical injury including the use of a CPB machine [7, 8]. To address these issues, we performed an explorative study and measured plasma levels of six well-established endothelial cell activation markers, released by Weibel-Palade bodies, in patients scheduled for mitral valve surgery. These markers were vWF, vWFpp, OPG, angiopoietin-2, sP-selectin (sP-selectin) and interleukin-8 (IL-8) [6, 9, 10].
MATERIALS AND METHODS
Patient population
In this 16-month prospective study, 40 patients undergoing cardiac valve surgery were included, of whom 20 had left ventricular dysfunction. Left ventricular dysfunction was defined as New York Heart Association (NYHA) function Class II to IV heart failure symptoms, moderate-to-severe mitral regurgitation and reduced left ventricular ejection fraction (<60%) [11]. All patients were scheduled for restrictive mitral annuloplasty ring implantation with or without additional procedures. This patient population was specifically selected since patients with and without left ventricular dysfunction both undergo this homogeneous procedure. In patients with left ventricular dysfunction, functional or ischaemic mitral regurgitation was observed. Valve regurgitation in patients without left ventricular dysfunction was caused by organic pathology, with a predominance of prolapse disease. Exclusion criteria were perioperative corticosteroid treatment, active infection, minimally invasive surgical procedures, emergency operations and previous cardiac surgery. Although there is no consensus in the literature about the definition of post-cardiac surgery vasoplegia, we used the definition of Colson et al. [12]. In the intensive care unit (ICU), dobutamine was used in order to obtain a cardiac index of ≥2.2 l/min/m2 as assessed by thermodilution (pulmonary artery catheter). A mean arterial blood pressure of <60 mmHg with a cardiac index of ≥2.2 l/min/m2 defined post-cardiac surgery vasoplegia in the first 12 h at the ICU and was treated with continuous intravenous administration of norepinephrine.
Although post-cardiac surgery vasoplegia occurs more often in patients with a low ejection fraction, it can also develop in those with a normal systolic function [13]. In daily clinical practice, vasoplegia is commonly observed in patients with prolonged aortic cross-clamp time, such as valve repair. Therefore, it is essential to unravel the underlying pathophysiological mechanisms to prevent it in all patients undergoing cardiac valve surgery, with or without systolic dysfunction. This study was approved by our local ethics committee, and written informed consent was obtained from each patient.
Anaesthesia and surgical procedures
All participating patients received standardized anaesthetic procedures, according to a fast-track protocol. A 5-Fr indwelling jugular vein catheter (PICC, Arrow International, Inc., REF PS-01651, USA) was inserted into the right atrium and placed in the coronary sinus by the surgeon. Since all patients underwent mitral valve surgery using a vertical trans-septal incision, the coronary sinus could be easily cannulated during the surgical procedure. An arterial catheter was routinely placed in the radial artery. Cardiac surgery was performed according to local standardized protocols. All surgical procedures were performed via a midline sternotomy under normothermic CPB (Jostra Maquet, Maquet, Hirrlingen, Germany) with intermittent antegrade warm-blood cardioplegia (flow ∼450 ml/min), heparin softline coating and a Quadrox-I adult oxygenator (Jostra Maquet). A uniform CPB protocol was designed, excluding autologous priming, ultrafiltration and pulsatile flow. The CPB system was primed with 1300 ml priming volume consisting of 1000 ml Voluven® 6%, 100 ml Mannitol 20% and 200 ml Ringer solution. In addition, 5000 IU heparin was added. Cardioplegia was infused in the aortic root for the duration of 2 min and repeated every 15–20 min.
Plasma measurements
Arteriovenous measurements
Arterial (radial artery) and myocardial venous blood samples (coronary sinus) were collected simultaneously over the reperfused heart as described previously [14, 15]. With this approach, accurate and specific measurements of locally ongoing processes were assessed the first hour after reperfusion. These paired blood samples were obtained during the early reperfusion phase: 0, 15, 30, 45; and 60 min after the start of reperfusion, i.e. after removing the aortic cross-clamp. Two additional samples were collected: at baseline (the day before surgery from the brachial vein) and 1-day post-reperfusion (radial artery). Samples were collected in precooled ethylenediaminetetraacetic acid tubes, immediately placed on melting ice, centrifuged twice and stored at −70°C until analysis.
Measurement of endothelial cell activation markers
Plasma levels of the following endothelial cell activation markers, released by Weibel-Palade bodies, were assessed: vWF, vWFpp, OPG, angiopoietin-2, sP-selectin and IL-8. These markers were selected as they are released from prestored pools, and thus provide an immediate read-out of endothelial cell activation.
vWF, vWFpp and OPG were determined using a semi-automated enzyme-linked immunosorbent assay (ELISA) on a TECAN Freedom Evo robot using the following commercial antibody duosets: rabbit anti-human vWF and peroxidase-conjugated rabbit anti-human vWF, A00082 and P0226, Dako, Glostrup, Denmark; vWFpp rabbit-anti-propeptide and rabbit-anti-propeptide–biotine [16]; OPG, duoset DY805, R&D systems, Abingdon, UK. Plasma levels of angiopoeitin-2 were measured manually (human angiopoeitin-2, DY623, R&D systems). Plasma levels of IL-8 were measured with a custom-made X-plex assay according to the instructions of the manufacturer (Biorad, Veenendaal, Netherlands). Plasma levels of sP-selectin were determined using a semi-automated ELISA on a TECAN Freedom Evo robot. ELISA plates (384 wells, Maxisorp, cat no. 460372) were coated overnight (4°C) with coating buffer. Established human ELISA antibody duosets were used (sP-selectin, duoset DY137, R&D systems).
