Abstract
Albendazole (ABZ), a broad-spectrum anthelmintic agent, is poorly absorbed after oral administration due to its low aqueous solubility. The aim of this study was to improve albendazole dissolution rate by formulating avicel pellets loaded with 10% w/w drug using extrusion/spheronization technique. In addition the wet masses were characterized by mix torque rheometry (MTR) prior to pelletization process. Different additives (i.e., lactose, Tween 80 and low molecular weight chitosan) were formulated with avicel to enhance the dissolution rate of ABZ from the produced pellets. Moreover, mix torque rheometer was used to quantitatively determine the suitable moisture content in the pastes before the extrusion process. The produced pellets were characterized for their ABZ content, particle size, particle shape, dissolution profile and thermal behaviors. The maximum consistencies (the peak torques) of the wet granules were obtained using 0.667–1.333 ml/g of water or water containing surfactant. Also, the produced pellets have size range from 1036 to 1246 μm. The calculated drug RDR30 for 10%, 30% and 50% lactose concentrations were 1.08, 1.08 and 2.03, respectively, while that calculated for 10%, 30% and 50% w/w chitosan concentrations were 1.71, 3.62 and 3.62, respectively. The results revealed also that increasing the weight ratio of lactose and chitosan was accompanied by a significant reduction of the peak torque magnitude and this was accompanied by an enhanced ABZ dissolution rate.
Keywords: Albendazole, Mix torque rheometry, Extrusion/spheronization, Lactose, Tween 80 and low molecular weight chitosan
1. Introduction
Albendazole (ABZ), methyl [5-(propylthio)-l-H-benzimidazol-2yl] carbamate, is undoubtedly the most effective of the broad-spectrum anthelmintic agents (Cook, 1990). The albendazole therapy is very important in systemic cestode infections specially in inoperable or disseminated cases of hydatidosis (Wen et al., 1993) and neurocysticercosis (Del-Brutto et al., 1993). The biggest problem of using ABZ, as a member of benzimidazoles, is its low availability and irritating effect as a result of its low aqueous solubility (Torrado et al., 1996).
This property is a major disadvantage for the use of ABZ in the treatment of systemic helminthiasis (Yasawy et al., 1993). Furthermore, the lack of water solubility reduces flexibility for ABZ formulation and administration. Therefore, the overcome of poor aqueous solubility of ABZ is important and needed.
ABZ solubility has been enhanced using different techniques. For example, solid dispersion systems of ABZ were mixed with varying concentrations of polyvinylpyrrolidone (PVP K 12) in an attempt to improve the solubility and dissolution rate of the drug (Torrado et al., 1996). The ABZ dissolution rate, expressed as the dissolution efficiency, and also the solubility coefficient were increased when the drug was mixed with PVP. Also, in our previous report showed (Alanazi et al., 2007) improving the dissolution rate of ABZ by producing microparticles with certain hydrophilic polymers such as hydroxypropyl methylcellulose (HPMC), polyvinyl alcohol (PVA), and polyvinyl pyrrolidone (PVP) using spray drying technique. Recently, formulation of drug in multiparticulate dosage forms (pellets) is considered as one of the solubility improving mechanisms due to the great surface area of pellets which enhanced drug solubility. Jachowicz et al. (2000) improved the dissolution rate of ketoprofen by incorporating the solid dispersion of the drug with macrogol and kollagen in the form of pellets.
The interest in pellets as dosage forms has been increasing continuously. Pellets as a drug delivery system offer therapeutic advantages such as less irritation of the gastro-intestinal tract and a lowered risk of side effects due to dose dumping (Bechgaard and Nielsen, 1978). In addition, technological advantages are present, for example, better flow properties less friable dosage form, narrow particle size distribution, ease of coating and uniform packing. The reproducibility of the drug blood levels (Eskilson, 1985) is an additional advantage to the use of a pellet formulation. The in vitro release rate of hydrochlorothiazide from Avicel PH101 pellets was enhanced by the incorporation of polyethylene glycol 400 and PEG-40 hydrogenated castor oil (Vervaet et al., 1994).
Extrusion and spheronization are currently one of the techniques used to produce pharmaceutical pellets. The preparation of spherical granules or pellets by extrusion and spheronization is now a more established method because of its advantages over the other methods (Chambliss, 1989; Robinso and Hollenbeck, 1991). With each production technique, pellets with specific characteristics are obtained. One of the important steps in preparing pellets with this method is optimal wet mass production. The amount of water added at the maximum torque should be comparable with that found for the optimum production of pellets during spheronization (Paul et al., 2009). Also, moisture content of the wet mass is the most critical variable for pellet growth in the extrusion spheronization process because it provides the required plasticity and cohesiveness to the powder to extrude the wet mass and spheronize it to give a spherical shape. An optimum quantity of moisture content should be there to obtain pellet of acceptable quality (Kleinebudde, 1993). It has been shown that the rheological properties of wet masses can be successfully monitored by a mixer torque rheometer (Parker et al., 1990a,b; Rowe and Parker, 1994; Chatlapalli and Rohera, 1998). The use of the mixer torque rheometer (MTR) as an upfront analytical tool can greatly reduce the number of development batches. This equipment has been shown to be an excellent tool for the evaluation of wet granulated systems and as a scale-up tool for high shear granulations (Hancock et al., 1991). Several authors have compared the rheological properties of different MCC grades (Rowe and Sadeghnejad, 1987; Parker and Rowe, 1991; Hancock et al., 1992), but the rheological properties of some additives on microcrystalline cellulose have not been studied previously.
