Abstract
In this study molecular imprinting technology was employed to prepare a specific affinity sorbent for the resolution of Cathine, a chiral drug product. The molecularly imprinted polymer (MIP) was prepared by non-covalent molecular imprinting with either (+) or (−)-Cathine (threo-2-amino-1-hydroxy-1-phenyl propane; norpseudoephedrine) as the template. Methacrylic acid and ethylene glycol di-methacrylate were copolymerized in the presence of the template molecule. The bulk polymerization was carried out in chloroform with 2,2′-azobisisobutyronitrile as the initiator, at 5 °C and under UV radiation. The resulting MIP was ground into powders, which were slurry packed into analytical columns. After removal of template molecules, the MIP-packed columns were found to be effective for the resolution of (±)-Cathine racemates. The separation factor for the enantiomers ranged between 1.5 and 2.4 when the column was packed with MIP prepared with (+)-Cathine as the template. A separation factor ranging from 1.6 to 2.9 could be achieved from the column packed with MIP, prepared with (−)-Cathine as the template. Although the separation factor was higher with that previously obtained from reversed-phase column chromatography following derivatization with a chiral agent, elution peaks were broader due to the heterogeneity of binding sites on MIP particles and the possible non-specific interaction.
Keywords: Enantiomer separation, Cathine, Chiral drug product, Molecularly imprinted polymers, Template
1. Introduction
Resolution of the enantiomers is usually carried out by chromatography of the racemic mixture on a chiral stationary phase (CSP), which commonly includes crown ether and several types of derivatives of cellulose and amylose. Stationary phases containing crown ether are particularly useful for the chiral resolution of amino containing compounds and their derivatives (Motellier and Wainer, 1990), and carbamates and benzoates of polysaccharides such as cellulose and amylose are effective and very popular for the optical resolution of racemic drugs (Okamoto and Kaida, 1994). Also frequently used as CSPs are cyclodextrin (Armstrong et al., 1986) and immobilized proteins such as bovine serum albumin (Harada et al., 1996) and α-acid glycoprotein (Hermansson, 1984). Pettersson and Schill (1981) have developed an ion-pair chromatographic method for the separation of a racemic mixture of various drugs including β-blockers. In their method a conventional reversed phase is used as the stationary phase. In this study we describe the use of a molecularly imprinted polymer (MIP) as the stationary phase for the separation of racemates. This type of stationary phase can be classified as a specific group of CSP. Molecular imprinting is a promising technique for the preparation of polymers which possess highly selective recognition properties and serve as separation media, especially for chiral molecules. The applications of molecularly imprinted polymer as separation media in liquid chromatography, capillary electrophoresis and capillary electro chromatography for chiral separation have been extensively reviewed (Wulff, 1986, Mosbach, 1994, Kempe and Mosbach, 1995, Andersson, 2000, Vallano and Remcho, 2000, Sellergren, 2001). Wulff and Vesper (1978) first reported the use of a molecularly imprinted polymer prepared by a covalent approach for the separation of enantiomers. In their study, the template 4-nitrophenyl-α-d-mannopyranoside was covalently linked to a monomer to form 4-nitrophenyl-α-d-mannoside-2,3,4,6-di-o-(4-vinylphenylboronate), which was then co-polymerized with styrene and divinylbenzene. However, non-covalently molecular imprinting has been reported to be a more direct and flexible approach because of its use of a larger range of compounds including chiral molecules that can be imprinted (Kempe and Mosbach, 1995). l-Phenylalanine anilide (Dauwe and Sellergren, 1996, O’Shannessy et al., 1989, Sajonz et al., 1998), and l-phenylalanine and its derivatives (Sellergren et al., 1985, Ramstrom et al., 1993, Kempe and Mosbach, 1994, Mayes and Mosbach, 1996) have been extensively used as the templates. In addition to various racemic amino acid derivatives (Mosbach, 1994, Kempe and Mosbach, 1995), other chiral drugs, including S-(2)-timolol Fischer et al., 1991, (S)-naproxen Kempe and Mosbach, 1994, (S)-a-methylbenzylamine (Matsui et al., 1996) and (2)-nicotine Andersson et al., 1999, have also been used as templates for imprinting. Methacrylic acid (MAA) has been usually used as the functional monomer. When l-phenylalanine anilide (l-PA) is used as the template, the carboxyl group of methacrylic acid can interact with the amino group of l-PA to form hydrogen bonds and also hydrophilically interact with the C O bond in l-PA (Dauwe and Sellergren, 1996). In the l-PA molecule there are two benzene groups involved in forming a complementary shape. Cathine, a somewhat simpler molecule than l-PA used as the imprinting template in this study, contains a single amino group that can interact with MAA and only one benzene group for shape formation. Cathine, also referred to as norpseudoephedrine, is a sympathomimetic drug. It, as well as its related compounds phenylephrine, ephedrine, norephedrine and pseudoephedrine, are adrenergic agents widely used in the treatment of asthma, ophthalmia, colds and allergies.
