Abstract
OBJECTIVE
To analyse the results after elective open total aortic arch replacement.
METHODS
We analysed 39 patients (median age 63 years, median logistic EuroSCORE 18.4) who underwent elective open total arch replacement between 2005 and 2012.
RESULTS
In-hospital mortality was 5.1% (n = 2) and perioperative neurological injury was 12.8% (n = 5). The indication for surgery was degenerative aneurysmal disease in 59% (n = 23) and late aneurysmal formation following previous surgery of type A aortic dissection in 35.9% (n = 14); 5.1% (n = 2) were due to anastomotical aneurysms after prior ascending repair. Fifty-nine percent (n = 23) of the patients had already undergone previous proximal thoracic aortic surgery. In 30.8% (n = 12) of them, a conventional elephant trunk was added to total arch replacement, in 28.2% (n = 11), root replacement was additionally performed. Median hypothermic circulatory arrest time was 42 min (21–54 min). Selective antegrade cerebral perfusion was used in 95% (n = 37) of patients. Median follow-up was 11 months [interquartile range (IQR) 1–20 months]. There was no late death and no need for reoperation during this period.
CONCLUSIONS
Open total aortic arch replacement shows very satisfying results. The number of patients undergoing total arch replacement as a redo procedure and as a part of a complex multisegmental aortic pathology is high. Future strategies will have to emphasize neurological protection in extensive simultaneous replacement of the aortic arch and adjacent segments.
Keywords: Aortic arch surgery, Multisegmental thoracic aortic pathology, Aneurysm, Dissection
INTRODUCTION
Refinements in perfusion technology, anaesthesiological handling as well as in surgical technique have made proximal thoracic aortic surgery a safe and reproducible procedure [1–3]. The use of the right axillary or subclavian artery for arterial return and routine application of selective antegrade cerebral perfusion have substantially contributed to the results obtained currently following surgical treatment [4, 5]. While the majority of reports deal with hemi-arch replacement, few series addressing the issue of total arch replacement are available [6, 7].
The aim of this study was to analyse the results after elective open total aortic arch replacement in our institution.
PATIENTS AND METHODS
Patients
We analysed 39 patients (median age 63 years, median logistic EuroSCORE 18.4) who underwent elective open total arch replacement as first operation or as redo operation after prior surgery on the proximal thoracic aorta as well as after prior surgery for aortic coarctation between 2005 and 2012. Early and mid-term outcomes were evaluated. The institutional review board of the University Hospital of Berne approved the study and waived the need for patient consent.
Definition of clinical parameters
Preoperative parameters were defined according to EuroSCORE I guidelines [8]. Mortality was defined as in-hospital death.
Stroke was defined as any new senso-motoric deficit (including those with subclinical manifestation) persisting at the time of discharge in combination with a morphological correlate in CT scan or MR imaging. Furthermore, stroke was subclassified as disabling and non-disabling, according to the VARC 2 criteria [9].
Definition of total arch replacement
Total arch replacement was defined as arch surgery requiring a circumferential anastomosis at the level of the aortic arch or the descending aorta with the need for separate reimplantation of at least one supra-aortic vessel. This definition was chosen by consent of an expert consensus panel (Vascular Domain of EACTS).
Surgical techniques
The preferred technique of supra-aortic vessel reimplantation was by the island technique. Further approaches consisted of patch reimplantation of the brachiocephalic trunk as well as the left common carotid artery with a separate graft to the left subclavian artery (Fig. 1). The third approach consisted of a branched graft to all three supra-aortic vessels (Fig. 2).