According to Parker et al. (1990a,b), the degree of liquid spreading and wetting as well as the substrate binder interaction will determine the relative positions of the peak values of mean line torque. Any change in the mean torque with the increase in the binder level at different concentrations either a sharp or an extended peak might influence the attributes of the produced pellets.
The objective of this study was to formulate ABZ-loaded pellets using extrusion/spheronization technique in order to improve drug dissolution rate. The prepared pellets were characterized for their particle sizes and morphology, drug content and the in vitro drug release as well. In addition, the effect of additives on the pellets properties was studied. The binder ratio required for the preparation of wet mass prior to the pelletization process was measured by the mix torque rheometer. Moreover, the relation between wet mass consistency with binder ratio and their impact on the dissolution behavior of ABZ from pellets was studied.
2. Materials and methods
2.1. Materials
Albendazole (ABZ) was kindly donated from Saudi Pharmaceutical Industries (Riyadh, SA). Microcrystalline cellulose (Avicel® PH101) was purchased from Serva Feinbiochemica (Heidelberg, Germany). Lactose monohydrate was purchased from Winlab (Leicestershire, UK). Polyoxyethylene (20) sorbitan monooleate (Tween® 80) was purchased from Merck Company (Muenchen, Germany). Chitosan, low MWt grade (Brookfield viscosity 20000 cp) was purchased from Sigma–Aldrich Chemie, GmbH (Steinheim, Germany). All other materials and solvents used are of reagent or analytical grade and they were used without further purification.
2.2. Methods
2.2.1. Wet massing studies using a mixer torque rheometer
Drug substance and excipients were mixed in turbula mixer (type S27, Erweka, Apparatebau, Germany). A 15 g sample of this dry blend was utilized in these studies. Five milliliters of granulating fluid (deionized. water with or without Tween® 80) were added in multiple additions over 7 wet massing intervals. Each wet massing interval consisted of a 1-min mixing period and a 20-s data logging (collection) period with the mixer torque rheometer (MTR) operating at 50 rpm (MTR-3, Caleva, Dorset, England). Mean line torque was monitored during the granulation process. The data acquisition and analyses were carried out by a personal computer using data acquisition system and software package supplied by the equipment manufacturer.
2.2.2. Preparation of ABZ-loaded pellets
Pellets were prepared from those wet masses showing highest mean torque. The tested additives (avicel, lactose and chitosan) and ABZ were mixed in turbula mixer at 9.5:0.5 weight ratio (type S27, Erweka, Apparatebau, Germany). The powder mixture was wetted with the granulation fluid (water containing different concentrations of the surfactant) depending on the composition of the formulation (Table 1). Next, the resulting wet mass was extruded at a speed of 90 rpm (Mini Screw Extruder, Model MSE1014, Caleva, Dorset, England), through 1 mm diameter die. Spheronization was performed in a spheronizer (Model 120, Caleva, Dorset, England) with a rotating plate of regular cross-hatch geometry, at a speed of 700 rpm, for 5 min. Pellets were then dried on a tray in a hot oven at 50–60 °C for 6 h.
Table 1.
Composition of pellets formulae containing 10% w/w albendazole.
| Formula | Ingredient (% w/w) |
||||
|---|---|---|---|---|---|
| Avicel PH101 | Lactose anhydrous | Chitosan (low MWt) | Tween 80a | Dist. water | |
| ABZ 1 | 100 | – | – | – | Q.S. |
| ABZ 2 | 90 | 10 | – | – | Q.S. |
| ABZ 3 | 70 | 30 | – | – | Q.S. |
| ABZ 4 | 50 | 50 | – | – | Q.S. |
| ABZ 5 | 50 | 50 | – | 5 | Q.S. |
| ABZ 6 | 50 | 50 | – | 10 | Q.S. |
| ABZ 7 | 50 | 50 | – | 20 | Q.S. |
| ABZ 8 | 90 | – | 10 | – | Q.S. |
| ABZ 9 | 70 | – | 30 | – | Q.S. |
| ABZ 10 | 50 | – | 50 | – | Q.S. |
Dissolved in water.
