Abstract
OBJECTIVES
Station 3A nodes have been commonly neglected in surgical practice. This retrospective study collected information on the incidence and risk factors of Station 3A node to ascertain the prognostic role of 3A nodal involvement.
METHODS
A total of 180 consecutive pN2 (stage IIIa) non-small-cell lung cancer (NSCLC) cases who underwent systemic lymphadenectomy and contained Station 3A nodes were enrolled. Survival rates were calculated according to the final pathology of Station 3A lymph node: Station 3A node (+) and Station 3A node (−). Statistical analysis was conducted using Kaplan–Meier and Cox regression models.
RESULTS
Station 3A nodal metastasis was validated in 32 cases, and the incidence of Station 3A node involvement was 17.8%. Station 3A nodes involvement was strongly associated with the metastatic status of Station 4R nodes and histological nature of pulmonary cancer. The overall 3-year survival was 53% and median survival time was 40.6 months. The 3-year survival difference was significant between Station 3A node (−) and Station 3A node (+) (63 vs 22%, χ2 = 16.426, P < 0.001). Moreover, the overall 3-year survival was closely related with the number of involved nodal zones (χ2 = 31.156, P < 0.001). Multivariate analysis showed two statistically significant risk factors for survival including metastasis of Station 3A node and the number of positive nodal zones (hazard ratios [HR]: 2.702; 95% confidence intervals [CI]: 1.008–7.242; P = 0.027; and HR: 7.404; 95% CI: 3.263–16.936, P < 0.001, respectively).
CONCLUSIONS
The involvement of Station 3A lymph nodes predicts poor prognosis of right-sided stage pIIIa-N2 NSCLC patients. Therefore, systemic lymphadenectomy for right-sided cancers should include Station 3A nodes when ascertaining a complete resection.
Keywords: Non-small-cell lung cancer, N2 stage, Lymphadenectomy, Prognosis, Lymph node metastases
INTRODUCTION
Although systemic lymphadenectomy has been well recognized as a vital part of complete resection for non-small-cell lung cancers (NSCLC), opinions concerning the essential range of nodal removal have not been unified. For right-sided lung cancers, general thought holds that the removal of nodal stations 2, 4, 7, 8 and 9 may be adequate [1]. However, the prognostic role of Station 3A nodes is largely neglected in routine clinical practice.
Node station 3A, also called the prevascular mediastinal lymph node, lies within the fatty tissue of the anterior mediastinum [2]. Specifically, the compartment containing 3A nodes is defined by the left innominate vein as the upper border, superior vena cava (SVC) as the back and the ascending aorta as the lateral border [3]. In general knowledge, Station 3A nodes may represent a side path of lymphatic spread because of SVC hindrance; therefore, cancerous involvement at this position may be especially uncommon. Accordingly, a clear definition of Station 3A nodes in the new TNM system [4] may reasonably raise additional questions such as the following: (i) What is the prognostic role of Station 3A nodes? (ii) Should these nodes be included in routine examinations before operations and dissection during operations? And (iii) What is their corresponding role in the complex web of mediastinal lymphatic drainage route?
We performed a retrospective study to collect information on the incidence and risk factors of Station 3A nodes to ascertain the prognostic role of 3A nodal involvement. Because Station 3A nodes represent a mediastinal nodal status, only NSCLC cases at stage IIIa were enrolled.
PATIENTS AND METHODS
Approval for the present study was obtained from the Ethics Committee of the Hospital.
Cases selection criteria
Strict criteria were applied to screen the surgical NSCLC database before performing data collection and statistical analysis. This screening was necessary considering that Station 3A nodal removal is not mandatory, even though aggressive lymph node dissection has been prevalent in our department in recent years. Since August 2008, a few treatment groups in our department have begun dissection of Station 3A node as routine and habitual practice. Therefore, in the present study, only cases from a few teams were considered to guarantee the ‘consecutive’ nature of the enrolled cases. Specifically, the selection criteria were as follows:
All data were retrieved from consecutive NSCLC cases when Station 3A node removal was routine at all stages (I-III) by each independent treatment group;
Only N2 (at stage IIIa) NSCLC cases were enrolled and therefore, were also consecutive;
All cases meet our prior administrative request of systemic lymph node dissection, which included removal of at least three mediastinal stations and more than six mediastinal lymph nodes, in addition to the resection of hilar nodes [5];
No prior history of induction chemo or radiotherapy;
Cases of exploratory thoracotomy, incomplete resection with cancerous retain or massive pleural dissemination were excluded from the present study.
This screening resulted in case enrolment from August 2008 to December 2012. A total of 180 consecutive stage N2-IIIa cases with confirmative information of Station 3A nodes were enrolled. All cases were re-staged according to the seventh edition of the TNM staging system for lung cancers [6], and the researchers considered the T and N definition shifts in the new staging system. The pathologist reconfirmed the histological diagnosis according to the 2004 World Health Organization (WHO) guidelines [7].
Preoperative evaluations and operations
All patients had undergone routine preoperative evaluations to exclude surgical contraindications. Remote metastasis was excluded by chest high-resolution computed tomography (HRCT) scan, upper abdominal ultrasonography (or CT), brain CT (or MRI) and whole-body bone scintigraphy. Although PET-CT, mediastinoscopy and E-BUS are not required as routine examinations, they have been adopted more frequently in recent years. Of note, radiologically bulky mediastinal lymphadenopathy, involvement of mediastinal lymph nodes >20 mm in short-axis diameter, is strictly contraindicated for surgical treatment in our department [8].
Major pulmonary resections, including pneumonectomy, (bi-)lobectomy, sleeve lobectomy and tracheal/bronchus resections, were performed with the aim to cure. Broncho-plastic procedures and sleeve lobectomies were adopted to spare functional pulmonary tissue when indicated. Further, bronchial stump underwent frozen section examinations in routine flavour to exclude possible cancerous retention.