Statistical analysis
Continuous data are presented as median with 25th and 75th percentiles (interquartile ranges, IQRs). Categorical data are expressed as frequencies and percentages. Patients were divided into two subgroups based on the occurrence of postoperative vasoplegia during follow-up. Perioperative data were evaluated between these groups with a Mann–Whitney U-test or χ2 test (or Fisher's exact test) whenever appropriate. Statistical tests that required normality (or rather symmetry) were applied on log-transformed data. Data were then back-transformed to the original data scales. Moreover, to assess the association between baseline log-transformed vWFpp and sP-selectin levels and vasoplegia, correlation analysis was performed using Pearson correlation coefficients. In addition, after performing the statistical tests, data were back-transformed to the original scales.
Receiver operating characteristic (ROC) curve analysis was also performed to determine the sensitivity and specificity for various cut-off values of baseline log-transformed vWFpp and sP-selectin levels for the prediction of vasoplegia after mitral valve surgery. Area under the curve (AUC) was calculated for the measurements in arterial and myocardial venous blood samples the first hour after reperfusion, and compared using a paired t-test. Arterial AUCs were compared between the two patient groups with an unpaired t-test. All statistical analyses were performed with the Statistical Package for the Social Sciences 20.0 (SPSS, Inc., Chicago, IL, USA).
RESULTS
This explorative study included 40 patients undergoing mitral valve surgery for severe mitral regurgitation. One patient without pre-existing heart failure was excluded since the left ventricular function was perioperatively worse than estimated on the preoperative echocardiogram. In the remaining population of 39 patients, 15 (38.5%) developed post-cardiac surgery vasoplegia after mitral valve surgery, of whom 12 were diagnosed with pre-existing left ventricular dysfunction. In 4 of these patients, the vasoplegia persisted up to 24 h after surgery. Two of these patients developed a more prolonged vasoplegia of >24 h post-surgery. One patient in the vasoplegia group died 41 days after surgery as a result of therapy-resistant heart failure. One patient in the non-vasoplegia group, with a poor left ventricular function, died 8 days after surgery as a result of ventricular fibrillation. Baseline, surgical and outcome characteristics of the patient population are summarized in Table 1. Detailed information about the surgical procedures is provided in Supplementary Table 1.
Table 1:
Perioperative patient characteristics
| All patients (N = 39) | Vasoplegia (N = 15) | No vasoplegia (N = 24) | P-value* | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (years) | 65 (57–74) | 71 (57–77) | 64 (56–71) | 0.36 |
| Male gender (n, %) | 24 (62%) | 11 (73%) | 13 (54%) | 0.23 |
| Body mass index (kg/m2) | 27 (24–29) | 27 (25–30) | 27 (24–29) | 0.48 |
| Systolic blood pressure (mmHg) | 125 (115–140) | 120 (105–130) | 128 (120–140) | 0.12 |
| Diastolic blood pressure (mmHg) | 70 (65–80) | 70 (60–80) | 73 (65–80) | 0.59 |
| Haemoglobin (mmol/l) | 8.6 (8.1–9.2) | 8.3 (7.8–9.2) | 8.6 (8.3–9.3) | 0.32 |
| Creatinine (μmol/l) | 86 (74–103) | 93 (83–109) | 81 (73–99) | 0.19 |
| NT-proBNP (ng/l) | 1242 (578–2295) | 1598 (660–2449) | 898 (364–2257) | 0.11 |
| Logistic EuroSCORE (%) | 5.97 (3.07–9.79) | 6.96 (5.58–14.20) | 4.29 (2.08–7.75) | 0.02 |
| NYHA functional class | 3 (2–3) | 3 (3–3) | 3 (2–3) | 0.01 |
| Comorbidities | ||||
| Diabetes (n, %) | 9 (23%) | 4 (27%) | 5 (21%) | 0.71 |
| COPD (n, %) | 7 (18%) | 3 (20%) | 4 (17%) | 1.00 |
| AF (n, %) | 11 (28%) | 6 (40%) | 5 (21%) | 0.20 |
| Medication | ||||
| ACE inhibitors/ARBs (n, %) | 27 (69%) | 12 (80%) | 15 (63%) | 0.25 |
| Beta-blockers (n, %) | 28 (72%) | 14 (93%) | 14 (58%) | 0.02 |