2.2.3. Determination of ABZ content in the prepared pellets
Albendazole content of the prepared pellets was determined spectrophotometrically (UV-9100 Spectrophotometer, Beijing Rayleigh Analytical Instruments Corp., China) at 290 nm in triplicate (Alanazi et al., 2007). Pellets were crushed in a porcelain mortar and about 25 mg of ABZ-loaded pellets were dispersed in 20 ml phosphate buffer (pH = 6.8) under sonication. The solution was filtered and ABZ amount was measured.
2.2.4. Particle size analysis of the prepared pellets
The size distribution of the prepared pellets was investigated using laser light diffraction particle sizer (Mastersizer Mastersizer Scirocco 2000, Malvern Instruments, Grovewood Road, UK). For a typical experiment, about 300 mg of pellets were loaded in the sample micro feeder. All samples were analyzed 5 times and average results were taken. The sizes below which 10% (d(0.1)), 50% (d(0.5)) and 90% (d(0.9)) of the pellets were used to characterize the pellets size distribution. The mean diameter was taken as the average of d(0.1), d(0.5) and d(0.9) values.
2.2.5. Morphological analysis of the prepared pellets
The morphological characteristics of prepared pellets were observed by scanning electron microscopy (SEM). The samples were sputter-coated with thin gold palladium layer under an argon atmosphere using a gold sputter module in a high-vacuum evaporator. The coated samples were then scanned and photomicrographs were taken with an SEM (Jeol JSM-1600, Tokyo, Japan).
2.2.6. Thermal analysis
Physical mixtures of ABZ with the tested pellet excipients (lactose, chitosan and Tween) as well as the individual components were investigated for their interactions. The samples (3–5 mg) were hermetically sealed in aluminum pans and heated at a constant rate of 10 °C/min, over a temperature range of 25–250 °C. Thermograms of the samples were obtained using differential scanning calorimetry (DSC-60, Shimadzu, Japan). Thermal analysis data were recorded using a TA 50I PC system with Shimadzu software programs. Indium standard was used to calibrate the DSC temperature and enthalpy scale. N2 was used as purging gas at the rate of 30 ml/min.
2.2.7. In vitro release studies of the prepared pellets
In vitro drug release was performed for the ABZ-loaded pellets using a USP dissolution test apparatus. The dissolution of ABZ from the prepared pellets was monitored using an automated dissolution tester (LOGAN Instrument Corp., Somerset, NJ, USA) coupled to an automated sample collector (SP-100 peristaltic pump, Somerset, NJ, USA). The USP apparatus I (basket method) was used at 100 rpm. The media used were 500 ml of 0.1 N HCl at a pH 1.2 maintained at 37 ± 0.5 °C. The amount of ABZ released from pellets was determined by UV spectrophotometer (UV-1800, Shimadzu, Tyoto, Japan) at 290 nm. The dissolution experiments were carried out in triplicate.
2.2.8. Kinetic assessment of the in vitro release of MCP from the prepared pellets
In order to determine the release model which best describes the pattern of drug release, the in vitro release data were fitted to zero order, and diffusion controlled release mechanisms according to the simplified Higuchi (1963) model.
3. Results and discussion
3.1. Wet massing studies
Experiments were performed in order to establish the massing binder ratio needed to reach an equilibrium torque response. Figs. 1 and 2 show the rheological profiles for Avicel PH101 when mixed at different ratios with lactose and chitosan, respectively. It is evident that there is an increase in torque with increasing liquid content, rising to a maximum, and then decreasing as larger volumes of binder are used.
Figure 1.
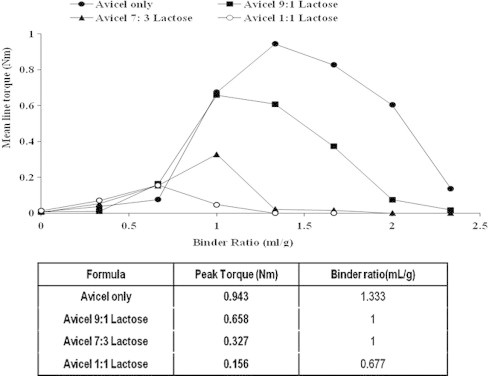
Effect of different avicel:lactose concentrations on mean torque the wet mass. Also, the table shows the relation between formula composition with binder ratio and peak torque.
Figure 2.
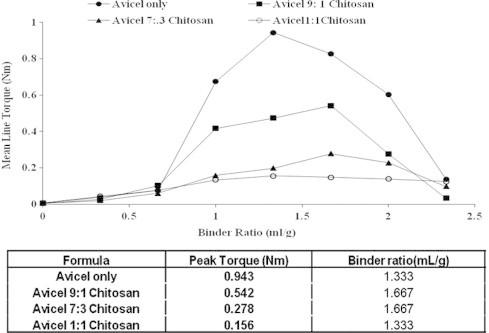
Effect of different avicel:chitosan concentrations on mean torque the wet mass. Also, the table shows the relation between formula composition with binder ratio and peak torque.