Few reports have been published on the chromatographic resolution of the enantiomers of these adrenergic agents. Norpseudoephedrine (Cathine) has recently been imprinted and the resultant MIP was used for enantiomer separation in the thin-layer chromatography (TLC) mode Suedee et al., 1999. Phenylephrine, phenylpropanolamine, ephedrine and pseudoephedrine have also been imprinted and the resultant MIP was used for liquid chromatography of enantiomer separation (Ramstrom et al., 1996). Konig and Benecke (1981) synthesized a chiral gas–liquid chromatography stationary phase and used it in a glass capillary column to separate the enantiomers of phenylpropanolamine. Gal (Gal and Sedman, 1984) describes the use of a reversed phase column for the resolution of the enantiomers of norephedrine. His method is based on the derivatization of analytes with the chiral reagent 2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl cyanate (GITC). The separation factor was, however, very small (1.12). The present paper demonstrates that the chiral resolution can be greatly enhanced by using the molecularly imprinted polymer as the stationary phase.
2. Materials and methods
2.1. Materials
Methacrylic acid (98%) and ethylene glycol dimethacrylate (EGDMA, 98%) were obtained from Merck (india) and used as received. 2,2′-azo-bisisobutyronitrile (AIBN) was obtained from sigma. Acetone, methanol and acetonitrile were from Merck India and all of HPLC grade. Chloroform and acetic acid were from sigma aldrich, while 1R,2S-(+)-Cathine (99%) and 1S,2R-(−)-Cathine (99%) were purchased from Sigma. All chemicals were used without further purification.
2.2. Preparation of molecular imprinting stationary phase
The MIP stationary phase was prepared by bulk polymerization at a low temperature. Briefly, Cathine (0.1 g), methacrylic acid (0.23 mL), EGDMA (2.5 mL) and AIBN (0.02 g) were dissolved in 5 mL of chloroform in conical flask. After degassing and nitrogen purging for 5 min, the flask was sealed and allowed to polymerize at 5 °C for 6 h under UV (254 nm, 100 W lamp) irradiation. For each preparation of MIP, either (+)-Cathine or (−)-Cathine was used as the template. Methacrylic acid was used here as the functional monomer, EGDMA as the cross linking monomer and AIBN as the free radical initiator. After polymerization, the chloroform was removed. The product in the form of a white solid was dried in a vacuum oven for 12 h at room temperature. The resultant bulk polymer was finally ground and sieved. The fraction of particles having an average size ranging from 5 to 10 μm was collected for packing in chromatographic column.
2.3. Liquid chromatography
MIP particles were suspended in methanol by sonication and then slurry packed into 15 cm × 0.46 cm i.d. stainless steel columns using an air-driven fluid pump with acetone as the solvent. The back-pressure for packing was 300 bar. Template molecules were removed from the columns by continuously washing with methanol–acetic acid (8:2, v/v) until a stable baseline was reached. For the HPLC analysis, a 20 μL sample solution was injected and eluted isocratically at a flow rate of 0.5 mL/min, using a mixture of water, acetic acid and acetonitrile (2:7:91, v/v) as the mobile phase. The temperature was kept at 20 °C. The effluent solution was constantly monitored by measuring the absorbance at 257 nm. Toluene was used as the non-retained component for the determination of the void fraction for each column. Capacity factors ( and ) were calculated according to standard chromatographic theory as = (t− − t0)/t0 and = (t+ − t0)/t0, where t+ and t− are the retention times of (+)-Cathine and (−)-Cathine, respectively, and the toluene retention time was used as the retention time of the non-retained component, t0 The separation factor (α) was defined as the ratio of these two capacity factors. For example, α = / when the chromatographic column was packed with MIP prepared by using (+)-Cathine as the template.
3. Results and discussion
3.1. Synthesis of stationary phase
To prepare the molecularly imprinted polymer, there should be interactions between the template molecule and methacrylic acid functional monomer. These interactions, including electrostatic binding and hydrogen bonding between the amino and the acid groups, are subject to collapse at elevated temperatures. Free radical polymerization was thus carried out at a very low temperature using UV (254 nm) irradiation. Two MIPs using (+)-Cathine and (−)-Cathine as the template molecules were successfully prepared. On the prepared MIP stationary phase, methacrylic acid was incorporated as the host molecule to the template (+)-Cathine or (−)-Cathine. Potential interactions for the formation of a complementary shape for template recognition in the chromatographic process included electrostatic binding and hydrogen bonding between the template and methacrylic acid, as shown in Fig. 1. The template was dissolved in carbon tetra chloride, which was the solvent for MIP polymerization, though a higher carbon tetra chloride content could lead to a resultant polymer too soft to use.
Figure 1.