| Statins (n, %) | 22 (56%) | 11 (73%) | 11 (46%) | 0.09 |
| Diuretics (n, %) | 28 (72%) | 13 (87%) | 15 (63%) | 0.10 |
| Digoxins (n, %) | 7 (18%) | 2 (13%) | 5 (21%) | 0.55 |
| Echocardiography | ||||
| LVESV (ml) | 92 (62–113) | 100 (92–120) | 71 (57–102) | 0.10 |
| LVEDV (ml) | 146 (135–194) | 156 (135–194) | 143 (133–191) | 0.57 |
| LVEF (%) | 39 (32–54) | 33 (30–38) | 50 (32–59) | 0.06 |
| Surgical characteristics | ||||
| Aortic cross-clamp time (min) | 120 (94–159) | 147 (115–186) | 108 (89–151) | 0.04 |
| CPB time (min) | 178 (140–212) | 205 (154–252) | 162 (135–198) | 0.01 |
| Surgery time (min) | 310 (255–352) | 376 (275–406) | 292 (226–322) | 0.003 |
| Postoperative characteristics | ||||
| Norepinephrine (µg/kg/min) | ||||
| 0–12 h at the ICU | 0.03 (0.01–0.10) | 0.10 (0.07–0.20) | 0.02 (0.00–0.05) | <0.001 |
| 12–24 h at the ICU | 0.00 (0.00–0.02) | 0.02 (0.01–0.08) | 0.00 (0.00–0.02) | 0.004 |
| Dobutamine (µg/kg/min) | ||||
| 0–12 h at the ICU | 3 (0–5) | 5 (3–8) | 2 (0–4) | 0.004 |
| 12–24 h at the ICU | 2 (0–5) | 4 (2–7) | 0 (0–4) | 0.005 |
| Creatinine 24 h after surgery (μmol/l) | 79 (64–105) | 96 (70–140) | 77 (58–96) | 0.06 |
| Total stay in ICU (hours) | 24 (21–69) | 47 (21–93) | 22 (21–25) | 0.07 |
| Total stay in hospital (days) | 13 (10–19) | 15 (11–21) | 13 (10–19) | 0.35 |
The differences at baseline between the groups, for example higher log EuroSCORE and NYHA class in patients with vasoplegia, reflect a more vulnerable patient population. Medians and IQRs are shown unless stated otherwise.
ACE: angiotensin-converting enzyme; AF: atrial fibrillation; ARB: angiotensin receptor blocker; COPD: chronic obstructive pulmonary disease; CPB: cardiopulmonary bypass; ICU: intensive care unit; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; NT-proBNP: N-terminal-proB-type natriuretic peptide; NYHA: New York Heart Association.
*P-values are given for the comparisons of patients with and without vasoplegia after mitral valve surgery.
Association between preoperative von Willebrand Factor propeptide and soluble P-selectin levels and vasoplegia
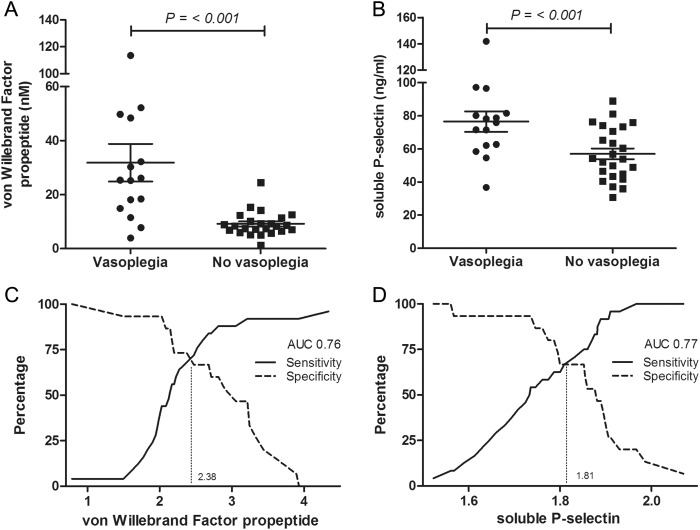
Baseline vWFpp levels (i.e. day before surgery) were elevated in patients with vasoplegia, compared with non-vasoplegia patients (25.3 [IQR 14.9–48.4] vs 8.4 [IQR 6.6–11.1] nM, P < 0.001). A correlation was observed between baseline vWFpp levels and vasoplegia (Spearman's rho correlation coefficient; r = 0.63, P < 0.001). According to ROC curve analysis, a cut-off value of 11.9 nM showed a good accuracy to identify vasoplegia (Fig. 1). Baseline sP-selectin levels were also higher in patients with vasoplegia compared with non-vasoplegia patients (76.0 [IQR 62.1–81.6] vs 54.0 [IQR 43.8–72.7] ng/ml, P = 0.006). A correlation was observed between baseline sP-selectin levels and vasoplegia (Spearman's rho coefficient; r = 0.45, P = 0.004). According to ROC curve analysis, a cut-off value of 64.4 ng/ml showed a good accuracy in identifying vasoplegia (Fig. 1). Baseline levels of vWF, OPG, angiopoeitin-2; and IL-8 were similar between the two groups (Table 2).
Figure 1:
vWFpp and sP-selectin in patients with and without vasoplegia after mitral valve surgery. Median and IQR concentrations of baseline vWFpp (A) and sP-selectin (B) in patients with and without vasoplegia. (C) ROC curve analysis demonstrated a sensitivity of 79% with a specificity of 80% to predict vasoplegia following cardiac valve surgery at a cut-off of log-transformed vWFpp of 1.08 at baseline (non-log-transformed value: 11.9 nM). AUC indicated the AUC of the ROC curve. (D) ROC curve analysis demonstrated a sensitivity of 67% with a specificity of 67% to predict vasoplegia following cardiac valve surgery at a cut-off of log-transformed sP-selectin of 1.81 at baseline (non-log-transformed value: 64.4 ng/ml). AUC indicated the AUC of the ROC curve.