The MTR behavior of avicel alone showed that the peak torque was 0.943 N m and was achieved by using a binder ratio of 1.333 mL/g. Considering avicel–lactose system, increasing the weight ratio of lactose was accompanied by both a reduction in the wet mass peak torque and at the same time a reduction in the binder ratio required for wet massing. For example, the peak torque of avicel:lactose (9:1 w/w) mixture was 0.658 N m with a binder ratio of 1 mL/g, while the peak torque of avicel:lactose (1:1 w/w) mixture was 0.156 N m with a binder ratio of 0.667 mL/g, Fig. 1.
The MTR characteristics of avicel–chitosan systems showed different profiles, in which the curves become more broad (higher binder ratios were consumed in the transformation from pendular and capillary phases). Also, increasing chitosan concentration resulted in decreasing the peak torque of the wet mass, Fig. 2. This might be due to the lower affinity of chitosan to absorb water when compared to avicel.
Furthermore, addition of Tween 80 to avicel:lactose (1:1 w/w) mixture system did not affect both the peak torque and the binder ratio of the wet masses, Fig. 3, with an exception of 5% w/v Tween 80 concentration. For example, the peak torque of avicel:lactose (1:1 w/w) mixture containing 10% Tween 80 was 0.158 N m while the peak torque of such mixture containing 20% Tween 80 was 0.159 N m.
Figure 3.
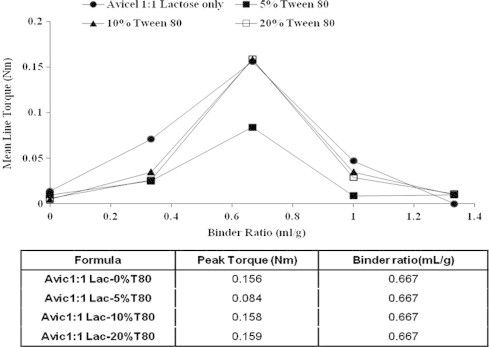
Effect of water containing different concentrations of Tween 80 on mean torque for avicel–lactose wet mass. Also, the table shows the relation between formula composition with binder ratio and peak torque.
The degree of liquid spreading and wetting as well as the substrate binder interaction determine the relative positions of the peak values of mean line torque. An increase in the mean torque with the increase in the binder level at different concentrations either a sharp or an extended peak followed by a drop in the torque as over-wetting of the powder mass occurred (Parker et al., 1990a,b). In addition, the pendular and funicular states are characterized by progressively increasing the network of liquid bridges. Both these stages cause an increase in cohesiveness of the powder mass, and hence an increased torque on the mixer (Parker et al., 1990a,b). The capillary state which is reached when all the air spaces in the granular material are filled with liquid occurs at the maximum on the curve. With further addition of liquid the torque decreases as slurry of particles dispersed in liquid is formed.
3.2. Drug content
The drug content data of 10% ABZ-loaded pellets are listed in Table 3. The data show that ABZ was efficiently loaded into the pellets and the actual ABZ content ranged between 9.66% and 10.45%. It is evident that there is an inverse proportionality between the concentration of Tween added and the pellet drug content. The addition of Tween 80 to the formula contains avicel 1:1 lactose (Formulae ABZ 5–7), during pellet preparation lowered the drug content. This decrease in drug content is directly proportional to the Tween concentration and might be due to that these Tween 80 did not evaporate during pellet drying. It is evident also that pellet drug content in the presence of chitosan or lactose shows no statistically difference than pellets prepared from avicel alone (p < 0.05).
Table 3.
Albendazole content, percentage albendazole dissolved after 30 min (D30) and 90 min (D90) as well as relative dissolution rate at 90 min (RDR90) from its loaded Avicel PH101 pellets.