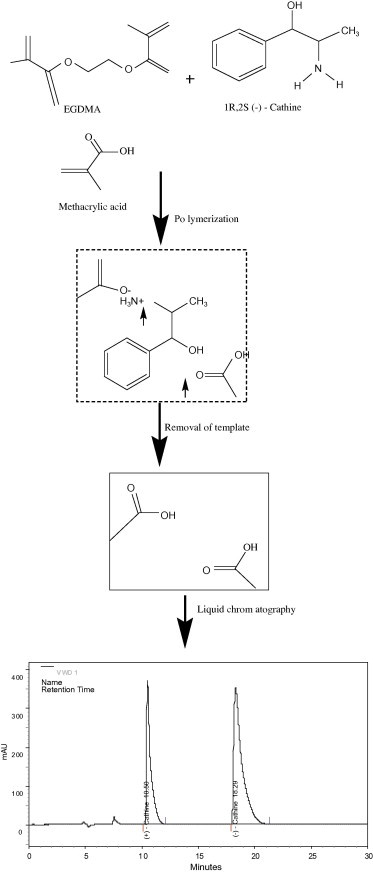
Schematic representation of hypothetical formation of molecularly imprinted polymer and chiral separation, using (−)-Cathine as template. Arrows represent potential electrostatic and hydrogen-bonding interactions between methacrylic acid and the template.
3.2. Resolution of enantiomers of Cathine
The structure of Cathine contains two asymmetric centers. The compounds of interest are a pair of enantiomers: 1R,2S-(−)-Cathine and 1S,2R-(+)-Cathine. Besides hydrogen, a hydroxyl group and a benzene ring are bound onto C1 of Cathine, while C2 has a methyl and an amino group. The retention of Cathine on the columns packed with MIPs was mainly based on the contribution of methacrylic acid functionality on the stationary phase. The interaction between the amino group in MIP and the acid group in the template, and hydrogen bonding between methacrylic acid and the hydroxyl group of the template, are thus the most important factors for the recognition of the template molecule by MIP, and the separation of the Cathine template from its enantiomer. Both Figure 2, Figure 3 indicate that enantiomers of Cathine could be well separated using the MIP made by using either (−)-Cathine or (+)-Cathine as the template. After a series of tests, the composition of the mobile phase to achieve the best resolution was found to be water–acetic acid–acetonitrile (2:7:91, v/v). The resolution, as shown in the chromatographic peaks, was very good. Table 1 indicates that the separation factor for these enantiomers ranged between 1.5 and 2.4 when the column was packed with MIP prepared using (+)-Cathine as the template. A separation factor ranging from 1.6 to 2.9 could be achieved from the column packed with MIP prepared using (−)-Cathine as the template (Table 2).
Figure 2.
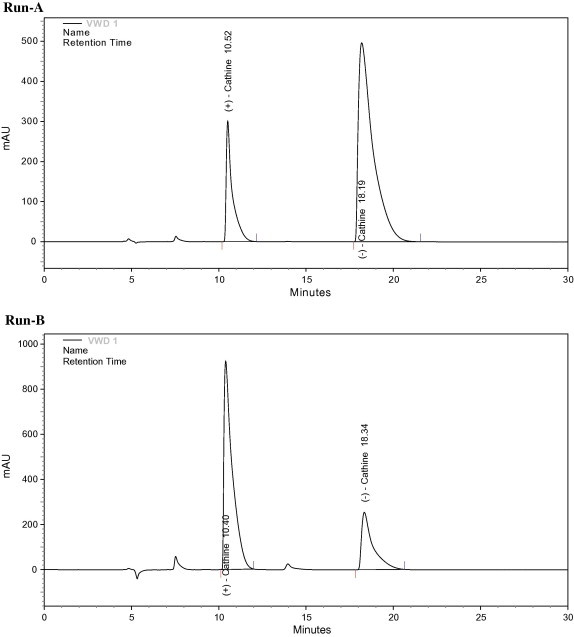
Separation of enantiomers of Cathine using molecularly imprinted polymer with (−)-Cathine as the template. Concentrations of (+)-Cathine and (−)-Cathine in samples are 1 and 2 mg/mL, respectively, for run A, and 3 and 1 mg/mL, respectively, for run B.
Figure 3.