Table 2:
Baseline endothelial cell activation markers
| All patients (N = 39) | Vasoplegia (N = 15) | No vasoplegia (N = 24) | P-value* | |
|---|---|---|---|---|
| von Willebrand Factor (nM) | 45.6 (32.1–64.1) | 47.5 (36.9–57.3) | 43.1 (25.6–66.4) | 0.77 |
| von Willebrand Factor propeptide (nM) | 10.1 (7.2–24.5) | 25.3 (14.9–48.4) | 8.4 (6.6–11.1) | <0.001 |
| Osteoprotegerin (pg/ml) | 36.2 (19.1–53.5) | 26.3 (15.5–46.0) | 38.9 (24.6–60.1) | 0.09 |
| Angiopoeitin-2 (ng/ml) | 2.4 (2.1–3.1) | 2.5 (2.1–3.5) | 2.4 (2.1–2.8) | 0.57 |
| Interleukin-8 (pg/ml) | 8.4 (3.5–13.9) | 8.3 (5.6–17.0) | 8.4 (2.9–13.3) | 0.45 |
| Soluble P-selectin (ng/ml) | 62.7 (49.0–76.4) | 76.0 (62.1–81.6) | 54.0 (43.8–72.7) | 0.006 |
Medians and IQRs are shown.
*P-values are given for the comparisons of patients with and without vasoplegia after mitral valve surgery.
Myocardial release of von Willebrand Factor propeptide and osteoprotegerin upon reperfusion
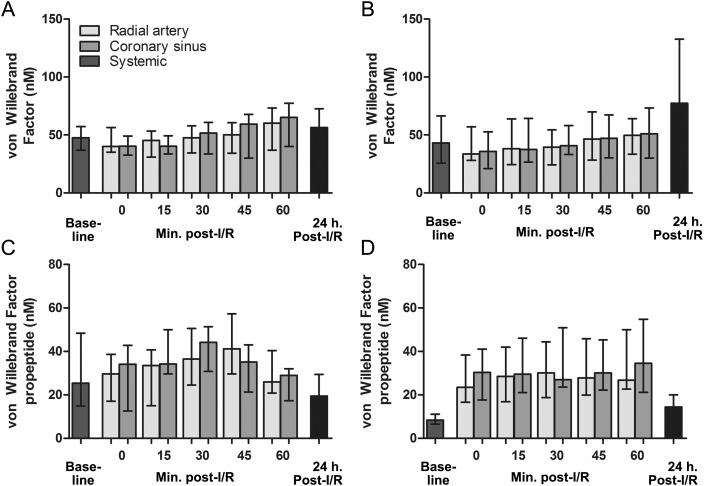
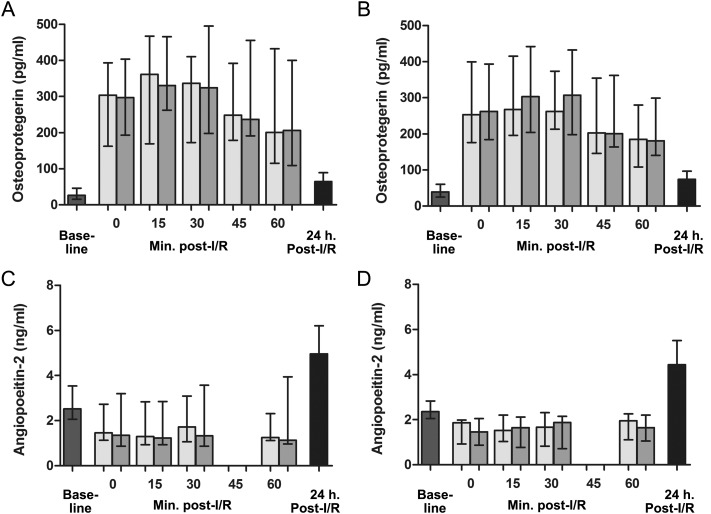
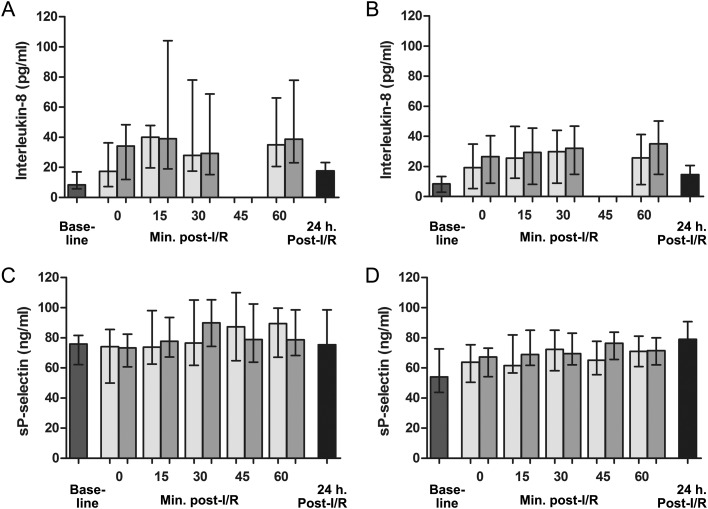
Arteriovenous differences the first hour after reperfusion were analysed in patients with and without vasoplegia after mitral valve surgery. The AUCs of the values of the endothelial cell activation markers, measured in blood collected from the coronary sinus and radial artery, were compared. In patients with vasoplegia, no significant myocardial release of vWF, vWFpp, OPG, angiopoeitin-2, IL-8 or sP-selectin was observed (104%, P = 0.46; 101%, P = 0.86; 103%, P = 0.18; 93%, P = 0.16; 104%, P = 0.22 and 102%, P = 0.53, respectively). In contrast, in patients without vasoplegia, myocardial levels of vWFpp, OPG and IL-8 were increased by 107% (P < 0.001), 106% (P = 0.02) and 116% (P = 0.009), respectively, compared with arterial levels the first hour after reperfusion, indicating a myocardial release of these markers. Myocardial venous vWF, angiopoeitin-2; and sP-selectin levels were similar compared with arterial levels in this patient group (increased by 99% (P = 0.60), 92% (P = 0.27) and 103% (P = 0.09), respectively).