| Pellets formulae | ABZ content (%w/w) | D30 | D90 | RDR90 |
|---|---|---|---|---|
| ABZ 1 | 10.20 ± 0.41 | 13.27 ± 1.17 | 27.66 ± 2.48 | – |
| ABZ 2 | 9.89 ± 0.37 | 14.16 ± 0.12 | 29.86 ± 0.01 | 1.08 |
| ABZ 3 | 9.78 ± 0.85 | 19.08 ± 1.78 | 38.40 ± 3.34 | 1.39 |
| ABZ 4 | 10.45 ± 0.56 | 31.35 ± 0.02 | 56.21 ± 1.17 | 2.03 |
| ABZ 5 | 9.73 ± 0.35 | 32.82 ± 1.50 | 57.01 ± 2.25 | 2.06 |
| ABZ 6 | 9.66 ± 0.26 | 30.56 ± 1.72 | 57.87 ± 2.55 | 2.09 |
| ABZ 7 | 7.81 ± 0.16 | 32.22 ± 1.23 | 62.54 ± 5.21 | 2.26 |
| ABZ 8 | 9.88 ± 0.59 | 21.42 ± 0.62 | 47.24 ± 0.94 | 1.71 |
| ABZ 9 | 10.12 ± 0.45 | 56.67 ± 2.80 | 100.00 ± 1.81 | 3.62 |
| ABZ 10 | 10.25 ± 0.67 | 57.89 ± 3.47 | 100.00 ± 6.03 | 3.62 |
3.3. Size of the pellets
The volume weighted mean particle size, the d(0.1), d(0.5) and d(0.9) values of different pellet formulae loaded with ABZ (10% w/w as determined by laser diffractometry) are listed in Table 2. Generally, the produced pellets have size range from 1036 to 1246 μm. Concerning avicel–lactose pellets, the increase in lactose concentration caused a pronounced increase in the pellets sizes. The size of pellet formula ABZ 2 (based on 10% lactose) is 1116.21 μm, for ABZ 3 (based on 30% lactose) was 1189.73 μm, while for formula ABZ 4 (based on 50% lactose) is 1246.80 μm as shown in Table 2. Upon the addition of Tween 80 in the manufacture of avicel–lactose pellets (formulae ABZ 5–ABZ 7), the pellet size became smaller noticeably (1016.34, 1093.49 and 1165.78 μm were recorded for formulae ABZ 5, ABZ 6 and ABZ 7, respectively). This result correlates well with the results of MTR, in which the mean torque value increases by increasing Tween 80 concentration and the pellet size becomes larger with the increase in mean line torque value, Fig. 4. This increase in the pellet size by increasing the wet mass torque (cohesiveness) might be due to the difficulty in the extrusion and spheronization of the wet mass of the higher torque value, which leads to a larger size, i.e., cutting of the particle becomes hard due to the high force of cohesiveness between particles. Avicel–chitosan pellets prepared from lower chitosan concentration showed particle sizes that are slightly larger than those prepared from higher chitosan levels, Table 2. This could be explained on the basis of the slightly lower peak torque of pellets prepared from higher chitosan levels, which result also in increasing ABZ dissolution rate.
Table 2.
Volume weighted mean particle size and the d(0.1), d(0.5) and d(0.9) values of different pellet formulae loaded with albendazole (10% w/w as determined by laser diffractometry.
| Pellets formulae | Mean (μm) | d(0.1) (μm) | d(0.5) (μm) | d(0.9) (μm) |
|---|---|---|---|---|
| ABZ 1 | 1113.59 | 792.73 | 1088.67 | 1468.12 |
| ABZ 2 | 1116.21 | 796.54 | 1091.29 | 1469.65 |
| ABZ 3 | 1189.73 | 865.73 | 1171.33 | 1550.12 |
| ABZ 4 | 1246.80 | 916.12 | 1232.14 | 1587.39 |
| ABZ 5 | 1016.34 | 737.03 | 988.98 | 1342.75 |
| ABZ 6 | 1093.49 | 694.38 | 1053.16 | 1556.31 |
| ABZ 7 | 1165.78 | 847.86 | 1145.82 | 1519.24 |
| ABZ 8 | 1115.19 | 794.39 | 1090.45 | 1469.36 |
| ABZ 9 | 1118.79 | 799.66 | 1094.42 | 1470.81 |
| ABZ 10 | 1036.00 | 708.50 | 1008.52 | 1401.89 |
Figure 4.
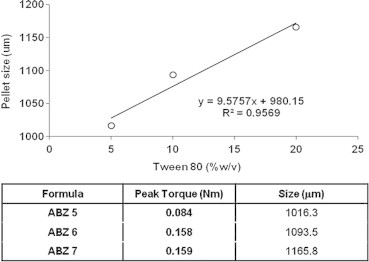
Effect of different Tween 80 concentrations on particle size (volume weighted mean) of pellets prepared from avicel:lactose (formulae ABZ 5–ABZ 7). The included table relates the mean line torque of wet mass and pellet particle size.
3.4. Morphological analysis of the prepared pellets
Scanning electron micrographs of ABZ pellets of different compositions are displayed in Fig. 5. The resulting pellets were spherical and intact in shape. The pellets showed slightly rough surfaces. It should be noted that pellets formulated from avicel only exhibited incomplete spherical shapes. In addition, pellets composed of avicel–chitosan showed highly rough surfaces, which could be explained on the base of the surface nature of chitosan powder and solubility in the massing liquid (water). However, lactose and Tween 80 (Formula ABZ 4 and 7, respectively) improved surface properties of the manufactured pellets. This might be due to the hydrophilic nature of lactose, in which lower mean torques as well as lower binder ratios were observed upon increasing the lactose weight ratio. Law and Deasy (1997) reported that the use of hydrophilic polymers with avicel favored more spherical and smooth pellets.
