Abstract
We present preliminary data on the flow-induced haemodynamic and structural loads exerted on a penetrating atherosclerotic aortic ulcer (PAU). Specifically, one-way fluid–structure interaction analysis was performed on the aortic model reconstructed from a 66-year-old male patient with a PAU that evolved into an intramural haematoma and rupture of the thoracic aorta. The results show that elevated blood pressure (117 mmHg) and low flow velocity at the aortic wall (0.15 m/s2) occurred in the region of the PAU. We also found a low value of time-averaged wall shear stress (1.24 N/m2) and a high value of the temporal oscillation in the wall shear stress (oscillatory shear index = 0.13) in the region of the PAU. After endovascular treatment, these haemodynamic parameters were distributed uniformly on the luminal surface of the stent graft. These findings suggest that wall shear stress could be considered one of the major haemodynamic factors indicating the structural fragility of the PAU wall, which ultimately lead to PAU growth and rupture.
Keywords: Penetrating atherosclerotic ulcer, Fluid–structure interaction, Wall shear stress
INTRODUCTION
Penetrating atherosclerotic ulcer (PAU) of the aorta is characterized by an ulceration that penetrates the vessel wall through the elastic lamina into the media, with a variable haematoma [1, 2]. When symptomatic, PAU requires endovascular stent graft treatment [3], and studies indicate differences with aortic dissections [4, 5]. The likelihood of rupture is reported to be up to 40%.
Identification of parameters that may predict the evolution of PAUs could justify early endovascular handling to prevent deleterious complications. Here, we report haemodynamic disturbances of a ruptured PAU derived by computational fluid–structure interaction analysis.
MATERIALS AND METHODS
Stent grafting and surgical procedure
A 66-year-old man was admitted to our hospital for sudden onset of chest pain radiating to the back and interscapular region. A history of untreated hypertension was recorded. A contrasted computed tomography (CT) scan confirmed a ruptured PAU distal to the left subclavian artery, with a large intramural haematoma involving the thoracic aorta and the distal aortic arch (Fig. 1). In consideration of these findings, emergent endovascular intervention was planned. A 42–150 mm thoracic endoprosthesis (Talent®; Medtronic, Santa Rosa, CA, USA) was implanted to seal the ruptured PAU. Postoperative CT confirmed PAU exclusion without contrast leak.
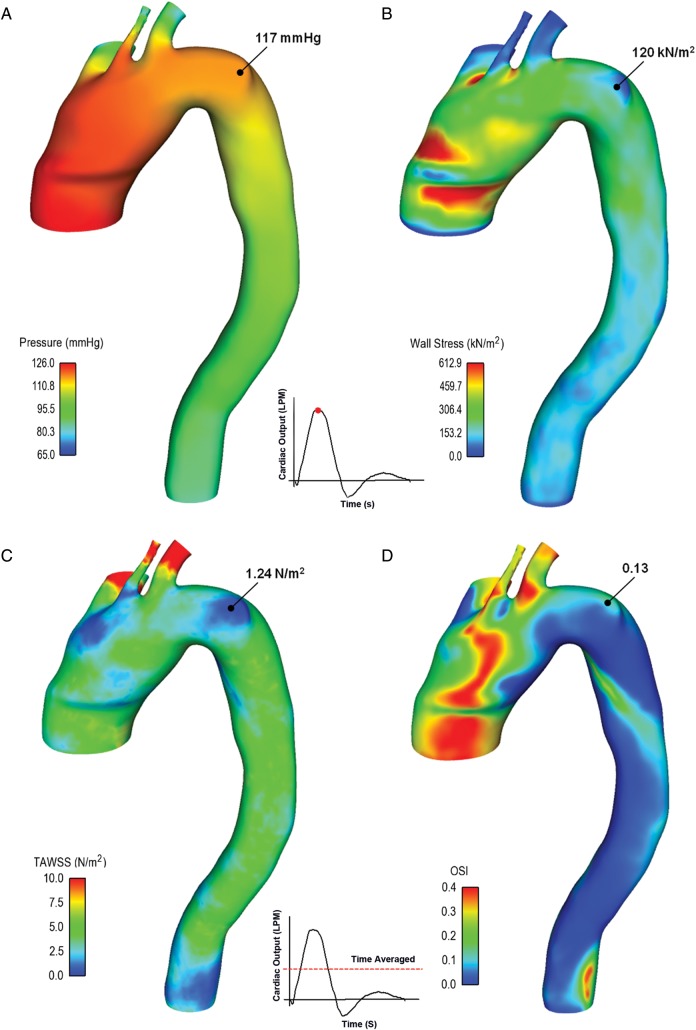
Figure 1:
Distribution of blood pressure (A) and wall stress (B) shown at peak of systole, while time-averaged wall shear stress (TAWSS; C) and oscillatory shear index (OSI; D) are shown over one cardiac cycle.