Radiological reading of Station 3A nodes
For chest CT images, lymph nodes with a short-axis diameter >10 mm were considered abnormal. The radiological manifestations of Station 3A nodes of all enrolled cases were re-measured, and subgroups were assigned according to Station 3A node diameters by 5 mm intervals. The radiological and histological findings were compared.
Follow-up
All patients were followed up at predetermined schedules that included outpatient clinic visits every 3 months during the first 2 postoperative years, every 6 months for the following 2 years and once a year thereafter. To obtain complete follow-up information, trained staff conducted either telephone interviews or mailings. The end-point of follow-up was the date of death or 31 December 2012. Patients known to be alive at the last contact were censored. If the patient or family member could not be reached on the follow-up date, the date and the information were censored on the date of the last follow-up.
Statistical analysis
Two groups were assigned according to the final pathology of Station 3A lymph node: Station 3A node (+) and Station 3A node (−). Statistical analysis was performed using a χ2 test for categorical variables and unpaired t-test for continuous variables. The numerical data were expressed in terms of frequency, mean and standard deviation (SD). The survival rate was calculated using the Kaplan–Meier and the life-table methods, and statistical significance was evaluated using the log-rank test. Binary logistic regression (odds ratios [OR] ± 95% confidence intervals [CI]) was used to analyse risk factors for Station 3A node metastasis. Hazard ratios (HR) ± 95% CI for Cox's regression were used for the multivariate survival analysis. A P-value of <0.05 was defined as statistically significant. All statistical analyses were performed in SPSS Released 2009 by PASW Statistics for Windows, Version 18.0 (SPSS, Inc., Chicago, IL, USA).
RESULTS
General information and operations
Among the 180 enrolled consecutive pT1-3N2M0 NSCLC cases, there were 124 males and 56 females. The right upper lobe was involved in 96 cases (53.3%), right middle lobe in 21 (11.7%), right lower lobe in 54 (30%) and right hilum in 9 (5%) (see Table 1). The average age was 57.7 ± 9.7 years (range: 31–80 years); 153 (85%) patients were under 70 years of age. Coexisting diseases, which included hypertension, coronary heart disease, diabetes, bronchiectasis, emphysema and arthritis, were documented in 51 patients.
Table 1:
General information of enrolled cases
| All patients (n = 180) | #3A positive (n = 32) | #3A negative (n = 148) | |
|---|---|---|---|
| Mean age (range) | 57.7 ± 9.7 (31–80) | 58.1 ± 11.5 (31–77) | 57.6 ± 9.4 (33–80) |
| Smoking | 63 (35.0%) | 11 (34.4%) | 52 (35.1%) |
| Gender | |||
| Female | 56 (31.1%) | 12 (37.5%) | 44 (29.7%) |
| Male | 124 (68.9%) | 20 (62.5%) | 104 (70.3%) |
| Tumour location | |||
| RUL | 96 (53.3%) | 20 (62.5%) | 76 (51.4%) |
| RML | 21 (11.7%) | 4 (12.5%) | 17 (11.5%) |
| RLL | 54(30%) | 7 (21.9%) | 47 (31.8%) |
| RH | 9(5%) | 1 (3.1%) | 8 (5.4%) |
| Histology | |||
| Sq | 81 (45%) | 5 (15.6%) | 76 (51.4%) |
| Ad | 82 (45.6%) | 18 (56.2%) | 64 (43.2%) |
| Others | 17 (9.4%) | 9 (28.1%) | 8 (5.4%) |
| pT stage | |||
| T1 | 14 (7.8%) | 1 (3.1%) | 13 (8.8%) |
| T2 | 146 (81.1%) | 31 (96.9%) | 115 (77.8%) |
| T3 | 20 (11.1%) | 0 | 20 (13.5%) |
| Involvement of N2 nodal station | |||
| Single station | 87 (48.3%) | 4 (12.5%) | 83 (56.1%) |
| Multistation | 93 (51.7%) | 28 (87.5%) | 65 (43.9%) |
| Involvement of N2 nodal zone | |||
| Single zone | 112 (62.2%) | 7 (21.9%) | 105 (70.9%) |
| Multizone | 68 (37.8%) | 25 (78.1%) | 43 (29.1%) |
RUL: right upper lobe; RML: right middle lobe; RLL: right lower lobe; Sq: squamous cell carcinoma; Ad: adenocarcinoma; #: the station number of mediastinal lymph nodes.
There were 15 pneumonectomies, 129 lobectomies and 27 bilobectomies; extended resections were performed in 9 cases (4 with angioplasty, and 5 cases with bronchi-tracheoplasty, of which 3 cases underwent extended sleeve lobectomy with additional wedge resections). No 30-day postoperative deaths occurred. No serious technical morbidity was recorded in this cohort. Additionally, no major operative bleeding occurred, and the mean duration of postoperative chest tube drainage was 2 ± 1.5 days. Postoperative drainage was 350 ± 120 (range: 200–550) ml. Major postoperative complications occurred in 5 cases: 4 cases of bronchopleural fistula with prolonged chest tube drainage under negative pressure (>2 weeks) and 1 case of postoperative infection, which was cured after an additional 2 weeks of antibiotic therapy.
Additionally, 173 patients received four-cycle adjuvant platinum-based chemotherapies. Seven patients failed to accomplish postoperative chemotherapies mostly due to poor performance status (PS) score (6 patients) and one patient died of cardiovascular accident before the final cycle of chemotherapy. By the time of final follow-up, 24 patients (13.3%) had died. Of these, remote metastasis (brain or bone) and cancer relapse was confirmed as the main cause of death in 22 cases.