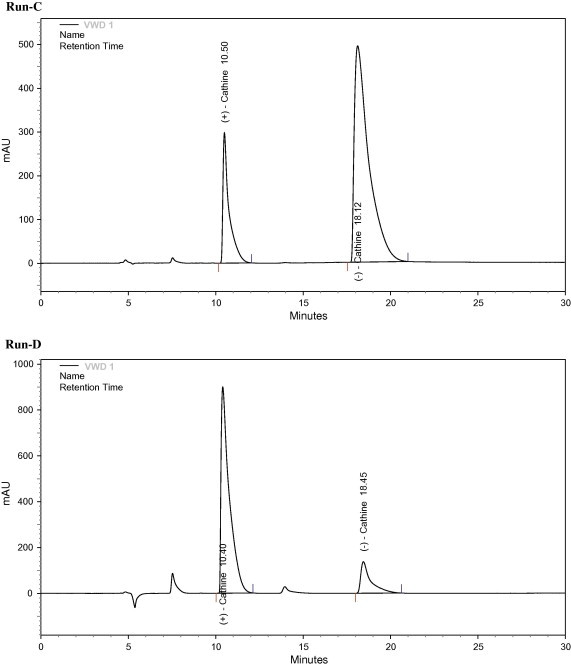
Separation of enantiomers of Cathine using molecularly imprinted polymer with (+)-Cathine as the template. Concentrations of (+)-Cathine and (−)-Cathine in samples are 1 and 2 mg/mL, respectively, for run C, and 8 and 1 mg/mL, respectively, for run D.
Table 1.
Resolution of enantiomers of Cathine using MIP with (+)-Cathine as the template.
| S. No. | Concentration in sample (mg/mL) |
Retention time, min (capacity factor) |
α = (/) | ||
|---|---|---|---|---|---|
| (−)-Cathine | (+)-Cathine | (−)-Cathine() | (+)-Cathine() | ||
| 1 | 8 | 0 | 9.46 (1.3) | – | – |
| 2 | 8 | 1 | 10.83 (1.6) | 14.19(2.5) | 1.6 |
| 3 | 2 | 1 | 11.11 (1.7) | 14.85(2.6) | 1.5 |
| 4 | 1 | 1 | 10.83 (1.6) | 16.37(3.0) | 1.9 |
| 5 | 1 | 2 | 10.69 (1.6) | 18.26(3.5) | 2.2 |
| 6 | 1 | 3 | 10.94 (1.7) | 17.27(3.2) | 1.9 |
| 7 | 1 | 8 | 10.39 (1.5) | 19.05(3.6) | 2.4 |
| 8 | 0 | 8 | – | 14.48(2.5) | – |
Table 2.
Resolution of enantiomers of Cathine using MIP with (−)-Cathine as the template.
| S. No. | Concentration in sample (mg/mL) |
Retention time, min (capacity factor) |
α = ( /) | ||
|---|---|---|---|---|---|
| (+)-Cathine | (−)-Cathine | (+)-Cathine() | (−)-Cathine() | ||
| 1 | 2 | 0 | 9.22 (1.2) | – | – |
| 2 | 2 | 1 | 10.02 (1.4) | 13.44 (2.3) | 1.6 |
| 3 | 1 | 1 | 10.86 (1.6) | 15.53 (2.8) | 1.8 |
| 4 | 1 | 2 | 11.01 (1.7) | 18.64 (3.5) | 2.1 |
| 5 | 1 | 3 | 10.41 (1.5) | 20.33 (4.0) | 2.7 |
| 6 | 1 | 8 | 10.76 (1.6) | 23.23 (4.7) | 2.9 |
| 7 | 0 | 8 | – | 14.55 (2.5) | – |
All the values of separation factor obtained in this work are much greater than the ones reported by Gal and Sedman (1984), who using a reversed-phase column and a method based on derivatization with the chiral reagent 2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl isothiocyanate, obtained the resolution of the enantiomers of Cathine with a separation factor of 1.12. This difference demonstrates the advantage of using MIP as the stationary phase for chiral resolution.
Chromatographic results indicate that the enantiomer of the template was also retained in the column due to the possible hydrophilic interaction between the enantiomer and the methacrylic acid in MIP. The presence of this interaction is very clear since a polar compound acetone could also be retained in the column with a retention time of 7.0 min. However, since the MIP did not contribute to the favorable retention of hydrophobic substances, toluene was thus considered as the non-retained component. In an imprinting of Nα-protected amino acids and derivatives using methacrylic acid and EGDMA, respectively as the functional and crosslinking monomers, Kempe and Mosbach (1994) also used toluene as the void marker.
In comparison with the template, its enantiomer was only slightly retained in the column by MIP and had a small increase of retention time with sample load. On the contrary, the retention of the template was strongly dependent on the sample loading. As shown in Table 1, Table 2, a longer retention of template molecules was obtained with a heavy sample load of the mixture. The separation factor was also a function of sample load. In the column using (−)-Cathine as the print molecule, the selectivity increased monotonously with the loading of print molecules in the sample, while in the column using (+)-Cathine the observed maximum in separation factor was present at medium sample loading. A similar finding that sample load increased capacity factor and changed selectivity has been previously reported in the system using (−)-nicotine as the print molecule (Andersson et al., 1999). According to Andersson et al. (1999), several mechanisms were likely to be acting in concert, and they proposed a hypothetical model, suggesting that different template functional monomer complex states are present and that a gradual increase in analyte concentration shifts the equilibrium from higher complex toward lower complex states and consequently causes an increase in chromatographic retention. Higher order template–template complexes during the imprinting process and in the eluent could also be present. The possible presence of template association during polymerization could explain the sample load optimum for enantiomer resolution that was observed in our study, since the print molecule was likely to have a larger increase of retention time with sample load, in comparison with its enantiomer in the mixture. Although the finding that the retention factor increased with increasing sample load could be explained by the published model, it should be noted that this finding is the opposite to that generally observed for imprinted polymers.