Computational analysis of fluid–structure interaction
Computational methodology was applied to present research following a technique previously reported by our group [6]. In brief, the three-dimensional aortic arch geometry created in our model was segmented from the patient's CT data using the vascular modelling toolkit VMTK v0.9.0 (http://www.vmtk.org). For the postrepair model, the geometry of the stent graft was placed into the three-dimensional model as it appeared in the CT images of the patient following PAU repair and modelled as a tubular protrusion into the aortic arch. Then, the aortic anatomy was meshed into small elements to estimate both fluid dynamics and structural loads. For the fluid analysis, the total cardiac output (i.e. inlet flow to aortic root) was assumed to be 5 l/min, and this flow was distributed between the supra-aortic vessels and the descending aorta with a ratio of 20 : 80 using resistance boundary conditions [7]. For the structural analysis, the aorta was assumed to have a uniform thickness of 1.72 mm, and it was modelled as a hyperelastic, homogeneous, incompressible and isotropic material using a finite strain constitutive model developed for modelling the human aorta [8].
Results
High pressure values with a maximum of 117 mmHg were found in the region of the aortic wall bulge induced by the penetrating ulcer (Fig. 1A). At the PAU lesion, the wall tension expressed in terms of maximum principal stress was 120 kN/m2, which is lower than that occurring near the ascending aorta and ostia of supra-aortic vessels (Fig. 1B).
The time-averaged wall shear stress over one cardiac cycle (TAWSS) and the temporal oscillations in the wall shear stress as described by oscillatory shear index (OSI) were also extracted by fluid simulation (Fig. 1C and D). Specifically, the region of the PAU exhibited a low value of TAWSS with a minimum of 1.24 N/m2. In contrast, the high value of OSI suggests flow reversal in proximity to the wall of the ulcerating lesion.
Table 1 summarizes the values of haemodynamic parameters both pre- and postrepair of the aorta near the bulged wall of the PAU lesion. Specifically, TAWSS increased whereas OSI reduced after endovascular treatment. At the PAU lesion, the aorta postrepair exhibited a lower peak systolic pressure than prerepair, while the end diastolic pressure was slightly changed.
Table 1:
Values of pre- and postrepair haemodynamic parameters found in proximity of penetrating atherosclerotic aortic ulcer lesion
| Time-averaged wall shear stress (kN/m2) | Oscillatory shear index | Systolic pressure (mmHg) | Diastolic pressure (mmHg) | Peak blood velocity (m/s2) | |
|---|---|---|---|---|---|
| Prerepair | 1.24 | 0.13 | 117 | 82.1 | 0.15 |
| Postrepair | 1.75 | 0.085 | 109 | 80.5 | 0.5 |
DISCUSSION
Our findings suggest that the blood stagnates in the region of the PAU, resulting in both an elevated intraluminal pressure and a low flow velocity at the level of the aortic wall. This may ultimately lead to the progression of the PAU due to the increased residence time of the blood in this region. Slow progression of the PAU is also evinced by the low magnitude of maximum principal stress.
In this study, we found low wall shear stress (i.e. TAWSS) and high shear stress oscillation (i.e. OSI) in the PAU region. This suggests that the ulcerating lesion results in mechanical damage. Specifically, exposure of the aortic wall to a relatively low wall shear stress may increase intercellular permeability and consequently increase the vulnerability of this region to PAU progression [9]. It has been demonstrated that both low shear stress and high oscillation of shear stress inhibit the release of factors from endothelial cells that promote coagulation and survival of the endothelial cells of the vessel [10]. In spite of the fact that high wall stress plays a key role on the onset of the initial lesion, low shear stress is responsible for its growth and vascular remodelling [10]. It is generally assumed that a wall shear stress of TAWSS = 2.0 N/m2 is suitable for maintaining the structure of the vessel, and a value lower than 1.5 N/m2 will result in degeneration of the endothelial cells via the apoptotic cell cycle. In our study, the TAWSS in the PAU region was barely ∼1.24 N/m2 and seems to be too low to maintain the regular cellular functions of endothelial cells, resulting in vessel permeability. Furthermore, the temporal oscillation in the wall shear stress in the PAU region was higher than that typically reported for a normal aorta with a laminar flow (see Table 1). After endovascular treatment, both TAWSS and OSI redistributed uniformly and showed adequate values for maintainance of the vascular structure.