Pathological information
As defined by the case selection criteria, all patients were staged pIIIa and consecutive in nature. In the recruited cases, 81 (45.0%) were squamous-cell carcinoma, 82 (45.6%) were adenocarcinoma and 17 (9.4%) were large-cell carcinoma and adenosquamous carcinoma. The tumour size was in the range 1.0–14.0 cm (average 3.4 ± 1.8 cm). In the current cohort, there were 14 cases of T1, 146 of T2 and 20 of T3. A total of 3131 lymph nodes (2453 mediastinal and 678 regional LNs) had been removed, at an average of 17.4 ± 5.9 nodes per patient. Among 596 positive mediastinal lymph nodes, 53 Station 3A nodes from 32 cases were cancerous at an incidence of 8.89%. Interestingly, non-squamous lung cancers yielded a higher incidence of Station 3A metastasis: the incidence were 84.4% (27 of 32 cases) in Station 3A node (+) and 48.6% (72 of 148 cases) in Station 3A node (−).
Predictive value of radiological measurement towards Station 3A involvement
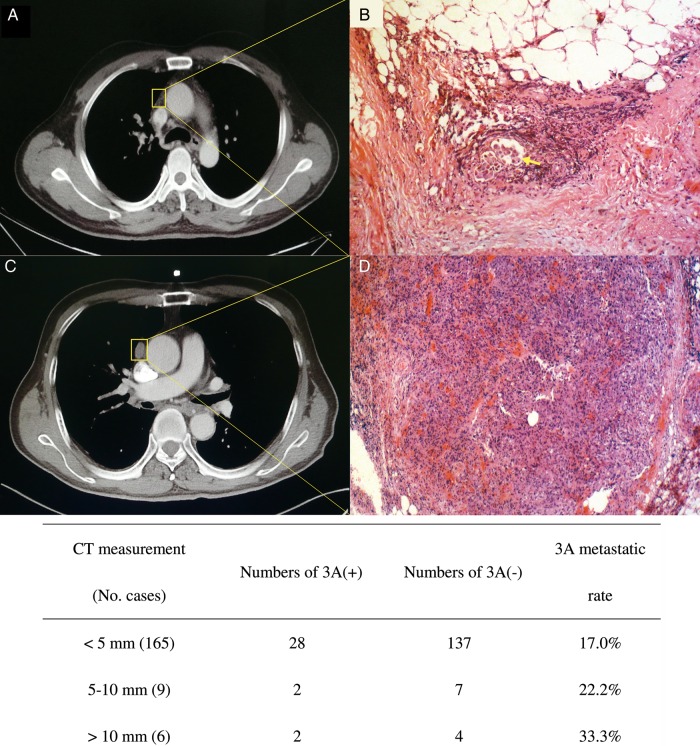
In the present study, 68 cases were suspected of cN2 before surgery (the short-axis diameter of mediastinal lymph node was between 5 and 10 mm); 48 cases were staged as cN2 (in 43 cases, the lymph node diameter was from 10 to 20 mm; and the mediastinal nodes were positive under FDG imaging in the remaining 5 cases). In this study, there were 64 (35.6%) undetected N2 cases. Our result shows that histologically 3A node involvement could be poorly predicted from radiological measurement results despite that 1 cm has been delineated as the common criterion of nodal metastasis. In this study, 3A nodes were visible on CT scans in a total of 29 cases, at 2–17 mm (6.7 ± 3.7 mm) of the short axis. However, of these 29 cases, pathologically positive 3A nodes were confirmed only in 7 patients with a 75.9% (22 of 29) false-positive rate. Although no suspected 3A nodes were found in the remaining 151 cases on preoperative CT, 25 cases yielded positive 3A nodes. The false-negative of HRCT was 16.6%. Moreover, the metastatic rate of 3A was 17.0%, even in cases with a nodal size of <5 mm, which is comparable with 33.3% in cases with nodes >10 mm (see Fig. 1).
Figure 1:
(A and B) A false-negative case on CT scan. A tiny Station 3A node (A) turned out to be tumour metastasis in the fatty tissue (B). These tumour cells formed loci within a lymph vessel (haematoxylin–eosin, original magnification, ×200, arrow). (C and D) shows a false positive case of CT scan. A distinctively enlarged Station 3A node (C 13 mm at short-axis diameter) was actually lymphoid hyperplasia in histopathological section (D haematoxylin–eosin, original magnification, ×100). Early signs of tumour cell implant into lymph nodes were noticed in 4 cases, manifesting in histology as tumour cells located only within the cortex of lymph node or lymph vessels.
