Abstract
Background
This study examined the efficacy, complications, and contracture recurrence in patients who received injectable collagenase clostridium histolyticum (CCH) for Dupuytren’s-induced metacarpophalangeal (MP) and proximal interphalangeal (PIP) joint contractures.
Methods
A retrospective chart review at one center compared the degree of MP and PIP joint contracture pre-injection, post-cord rupture, and at final follow-up after a minimum duration of 6 months. Recurrence was defined as a 20 ° or greater increase in contracture above the minimum value achieved.
Results
Of 102 eligible patients, 48 patients (47 %) (31 males, 17 females) were available for review. 53 digits and 64 joints (46 MP joints and 18 PIP joints) were studied. The mean patient age was 66 years (range, 48–87 years) and mean follow-up duration was 15 months (range, 6 to 25 months). The mean MP joint contracture was 51 ± 20 ° at baseline, 4 ± 8 ° post-cord rupture, and 9 ± 15 ° at latest follow-up. The mean PIP joint contracture was 39 ± 23 ° at baseline, 14 ± 14 ° at cord rupture, and 29 ± 20 ° at latest follow-up. Of the 46 MP joints and 18 PIP joints, 11 MP (24 %) and 7 (39 %) PIP joints met the recurrence criteria. Of 102 patients, 1 patient had a small finger flexor tendon rupture.
Conclusions
Despite the dramatic initial reduction in contracture, recurrence developed in a high proportion of patients over the study period. While initially effective, CCH may not provide durable contracture reduction. However, CCH remains a viable nonsurgical treatment for Dupuytren’s disease.
Keywords: Collagenase, Dupuytren’s disease, Recurrence
Introduction
Dupuytren’s disease is a complex, progressive, genetic disorder, characterized by an abnormal balance of collagen on the palmar surface of the hand [7, 12]. The estimated global prevalence of this disease ranges between 3 and 6 %, with the highest incidence in males of northern European descent [10]. Current evidence suggests that it is inherited in an autosomal dominant fashion with variable penetrance [10]. Dupuytren’s disease generally begins as a hard nodule on the palm and slowly progresses into a fibrous cord that pulls the affected digit into a flexed contracture. The digits most commonly affected are the ring and small fingers, and contractures usually occur at either the metacarpophalangeal (MP) joint, proximal interphalangeal (PIP) joint, or rarely, the distal interphalangeal joint. Although Dupuytren’s disease may be quite debilitating across a range of manual tasks, it is generally painless.
Several treatment options are available to patients with Dupuytren’s disease, including open fasciectomy, limited or minimally invasive fasciectomy, radiation therapy, and needle aponeurotomy. Each technique has its unique advantages and disadvantages [3, 5, 6, 8, 14]. A recent addition to the treatment options for Dupuytren’s disease is a nonsurgical, injectable collagenase clostridium histolyticum (CCH) enzyme [11, 16]. This drug was approved by the Food and Drug Administration in February 2010 and marketed under the name Xiaflex® in the United States, (Auxilium Pharmaceuticals, Malvern, PA). This bacteria-derived enzyme functions by preferentially lysing type I and type III collagen, which are the major constituents of the diseased tissue [2, 17].
Early results of CCH treatment have been encouraging [9, 11, 22]. Recently, outcomes focused on the recurrence rates following CCH have become available, and indicate that recurrence occurs at varying rates depending on the joint involved and the severity of the baseline contracture [13, 20]. The purpose of this study is to present our experience with the use of CCH injection after a minimum follow-up duration of 6 months, with particular emphasis on recurrence and subjective patient outcomes.
Materials and Methods
This was a retrospective chart review that involved prospective patient recall. The study was approved by our institutional review board and written consent was obtained from all participants. An internal database was queried to identify all patients who were treated with CCH between June 2010 and June 2012. All eligible patients received the CCH injection by one of seven different board certified hand surgeons and returned 24 hours later for digital manipulation and cord rupture. Digital block with 1–2 % Xylocaine (lidocaine HCl) was used just prior to digit manipulation to obtain pain relief, with the aim of achieving a more complete cord rupture. Patients were then referred to a hand therapist, who fitted them with a customized thermoplastic dorsal- or volar-based orthosis, and provided home therapy exercise education. Similar to the Collagenase Option for the Reduction of Dupuytren’s (CORD I and II) studies, splinting and home therapy were not monitored or enforced. No specific disease patterns or treatment protocols were used to determine which patients received CCH. All patients were offered other interventions, which in our practice typically includes mini-incision fasciectomy and when warranted, open fasciectomy. The presence of disease in multiple digits may have motivated patients to proceed with formal surgical intervention as the possibility of multiple CCH injections to address pathology at adjacent digits and joints was discussed prior to CCH. Family history did not play a role in the decision algorithm for CCH treatment.