In addition to the interactions involving in the recognition, a weak interaction might involve in the retention of the template. Template molecules were strongly retained by the MIP due to the recognition, and also by the non-specific weak interaction, the latter resulting in a broader peak shape. The non- specific binding became more significant when the amount of template in the sample increased. The time to conclude the chromatography was thus longer and the elution peak became more tailing. It is believed that the recognition and the non-specific interactions were all caused by the methacrylic acid functionality.
Although the selectivity (shown in Table 1, Table 2) was very high, the elution of broad peaks with a little tailing was usually obtained. This phenomenon is not unique, but rather very common in MIP systems (Sellergren and Shea, 1995). This poor performance could be attributed to many possible reasons. In addition to the above- mentioned non-specific interaction, the heterogeneity of the adsorbent and relatively high degree of crosslinking could result in peak spreading and tailing. The high degree of crosslinking needed to capture a specific arrangement of functional monomers in MIP can hinder the diffusion of analytes in the particles, resulting in band spreading (Plunkett and Arnold, 1995). According to a review by Sajonz et al. (1998), a heterogeneous population of binding sites formed in the imprinted polymer is usually the result of a partially incomplete monomer–template association and the amorphous nature of the polymer structure. The peak asymmetry could originate from a combination of mass overloading and slow mass transfer rate that is associated with high energy binding sites, forming from the higher complexes of template with functional monomer. The peak broadening observed in the present work could be caused largely by this heterogeneous population of imprints with varying complementarity, ranging from high to low, to the analyte.
Like a typical chromatographic column packed with MIP, the use of MIP particles prepared from the bulk polymerization might also contribute to the dispersion in chromatographic peaks as shown in Figure 2, Figure 3. The particles that resulted from this method were irregular in particle shape and dispersed in particle size distribution. Therefore, advances on the polymerization technique for the MIP preparation are needed While the use of a two-phase system for MIP polymerization (suspension polymerization, for example) can yield particles in one step (Mayes and Mosbach, 1996, Ansell and Mosbach, 1997), an example on the resolution of tert-butoxycarbonyl d,l-phenylalanine using MIP prepared by suspension polymerization in a perfluorocarbon liquid shows that the elution peaks are still very dispersed (Ansell and Mosbach, 1997), meaning that there is significant room for improvement in the preparation of imprinted materials. For the practical application of enantiomer separation, the resolution of peaks presented in Figure 2, Figure 3 needs to be improved by optimizing the preparation conditions of MIP. For example, the amounts and types of monomers and cross-linkers, the mass ratio of template to total amount of monomers and crosslinkers, and the volume of solvent should be carefully optimized (Ansell, 2005).
Although Cathine is commonly used in a racemic form, the enantiomers are different from each other in their pharmacological actions (Young et al., 1999, Wang et al., 2005). The resolution of these enantiomers will be very helpful for studying the pharmacological effect of each enantiomer (Glennon et al., 1984, Sporkert et al., 2003, Boysen and Hearn, 2010). Despite the dispersion of elution peaks, the chromatographic columns prepared in this study were effective for the quantitative analysis, based on the evaluation of peak areas. As shown in Fig. 4, a linear calibration up to a load of 90 μg of template and its enantiomer in the sample (20 μL) could be obtained. The slope for (+)-Cathine was slightly larger than that for (−)-Cathine, as the former had a higher purity.
Figure 4.
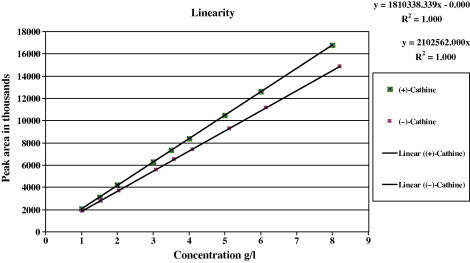
Peak areas of (+)-Cathine  and (−)-Cathine
and (−)-Cathine as functions of sample loading on a column using molecularly imprinted polymer with (+)-Cathine as the template.
as functions of sample loading on a column using molecularly imprinted polymer with (+)-Cathine as the template.
3.3. Influence of mobile composition on the retention of Cathine
Since the template employed for MIP preparation in this work has a simple structure, the mobile phase played a very important role on the resolution of template and its enantiomer. A mobile phase containing polar substances was used to weaken the binding of target (template) molecules and consequently to release them on the imprinting cavity of the stationary phase. The mobile phase consisted of HPLC grade water, acetic acid and acetonitrile. In the absence of water, both template and its enantiomer were eluted in a single peak by a mobile phase selected from several combinations of acetic acid and acetonitrile concentrations. Although water must be present in order to yield resolved peaks, the water concentration did not significantly influence the retention times of the eluted peaks (Table 3).