Figure 1:
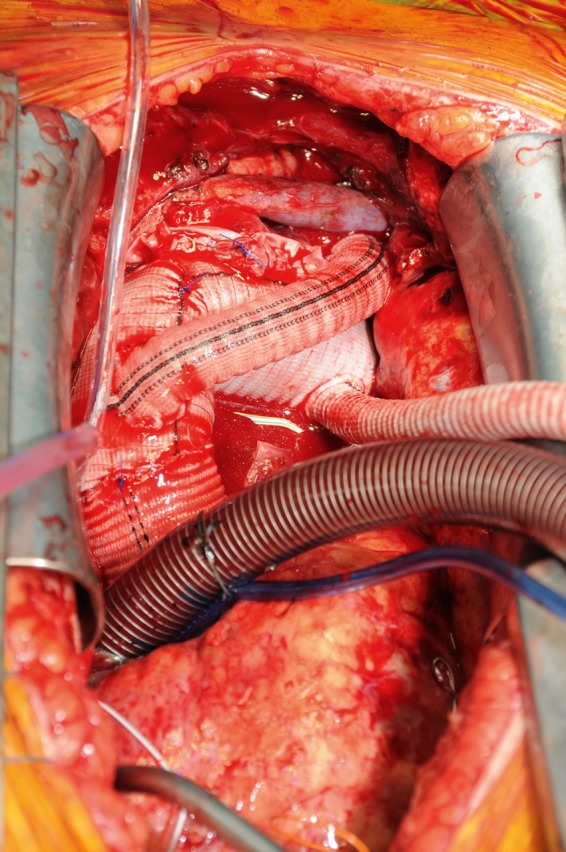
Completed arch repair with Dacron graft to left subclavian artery and island reimplantation of left brachiocephalic trunk and left common carotid artery.
Figure 2:
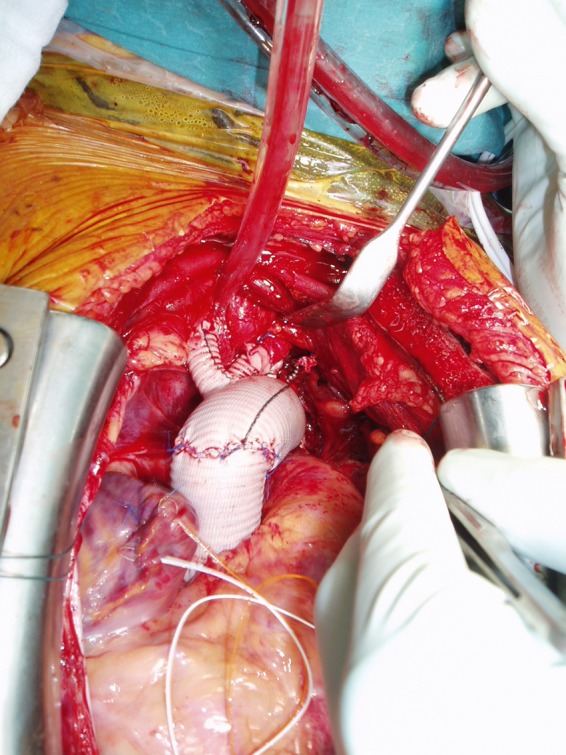
Completed arch repair with branched graft to all three supra-aortic vessels.
Data collection and follow-up protocol
Data were collected prospectively. After surgery, patients were seen in our aortic outpatient clinic at regular intervals. Those who did not show up in the outpatient consultation were contacted via general practitioners or directly via phone calls. Consequently, follow-up was complete in all patients.
Extracorporeal circulation and myocardial protection strategy
Patients were cooled to bilateral 20°C tympanic temperature and 26°C bladder temperature. Vasodilators such as sodium nitroprusside and phentolamine were used to achieve homogenous cooling by reducing peripheral vascular resistance. During rewarming, target temperatures were bilateral 36°C tympanic temperature as well as 35°C core temperature. Cerebral protection was achieved with either selective antegrade perfusion using two perfusion catheters in the innominate artery and the left common carotid artery or antegrade perfusion through the right subclavian artery (cannulation site) and an additional catheter in the left common carotid artery in the majority of cases. Temperature of the cerebral perfusate was 20°C. Total cerebral flow was between 500 and 750 ml/min and targeted according to the anticipated resistance of the cannulas. Most importantly, resistance of 50 mmHg at the level of the individual cannula was not exceeded in order not to expose the brain to episodes of excessive pressure. Myocardial protection was performed using a low-volume cardioplegic solution (Cardioplexol®) as induction cardioplegia with intermittent modified Buckberg cold blood cardioplegia every 20–30 min. Before coronary reperfusion, a modified Buckberg warm blood cardioplegia was administered.