The inclusion criteria were (1) aged at least 18 years at the time of injection and (2) a minimum of 6 months between the time of injection and the research query. Patients who were enrolled in a separate study that involved a regimented hand therapy program for severe PIP joint contractures were excluded [15]. Basic demographic data and information on the hand, digit, and joint treated, pre-injection degree of contracture, minimum degree of contracture achieved immediately post-cord rupture, and side effects or complications experienced were collected via chart review. Patients who met the inclusion criteria were then asked to return to the clinic to have their finger contractures measured. One observer collected the degree of passive contracture using a finger goniometer. We defined contracture recurrence as a 20 ° or greater increase in contracture relative to the most corrected measurement achieved at cord rupture.
A total of 102 patients met the inclusion criteria and were contacted. Of these patients, 29 did not respond or were unreachable and 25 were reached, but declined participation, which left 48 patients for review (47 %). Among this subgroup, there were 53 digits (27 small, 19 ring, 5 long, and 2 index), and 64 joints. These patients had a mean age of 66 years (range, 48–87) and there were 31 males and 17 females. Of the 64 treated joints, 46 were MP joints and 18 were PIP joints. The mean length of follow-up was 15 months (range, 6–25 months). Complications or post-injection side effects were noted for all 48 patients. Additional treatment, or major complications (flexor tendon or pulley rupture) following injection were assessed in all 102 patients.
During the follow-up appointment, the Quick Disabilities of the Arm, Shoulder, and Hand (Quick DASH) questionnaire was also administered. Patients were also asked whether they would recommend collagenase to other individuals with Dupuytren’s disease (yes/no answer); asked whether they would have another injection (yes/no answer); and asked to rate their satisfaction with treatment on a scale of 1–10, with 1 indicating complete dissatisfaction and 10 indicating complete satisfaction. Those patients whose follow-up appointment was with their surgeon, rather than the research team did not receive either questionnaire.
A one-way ANOVA with Bonferroni post hoc analysis was used to determine the differences in means at the three time points: ( 1) pre-injection, (2) post-cord rupture, and (3) latest follow-up. A p value less than 0.05 was considered statistically significant.
Results
For the 64 treated joints, the mean initial degree of contracture was 48 ± 21 °. After cord rupture, the mean contracture decreased to 7 ± 11 °, and the mean contracture at latest follow-up became 15 ± 19 °. The mean baseline contracture for the 46 MP joints was 51 ± 20 °. Post-cord rupture mean contracture was reduced to 4 ± 8 °, and at latest follow-up, the contracture became 9 ± 15 °. The differences in means were statistically significant at all time points (p <0.05). The mean pre-injection contracture for the 18 treated PIP joints was 39 ± 23 °. Immediately following cord rupture, the mean contracture became 14 ± 14 °, and at latest follow-up, the average PIP contracture became 29 ± 20 °. The differences in means were also statistically significant at all time points (p <0.05).
Of the patients, 15 of 102 required additional interventions in the form of either another CCH injection (n = 10) or surgical release (n = 5). Based on the values obtained from patients with at least 6 months of follow-up, 18 of the 64 joints (28 %) had contracture recurrence during the study period, according to our definition. Of the 46 MP joints treated, 11 (24 %) recurred, while 7 of 18 (39 %) of PIP joints recurred.
Out of 102 patients, there was one major complication, which was a flexor tendon rupture in one patient treated for a small finger PIP joint contracture. Within the subgroup of patients with 6-month follow-up data, minor adverse events experienced included: ecchymosis in 56 of 64 (88 %) treated joints, localized or palmar edema in 25 of 64 (39 %), a skin tear following manipulation in 11 of 64 (17 %), and swelling or tenderness of the axillary lymph nodes in 4 of 64 (6 %) cases.
Quick DASH scores were obtained for 36 of 48 patients (75 %) and the average score at latest follow-up was 3 (range, 0–18). Out of 32 respondents, 28 (88 %) indicated that they would recommend the procedure to another individual with Dupuytren’s disease. Also, 28 of 32 responders (88 %) indicated that they would repeat the procedure. Lastly, 42 of 48 (88 %) patients responded to the satisfaction questionnaire and the mean satisfaction score was 8.5 (range, 1–10).