Figure 5.
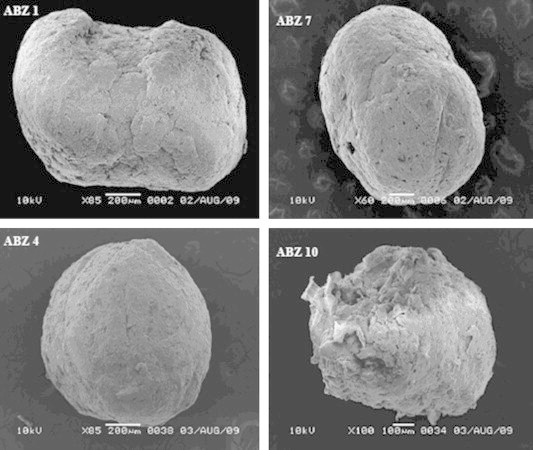
Scanning electron micrographs of different ABZ pellet formulations.
3.5. Thermal analysis
The DSC thermograms of pure ABZ (Fig. 6) show an endothermic peak at 218 °C with a shoulder at 198 °C due to the drug melting (Alanazi et al., 2007). In addition, two peaks were recorded upon DSC scanning of lactose at 144 and 231 °C. However, no melting peak was detected for avicel. The DSC thermographs of ABZ when formulated as pellets with different excipients show that the characteristic drug peak was completely disappeared, which could be attributed to the solubility of ABZ in the molten polymers (Mahrous et al., 2010).
Figure 6.
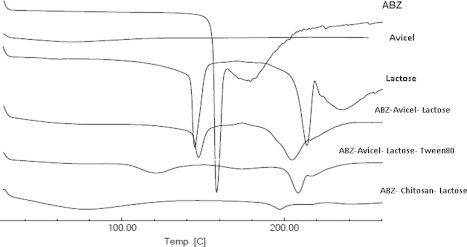
DSC curves of ABZ pellet formulations compared to the individual components.
3.6. In vitro release of ABZ from the prepared pellets
The in vitro dissolution profiles of ABZ of the avicel pellet formulations containing different concentrations of lactose are illustrated in Fig. 7. It is clear from the figure that the release rate of ABZ from pellets prepared from avicel only was very slow (only 27.66% ± 2.48 was dissoluted after 90 min). The addition of lactose improved the dissolution rate of the drug, especially at higher lactose concentrations. The calculated drug RDR30 for 10%, 30% and 50% lactose concentrations were 1.08, 1.08 and 2.03, respectively, Table 3. The enhanced dissolution rate of ABZ upon addition of lactose might be due to the hydrophilic nature of lactose (Jamzad and Fassihi, 2006).
Figure 7.
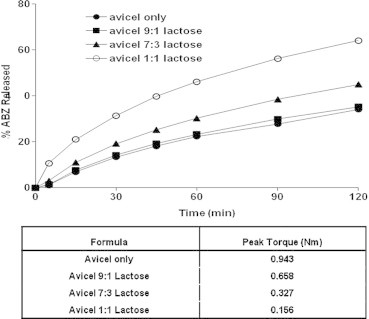
Effect of different avicel:lactose concentrations on the in vitro release profiles of ABZ from pellet formulations.
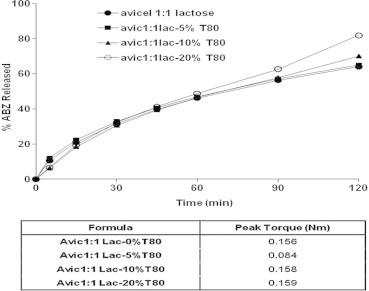
One could see that increasing the weight ratio of lactose was accompanied by a significant reduction of the peak torque magnitude and this was accompanied by an enhanced ABZ dissolution rate. When different Tween 80 concentrations were added to avicel–lactose pellets (50:50), the in vitro ABZ dissolution was not significantly affected, Fig. 8. The recorded D90 for 5%, 10% and 20% w/w of Tween were 57.01 ± 2.25, 57.87 ± 2.25 and 62.54 ± 5.21, respectively, while 56.21% ± 1.17 dissoluted in the absence of Tween 80, Table 3. This might be due to a fact that increasing the weight ratio of Tween 80 did not affect the value of peak toque of avicel–lactose (50:50) paste during pellet formulation, which correlates with the ABZ dissolution data.
Figure 8.
Effect of different concentrations of Tween 80 (%w/w) on the in vitro release profiles of ABZ from ABZ 4 pellet formulation.