Our hypothesis should be tested on a larger group to establish whether these haemodynamic characteristics are putative factors for PAU evolution or whether they are a consequence of the PAU. A major limitation of the present study is that, to simplify the model, we assumed uniform distributions of aortic wall thickness and elasticity.
In conclusion, low shear stress and high oscillation of shear stress over the cardiac cycle could be considered key haemodynamic factors that indicate structural fragility of the PAU wall and its eventual evolution towards rupture. In the future, PAUs with significantly altered values of wall shear stress parameters could be treated preventively to anticipate emergent evolution.
Funding
This work was supported by a grant from Fondazione RiMED provided to S. Pasta.
Conflict of interests: none declared.
REFERENCES
- 1.Welch TJ, Stanson AW, Sheedy PF, 2nd, Johnson CM, McKusick MA. Radiologic evaluation of penetrating aortic atherosclerotic ulcer. Radiographics. 1990;10:675–85. doi: 10.1148/radiographics.10.4.2377766. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi H, Matsuoka Y, Sakamoto I, Sueyoshi E, Okimoto T, Hayashi K, et al. Penetrating atherosclerotic ulcer of the aorta: imaging features and disease concept. Radiographics. 2000;20:995–1005. doi: 10.1148/radiographics.20.4.g00jl01995. [DOI] [PubMed] [Google Scholar]
- 3.Pauls S, Orend KH, Sunder-Plassmann L, Kick J, Schelzig H. Endovascular repair of symptomatic penetrating atherosclerotic ulcer of the thoracic aorta. Eur J Vasc Endovasc Surg. 2007;34:66–73. doi: 10.1016/j.ejvs.2006.12.029. doi:10.1016/j.ejvs.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 4.Cho KR, Stanson AW, Potter DD, Cherry KJ, Schaff HV, Sundt TM., 3rd Penetrating atherosclerotic ulcer of the descending thoracic aorta and arch. J Thorac Cardiovasc Surg. 2004;127:1393–9. doi: 10.1016/j.jtcvs.2003.11.050. discussion 1399–401 doi:10.1016/j.jtcvs.2003.11.050. [DOI] [PubMed] [Google Scholar]
- 5.Macura KJ, Corl FM, Fishman EK, Bluemke DA. Pathogenesis in acute aortic syndromes: aortic dissection, intramural hematoma, and penetrating atherosclerotic aortic ulcer. AJR Am J Roentgenol. 2003;181:309–16. doi: 10.2214/ajr.181.2.1810309. doi:10.2214/ajr.181.2.1810309. [DOI] [PubMed] [Google Scholar]
- 6.Pasta S, Cho JS, Dur O, Pekkan K, Vorp DA. Computer modeling for the prediction of thoracic aortic stent graft collapse. J Vasc Surg. 2013;57:1353–61. doi: 10.1016/j.jvs.2012.09.063. [DOI] [PubMed] [Google Scholar]
- 7.Pekkan K, Dur O, Sundareswaran K, Kanter K, Fogel M, Yoganathan A, et al. Neonatal aortic arch hemodynamics and perfusion during cardiopulmonary bypass. J Biomech Eng. 2008;130:061012. doi: 10.1115/1.2978988. doi:10.1115/1.2978988. [DOI] [PubMed] [Google Scholar]
- 8.Raghavan ML, Vorp DA. Toward a biomechanical tool to evaluate rupture potential of abdominal aortic aneurysm: identification of a finite strain constitutive model and evaluation of its applicability. J Biomech. 2000;33:475–82. doi: 10.1016/s0021-9290(99)00201-8. doi:10.1016/S0021-9290(99)00201-8. [DOI] [PubMed] [Google Scholar]
- 9.Okano M, Yoshida Y. Junction complexes of endothelial cells in atherosclerosis-prone and atherosclerosis-resistant regions on flow dividers of brachiocephalic bifurcations in the rabbit aorta. Biorheology. 1994;31:155–61. doi: 10.3233/bir-1994-31203. [DOI] [PubMed] [Google Scholar]
- 10.Shaaban AM, Duerinckx AJ. Wall shear stress and early atherosclerosis: a review. AJR Am J Roentgenol. 2000;174:1657–65. doi: 10.2214/ajr.174.6.1741657. doi:10.2214/ajr.174.6.1741657. [DOI] [PubMed] [Google Scholar]