Abstract
As a new potential inflammatory mediator, visfatin plays an important role in inflammation and atherosclerosis. The formation of macrophage-derived foam cells occurs at the early stage of atherosclerosis and underlies the visible fatty streak. Recent studies have indicated that visfatin may be associated with the development of foam cells, but its exact effect and molecular mechanism remain unknown. This study aims to study the effect of visfatin on foamy cell formation and its underlying molecular mechanism. Visfatin levels were determined in apolipoprotein E (ApoE) knockout (KO) mice on a western diet for 16 weeks. Effects of visfatin in cholesterol accumulation were studied both in vivo and in vitro. The levels of scavenger receptors located in macrophage surface were measured in RAW264.7 cells after treatment with visfatin. Visfatin levels were much higher in ApoE KO mice than that in the control mice. Meanwhile, oxidized low-density lipoprotein induces both visfatin release from RAW264.7 cells and its cellular levels within 24 h. Visfatin promotes lipid accumulation mainly through excessive cholesterol uptake not only in RAW264.7 cells but also in peritoneal macrophages isolated from ApoE KO mice. Furthermore, visfatin induces the activation of scavenger receptors (SR)-A and cluster of differentiation (CD)36, but not that of SR-BI, ATP-binding cassette transporter (ABC)A1, or ABCG1 in RAW264.7 cells. Both transcriptional and posttranscriptional regulation may work in concert to mediate the expression of SR-A and CD36 in visfatin-treated cells. Visfatin induces cholesterol accumulation in macrophages and accelerates the process of atherosclerosis mainly through modulating the expression of SR-A and CD36.
Keywords: Atherosclerosis, Visfatin, Foam cell, Scavenger receptor
Introduction
Atherosclerosis, the most common causes of death from coronary artery disease and cerebrovascular disease in developed countries, is characterized by chronic inflammation and redundant cholesterol accumulation within the artery wall, especially in the coronary artery and aorta (Glass and Witztum 2001; Lusis 2000). The fatty streak is the earliest visible atherosclerotic lesion and plays a key role in the development of atherosclerosis (Berliner and Heinecke 1996). The atherosclerotic plaque progression, rupture, and thrombosis always lead to acute coronary syndrome and stoke (Lusis 2000; Ross 1999; Kleemann et al. 2008). The functional disorder of arterial intima induced by kinds of impaired factors, following the migration and differentiation of monocytes here, is a crucial step in the initiation of atherosclerosis (Lusis 2000; Wu et al. 1992; Castagna et al. 2012). The monocyte-derived macrophages swallow unwanted self-modified lipoprotein via scavenger receptors (SRs) located on its surface resulting in foamy cell formation, which underlies the visible fatty streak (Kleemann et al. 2008; Li and Glass 2002). Cholesterol accumulation in macrophage-derived foam cells is due to the broken balance between uptake and reverse transport of cholesterol, which is mainly because of uncontrolled lipid uptake and reduced cholesterol efflux (Kleemann et al. 2008; Collot-Teixeira et al. 2007; Cheng et al. 2011). There are several classes of SRs on macrophage membrane, in which class A (SR-A) and B (SR-B) attract more attention. SR-A and cluster of differentiation (CD)36 are mainly responsible for the uptake of oxidized low-density lipoprotein (ox-LDL) (Collot-Teixeira et al. 2007; Kunjathoor et al. 2002), while SR-B type I (SR-BI) mediates reverse cholesterol transport (Ji et al. 2011). Meanwhile, both ATP-binding cassette transporter A1 and G1 (ABCA1 and ABCG1) participate in the process of efflux of intracellular cholesterol to high-density lipoprotein (HDL) (Cheng et al. 2011; Chen et al. 2011). Thus, the formation of foam cells is predominantly regulated by these SRs and reverse cholesterol transporters (RCTs). Recently, a growing body of evidences has shown that some kinds of adipokine, such as adiponectin, leptin, and vaspin, may affect cholesterol accumulation in macrophages and, thus, accelerate or retard the process of atherosclerosis through modulation of SRs or RCTs (Chen et al. 2010; Spiroglou et al. 2010; Kopff and Jegier 2005; Kjerrulf et al. 2006).