Systemic levels of endothelial cell activation markers
In contrast to the pre-existing differences of baseline vWFpp and sP-selectin between the 2 patient groups, no differences were observed up to 1 h after reperfusion, comparing patients with and without vasoplegia for AUCs of systemic levels of vWF (101%, P = 0.96), vWFpp (104%, P = 0.84), OPG (104%, P = 0.80), angiopoeitin-2 (90%, P = 0.81) and IL-8 (151%, P = 0.16) the first hour after reperfusion, respectively. However, sP-selectin levels were higher in patients who experienced vasoplegia post-reperfusion when compared with those without this vasoplegia upon reperfusion (120%, P = 0.03).
Similar levels of all markers were found in patients with and without vasoplegia 1 day post-reperfusion: vWF (56.4 [IQR 31.1–72.7] vs 77.3 [IQR 43.4–132.5] nM, P = 0.52), vWFpp (19.4 [IQR 10.9–29.4] vs 14.4 [IQR 10.9–20.1] nM, P = 0.18), OPG (64.8 [IQR 38.8–89.0] vs 73.9 [IQR 54.1–96.7] pg/ml, P = 0.51), angiopoeitin-2 (5.0 [IQR 3.8–6.2] vs 4.4 [IQR 3.6–5.5] ng/ml, P = 0.40), IL-8 (17.5 [IQR 5.4–23.1] vs 14.6 [IQR 4.0–20.6] pg/ml, P = 0.47) and sP-selectin (75.5 [IQR 66.3–98.7] vs 78.9 [IQR 63.4–90.8] ng/ml, P = 0.16). Overall, reperfusion was characterized by a decrease in plasma vWF and angiopoeitin-2 levels, and increased levels of vWFpp, OPG and IL-8. sP-selectin levels were relatively stable upon reperfusion when compared with baseline levels. Moreover, a steep increase in angiopoeitin-2 was observed 1 day post-reperfusion (Figs 2–4).
Figure 2:
Systemic and myocardial venous plasma concentrations of (A and B) vWF, and (C and D) vWFpp at baseline, the first hour after reperfusion, and 1 day post-reperfusion in, respectively, patients with and without vasoplegia. Baseline vWFpp levels (i.e. day before surgery) were elevated in patients with vasoplegia compared with non-vasoplegia patients (25.3 [IQR 14.9–48.4] vs 8.4 [IQR 6.6–11.1] nM, P < 0.001). In patients with vasoplegia, no significant myocardial release of vWFpp was observed. In contrast, in patients without vasoplegia, myocardial levels of vWFpp were 107% (P < 0.001) increased compared with arterial levels the first hour after reperfusion, indicating a myocardial release of these markers. Myocardial venous vWF levels were similar compared with arterial levels in this patient group. Similar levels of all markers were found in patients with and without vasoplegia 1 day post-reperfusion. Graph error bars indicate medians with IQRs.
Figure 3:
Systemic and myocardial venous plasma concentrations of (A and B) OPG, and (C and D) angiopoeitin-2 at baseline, the first hour after reperfusion, and 1-day post-reperfusion in, respectively, patients with and without vasoplegia. In patients with vasoplegia, no significant myocardial release of OPG or angiopoeitin-2 was observed. In contrast, in patients without vasoplegia, myocardial levels of OPG were 106% (P = 0.02) increased compared with arterial levels the first hour after reperfusion, indicating a myocardial release of these markers. Myocardial venous angiopoeitin-2 levels were similar compared with arterial levels in this patient group. Similar levels of all markers were found in patients with and without vasoplegia 1-day post-reperfusion. Graph error bars indicate medians with IQRs.
Figure 4:
Systemic and myocardial venous plasma concentrations of (A and B) IL-8, and (C and D) sP-selectin at baseline, the first hour after reperfusion, and 1-day post-reperfusion in, respectively, patients with and without vasoplegia. Baseline sP-selectin levels (i.e. day before surgery) were elevated in patients with vasoplegia compared with non-vasoplegia patients (76.0 [IQR 62.1–81.6] vs 54.0 [IQR 43.8–72.7] ng/ml, P = 0.006). In patients with vasoplegia, no significant myocardial release of IL-8 or sP-selectin was observed. In contrast, in patients without vasoplegia, myocardial levels of IL-8 were 116% (P = 0.009) increased compared with arterial levels the first hour after reperfusion, indicating a myocardial release of these markers. Myocardial venous sP-selectin levels were similar compared with arterial levels in this patient group. Similar levels of all markers were found in patients with and without vasoplegia 1-day post-reperfusion. Graph error bars indicate medians with IQRs.