Incidence of Station 3A node involvement in the mediastinal lymph drainage network
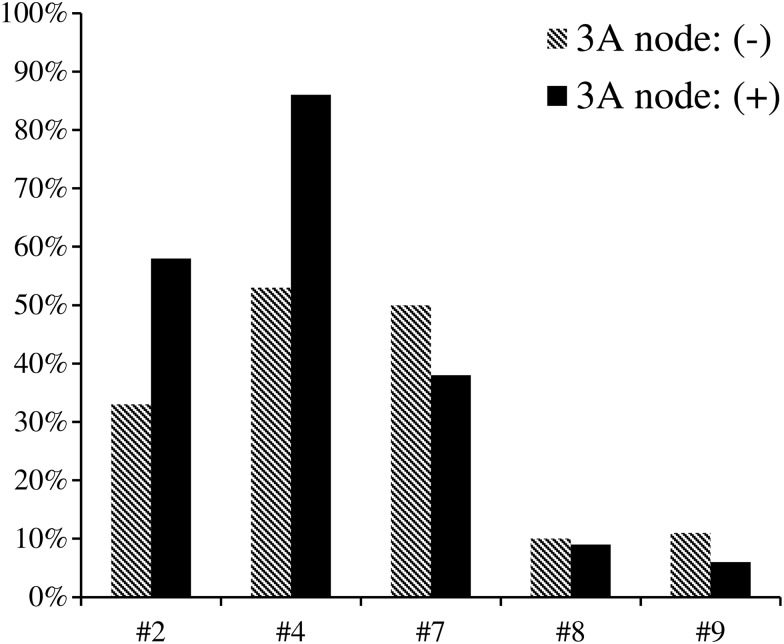
Station 3A metastasis seemed to occur more frequently on occasions of multiple station involvement. In the 99 cases with multiple-station involvement, positive 3A was confirmed in 28 (28.3%). In two-station and three- or more-station metastasis, the incidences were 25% (7 of 28) and 75% (21 of 28), respectively. A χ2 test was used to compare the correlation of lymph node metastasis between each mediastinal lymph node and 3A node. The findings of this test suggests that stations 2R and 4R might be highly associated with 3A nodal metastasis (χ2 = 6.303, P = 0.001, and χ2 = 10.442, P < 0.001, respectively, see Fig. 2). It is surprising that single 3A node involvement occurred in 4 cases, which indicates that lymphatic drainage to 3A nodes might follow a ‘skip’ route in some cases.
Figure 2:
Relationship between 3A node involvement and status of other mediastinal stations. The upper nodal zone (station #2 LN and #4 LN) was highly related to 3A metastasis (χ2 = 6.303, P = 0.001, and χ2 = 10.442, P < 0.001, respectively).
Clinical indicators of 3A nodal metastasis
Univariate analysis revealed that histopathology and metastasis of station 2 LNs and station 4 LNs were significant risk factors for Station 3A node involvement (see Table 2). Multivariate analysis demonstrated that tumour histopathology (non-squamous NSCLC) and metastasis of Station 4 nodes were both strongly associated with 3A node involvement (OR = 0.193, P = 0.002 and OR = 4.063, P = 0.009, respectively). While the metastasis of Station 2 LNs were not statistically of significant relevance (Table 3).
Table 2:
Univariate analysis for factors associated with Station 3A lymph node metastasis
| Variables | OR | 95% CI | P-value |
|---|---|---|---|
| Gender | |||
| Male | 0.705 | 0.318, 1.566 | 0.391 |
| Female | |||
| Age (years) | |||
| <50 | 1.927 | 0.589, 6.289 | 0.278 |
| 50–70 | 2.745 | 0.976, 7.720 | 0.156 |
| >70* | |||
| Tumour location | |||
| RUL | 0.475 | 0.056, 4.023 | 0.495 |
| RML | 0.531 | 0.051, 5.553 | 0.579 |
| RLL | 0.839 | 0.091, 7.769 | 0.877 |
| RH* | |||
| Histopathology | |||
| Sq | 0.175 | 0.064, 0.480 | 0.001 |
| Non-Sq | |||
| Tumour size (cm) | |||
| <2 | 1.829 | 0.293, 11.427 | 0.519 |
| 2–3 | 1.782 | 0.305, 10.413 | 0.521 |
| 3–5 | 2.089 | 0.349, 12.489 | 0.419 |
| >5* | |||
| Skipping metastasis | |||
| Skipping | 0.585 | 0.271, 1.261 | 0.171 |
| Non-skipping | |||
| Metastasis of #2 LNs | |||
| Positive | 2.598 | 1.193, 5.654 | 0.016 |
| Negative | |||
| Metastasis of #4 LNs | |||
| Positive | 4.716 | 1.722, 12.916 | 0.003 |
| Negative | |||
| Metastasis of #7 LNs | |||
| Positive | 0.660 | 0.274, 1.315 | 0.202 |
| Negative | |||
| Metastasis of #8 LNs | |||
| Positive | 0.917 | 0.249, 3.376 | 0.897 |
| Negative | |||
| Metastasis of #9 LNs | |||
| Positive | 0.481 | 0.106, 2.188 | 0.344 |
| Negative | |||
RUL: right upper lobe; RML: right middle lobe; RLL: right lower lobe; RH: right hilum; Sq: squamous cell carcinoma; non-Sq: non-squamous cell carcinoma; OR: odds ratios; CI: confidence intervals; LN: lymph node; #: the station number of mediastinal lymph nodes;
*Reference category.
Table 3:
Multivariate analysis of risk factors of #3A lymph node involvement
| Variables | OR | 95% CI |
P-value | |
|---|---|---|---|---|
| Lower CI | Upper CI | |||
| Histopathology | 0.193 | 0.068 | 0.541 | 0.002 |
| Metastasis of #4 LNs | 4.063 | 1.420 | 11.624 | 0.009 |
| Metastasis of #2 LNs | 1.647 | 0.708 | 3.833 | 0.246 |
OR: odds ratios; CI: confidence intervals; LN: lymph node; #: the station number of mediastinal lymph nodes.