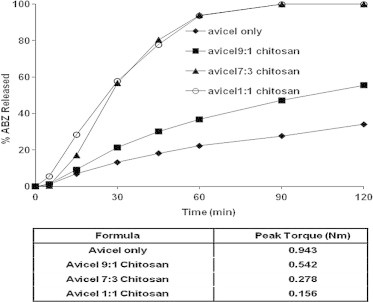
The in vitro ABZ dissolution profiles from pellet formulations prepared from avicel and chitosan at different weight ratios was investigated and the results are displayed in Fig. 9. ABZ exhibited the highest dissolution rate from avicel–chitosan pellet formulations containing 30% and 50% w/w chitosan. The recorded D90 values for 10%, 30% and 50% w/w chitosan concentrations were 47.24 ± 0.94, 100.00 ± 1.81 and 100.00 ± 6.03, Table 3. It is worthy to note that at the first 30 min, chitosan pellets formulated with 30% and 50% w/w chitosan began to rupture and completely disintegrated after 90 min. This phenomenon might be the cause of the rapid dissolution rate from these formulae. Some factors may contribute to the enhanced dissolution rate of ABZ from chitosan pellets especially those prepared from% 30 and 50% w/w chitosan. First, the lower torque values recorded for avicel–chitosan pellets (0.278 and 0.156 N m for avicel 70:30 chitosan and avicel 50:50 chitosan, respectively, Fig. 6). Second, the dispersion of chitosan in the acidic dissolution buffer (Majeti and Kumar, 2000) facilitates the dissolution rate of ABZ.
Figure 9.
Effect of different avicel:chitosan concentrations on the in vitro release profiles of ABZ from pellet formulations.
3.7. Kinetic assessment of the in vitro dissolution of ABZ from the prepared pellets
The correlation coefficient (r), and the first order release rate constant of fitting the release data to zero, first and Higuchi diffusion models for spheres are listed in Table 4. The higher correlation coefficient (r) values for first order model were obtained in all formulae that indicate a good fitting with the first order mechanism. It should be mentioned that the tested pellet formulae did not show any swelling during the dissolution period. Only chitosan pellets containing higher chitosan levels were ruptured and disintegrated during dissolution as mentioned before. The values of first order release rate constant (K1) of ABZ dissolution from tablet formulae showed that chitosan pellets exhibited the most pronounced enhancing action on ABZ dissolution. The recorded values of K1 were 463.6 × 104 and 439.8 × 104 min−1 for pellets containing 30% and 50% w/w chitosan, Table 4.
Table 4.
Kinetic modeling and first order release rate constant values for the prepared albendazole pellets.
| Formula | Correlation coefficient |
K1 (min−1) 104 | ||
|---|---|---|---|---|
| Zero order | First order | Higuchi model | ||
| ABZ 1 | 0.9806 | 0.9904 | 0.7951 | 34.8 |
| ABZ 2 | 0.9785 | 0.9894 | 0.8013 | 36.7 |
| ABZ 3 | 0.9754 | 0.9907 | 0.8175 | 49.8 |
| ABZ 4 | 0.8588 | 0.9902 | 0.8680 | 82.1 |
| ABZ 5 | 0.9552 | 0.9891 | 0.8773 | 83.2 |
| ABZ 6 | 0.9748 | 0.9973 | 0.8286 | 96.8 |
| ABZ 7 | 0.9858 | 0.9846 | 0.8063 | 129.9 |
| ABZ 8 | 0.9790 | 0.9955 | 0.7840 | 69.5 |
| ABZ 9 | 0.8952 | 0.9759 | 0.8060 | 463.6 |
| ABZ 10 | 0.9011 | 0.9761 | 0.8416 | 439.8 |
4. Conclusion
From the previous results, it could be concluded that the wet mass behavior could be helpful in predicting pellets’ shapes and sizes. Also, the mean line torque of the wet masses could draw a good picture about the dissolution rate of the drugs from the prepared pellets. In addition, the presence of hydrophilic additives as lactose and Tween 80 enhanced the drug dissolution rate from the manufactured pellets.