Visfatin (also known as pre-B cell-enhancing factor or PBEF) is an adipokine of which early studies have mainly focused on the metabolic-associated diseases (e.g., obesity and diabetes) (Paschou et al. 2010; De Luis et al. 2008). With the discovery of pro-inflammatory role of visfatin, its potential effect in inflammation has gradually attracted much attention, especially in atherosclerosis, which is closely related with both lipid metabolism and inflammation (Yan et al. 2010; Liu et al. 2009). One of the most related studies has reported that visfatin should be considered as an inflammatory mediator, localized to foam cell macrophages within unstable atherosclerotic lesions, which potentially plays a role in plaque destabilization (Dahl et al. 2007). Although it has been reported that visfatin is participated in the pathogenesis of atherosclerosis and may be involved in the early formation of foam cells, its exact effect is largely unknown. There are still no reports whether visfatin could regulate SRs or RCTs.
The present study aimed to investigate the expression of visfatin in macrophage foam cells in vivo and in vitro. We also studied the impact of visfatin on foamy cell formation and further explored its molecular mechanism.
Materials and methods
Reagents
The reagents used were human-recombined visfatin (Peprotech, USA), anti-CD36, SR-BI, SR-A, ABCG1 (Santa Cruz, USA), anti-visfatin, ABCA1 (Abcam, USA), secondary antibody (Cell Signaling, USA), visfatin ELISA Kit (Raybiotech, USA), Oil red O (ORO; Sigma, USA), fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), penicillin, streptomycin (Gibco, USA), ox-LDL (Yiyuan Biotech, China), Amplex Red Cholesterol Assay Kit, BCA Protein Assay Kit, total cholesterol (TC), triglycerides (TG), high density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) Reagent Kits (Invitrogen, USA), SYBR Green qPCR Supermix (Takara, Japan), small interfering RNA (siRNA) against mouse visfatin, and Transfection Reagent (Ambion, USA).
Animal experiments
Animals Six-week-old male apolipoprotein E (ApoE) knockout (KO) mice and C57BL/6 J mice from Vital River, which were introduced from Jackson Laboratory, were housed at 22 ± 2 °C, 55 ± 5 % relative humidity, with a 12-h light/dark cycle. ApoE KO mice were fed with a western diet (21 % fat and 0.15 % cholesterol) for 16 weeks to establish atherosclerosis model, while C57BL/6 J mice on a common diet as a control group. All experimental mice were allowed access to food and water ad libitum and weighed every 4 weeks.
Serum lipid measurement The mice were anesthetized by intraperitoneal injection with 10 % chloral hydrate (350 mg/kg), following eyeball extirpation to collect their blood after a 16-week experiment. Serum was prepared by a conventional method and subjected to measure the concentrations of TC, TG, HDL-C, and LDL-C by enzymatic procedures.
The protein levels of visfatin in mice To determine the circulating levels of visfatin, mice plasma was prepared and then analyzed using an enzyme-linked immunosorbent assay (ELISA) method according to the operating instructions of visfatin ELISA kit. Meanwhile, the proteins of mice aorta were isolated and measured using Western blotting methods.
Assessment of atherosclerotic lesion area After feeding on a fat-rich diet for 16 weeks, all mice were sacrificed, and hearts were perfused with iced phosphate-buffered saline (PBS) for 5 min. The aortic sinus was embedded and then made into serial cryosections of 7-μm thickness. The atherosclerotic lesions at aortic sinus were stained with ORO as previously described (Van Eck et al. 2000). The mean lesion area for each mouse was calculated from four sections. Whole aortic atherosclerotic lesions were determined by en face preparation followed by ORO staining as previously described (Kamari et al. 2011).
All animal experiments were carried out in accordance with the international guidelines and approved by the Ethics Committee of Southern Medical University.