Survival analysis
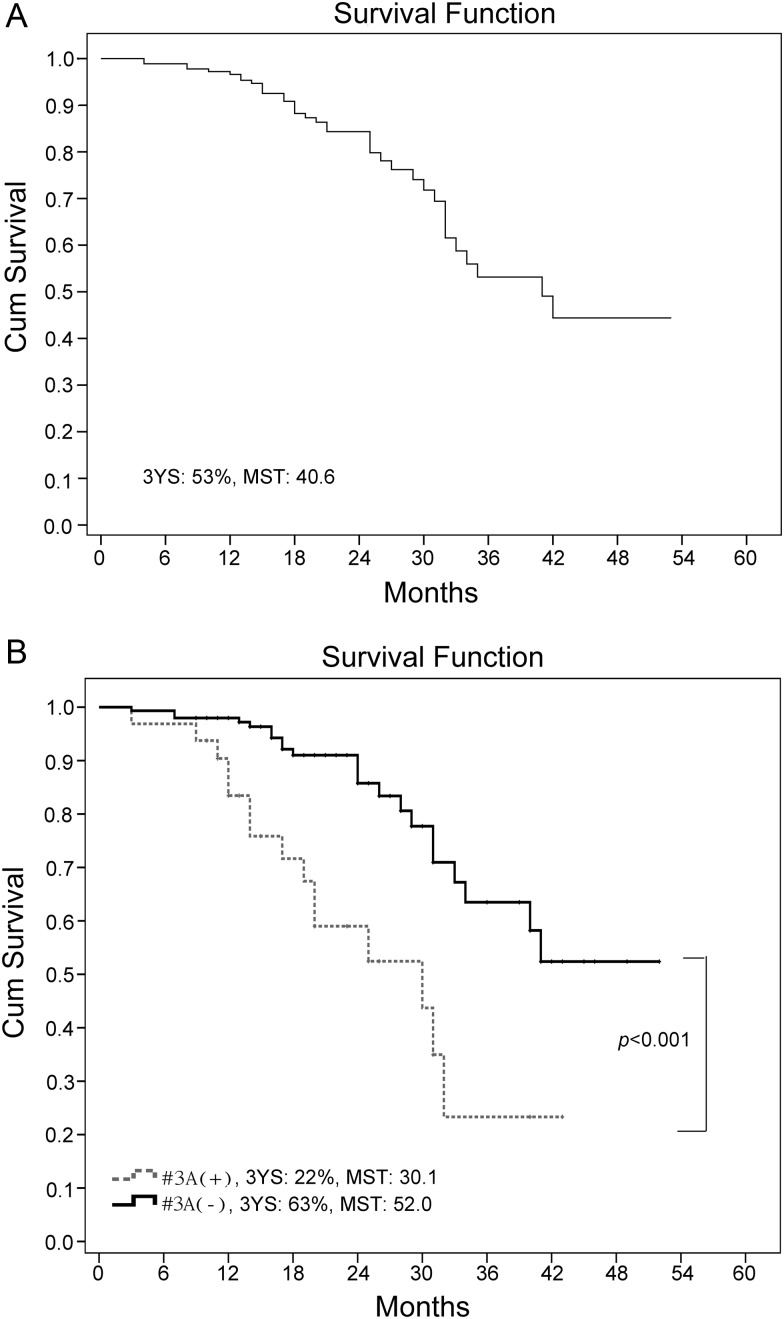
The median follow-up period was 20.5 months (range: 3–52 months). The overall 3-year survival was 53.0% with a median survival time (MST) of 40.6 months for the entire patient cohort. In Station 3A node (+), 15 (46.9%) patients died; while in Station 3A node (−), 22 (14.9%) patients died. Moreover, the MST and 3-year survival were 30.1 months and 22% for the 32 cases of Station 3A node (+) and 52.0 months and 63% for the 148 patients in Station 3A node (−) (see Fig. 3). The log-rank test confirmed a prominent survival difference between the two study groups (χ2 = 16.426, P < 0.001).
Figure 3:
Overall survival of our patient cohort by Kaplan–Meier (A); survival difference was significant between Station 3A node (+) and Station 3A node (−) (B). 3-YS: 3-year survival, MST: median survival time.
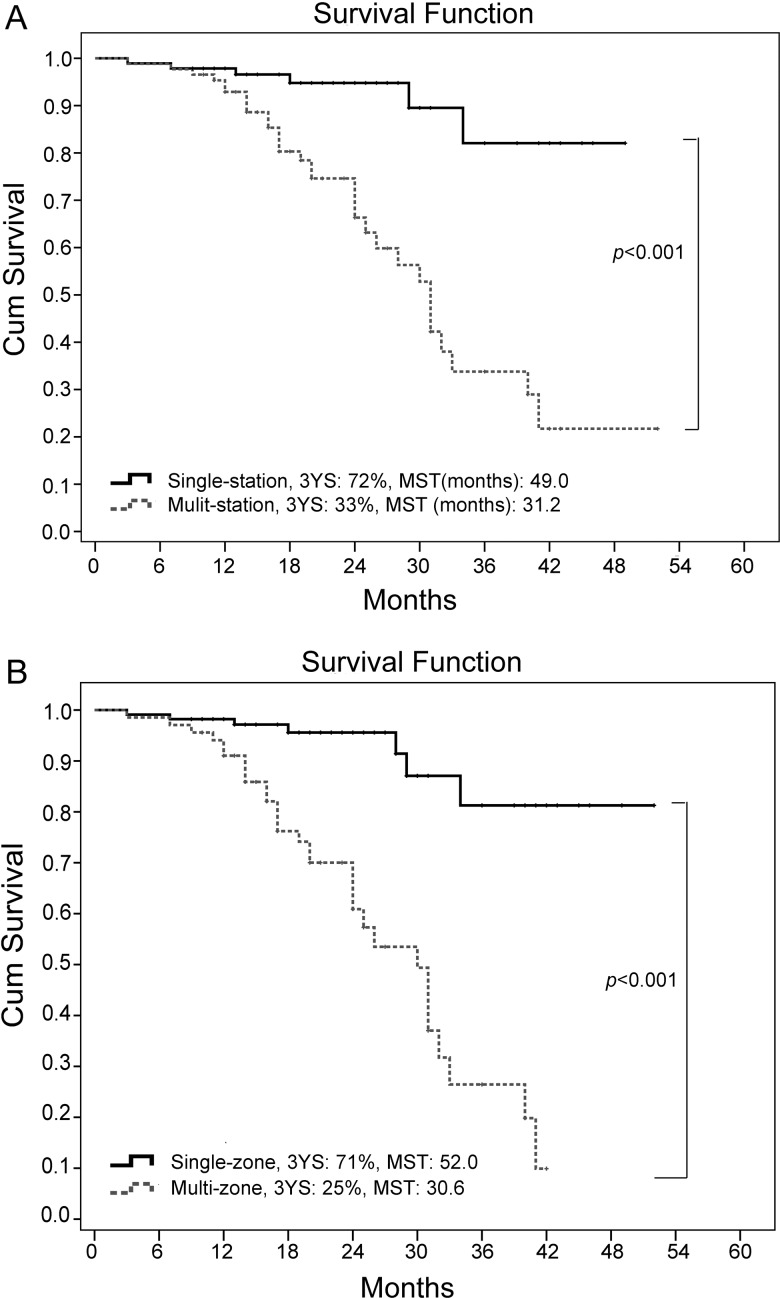
Additional analysis revealed that a prominent difference of 3-year survival rates between single and multistation nodal involvement (72 vs 33%, χ2 = 20.186, P < 0.001). The result also revealed a significant difference between cases with single-zone and multizone involvement (71 vs 25%, χ2 = 31.156, P < 0.001); the median survival times were 52 and 30.6 months, respectively (see Fig. 4). These results demonstrate that patients with single-zone nodal involvement even if multistation involvement is included have survival outcomes similar to those of patients with single-station nodal involvement only. These findings are highly consistent with prior studies of risk factors of N2 disease [4].