After weaning from cardiopulmonary bypass, reversal of heparin with protamine ratio 1:1 (1 mg protamine per 100 IU heparin) was performed. Intraoperative autologous transfusion using a cell-saver device was used in all patients. Intraoperative and postoperative transfusion thresholds were guided by in-hospital standards supplemented by rotational thromboelastometry (ROTEM, Pentapharm GmbH, Munich, Germany) and analysis of selected parameters of coagulation. During the entire operation, neuro-monitoring was performed by near infrared spectroscopy (NIRS) [4].
Statistical methods
Continuous data are presented as the median and interquartile range (IQR range from the 25th to the 75th percentile). Discrete data are given as counts and percentages. Overall survival and freedom from reintervention were calculated according to the method of Kaplan and Meier. All calculations were performed with SPSS 20.0 software for MacOSX (IBM, Inc., Somers, NY, USA).
Results
Descriptive characteristics of the cohort
Descriptive characteristics of the cohort are shown in Table 1. Median age was 63 years (IQR 55–70 years, 20.5% female). Thirty-six percent suffered from coronary artery disease (n = 14). In 7.7% (n = 3), neurological injury had occurred prior to surgery. Median logistic EuroSCORE I was 18.4 (IQR 11.6–26). Fifty-nine percent (n = 23) had already undergone previous proximal thoracic aortic surgery. The median body surface area of our patients was 1.92 ± 0.21 and the body mass index was 25.5 ± 4.0.
Table 1:
Descriptive characteristics of the cohort
| Overall (n =39) |
||
|---|---|---|
| Demographics | ||
| Age in years, median (IQR) | 63 | (55–70) |
| Female, n (%) | 8 | (20.5) |
| Chronic health conditions and risk factors | ||
| Hypertension, n (%) | 36 | (92.3) |
| Chronic obstructive pulmonary disease, n (%) | 1 | (2.6) |
| Diabetes mellitus, n (%) | 3 | (7.7) |
| Serum creatinine >200 mmol/l, n (%) | 2 | (5.1) |
| Coronary artery disease, n (%) | 14 | (35.9) |
| Extracardiac arteriopathy, n (%) | 3 | (7.7) |
| Pulmonary hypertension, n (%) | 1 | (2.6) |
| Permanent neurological deficit, n (%) | 3 | (7.7) |
| Logistic EuroSCORE, median (IQR) | 18.4 | (11.6–26) |
| Additive EuroSCORE, median (IQR) | 10 | (8–11) |
| Redo surgery, n (%) | 23 | (59) |
| Previous surgical approach | ||
| Ascending aortic replacement, n (%) | 13 | (56.5) |
| Root replacement, n (%) | 3 | (13) |
| AVR/ascending, n (%) | 2 | (8.7) |
| Coarctational repair, n (%) | 2 | (8.7) |
| Rerouting/TEVAR, n (%) | 2 | (8.7) |
| Descending aortic replacement, n (%) | 1 | (4.4) |
Unless otherwise indicated, data are numbers (%).
Classification of chronic health conditions and risk factors according to EuroSCORE criteria.
IQR: interquartile range; AVR: aortic valve replacement; TEVAR: thoracic aortic endovascular repair.
Type of previous aortic operations performed were as follows. In 56.5% (n = 13), ascending ± hemi-arch replacement was performed, 13% (n = 3) had already undergone aortic root replacement. Prior aortic valve with concomitant ascending aortic replacement, aortic coarctation repair and supra-aortic rerouting with TEVAR were performed in 8.7% (n = 2) each. Finally, prior descending aortic replacement was performed in 4.4% (Table 1).
Reasons for proximal thoracic aortic redo surgery
The majority of patients underwent total aortic arch replacement due to chronic aneurysmal formation (59%, n = 23), whereas the development of an aneurysm following repair of previous acute aortic dissection type A was the indication for reoperation in 35.9% (n = 14). Anastomotic aneurysms after previous ascending aortic replacement accounted for 5.1% (n = 2) (Table 2).