DISCUSSION
Our results show that pre-existing endothelial cell activation is a predisposing factor for vasoplegia after mitral valve surgery, identified with high baseline vWFpp and sP-selectin levels. Effective treatment strategies for this vasoplegic syndrome after cardiac surgery are currently lacking, although these markers may be of prognostical value in patients undergoing mitral valve surgery. This pre-existing activation could be a compensatory mechanism in order to maintain homeostasis, but it may also reflect a pathological state with persistent endothelial cell activation. These markers may be used as an opportunity to develop preoperative interventions aimed at stabilizing the vascular tone, such as discontinuing the intake of angiotensin-converting enzyme inhibitors preoperatively and use of alpha blockers to prevent alpha-adrenergic receptor-mediated vasoconstriction [17, 18]. No myocardial release of endothelial cell activation markers was observed the first hour upon reperfusion in patients with vasoplegia. In contrast, patients without vasoplegia showed a myocardial release of vWFpp, OPG; and IL-8 during this time frame. This myocardial release of endothelial cell activation markers post-surgery in non-vasoplegia patients may appear counterintuitive, but may reflect the more prominent ability of the endothelium to respond to injury. The pre-existing systemic as well as myocardial endothelial cell activation in patients with vasoplegia may explain the absence of this myocardial release after surgery.
The incidence of postoperative vasoplegic syndrome is different between the various surgical procedures. In our study, we focused on patients undergoing mitral valve surgery, a surgical procedure performed in both patients with and without left ventricular dysfunction. However, this specific patient population limited the number of patients included. Previous studies have described other patient populations in the light of the vasoplegic syndrome. Sun et al. [19] showed that the incidence of postoperative vasoplegic syndrome defined as a mean arterial pressure of ≤70 mmHg, indexed systemic vascular resistance of <1400 dynes × cm5/m2, cardiac index of ≥2.5 l/min/m2 and central venous pressure of ≥10 mmHg was significantly lower after off-pump coronary artery bypass surgery (2.8%) than after on-pump coronary artery bypass surgery (6.9%). Moreover, in another study of Sun et al. [20], it has been described that postoperative vasoplegic syndrome occurred less often after isolated coronary artery bypass graft surgery than after open-heart surgery (incidence 6.9 vs 17.0%, respectively). They also showed that advanced age and low preoperative ejection fraction strongly predicted postoperative vasoplegic syndrome. Levin et al. [2] showed that the only cardiac surgery subset that was not at increased risk for vasoplegia vs coronary artery bypass graft surgery was the group undergoing thoracic aortic surgery, likely as a result of hypothermic perfusion. They defined the amount of vasopressor support required to separate from CPB as a surrogate marker of vasoplegia.
As indicated, elevated baseline levels of vWFpp were observed in patients who later developed vasoplegia. vWF and vWFpp are stored in, and released from, activated Weibel-Palade bodies, and are therefore markers of acute endothelial cell injury [21]. vWF, in contrast to vWFpp, is a substrate for platelet aggregation [22]. Moreover, the multimeric vWF is cleaved by a specific metalloproteinase (ADAMTS13, disintegrin-like and metalloproteinase with thrombospondin Type I motifs 13), resulting in the consumption of vWF [22, 23]. As a result, plasma vWFpp levels more directly reflect vascular endothelial cell injury than that of vWF [22].
Another difference between vWF and vWFpp was observed with the measurement of arteriovenous concentration differences to discriminate between myocardial and systemic release of endothelial cell activation markers. Our results indicate a myocardial release of vWFpp, but not vWF, upon reperfusion in patients without vasoplegia after mitral valve surgery. A possible hypothesis for this discrepancy is that the larger vWF molecules remain inside the heart, whereas vWFpp is released into the circulation. In line with our study, no myocardial release of vWF was observed in previous studies [24, 25]. Moreover, differences in plasma kinetics between vWF and vWFpp were observed: vWF levels increase much slower, but are more sustained than vWFpp. Our results regarding these biokinetic properties are in line with a previous in vivo study of experimental endotoxaemia [16]. Under ‘physiological’ conditions, the molar concentration of vWFpp in normal plasma is about one-tenth of the concentration of mature vWF. Yet, under ‘pathophysiological’ conditions, the molar ratio of vWFpp to mature vWF can increase 4- to 5-fold compared with baseline, presumably due to the compartmentalization and/or catabolism of the mature vWF.
Limitations
This explorative study has some limitations. The small sample size might have limited the detection of minor differences, like a trend towards significance for elevated angiopoeitin-2 levels 1 day post-reperfusion in patients with vasoplegia (Type II error). Moreover, no subgroup analysis could be performed, and neither was correction for haemodilution applied as a result of the small sample size. Only patients scheduled for mitral valve surgery were included to minimize variability. However, this may impact the extrapolation of our data. The differences at baseline between the groups, for example the higher logistic EuroSCORE and NYHA class in patients with vasoplegia, reflect a more vulnerable patient population. Moreover, the mortality rate was low and not significantly different between patients with and without vasoplegia. This limits the potential for conclusions regarding the prognostic value of plasma concentrations of vWFpp and sP-selectin for clinical outcomes. Arteriovenous measurements in our study were restricted to an hour after reperfusion. This time window should be adequate as it is generally assumed that ischaemia/reperfusion injury and the release of Weibel-Palade bodies are acute processes. In addition, there is no consensus regarding the definition of post-cardiac surgery vasoplegia in the current literature, although we decided to use a recent one of Colson et al. [12].