Discussion
The goals of this study were (1) to determine the efficacy of CCH injection for patients with Dupuytren’s contracture and (2) to examine the extent of contracture recurrence within the study period. The main difference between our patient population and those in the CORD I and II, Joint I and II, and the CORD Long-Term Evaluation Safety Study (CORDLESS) studies, was the use of local anesthesia for digital block during cord rupture. This may have allowed our patients to tolerate manipulation more effectively and could have allowed for a more complete cord rupture. However, this practice probably also lead to an increase in the rate of skin tears relative to previous studies. We observed a skin tear incidence of 17 %, relative to 11 % in the CORD I study and 9 % in the Joint studies [11, 22].
Joint I and II reported that a total of 71 % of patients were “very satisfied” and 21 % were “quite satisfied” with collagenase treatment [22]. We found that the mean satisfaction score in our patients was 8.5 (range, 1–10). The data from both studies seem to indicate that the majority of patients were pleased with the outcome.
Based on the recurrence data presented in various studies, there appears to be a positive correlation between follow-up duration and the development of recurrence (Table 1). However, it is difficult to directly compare results given the use of various definitions for recurrence. Despite our smaller cohort and shorter duration of follow-up, our patients showed similar rates of contracture recurrence compared to the CORDLESS study, which had a longer follow-up duration, and a larger sample size [13]. Ideally, the application and use of a standard definition for recurrence may better allow for direct comparison of the data between studies [5, 21]. A recent study that addressed the issue of “recurrence” definitions analyzed 20 studies, each of which utilized a different definition, with only one applying a quantitative measure of recurrence [21]. The study found that a commonly used qualitative definition of recurrence was “the reappearance of Dupuytren’s tissue in the operative field,” whereas quantitative definitions, such as “worsening of total passive extension deficit greater than 30 °,” were much less frequently applied [18, 19, 21]. For the purposes of this study, contracture recurrence was defined as a 20-degree or greater increase of the joint contracture relative to the minimum contracture measured at the time of cord rupture. This definition was selected because it is similar to the definition used in the CORD, Joint, and CORDLESS studies, and is probably of reasonable magnitude to alert patients and providers to a gross appearance of recurrence. The presence of a palpable cord however, was not documented in our medical records and therefore not incorporated into our definition, making it slightly different from the definition used in the aforementioned studies [9, 11, 13, 22]. Furthermore, we assessed recurrence in all patients, and not in a subgroup of patients. The definition for recurrence in the CORD, Joint, and CORDLESS studies is “an increase in joint contracture to 20 degrees or more in the presence of a palpable cord at any time during the study, evaluated in primary joints that reached the primary endpoint” (where the primary endpoint is a reduction of joint contracture to 0–5 °) [9, 11, 13, 22]. We believe that this definition introduces a type of selection bias and may mislead patients, researchers, and physicians during the assessment of outcomes of Dupuytren’s disease, particularly because joints with a low baseline contracture severity respond better to injection with collagenase than those with high baseline contracture severity [9, 22]. Furthermore, it is rather uncommon for severe PIP joint contractures to correct to 0–5 ° of passive extension despite surgical or nonsurgical treatment (Fig. 1) [1, 15]. According to the CORD, Joint, and CORDLESS definition, outcomes that do not reach the primary endpoint, i.e., 0–5 °, would be excluded from the pool that would eventually be evaluated for recurrence [9, 11, 13, 22]. Patients who do not attain a contracture less than 5 °, but have a measurable improvement greater than 20 °, will be evaluated for “durability of response” and cast into the “non-durable” category of outcomes, which we believe is a superfluous category that may confuse patients during counseling [13]. In effect, according to the CORD, Joint, and CORDLESS definition of recurrence, patients with a lower baseline contracture and a favorable response to treatment are filtered into a pool that will be assessed for recurrence, giving a falsely low recurrence rate. It is important to highlight that compared to the CORD, Joint, and CORDLESS studies, our inclusive reporting offers both providers and patients a more complete analysis of recurrence.
Table 1.