Figure 4:
Survival differences were significant for single and multiple nodal station involvement (A) between one and multizone involvement (B) 3-YS: 3-year survival, MST: median survival time.
Risk factors of survival
A univariate analysis of prognostic factors of survival was performed first by a log-rank test. In addition to involvement of nodal station/nodal zone (P < 0.001) and metastasis of Station 3A (P < 0.001), Station 4 (χ2 = 9.335, P = 0.002), Station 7 lymph node (χ2 = 4.401, P = 0.046) and histopathology (χ2 = 0.149, P = 0.026) were statistically significant risk factors for mid-term survival. The multivariate analysis showed that the independent risk factors for mid-term survival were as follows: 3A node involvement and multizone nodal involvement (P = 0.027 and P < 0.001, respectively). The remaining variables, including involvement of nodal station, histopathology and metastasis of Station 4 and Station 7 lymph node, were of no statistical significance (see Table 4).
Table 4:
Multivariate analysis of risk factors for survival
| Variables | HR | 95% CI |
P-value | |
|---|---|---|---|---|
| Lower CI | Upper CI | |||
| Involvement of nodal zone | 7.404 | 3.263 | 16.936 | <0.001 |
| Metastasis of #3A LNs | 2.702 | 1.008 | 7.242 | 0.027 |
| Involvement of nodal station | 0.908 | 0.091 | 9.014 | 0.934 |
| Metastasis of #4 LNs | 0.935 | 0.308 | 2.838 | 0.626 |
| Metastasis of #7 LNs | 0.811 | 0.350 | 1.883 | 0.905 |
| Histopathology | 0.704 | 0.339 | 1.460 | 0.346 |
HR: hazard ratios; CI: confidence intervals; #: the station number of mediastinal lymph nodes; LNs: lymph nodes.
DISCUSSION
For NSCLC, N2 status is generally acknowledged as a heterogeneous disease category due to its widely dispersed 5-year survival at 9–42% after surgical intervention [9, 10]. Ascertaining corresponding risk factors of survival has been especially important for prognosis and decision-making and planning of multidisciplinary treatment. Fukui et al. proposed that both the number and comparative ratio of metastatic lymph nodes are prognostic factors. The 5-year survival rate was 58% for patients with one to three positive nodes, 42% for those with four to six and 6% for those with more than seven. Additionally, multiple stations metastasis was associated with worse outcomes than was single station (5 : 14.7 vs 23.8%) [11, 12]. Considering the number of metastatic lymph nodes, Lee et al. reported that the number of N category was a significant prognostic indicator similar to the pathological N category (κ = 0.723; P < 0.001) [13]. The concept of lymph node zones was proposed by the IASLC staging project in 2009. Of note, the 5-year survival was also significantly better for single nodal-zone involvement compared with multizones [4]. The difference between ‘zone’ and ‘station’ is that single zone may include multistations. Research has demonstrated that patients with single-zone nodal involvement even if multistation involvement is included have a survival outcome similar to those of patients with single-station nodal involvement only in our prior study [14]. Nwogu et al. found that patients with low ratios of node involvement (nN) yielded significantly better prognoses than did those with high ratios. Additionally, more resected lymph nodes and lower ratio of positive nodes were prominently associated with better survival [15]. This finding suggests that the absolute number and ratio of mediastinal nodal involvement and locations of positive lymph nodes are closely associated with worse prognosis.
Moreover, the locations of the lymph nodes involved may have prognostic significance. Sakao et al. proposed that the highest mediastinal lymph node had prominent prognostic significance. In Sakao et al.'s [16] 53 N2-IIIa NSCLC case series, the 3-year survival rates were 52% for patients with negative highest nodes and 21% for those with positive highest nodes (P < 0.001). Tumours on the left upper lobe with positive Stations 5–6 had survival outcomes similar to those of N1 [17]. Okada et al. [18] suggested that the subcarinal lymph node (Station 7) predicted worse prognosis: Specifically, the 5-year survival was 9.0% for patients with positive subcarinal nodes, which was significantly lower than those with only superior mediastinal metastases (32.0%) [19]. However, the prognostic role of Station 3A nodes has been largely neglected.