Table 2:
Surgical characteristics of the cohort
| Overall (n = 39) |
||
|---|---|---|
| Surgical indications | ||
| Post-dissection aneurysm, n (%) | 14 | (35.9) |
| Non-dissecting aneurysm, n (%) | 23 | (59) |
| Anastomotic aneurysm, n (%) | 2 | (5.1) |
| Arterial cannulation sites and extent of aortic replacement | ||
| Axillary/subclavian cannulation, n (%) | 16 | (41) |
| Femoral cannulation, n (%) | 2 | (5.1) |
| Direct aortic/prosthetic cannulation, n (%) | 20 | (51.3) |
| Other, n (%) | 1 | (2.6) |
| Elephant trunk, n (%) | 12 | (30.8) |
| Root replacement, n (%) | 11 | (28.2) |
| Reimplantation of BC trunk and LCC, n (%) | 9 | (23.1) |
| Reimplantation of all three supra-aortic vessels, n (%) | 30 | (76.9) |
| Additional procedures | ||
| CABG, n (%) | 12 | (30.8) |
| Aortic valve replacement, n (%) | 13 | (33.3) |
| Mitral valve repair/replacement, n (%) | 2 | (5.1) |
| ECC and HCA times | ||
| ECC in minutes, median (IQR) | 166 | (135–222) |
| Aortic cross-clamp times in minutes, median (IQR) | 93 | (73–122) |
| HCA in minutes, median (IQR) | 42 | (21–54) |
| HCA longer than 40 min, n (%) | 20 | (51.3) |
| SACP, n (%) | 37 | (95) |
Unless otherwise indicated, data are numbers (%).
Classification of chronic health conditions and risk factors according to EuroSCORE I criteria.
IQR, interquartile range; BC trunk: brachiocephalic trunk; LCC: left common carotid artery; ECC: extracorporeal circulation; HCA: hypothermic circulatory arrest; SACP: selective antegrade cerebral perfusion.
Surgical strategy during reintervention, extension of arch surgery to adjacent aortic segments and additional cardiac procedures
Sites of cannulation for arterial return during redo surgery are shown in Table 2. In 51.3% (n = 20), a direct cannulation approach via the native ascending aorta or via a previously implanted prosthesis was used. In 41% (n = 16), the right axillary/subclavian artery was used. Other cannulation sites were rare (Table 2).
In 30.8% (n = 12), a conventional elephant trunk technique was performed to facilitate later potential thoraco-abdominal aortic replacement. No frozen elephant trunk implantations were performed in this series. In 28.2% (n = 11), aortic root replacement was performed. In 76.9% (n = 30), the distal anastomosis was accomplished distal to the left subclavian artery with reimplantation of all three arch vessels, whereas in 23.1% (n = 9), a circumferential arch anastomosis was done between the left common carotid artery and the left subclavian artery with island reimplantation of the brachiocephalic trunk and the left common carotid artery (Table 2). Additional cardiac procedures are shown in Table 2.
Extracorporeal circulation data
Median extracorporeal circulation time was 166 min (IQR 135–222), median aortic cross-clamp time was 93 min (IQR 73–122) and hypothermic circulatory arrest times with antegrade selective cerebral perfusion was 42 min (IQR 21–54) (Table 3).
Table 3:
Outcome characteristics of the cohort
| Overall (n = 39) |
||
|---|---|---|
| Early in-hospital complications | ||
| In-hospital mortality, n (%) | 2 | (5.1) |
| Acute renal failure, n (%) | 5 | (12.8) |
| Pulmonary complications, n (%) | 2 | (5.1) |
| Acute myocardial infarction, n (%) | 3 | (7.7) |
| Neurological injury, n (%) | 5 | (12.8) |
| Late outcome | ||
| Follow-up in months, median (IQR) | 11 | (1–20) |
| Late death, n (%) | 0 | (0) |
| Redo surgery, n (%) | 0 | (0) |
Unless otherwise indicated, data are numbers (%).
Classification of complications according to STS criteria.
IQR: interquartile range.
Outcome characteristics of the cohort
In-hospital mortality was 5.1% (n = 2). These 2 patients died due to multiorgan failure. New onset of neurological injury was 12.8% (n = 5). Neurological injury was subclassified into two categories according to the VARC 2 criteria—disabling and non-disabling neurological injury. Thereby, the incidence of disabling stroke was 5.1% (n = 2) and of non-disabling stroke was 7.7% (n = 3). Temporary neurological dysfunction was observed in 1 patient.