Acknowledgment
The author greatly acknowledges the financial support from Sabic (Grant number MED-30-17), Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alanazi F.K., El-Badry M., Ahmed M.O., Alsarra I.A. Improvement of albendazole dissolution by preparing microparticles using spray-drying technique. Sci. Pharm. 2007;75:63–79. [Google Scholar]
- Bechgaard H., Nielsen G.H. Controlled-release multiple-units and single-unit doses. A literature review. Drug Dev. Ind. Pharm. 1978;4:53–67. [Google Scholar]
- Chambliss W.G. In: Pharmaceutical Pelletization Technology. first ed. Ghebre-Sellassie Isaac., editor. Marcel Dekker; New York: 1989. pp. 15–38. [Google Scholar]
- Chatlapalli R., Rohera B.D. Rheological characterization of diltiazem HCl:cellulose wet masses using a mixer torque rheometer. Int. J. Pharm. 1998;175:47–59. doi: 10.1016/s0378-5173(02)00065-0. [DOI] [PubMed] [Google Scholar]
- Cook G.C. Use of benzimidazole chemotherapy in human helminthiases: indications and efficacy. Parasitol. Today. 1990;6:133–136. doi: 10.1016/0169-4758(90)90232-s. [DOI] [PubMed] [Google Scholar]
- Del-Brutto O.H., Sotelo J., Roman G.C. Therapy for neurocysticercosis: a reappraisal. Clin. Infect. Dis. 1993;17:730–735. doi: 10.1093/clinids/17.4.730. [DOI] [PubMed] [Google Scholar]
- Eskilson C. Controlled release by microencapsulation. Manuf. Chem. 1985;56:33–39. [Google Scholar]
- Hancock B.C., York P., Rowe R.C. Characterization of wet masses using a mixer torque rheometer: 1: effect of instrument geometry. Int. J. Pharm. 1991;76:239–245. [Google Scholar]
- Hancock B.C., York P., Rowe R.C. Characterization of wet masses using a mixer torque rheometer: 2. Mixing kinetics. Int. J. Pharm. 1992;83:147–153. [Google Scholar]
- Higuchi T. Mechanism of sustained-action medication: theoretical analysis of rate of release of solid drug dispersed in solid matrices. J. Pharm. Sci. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- Jachowicz R., Nürnberg E., Pieszczek B., Kluczykowska B., Maciejewska A. Solid dispersion of ketoprofen in pellets. Int. J. Pharm. 2000;206:13–21. doi: 10.1016/s0378-5173(00)00437-3. [DOI] [PubMed] [Google Scholar]
- Jamzad S., Fassihi R. Development of a controlled release low dose class II drug-Glipizide. Int. J. Pharm. 2006;312:24–32. doi: 10.1016/j.ijpharm.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Kleinebudde P. Application of low substituted hydroxypropyl cellulose (L-HPC) in the production of pellets using extrusion/spheronization. Int. J. Pharm. 1993;96:119–128. [Google Scholar]
- Law M.F.L., Deasy P.B. Use of canonical and other analyses for the optimization of an extrusion–spheronization process for indomethacin. Int. J. Pharm. 1997;146:1–9. [Google Scholar]
- Mahrous G.M., Ibarhim M.A., El-Badry M., Al-Anazi F.K. Indomethacin sustained release pellets prepared by extrusion–spheronization. J. Drug Delivery Sci. Technol. 2010;20:119–125. [Google Scholar]
- Majeti N.V., Kumar R. A review of chitin and chitosan applications. React. Funct. Polym. 2000;46:1–27. [Google Scholar]
- Parker M.D., Rowe R.C. Source variation in the wet massing (granulation) of some microcrystalline celluloses. Powder Technol. 1991;65:273–281. [Google Scholar]
- Parker M.D., Rowe R.C., Upjohn N.G. Mixer torque rheometry: a method for quantifying the consistency of wet granulation’s. Pharm. Tech. Int. 1990;2:50–64. [Google Scholar]
- Parker M.D., York P., Rowe R.C. Binder-substrate interactions in wet granulation. 1: The effect of binder characteristics. Int. J. Pharm. 1990;64:207–216. [Google Scholar]
- Paul K.E., Fridrun P., Michael N.J. The rheological properties of modified microcrystalline cellulose containing high levels of model drugs. J. Pharm. Sci. 2009;98:2160–2169. doi: 10.1002/jps.21587. [DOI] [PubMed] [Google Scholar]
- Robinso n R.L., Hollenbeck R.G. Manufacture of spherical acetaminophen pellets: comparison of rotary processing with multi-step extrusion and spheronization. Pharm. Technol. 1991;15:48–56. [Google Scholar]
- Rowe R.C., Parker M.D. Mixer torque rheometry – an update. Pharm. Technol. Eur. 1994;6:27–36. [Google Scholar]
- Rowe R.C., Sadeghnejad G.R. The rheology of microcrystalline cellulose powder:water mixes-measurement using a mixer torque rheometer. Int. J. Pharm. 1987;38:227–229. [Google Scholar]
- Torrado S., Torrado S., Torrado J.J., Cadórniga R. Preparation, dissolution and characterization of Albendazole solid dispersions. Int. J. Pharm. 1996;140:247–250. [Google Scholar]
- Vervaet C., Baert L., Remon J.P. Enhancement of in vitro drug release by using polyethylene glycol 400 and PEG-40 hydrogenated castor oil in pellets made by extrusion/spheronisation. Int. J. Pharm. 1994;108:207–212. [Google Scholar]
- Wen H., New R.R.C., Craig P.S. Diagnosis and treatment of human hydatidosis. Br. J. Clin. Pharm. 1993;35:565–574. doi: 10.1111/j.1365-2125.1993.tb04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasawy M.I., Al-Karawi M.A., Mohamed A.R. Combination of praziquantel and albendazole in the treatment of hydatid diseases. Trop. Med. Parasitol. 1993;44:192–194. [PubMed] [Google Scholar]