The results of the present study provide unexpected but interesting evidence that 3A nodal metastasis is associated with worse mid-term survival. This finding was validated by survival data (see Fig. 3) and subsequent univariate and multivariate analyses. The underlying reason for these results may be that 3A nodal involvement indicates a wider range of mediastinal metastasis and widespread micro-metastasis via the lymphatic network. Therefore, future subclassification for N2-IIIa cases could better integrate such information and include multistation/multizone nodal involvement, ratio of involved nodes and metastatic status of 3A nodes.
3A nodes have been neglected in prior studies for several reasons. First, the hindrance of the SVC and phrenic nerve make this area an ‘uncommon’ place for lymphatic spread. Additionally, abundant communications between Stations 4R, 2R and the superior 10R make the 3A node a rare participant in the mediastinal lymphatic drainage system [20]. Second, the position of 3A nodes in the mediastinal lymph drainage network is unclear. Riquet et al. [21] proposed that the frequency of 3A/3P nodal involvement is low (1.7% in their early autopsy study); therefore, rarely participates in the lymphatic drainage of the lung. Third, 3A nodal removal has not been requested under the current guidelines of systematic lymphadenectomy. Despite this information, 3A nodes lie within a shallow and planar area and can be removed easily and safely.
It was also surprising to find that 3A lymph nodes were involved at a high incidence (17.8%, 32 of 180) in stage N2-IIIa NSCLCs. In contrast to the phenomenon that 3A nodal involvement is experientially uncommon, such a high incidence reflects the virtual situation that research has commonly neglected. To guarantee that this is the factorial data concerning surgical N2 candidates, the authors double checked all case charts, radiological images and the data bank. All the enrolled cases strictly met surgical indications as previously mentioned and all the cases were consecutive. Therefore, we believe that our results are an accurate calculation of 3A metastasis incidence among N2-IIIa cases. A side proof is that involvement of other stations also reached a similar high incidence rate, e.g. 26% for Station 4R and 37% for Station 7. Considering these high metastasis rates, 3A nodes should be removed routinely during lung cancer operations, especially for those with the risk factors listed in Table 3.
Preoperative radiological examinations offer only limited accuracy for picking out metastatic 3A nodes. The sensitivities and specificities of CT examinations for metastatic lymph nodes range from 52 to 66% and 69 to 93%, respectively [22]. Approximately 21% of metastases occurred in normal-sized lymph nodes, whereas no malignancy was detected in 40% of enlarged nodes [23]. The situation was similar with our data as the true positive rate was only 26.7% (4 of 19). Moreover, single 3A nodal involvement was interestingly confirmed in 4 patients. Before an accurate preoperative evaluation method can be undertaken, an aggressive surgical dissection on the 3A field is seemingly necessary.
The researchers also examined clinical indicators of Station 3A nodal involvement. Results revealed that Station 4 nodal involvement, histopathology (non-squamous cancers), and multizone mediastinal lymph node involvement were the most important. Although Stations 2R and 4R were also associated with 3A metastasis, only the status of Station 4 nodes was identified as an independent risk factor (P = 0.009). These findings suggest wide links between 3A and other superior mediastinal lymph nodes, rather than being an isolated condition. Moreover, the communication between Station 4 and 3A nodes was common.
To our knowledge, the present study is the first to document imaging, pathology and prognostic roles of 3A nodes in patients with stage IIIA-N2 NSCLC. Despite the lack of this type of study, only 4-year follow-up information was obtained in the present study, instead of 5-year information. However, at the 4-year follow-up, it was clear that 3A nodes provided a prominent prognostic significance.
Several limitations of our study must be noted. First, this was a retrospective single-institution study. A retrospective analysis is susceptible to various sources of bias, which may not have been identified and controlled. Second, preoperative PET scanning, mediastinoscopy and E-BUS are not routinely performed as pathological staging of suspicious nodes before resection, which is a source of potential weakness because some patients with a poor prognosis may be enrolled in this study. Third, the follow-up period for this study was relatively short and had a mean follow-up of approximately a year and a half, which suggests a possible influence on the overall survival rate. In any case, because our study used a small series, additional prospective randomized controlled trials with the same background need to be conducted in larger series.
In conclusion, metastatic status of Station 3A nodes is an independent prognostic factor. These nodes occur at a high incidence in right-side stage pIIIa-N2 NSCLC patients. Further, 3A nodal involvement is closely associated with Station 4 nodal metastasis and non-squamous nature of the cancer. Dissection of 3A lymph nodes is highly recommended, especially for high-risk patients.
FUNDING
This work was supported by Shanghai Municipal Committee of science and technology research projects (11411951300 and 11DZ1973202).
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
The authors thank Li-ling Zou (Department of Statistics, Tongji University School of Medicine) for her assistance with the statistical analysis.