Acute renal failure requiring intermittent haemodialysis was observed in 12.8% (n = 5), pulmonary complications requiring tracheostomy were seen in 5.1% (n = 2). The remaining outcome characteristics are shown in Table 3.
Follow-up
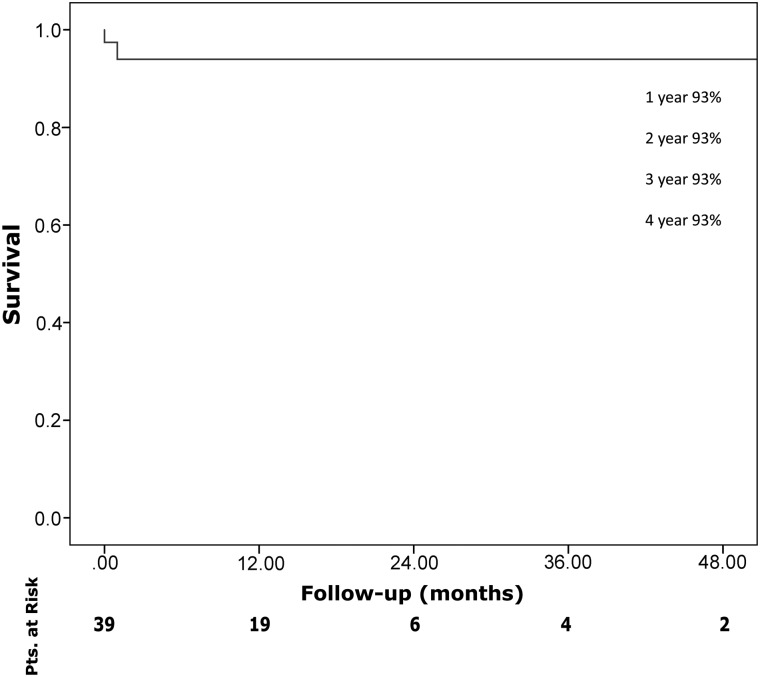
Median follow-up was 11 months (IQR 1–20). One-year and 4-year actuarial survival rates were 93% each (Fig. 3). Freedom from reoperation during follow-up was 100%.
Figure 3:
Kaplan–Meier survival curve.
Comment
Open total aortic arch replacement shows very satisfying results. The proportion of patients undergoing total arch replacement as a redo procedure and as a part of a complex multisegmental aortic pathology is high. Future strategies will have to improve neurological protection for extensive simultaneous replacement of the aortic arch and adjacent segments.
Median age of patients was rather young and strongly represents the fact that patients with proximal thoracic aortic disease present at a younger age when compared with patients with multisegemental descending thoracic aortic disease due to the different underlying pathology [10]. Furthermore, patients sustaining acute type A aortic dissection show a median age of 60 years, and therefore, some of them are likely to come back with chronic aneurysmal formation in the first 5 years following dissection repair [11]. Interestingly, an unexpectedly high number of patients suffered from coronary artery disease, which is rather unusual in those with dilatative arteriopathy.
The majority of patients undergoing open total aortic arch surgery as a redo procedure had undergone prior ascending and/or hemi-arch replacement. Interestingly, we had 2 patients with late aneurysmal formation after repair of aortic coarctation in whom total proximal thoracic aortic repair was necessary because of aneurysmal dilatation of the ascending aorta and bicuspid aortic valve. Finally, 2 patients after prior supra-aortic rerouting and TEVAR were operated on. This constellation requires a specific surgical approach as the anastomosis between the proximal portion of the stent-graft and a conventional Dacron prosthesis can be very challenging, due to the handling of the rigid stent-graft fabric and the presence of bare springs [12]. Primary removal of these bare springs may be dangerous as very sharp edges remain and might cause subsequent rupture of the suture line. As a technical solution, we do recommend the approximation of the bare springs via a suture and a tourniquet in order to facilitate this anastomosis. As soon as a certain part of the circumference is accomplished, the corresponding bare springs may be removed.