A comparison of definitions and recurrence rates following CCH injection
| Study | Definition of Recurrence in study that used CCH | Recurrence rate all joints | Recurrence rate MP joints | Recurrence rate PIP joints | Duration of follow-up |
|---|---|---|---|---|---|
| CORD I [11] | An increase in joint contracture to 20 ° or more in the presence of a palpable cord at any time during the study, evaluated in primary joints that reached the primary endpointa | 0 | 0 | 0 | 90 days |
| CORD II [9] | An increase in joint contracture to 20 ° or more in the presence of a palpable cord at any time during the study, evaluated in primary joints that reached the primary endpointa | 0 | 0 | 0 | 12 months |
| Joint I and II [22] | An increase in joint contracture to 20 ° or more in the presence of a palpable cord at any time during the study, evaluated in primary joints that reached primary endpointa. | 19 of 497 joints (4 %) | Not reported | Not reported | 9 months |
| CORDLESS [13] | An increase in joint contracture to 20 ° or more in the presence of a palpable cord at any time during the study, evaluated in primary joints that reached primary endpointa | 217 of 623 joints (35 %) | 121 of 451 joints (27 %) | 96 of 172 joints (56 %) | 3 years |
| Watt et al. [20] | Any increase in the degree of contracture of injected joint compared with maximum extension achieved after injection | 6 of 8 joints (75 %) | 4 of 6 joints (66 %) | 2 of 2 joints (100 %) | 8 years |
| Current study | An increase in joint contracture to 20 ° or more from the minimum contracture reached at cord rupture. | 18 of 64 joints (28 %) | 11 of 46 joints (24 %) | 7 of 18 joints (39 %) | 15 months average; minimum of 6 months |
aPrimary endpoint defined as a reduction of contracture to 0–5 º of extension
Fig. 1.
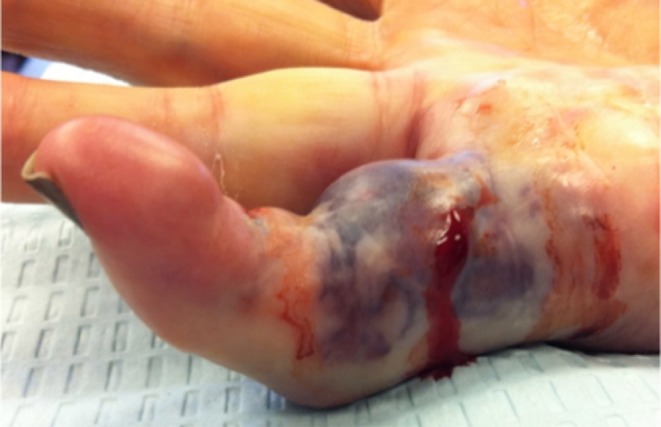
PIP joint following injection and manipulation with residual flexion contracture that is unlikely to improve to 0–5 º of extension without a rigorous rehabilitation protocol. (Courtesy of Terri M. Skirven, OTR/L, CHT, Philadelphia Hand Center)
The limitations of our study include the retrospective design, which likely yielded inconsistencies in pre and post-injection contracture measurements given the large number of physicians, therapists, and researchers involved with data measurements. The study’s retrospective nature also made precise determination of when exactly recurrence began to be difficult for each patient. The unusually high proportion of women in the study group reflects the fact that fewer of the women in the initial cohort of 102 patients were lost to follow-up. Seventeen of 23 women (74 %) returned to participate in the study, while only 31 of 79 men (39 %) returned for follow-up. Additionally, the splinting and home exercise therapy components were not monitored, similar to the CORD and JOINT studies, making it impossible to determine patient compliance rates and the value of rehabilitation as an intervention. The Quick DASH was used as an outcome assessment tool for our patients. Although some investigators have attempted to validate this questionnaire for Dupuytren’s disease, it has not been found to be particularly reflective of clinically meaningful changes [4]. At the current time, there does not seem to be any consensus about the utility of any particular patient rated outcome measure for Dupuytren’s disease.
Overall, the results from this study suggest that while initially effective at reducing contractures, CCH may not provide durable contracture reduction in a significant portion of patients, as demonstrated by the 28 % of patients who experienced recurrence within the mean 15 months of follow-up. However, CCH remains a viable nonsurgical alternative for the treatment of Dupuytren’s disease.
Acknowledgments
We would like to acknowledge Mrs. Jennifer Kuruc for her assistance with data acquisition.
Conflict of Interests
A. Lee Osterman: Consultant and speaker on bureau for Auxilium Pharmaceuticals; Randall W. Culp: Paid speaker for Auxilium Pharmaceuticals; Sidney M. Jacoby: Paid speaker for Auxilium Pharmaceuticals. None of the other coauthors have any conflicts of interest.