Table 3.
Effect of acetic acid concentration in the mobile phase on the retention of enantiomers in the column packed with MIP particles using (−)-Cathine as the template.
| S. No. | Mobile phase composition (% v/v) |
Retention time (min) |
|||
|---|---|---|---|---|---|
| Water | CH3COOH | CH3CN | (+)-Cathine | (−)-Cathine | |
| 1 | 2.0 | 2.0 | 96.0 | 16.3 | 59.6 |
| 2 | 2.0 | 3.0 | 95.0 | 15.1 | 59.7 |
| 3 | 2.0 | 4.0 | 94.0 | 15.8 | 44.9 |
| 4 | 2.0 | 5.0 | 93.0 | 15.6 | 40.9 |
| 5 | 2.0 | 6.0 | 92.0 | 15.0 | 29.4 |
| 6 | 2.0 | 7.0 | 91.0 | 10.5 | 18.3 |
| 7 | 1.0 | 2.0 | 97.0 | 15.7 | 58.8 |
| 8 | 1.5 | 2.0 | 96.5 | 15.9 | 59.2 |
| 9 | 3.0 | 5.5 | 91.5 | 15.6 | 28.6 |
| 10 | 5.5 | 5.5 | 89.0 | 15.0 | 26.6 |
| 11 | 2.0 | 8.0 | 90.0 | No resolved peaks | |
To investigate the role that acetic acid played on the recognition and binding of template molecule to MIP, chromatographic runs were carried out using different acetic acid content levels in mobile phase with constant fraction of water (2.0%, v/v) the results shown in Table 3 indicate that using a higher concentration of acetic acid could significantly reduce the retention of template molecule, while acetic acid had only a small effect on the non-template enantiomer. Since acetone, a polar solvent, could be retained in the MIP columns with a retention time of 7.0 min, it is believed that acetic acid molecules were likely to bind onto the MIP and displace the target molecules. The acid group in acetic acid could interact with the template, in competition with the immobilized methacrylic acid, and as a result template molecules finally eluted out from the column. As the acetic acid increased up to 8.0% or higher, no resolved peaks were obtainable. Both the template and its enantiomer were eluted together out from the column due to the displacement of acetic acid.
A small amount of water had to be present in the mobile phase, since the racemates could not be satisfactorily resolved without water. This allowed the interaction to occur between methacrylic acid and the template. The polar components in the mobile phase were used to break down the hydrogen bonding and electrostatic binding between the stationary phase and template molecule. The presence of acetic acid lowered the strength of the electrostatic interaction between the amine of Cathine and the carboxyl group in the stationary phase. This electrostatic interaction is the major factor in the recognition of the target molecule (template) in the MIP. On the column packed with the MIP using (−)-Cathine as the template molecule, the retention of (+)-Cathine was weak due to mismatching of the complementary shape. However, (−)-Cathine molecule was more strongly retained due to electrostatic interaction. As the concentration of acetic acid increased to 7.0% (v/v), sharp peaks were obtained and the tailing was also improved. However, acetic concentrations greater than this value resulted in a poor resolution of racemates and overlapping peaks. The polarity of the acetic acid directly influenced the partition of (−)-Cathine in the stationary phase. A pronounced selectivity for (−)-Cathine on the MIP column was thus evident.
4. Conclusion
The increasing demand for optically pure drug formulations has resulted in an interest in developing tools for efficient chiral separation. Since most drug products on the market are asymmetric molecules and administered as racemates. In the present study we have used a simple molecule, Cathine as the template for the preparation of MIP. Chromatographic columns packed with those MIP particles were effective for the resolution of Cathine enantiomers. The recognition and binding of template molecules was based on interactions between amino and hydroxyl groups of the template and the carboxyl group of methacrylic acid, a host molecule in the MIP. Higher values of separation factor obtained in the present work suggest that non-covalent molecular imprinting is a promising method for the resolution of chiral compound with a simple structure.
Acknowledgments
This study was supported by the Malladi Drugs and Pharmaceuticals Ltd., India the authors are grateful to PRIST university chemistry department and helpful discussions.