CONCLUSION
Higher baseline vWFpp and sP-selectin levels were measured in patients who developed vasoplegia after mitral valve surgery, reflecting a pre-existing endothelial cell activation. These markers may be useful for the identification of patients at increased risk of developing vasoplegia postoperatively. In contrast to the preoperatively measured effects, no myocardial release of endothelial cell activation markers was observed upon reperfusion in patients with vasoplegia. In contrast, patients without vasoplegia showed a myocardial release of vWFpp, OPG and IL-8. This myocardial release may appear counterintuitive, but may reflect the more prominent ability of the endothelium to respond to injury in this patient group. The pre-existing endothelial cell activation may have resulted in desensibilization of the endothelium in patients who develop the vasoplegic syndrome, resulting in no (additional) myocardial release of endothelial cell activation markers after surgery. Further research is essential to unravel the consequences of long-term systemic endothelial cell activation for the prognosis of patients undergoing mitral valve surgery.
SUPPLEMENTARY MATERIAL
Funding
This work was supported by the Dutch Heart Foundation, Netherlands (grant 2007B150 to R.K.).
Conflict of interest: none declared.
Supplementary Material
REFERENCES
- 1.Byrne JG, Leacche M, Paul S, Mihaljevic T, Rawn JD, Shernan SK, et al. Risk factors and outcomes for ‘vasoplegia syndrome’ following cardiac transplantation. Eur J Cardiothorac Surg. 2004;25:327–32. doi: 10.1016/j.ejcts.2003.11.032. doi:10.1016/j.ejcts.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 2.Levin MA, Lin HM, Castillo JG, Adams DH, Reich DL, Fischer GW. Early on-cardiopulmonary bypass hypotension and other factors associated with vasoplegic syndrome. Circulation. 2009;120:1664–71. doi: 10.1161/CIRCULATIONAHA.108.814533. doi:10.1161/CIRCULATIONAHA.108.814533. [DOI] [PubMed] [Google Scholar]
- 3.Leyh RG, Kofidis T, Struber M, Fischer S, Knobloch K, Wachsmann B, et al. Methylene blue: the drug of choice for catecholamine-refractory vasoplegia after cardiopulmonary bypass? J Thorac Cardiovasc Surg. 2003;125:1426–31. doi: 10.1016/s0022-5223(02)73284-4. doi:10.1016/S0022-5223(02)73284-4. [DOI] [PubMed] [Google Scholar]
- 4.Gomes WJ, Carvalho AC, Palma JH, Teles CA, Branco JN, Silas MG, et al. Vasoplegic syndrome after open heart surgery. J Cardiovasc Surg (Torino) 1998;39:619–23. [PubMed] [Google Scholar]
- 5.Lowenstein CJ, Morrell CN, Yamakuchi M. Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc Med. 2005;15:302–8. doi: 10.1016/j.tcm.2005.09.005. doi:10.1016/j.tcm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 6.van Mourik JA, Romani de Wit T, Voorberg J. Biogenesis and exocytosis of Weibel-Palade bodies. Histochem Cell Biol. 2002;117:113–22. doi: 10.1007/s00418-001-0368-9. doi:10.1007/s00418-001-0368-9. [DOI] [PubMed] [Google Scholar]
- 7.Bertuglia S, Ichimura H, Fossati G, Parthasarathi K, Leoni F, Modena D, et al. ITF1697, a stable Lys-Pro-containing peptide, inhibits Weibel-Palade body exocytosis induced by ischemia/reperfusion and pressure elevation. Mol Med. 2007;13:615–24. doi: 10.2119/2007-00079.Bertuglia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle EM, Jr, Pohlman TH, Johnson MC, Verrier ED. Endothelial cell injury in cardiovascular surgery: the systemic inflammatory response. Ann Thorac Surg. 1997;63:277–84. doi: 10.1016/s0003-4975(96)01061-2. doi:10.1016/S0003-4975(96)01061-2. [DOI] [PubMed] [Google Scholar]
- 9.Zannettino AC, Holding CA, Diamond P, Atkins GJ, Kostakis P, Farrugia A, et al. Osteoprotegerin (OPG) is localized to the Weibel-Palade bodies of human vascular endothelial cells and is physically associated with von Willebrand factor. J Cell Physiol. 2005;204:714–23. doi: 10.1002/jcp.20354. doi:10.1002/jcp.20354. [DOI] [PubMed] [Google Scholar]
- 10.Lip GY, Blann A. von Willebrand factor: a marker of endothelial dysfunction in vascular disorders? Cardiovasc Res. 1997;34:255–65. doi: 10.1016/s0008-6363(97)00039-4. doi:10.1016/S0008-6363(97)00039-4. [DOI] [PubMed] [Google Scholar]
- 11.Bonow RO. Chronic mitral regurgitation and aortic regurgitation: have indications for surgery changed? J Am Coll Cardiol. 2013;61:693–701. doi: 10.1016/j.jacc.2012.08.1025. doi:10.1016/j.jacc.2012.08.1025. [DOI] [PubMed] [Google Scholar]