References
- 1.Andrew JG. Contracture of the proximal interphalangeal joint in Dupuytren’s disease. J Hand Surg Br. 1991;16:446–448. doi: 10.1016/0266-7681(91)90024-I. [DOI] [PubMed] [Google Scholar]
- 2.Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: Non-operative treatment of Dupuytren’s disease. J Hand Surg Am. 2002;27:788–798. doi: 10.1053/jhsu.2002.35299. [DOI] [PubMed] [Google Scholar]
- 3.Betz N, Ott OJ, Adamietz B, et al. Radiotherapy in early-stage Dupuytren’s contracture. Long-term results after 13 years. Strahlenther Onkol. 2010;186:82–90. doi: 10.1007/s00066-010-2063-z. [DOI] [PubMed] [Google Scholar]
- 4.Budd HR, Larson D, Chojnowski A, et al. The Quick DASH score: a patient-reported outcome measure for Dupuytren’s surgery. J Hand Ther. 2011;24:15–20. doi: 10.1016/j.jht.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Crean SM, Gerber RA, Le Graverand MPH, et al. The efficacy and safety of fasciectomy and fasciotomy for Dupuytren’s contracture in European patients: a structured review of published studies. J Hand Surg Eur. 2011;36:396–407. doi: 10.1177/1753193410397971. [DOI] [PubMed] [Google Scholar]
- 6.Eaton C. Percutaneous fasciotomy for Dupuytren’s contracture. J Hand Surg Am. 2011;36:910–915. doi: 10.1016/j.jhsa.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Gelberman R, Amiel D, Rudolph R, et al. Dupuytren’s contracture: an electron microscopic, biochemical, and clinical correlative. J Bone Joint Surg Am. 1980;62:425–32. [PubMed] [Google Scholar]
- 8.Gelman S, Schlenker R, Bachoura A, et al. Minimally invasive partial fasciectomy for Dupuytren’s contractures. Hand. 2012;7:269–364. doi: 10.1007/s11552-012-9461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilpin D, Coleman S, Hall S, et al. Injectable collagenase Clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am. 2010;35:2027–2038. doi: 10.1016/j.jhsa.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Hu FZ, Nystrom A, Ahmed A, et al. Mapping of an autosomal dominant gene for Dupuytren’s contracture to chromosome 16q in a Swedish family. Clin Genet. 2005;68:424–429. doi: 10.1111/j.1399-0004.2005.00504.x. [DOI] [PubMed] [Google Scholar]
- 11.Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968–79. [DOI] [PubMed]
- 12.Murrell GA, Francis MJ, Bromley L. The Collagen changes of Dupuytren’s contracture. J Hand Surg Br. 1991;16:263–266. doi: 10.1016/0266-7681(91)90050-X. [DOI] [PubMed] [Google Scholar]
- 13.Peimer CA, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with collagenase Clostridium histolyticum (CORDLESS Study): 3-year data. J Hand Surg Am. 2013;38:12–22. doi: 10.1016/j.jhsa.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Shin EK, Jones NF. Minimally invasive technique for release of Dupuytren’s contracture: segmental fasciectomy through multiple transverse incisions. Hand. 2011;6:256–259. doi: 10.1007/s11552-011-9336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skirven T, Bachoura A, Jacoby SM, et al. The effect of a therapy protocol for increasing correction of severely contracted proximal interphalangeal joints Caused by Dupuytren’s disease and treated with collagenase injection. J Hand Surg Am. 2013;38:684–9. [DOI] [PubMed]
- 16.Starkweather KD, Lattuga S, Hurst LC, et al. Collagenase in the treatment of Dupuytren’s disease: an in vitro study. J Hand Surg Am. 1996;21:490–495. doi: 10.1016/S0363-5023(96)80368-6. [DOI] [PubMed] [Google Scholar]
- 17.Syed F, Thomas AN, Singh S, et al. In vitro study of novel collagenase (XIAFLEX®) on Dupuytren’s disease fibroblasts displays unique drug related properties. PLoS One. 2012;7:e31430. doi: 10.1371/journal.pone.0031430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Rijssen AL, Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129:469–477. doi: 10.1097/PRS.0b013e31823aea95. [DOI] [PubMed] [Google Scholar]
- 19.van Rijssen AL, Werker PM. Percutaneous needle fasciotomy in Dupuytren’s disease. J Hand Surg Eur. 2006;31:498–501. doi: 10.1016/j.jhsb.2006.03.174. [DOI] [PubMed] [Google Scholar]
- 20.Watt AJ, Curtin CM, Hentz VR. Collagenase injection as nonsurgical treatment of Dupuytren’s disease: 8-year follow-up. J Hand Surg Am. 2010;35:534–539. doi: 10.1016/j.jhsa.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Werker PM, Pess GM, van Rijssen AL, et al. Correction of contracture and recurrence rates of Dupuytren’s contracture following invasive treatment: the importance of clear definitions. J Hand Surg Am. 2012;37:2095–2105. doi: 10.1016/j.jhsa.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 22.Witthaut J, Jones G, Skrepnik N, et al. Efficacy and safety of collagenase clostridium histolyticum injection for Dupuytren contracture: short-term results from 2 open-label studies. J Hand Surg Am. 2013;38:2–11. [DOI] [PubMed]