In this series, open total arch replacement was not an isolated operation but was part of an extensive reconstruction of segments proximal and distal to the aortic arch in the majority of patients. This is reflected by the high number of patients receiving a conventional elephant trunk to facilitate potential later thoraco-abdominal surgery. Furthermore, a respectable number of patients underwent aortic root replacement. As many of these underwent prior repair of acute type A aortic dissection, the question arises whether a more extensive surgical approach during primary surgery would have prevented the need for redo surgery. It is our policy to perform root replacement during primary surgery if the retrograde component of the dissection involves more than the non-coronary sinus in order to prevent the need for late reoperation in this segment [13]. Regarding the extent of arch surgery, our own data indicate that if the primary entry tear is eliminated during surgery for acute type A aortic dissection, the need for later arch or thoraco-abdominal reoperation is very low. However, in case of the intimal tear or in case of a huge communication between both lumina in the arch or the descending aorta, the persistence of such a communication is an independent risk factor for the need for late repair due to aneurysmal degeneration [14].
Perioperative mortality was low and well comparable to other recently published series, which should serve as the benchmark for aortic arch surgery. However, the rate of perioperative neurological injury was significant. After analysing all 5 cases of neurological injury, we realized that causes were multifactorial. Two patients had thrombotic masses within the aortic arch and most likely sustained embolization. The third patient had an initially uneventful course, then sustained surgical bleeding and had neurological injury due to prolonged hypotension but without any functional sequelae during follow-up. A fourth patient underwent redo surgery due to endocarditis and had a prolonged cardiopulmonary bypass run and suffered from a diffuse morphological pattern of brain injury. The last patient had an initially uneventful course and sustained a stroke while being on the ward without any functional sequelae at the time of discharge. There was no need for redo surgery in the treated aortic segments during follow-up.
As combined surgical and endovascular techniques as well as total endovascular branched arch repair are progressing, the evaluation of conventional arch surgery in the context of these new alternatives is warranted. In the present series, we are convinced that the majority of patients would not have been candidates for combined surgical/endovascular or total endovascular repair due to several reasons. A significant proportion needed root surgery and the latter combined with endovascular procedures is currently mutually exclusive. Furthermore, aneurysmal formation on the basis of a chronic dissection is a weak indication for a solely endovascular approach, and is successful only under specific morphological and functional conditions [15, 16]. Finally, some patients required concomitant treatment for structural heart disease.
Limitations of the study
Without doubt, this series contains all the drawbacks of a retrospective single-centre analysis. The number of patients in this series seems small but it represents a highly selected cohort of patients. We do perform on average 250 thoracic aortic cases in Berne yearly, including an average of 35 open thoraco-abdominal replacements. Therefore, the overall experience of our centre is large for European dimensions. With this article, we intended to depict that aortic arch surgery in the current era has become an adjunct of a complex multistep procedure and is not an isolated operation, at least in our setting.
In summary, open total aortic arch replacement shows very satisfying results. A significant proportion of patients undergo total arch replacement as a redo procedure and as a part of a complex multisegmental aortic pathology.
Conflict of interest: none declared.