- 12.Colson PH, Bernard C, Struck J, Morgenthaler NG, Albat B, Guillon G. Post cardiac surgery vasoplegia is associated with high preoperative copeptin plasma concentration. Crit Care. 2011;15:R225–62. doi: 10.1186/cc10516. doi:10.1186/cc10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mekontso-Dessap A, Houel R, Soustelle C, Kirsch M, Thebert D, Loisance DY. Risk factors for post-cardiopulmonary bypass vasoplegia in patients with preserved left ventricular function. Ann Thorac Surg. 2001;71:1428–32. doi: 10.1016/s0003-4975(01)02486-9. doi:10.1016/S0003-4975(01)02486-9. [DOI] [PubMed] [Google Scholar]
- 14.Kortekaas KA, van der Pol P, Lindeman JH, Baan CC, van Kooten C, Klautz RJ. No prominent role for terminal complement activation in the early myocardial reperfusion phase following cardiac surgery. Eur J Cardiothorac Surg. 2012;41:117–25. doi: 10.1093/ejcts/ezs088. doi:10.1093/ejcts/ezs088. [DOI] [PubMed] [Google Scholar]
- 15.Kortekaas KA, Lindeman JH, Versteegh MI, van Beelen E, Kleemann R, Klautz RJ. Heart failure determines the myocardial inflammatory response to injury. Eur J Heart Fail. 2013;15:400–7. doi: 10.1093/eurjhf/hfs183. doi:10.1093/eurjhf/hfs183. [DOI] [PubMed] [Google Scholar]
- 16.Borchiellini A, Fijnvandraat K, ten Cate JW, Pajkrt D, van Deventer SJ, Pasterkamp G, et al. Quantitative analysis of von Willebrand factor propeptide release in vivo: effect of experimental endotoxemia and administration of 1-deamino-8-d-arginine vasopressin in humans. Blood. 1996;88:2951–8. [PubMed] [Google Scholar]
- 17.Argenziano M, Chen JM, Choudhri AF, Cullinane S, Garfein E, Weinberg AD, et al. Management of vasodilatory shock after cardiac surgery: identification of predisposing factors and use of a novel pressor agent. J Thorac Cardiovasc Surg. 1998;116:973–80. doi: 10.1016/S0022-5223(98)70049-2. doi:10.1016/S0022-5223(98)70049-2. [DOI] [PubMed] [Google Scholar]
- 18.Sugawara J, Komine H, Hayashi K, Yoshizawa M, Otsuki T, Shimojo N, et al. Reduction in alpha-adrenergic receptor-mediated vascular tone contributes to improved arterial compliance with endurance training. Int J Cardiol. 2009;135:346–52. doi: 10.1016/j.ijcard.2008.04.007. doi:10.1016/j.ijcard.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X, Zhang L, Hill PC, Lowery R, Lee AT, Molyneaux RE, et al. Is incidence of postoperative vasoplegic syndrome different between off-pump and on-pump coronary artery bypass grafting surgery? Eur J Cardiothorac Surg. 2008;34:820–5. doi: 10.1016/j.ejcts.2008.07.012. doi:10.1016/j.ejcts.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Sun X, Boyce SW, Herr DL, Hill PC, Zhang L, Corso PJ, et al. Is vasoplegic syndrome more prevalent with open-heart procedures compared with isolated on-pump CABG surgery? Cardiovasc Revasc Med. 2011;12:203–9. doi: 10.1016/j.carrev.2010.10.004. doi:10.1016/j.carrev.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Wagner DD, Fay PJ, Sporn LA, Sinha S, Lawrence SO, Marder VJ. Divergent fates of von Willebrand factor and its propolypeptide (von Willebrand antigen II) after secretion from endothelial cells. Proc Natl Acad Sci USA. 1987;84:1955–9. doi: 10.1073/pnas.84.7.1955. doi:10.1073/pnas.84.7.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Habe K, Wada H, Ito-Habe N, Hatada T, Matsumoto T, Ohishi K, et al. Plasma ADAMTS13, von Willebrand factor (VWF) and VWF propeptide profiles in patients with DIC and related diseases. Thromb Res. 2012;129:598–602. doi: 10.1016/j.thromres.2011.10.011. doi:10.1016/j.thromres.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Claus RA, Bockmeyer CL, Sossdorf M, Losche W. The balance between von-Willebrand factor and its cleaving protease ADAMTS13: biomarker in systemic inflammation and development of organ failure? Curr Mol Med. 2010;10:236–48. doi: 10.2174/156652410790963367. doi:10.2174/156652410790963367. [DOI] [PubMed] [Google Scholar]
- 24.Holdright DR, Hunt BJ, Parratt R, Segal H, Clarke D, Taggart D, et al. The effects of cardiopulmonary bypass on systemic and coronary levels of von Willebrand factor. Eur J Cardiothorac Surg. 1995;9:18–21. doi: 10.1016/s1010-7940(05)80043-0. doi:10.1016/S1010-7940(05)80043-0. [DOI] [PubMed] [Google Scholar]
- 25.Valen G, Blomback M, Sellei P, Lindblom D, Vaage J. Release of von Willebrand factor by cardiopulmonary bypass, but not by cardioplegia in open heart surgery. Thromb Res. 1994;73:21–9. doi: 10.1016/0049-3848(94)90050-7. doi:10.1016/0049-3848(94)90050-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.