References
- Andersson Lars I. Molecular imprinting: developments and applications in the analytical chemistry field. J. Chromatogr. B. 2000;745:3–13. doi: 10.1016/s0378-4347(00)00135-3. [DOI] [PubMed] [Google Scholar]
- Andersson H.S., Karlsson J.G., Piletsky S.A., Koch-Schmidt A.-C., Mosbach K., Nicholls I.A. Study of the nature of recognition in molecularly imprinted polymers, II: influence of monomer–template ratio and sample load on retention and selectivity. J. Chromatogr. A. 1999;848:39–49. [Google Scholar]
- Ansell Richard J. Molecularly imprinted polymers for the enantioseparation of chiral drugs. Ad. Drug Delivery Rev. 2005;57:1809–1835. doi: 10.1016/j.addr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Ansell R.J., Mosbach K. Molecularly imprinted polymers by suspension polymerisation in perfluorocarbon liquids, with emphasis on the influence of the porogenic solvent. J. Chromatogr. A. 1997;787:55. [Google Scholar]
- Armstrong D.W., Ward T.J., Armstrong R.D., Beesley T.E. Separation of drug stereoisomers by the formation of β-cyclodextrin inclusion complexes. Science. 1986;232:1132. doi: 10.1126/science.3704640. [DOI] [PubMed] [Google Scholar]
- Boysen Reinhard I., Hearn Milton T.W. Elsevier Science Publishing Company; New Jersey: 2010. Comprehensive Natural Products II. (Chapter 9.02, pp. 5-49) [Google Scholar]
- Dauwe Christian, Sellergren Börje. Influence of template basicity and hydrophobicity on the molecular recognition properties of molecularly imprinted polymers. J. Chromatogr. A. 1996;753:191–200. [Google Scholar]
- Fischer L., Muller R., Ekberg B., Mosbach K. Direct enantioseparation of beta-adrenergic blockers using a chiral stationary phase prepared by molecular imprinting. J. Am. Chem. Soc. 1991;113:9358–9360. [Google Scholar]
- Gal Joseph, Sedman Allen J. R-á-methylbenzyl isothiocyanate, a new and convenient chiral derivatizing agent for the separation of enantiomeric amino compounds by high-performance liquid chromatography. J. Chromatogr.A. 1984;314:275–281. [Google Scholar]
- Glennon Richard A., Schechter Martin D., Rosecrans John A. Discriminative stimulus properties of S(−)- and R(+)-cathinone, (+)-cathine and several structural modifications. Pharmacol. Biochem. Behav. 1984;21:1–3. doi: 10.1016/0091-3057(84)90121-7. [DOI] [PubMed] [Google Scholar]
- Harada Kumiko, Yuan Qun, Nakayama Morio, Sugii Atsushi. Effects of organic modifiers on the chiral recognition by different types of silica-immobilized bovine serum albumin. J. Chromatogr. A. 1996;740:207–213. doi: 10.1016/0021-9673(96)00128-8. [DOI] [PubMed] [Google Scholar]
- Hermansson J. Liquid chromatographic resolution of racemic drugs using chiral α1-acid glycoprotein column. J. Chromatogr. 1984;298:67–68. [Google Scholar]
- Kempe M., Mosbach K. Chiral recognition of N alpha-protected amino acids and derivatives in non-covalently molecularly imprinted polymers. Int. J. Pept. Protein Res. 1994;44:603–606. doi: 10.1111/j.1399-3011.1994.tb01150.x. [DOI] [PubMed] [Google Scholar]
- Kempe Maria, Mosbach Klaus. Direct resolution of naproxen on a non-covalently molecularly imprinted chiral stationary phase. J. Chromatogr. A. 1994;664:276–279. [Google Scholar]
- Kempe Maria, Mosbach Klaus. Separation of amino acids, peptides and proteins on molecularly imprinted stationary phases. J. Chromatogr. A. 1995;691:317–323. doi: 10.1016/0021-9673(94)00820-y. [DOI] [PubMed] [Google Scholar]
- Kempe M., Mosbach K. Molecular imprinting used for chiral separations. J. Chromatogr. A. 1995;694:3–13. [Google Scholar]
- Konig W.A., Benecke I. Gas chromatographic separation of enantiomers of amines and amino alcohols on chiral stationary phases. J. Chromatogr. 1981;209:91–95. [Google Scholar]
- Matsui Jun, Kaneko Akio, Miyoshi Yoko, Yokoyama Kenji, Tamiya Eiichi, Takeuchi Toshifumi. A molecularly imprinted nicotine-selective polymer. Anal. Lett. 1996;29:2071–2078. [Google Scholar]
- Mayes A.G., Mosbach K. Molecularly imprinted polymer beads: suspension polymerization using a liquid perfluorocarbon as the dispersing phase. Anal. Chem. 1996;68:3769–3774. doi: 10.1021/ac960363a. [DOI] [PubMed] [Google Scholar]