REFERENCES
- 1.Bachet J, Guilmet D, Goudot B, Dreyfus GD, Delentdecker P, Brodaty D, et al. Antegrade cerebral perfusion with cold blood: a 13-year experience. Ann Thorac Surg. 1999;67:1874–8. doi: 10.1016/s0003-4975(99)00411-7. [DOI] [PubMed] [Google Scholar]
- 2.Lei Q, Chen L, Zhang Y, Fang N, Cheng W, Li L. Predictors of prolonged mechanical ventilation after aortic arch surgery with deep hypothermic circulatory arrest plus antegrade selective cerebral perfusion. J Cardiothorac Vasc Anesth. 2009;23:495–500. doi: 10.1053/j.jvca.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Spielvogel D, Etz CD, Silovitz D, Lansman SL, Griepp RB. Aortic arch replacement with a trifurcated graft. Ann Thorac Surg. 2007;83:S791–5. doi: 10.1016/j.athoracsur.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Czerny M, Krähenbühl E, Reineke D, Sodeck G, Englberger L, Weber A, et al. Mortality and neurologic injury after surgical repair with hypothermic circulatory arrest in acute and chronic proximal thoracic aortic pathology: effect of age on outcome. Circulation. 2011;124:1407–13. doi: 10.1161/CIRCULATIONAHA.110.010124. [DOI] [PubMed] [Google Scholar]
- 5.Kazui T, Yamashita K, Washiyama N, Terada H, Bashar AH, Suzuki K, et al. Aortic arch replacement using selective cerebral perfusion. Ann Thorac Surg. 2007;83:S796–8. doi: 10.1016/j.athoracsur.2006.10.082. [DOI] [PubMed] [Google Scholar]
- 6.Sundt TM, 3rd, Orszulak TA, Cook DJ, Schaff HV. Improving results of open arch replacement. Ann Thorac Surg. 2008;86:787–96. doi: 10.1016/j.athoracsur.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Iba Y, Minatoya K, Matsuda H, Sasaki H, Tanaka H, Kobayashi J, et al. Contemporary open aortic arch repair with selective cerebral perfusion in the era of endovascular aortic repair. J Thorac Cardiovasc Surg. 2013;145(Suppl 3):S72–7. doi: 10.1016/j.jtcvs.2012.11.047. [DOI] [PubMed] [Google Scholar]
- 8.Roques F, Nashef SA, Michel P, Gauducheau E, de Vincentiis C, Baudet E, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15:816–22. doi: 10.1016/s1010-7940(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 9.Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2) Eur J Cardiothorac Surg. 2012;42:S45–60. doi: 10.1093/ejcts/ezs533. [DOI] [PubMed] [Google Scholar]
- 10.Gottardi R, Funovics M, Eggers N, Hirner A, Dorfmeister M, Holfeld J, et al. Supra-aortic transposition for combined vascular and endovascular repair of aortic arch pathology. Ann Thorac Surg. 2008;86:1524–9. doi: 10.1016/j.athoracsur.2008.06.075. [DOI] [PubMed] [Google Scholar]
- 11.Krüger T, Weigang E, Hoffmann I, Blettner M, Aebert H GERAADA Investigators. Cerebral protection during surgery for acute aortic dissection type A: results of the German Registry for Acute Aortic Dissection Type A (GERAADA) Circulation. 2011;124:434–43. doi: 10.1161/CIRCULATIONAHA.110.009282. [DOI] [PubMed] [Google Scholar]
- 12.Dumfarth J, Michel M, Schmidli J, Sodeck G, Ehrlich M, Grimm M, et al. Mechanisms of failure and outcome of secondary surgical interventions after thoracic endovascular aortic repair (TEVAR) Ann Thorac Surg. 2011;91:1141–6. doi: 10.1016/j.athoracsur.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 13.Czerny M, Barchichat I, Meszaros K, Sodeck GH, Reineke D, Englberger L, et al. Long-term results after proximal thoracic aortic redo surgery. PLOS One. 2013;8:e57713. doi: 10.1371/journal.pone.0057713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krähenbühl E, Maksimovic S, Sodeck G, Reineke D, Schoenhoff F, Schmidli J, et al. What makes the difference between the natural course of a remaining type B dissection after type A repair and a primary type B aortic dissection? Eur J Cardiothorac Surg. 2012;41:e110–5. doi: 10.1093/ejcts/ezs121. [DOI] [PubMed] [Google Scholar]
- 15.Czerny M, Roedler S, Fakhimi S, Sodeck G, Funovics M, Dumfarth J, et al. Midterm results of thoracic endovascular aortic repair in patients with aneurysms involving the descending aorta originating from chronic type B dissections. Ann Thorac Surg. 2010;90:90–4. doi: 10.1016/j.athoracsur.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Rheaume P, Perini P, Daligault M, Maurel B, Sobocinski J, Azzaoui R, et al. Total endovascular repair of an aortic arch aneurysm using an externalized transseptal guidewire technique. Ann Thorac Surg. 2012;93:1710–3. doi: 10.1016/j.athoracsur.2011.10.030. [DOI] [PubMed] [Google Scholar]