REFERENCES
- 1.Veronesi G, Maisonneuve P, Pelosi G, Casiraghi M, Agoglia BG, Borri A, et al. Screening-detected lung cancers: is systematic nodal dissection always essential? J Thorac Oncol. 2011;6:525–30. doi: 10.1097/JTO.0b013e318206dbcc. [DOI] [PubMed] [Google Scholar]
- 2.Zieliński M, Rami-Porta R. Proposals for changes in the Mountain and Dresler mediastinal and pulmonary lymph node map. J Thorac Oncol. 2007;2:3–6. doi: 10.1097/JTO.0b013e31802bff98. [DOI] [PubMed] [Google Scholar]
- 3.Zieliński M. Technical pitfalls of transcervical extended mediastinal lymphadenectomy—how to avoid them and to manage intraoperative complications. Semin Thorac Cardiovasc Surg. 2010;22:236–43. doi: 10.1053/j.semtcvs.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–77. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- 5.Whitson BA, Groth SS, Maddaus MA. Surgical assessment and intraoperative management of mediastinal lymph nodes in non-small cell lung cancer. Ann Thorac Surg. 2007;84:1059–65. doi: 10.1016/j.athoracsur.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 6.Tanoue LT, Detterbeck FC. New TNM classification for non-small-cell lung cancer. Expert Rev Anticancer Ther. 2009;9:413–23. doi: 10.1586/era.09.11. [DOI] [PubMed] [Google Scholar]
- 7.Travis WD, Brambilla E, Muller-Hermlink H, Harris C. World Health Organization. Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Phymus and Heart. Lyon: IARC Press; 2004. [Google Scholar]
- 8.Robinson LA, Ruckdeschel JC, Wagner HW, Stevens CW. Treatment of non-small cell lung cancer stage IIIA. ACCP evidence-based clinical practice guidelines (2nd editon) Chest. 2007;132:243S–65S. doi: 10.1378/chest.07-1379. [DOI] [PubMed] [Google Scholar]
- 9.Kim KJ, Ahn YC, Lim do H, Han J, Park K, Park JO, et al. Analyses on prognostic factors following tri-modality therapy for stage IIIa non-small cell lung cancer. Lung Cancer. 2007;55:329–36. doi: 10.1016/j.lungcan.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Kang CH, Ra YJ, Kim YT, Jheon SH, Sung SW, Kim JH. The impact of multiple metastatic nodal stations on survival in patients with resectable N1 and N2 nonsmall-cell lung cancer. Ann Thorac Surg. 2008;86:1092–7. doi: 10.1016/j.athoracsur.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 11.Fukui T, Mori S, Yokoi K, Mitsudomi T. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol. 2006;1:120–5. [PubMed] [Google Scholar]
- 12.Casali C, Stefani A, Natali P, Rossi G, Morandi U. Prognostic factors in surgically resected N2 non-small cell lung cancer: the importance of patterns of mediastinal lymph nodes metastases. Eur J Cardiothorac Surg. 2005;28:33–8. doi: 10.1016/j.ejcts.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Lee JG, Lee CY, Park IK, Kim DJ, Park SY, Kim KD, et al. Number of metastatic lymph nodes in resected non-small cell lung cancer predicts patient survival. Ann Thorac Surg. 2008;85:211–5. doi: 10.1016/j.athoracsur.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Zheng H, Wang LM, Bao F, Jiang GN, Xie HK, Ding JA, et al. Re-appraisal of N2 disease by lymphatic drainage pattern for non-small-cell lung cancers: by terms of nodal stations, zones, chains, and a composite. Lung Cancer. 2011;74:497–503. doi: 10.1016/j.lungcan.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Nwogu CE, Groman A, Fahey D, Yendamuri S, Dexter E, Demmy TL, et al. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann Thorac Surg. 2012;93:1614–9. doi: 10.1016/j.athoracsur.2012.01.065. discussion 1619–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakao Y, Miyamoto H, Yamazaki A, Oh T, Fukai R, Shiomi K, et al. Prognostic significance of metastasis to the highest mediastinal lymph node in nonsmall cell lung cancer. Ann Thorac Surg. 2006;81:292–7. doi: 10.1016/j.athoracsur.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 17.Sakao Y, Miyamoto H, Yamazaki A, Ou S, Shiomi K, Sonobe S, et al. The spread of metastatic lymph nodes to the mediastinum from left upper lobe cancer: results of superior mediastinal nodal dissection through a median sternotomy. Eur J Cardiothorac Surg. 2006;30:543–7. doi: 10.1016/j.ejcts.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Okada M, Tsubota N, Yoshimura M, Miyamoto Y, Matsuoka H. Prognosis of completely resected pN2 non-small cell lung carcinomas: What is the significant node that affects survival? J Thorac Cardiovasc Surg. 1999;118:270–5. doi: 10.1016/S0022-5223(99)70217-5. [DOI] [PubMed] [Google Scholar]
- 19.Aokage K, Yoshida J, Ishii G, Hishida T, Nishimura M, Nagai K. Subcarinal lymph node in upper lobe non-small cell lung cancer patients: is selective lymph node dissection valid? Lung Cancer. 2010;70:163–7. doi: 10.1016/j.lungcan.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Riquet M, Arame A, Foucault C, Le Pimpec Barthes F. Prognostic classifications of lymph node involvement in lung cancer and current International Association for the Study of Lung Cancer descriptive classification in zones. Interact CardioVasc Thorac Surg. 2010;11:260–4. doi: 10.1510/icvts.2010.236349. [DOI] [PubMed] [Google Scholar]
- 21.Riquet M, Manac'h D, Dupont P, Dujon A, Hidden G, Debesse B. Anatomic basis of lymphatic spread of lung carcinoma to the mediastinum: anatomo-clinical correlations. Surg Radiol Anat. 1994;16:229–38. doi: 10.1007/BF01627676. [DOI] [PubMed] [Google Scholar]
- 22.Shim SS, Lee KS, Kim BT, Chung MJ, Lee EJ, Han J, et al. Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology. 2005;236:1011–9. doi: 10.1148/radiol.2363041310. [DOI] [PubMed] [Google Scholar]
- 23.Perigaud C, Bridji B, Roussel JC, Sagan C, Mugniot A, Duveau D, et al. Prospective preoperative mediastinal lymph node staging by integrated positron emission tomography-computerised tomography in patients with non-small-cell lung cancer. Eur J Cardiothorac Surg. 2009;36:731–6. doi: 10.1016/j.ejcts.2009.05.044. [DOI] [PubMed] [Google Scholar]