- Mosbach K. Molecular imprinting. Trends Biochem. Sci. 1994;19:9–14. doi: 10.1016/0968-0004(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Motellier S., Wainer I.W. Direct stereochemical resolution of aspartame stereoisomers and their degradation products by high-performance liquid chromatography on a chiral crown ether based stationary phase. J. Chromatogr. A. 1990;516:365. doi: 10.1016/s0021-9673(01)89277-3. [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Kaida Y. Resolution by high-performance liquid chromatography using polysaccharide carbamates and benzoates as chiral stationary phases. J. Chromatogr. A. 1994;666:403–419. [Google Scholar]
- O’Shannessy D.J., Ekberg B., Andersson L.I., Mosbach K. Recent advances in the preparation and use of molecularly imprinted polymers for enantiomeric resolution of amino acid derivatives. J. Chromatogr. A. 1989;470:391–399. [Google Scholar]
- Pettersson C., Schill G. Separation of enantiomeric amines by ion-pair chromatography. J. Chromatogr. 1981;204:179–183. doi: 10.1016/s0021-9673(00)81656-8. [DOI] [PubMed] [Google Scholar]
- Plunkett S., Arnold F.H. Molecularly imprinted polymers on silica: selective supports for high-performance ligand-exchange chromatography. J. Chromatogr. A. 1995;708:19. doi: 10.1016/0021-9673(95)00378-z. [DOI] [PubMed] [Google Scholar]
- Ramstrom O., Andersson L.I., Mosbach K., Ramstroem Olof, Andersson Lars I., Mosbach K. Recognition sites incorporating both pyridinyl and carboxy functionalities prepared by molecular imprinting. J. Org. Chem. 1993;58:7562–7564. [Google Scholar]
- Ramstrom O., Yu C., Mosbach K. Chiral recognition in adrenergic receptor binding mimics prepared by molecular imprinting. J. Mol. Recognit. 1996;9:691–696. doi: 10.1002/(sici)1099-1352(199634/12)9:5/6<691::aid-jmr322>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Sajonz P., Kele M., Zhong G., Sellergren B., Guiochon G. Study of the thermodynamics and mass transfer kinetics of two enantiomers on a polymeric imprinted stationary phase. J. Chromatogr. A. 1998;810:1. [Google Scholar]
- Sajonz P., Kele M., Zhong G., Sellergren B., Guiochon G. Study of the thermodynamics and mass transfer kinetics of two enantiomers on a polymeric imprinted stationary phase. J.Chromatogr. A. 1998;810:1–17. [Google Scholar]
- Sellergren B. Imprinted chiral stationary phases in high-performance liquid chromatography. J. Chromatogr. A. 2001;906:227–252. doi: 10.1016/s0021-9673(00)00929-8. [DOI] [PubMed] [Google Scholar]
- Sellergren B., Shea K.J. Origin of peak asymmetry and the effect of temperature on solute retention in enantiomer separations on imprinted chiral stationary phases. J. Chromatogr. A. 1995;690:29–39. [Google Scholar]
- Sellergren B., Ekberg B., Mosbach K. Molecular imprinting of amino acid derivatives in macroporous polymers: demonstration of substrate- and enantio-selectivity by chromatographic resolution of racemic mixtures of amino acid derivatives. J. Chromatogr. A. 1985;347:1–10. [Google Scholar]
- Sporkert Frank, Pragst Fritz, Bachus Rainer, Masuhr Florian, Harms Lutz. Determination of cathinone, cathine and norephedrine in hair of Yemenite khat chewers. Forensic Sci. Int. 2003;133:39–46. doi: 10.1016/s0379-0738(03)00048-3. [DOI] [PubMed] [Google Scholar]
- Suedee R., Songkram C., Petmoreekul A., Sangkunakup S., Sankasa S., Kongyarit N. Direct enantioseparation of adrenergic drugs via thin-layer chromatography using molecularly imprinted polymers. J. Pharm. Biomed. Anal. 1999;19:519–527. doi: 10.1016/s0731-7085(98)00248-9. [DOI] [PubMed] [Google Scholar]
- Vallano Patrick.T., Remcho Vincent.T. Highly selective separations by capillary electrochromatography: molecular imprint polymer sorbents. J. Chromatogr. A. 2000;887:125–135. doi: 10.1016/s0021-9673(99)01199-1. [DOI] [PubMed] [Google Scholar]
- Wang Sheng-Meng, Lewis Russell J., Canfield Dennis, Li Tien-Lai, Chen Chang-Yu, Liu Ray H. Enantiomeric determination of ephedrines and norephedrines by chiral derivatization gas chromatography–mass spectrometry approaches. J. Chromatogr B. 2005;825:88–95. doi: 10.1016/j.jchromb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Wulff G. Molecular recognition in polymers prepared by imprinting with templates. ACS Symp. Ser. 1986;308:186. [Google Scholar]
- Wulff G., Vesper W. Preparation of chromatographic sorbents with chiral cavities for racemic resolution. J. Chromatogr. 1978;167:171. [Google Scholar]
- Young Richard, Bondarev Mikhail, Glennon Richard A. An examination of isomeric phenylpropanolamines in (−)ephedrine-trained rats. Drug Alcohol Depend. 1999;57:1–6. doi: 10.1016/s0376-8716(99)00052-6. [DOI] [PubMed] [Google Scholar]