Cell culture
To check foamy cell formation in vivo, peritoneal macrophages were isolated from ApoE KO mice after a 4-week high-fat diet as previously described (Takahashi et al. 2003). Briefly, the mice were injected intraperitoneally with 3 % thioglycollate, and peritoneal exudates were collected after 4 days. Macrophages were cultured in DMEM containing 10 % FBS. The nonadherent cells were removed after incubation for 3 h at 37 °C. The adherent cells were collected for ORO staining and cholesterol measurement. Murine macrophage RAW264.7 cells were cultured in DMEM supplemented with 10 % FBS, 1 % penicillin, and streptomycin.
Transfection of small interfering RNA
The sequences of siRNA against mouse visfatin were as follows: siRNA forward 5′-GGCACCACUAAUCAUCAGAtt-3′ and reverse5′-UCUGAUGAUUAGUGGUGCCtc-3′. Transfections of siRNA were performed as described for manual of transfection reagent. RAW264.7 cells were incubated for 24 h with siRNA and transfection reagent. Efficiency of visfatin silencing was analyzed at protein levels 2 days after transfection of siRNA.
ORO staining
Cells were washed by PBS and then fixed with 4 % paraformaldehyde for 15 min. After the removal of paraformaldehyde, cells were pretreated with 60 % isopropanol for several seconds and then stained with 0.3 % ORO for 10 min to visualize cellular lipid accumulation. Hematoxylin was used as counterstaining. Staining was recorded on a Nikon microscope equipped with a digital camera. The density of lipid content was evaluated by alcohol extraction after ORO staining. The absorbance at 540 nm was determined with a microplate reader.
Cholesterol measurement
RAW264.7 cells were treated with various concentrations of visfatin for 24 h and then washed twice with PBS. The cellular TC was extracted by hexane/isopropanol. After centrifuging at 12,000 × g, the supernatants were captured and then dried under nitrogen flush and redissolved in isopropanol. The content of TC was determined with cholesterol assay kit. The cellular total protein was measured by BCA assay kit.
Western blotting
The mice aorta or cultured cells were lysed in a lysis buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1 % NP-40, and 1 mM PMSF for 30 min on ice. After centrifugation at 12,000 × g for 15 min at 4 °C, the supernatants were collected as total proteins in aorta or cells. The protein concentrations were also determined with a BCA assay kit. Aliquots (50 μg) of protein samples were separated on 10 % SDS-PAGE and electro-transferred to polyvinylidene fluoride membranes (PVDF, Millipore, Bedford, MA). The PVDF membranes were incubated with primary antibodies (Abs) overnight at 4 °C after being blocked with 5 % nonfat milk and then subjected to secondary Abs for 1 ~ 2 h at 37 °C. The protein bands were detected with an enhanced chemiluminescence system (ECL, CST) on Kodak 2000MM. Densitometric analysis was conducted by Molecular Imaging Software Version 4.0. Actin proteins were detected as a control.
Quantitative real-time PCR
Total cellular RNA was isolated from RAW264.7 cells using TRIzol reagent and converted into cDNAs by Thermoscript reverse transcriptase PCR system. cDNAs were quantified using SYBR Green qPCR Supermix supplemented with specific primers for mouse SR-A (5′-TGGTCCACCTGGTGCTCC-3′ forward and 5′-ACCTCCAGGGAAGCCAAT TT-3′ reverse), CD36 (5′-CAGTTGGAGACCTGCTTATCC-3′ forward and 5′-GCGTCCTGGGTTACATTTTC-3′ reverse) and mouse β-actin (5′-TTGTCCCTGTATGCCTCTGG-3′ forward and 5′-GAGGTCTTTACGGATGTCAACG-3′ reverse) on MX3005P quantitative real-time PCR (qRT-PCR) system. Expression of target genes was measured in triplicate and then normalized to the level of the housekeeping gene β-actin. All the data were analyzed by MXPro 4.01 software.
Statistical analysis
All values are reported as mean ± SEM values and analyzed with SPSS 13.0 version. Student t test was used to compare two independent groups, while one-way analysis of variance followed by student Newman–Keuls q post hoc analyses for multiple groups. Differences were considered significant at P < 0.05.
Results
Expression of visfatin in plasma and atherosclerotic lesions in ApoE KO mice
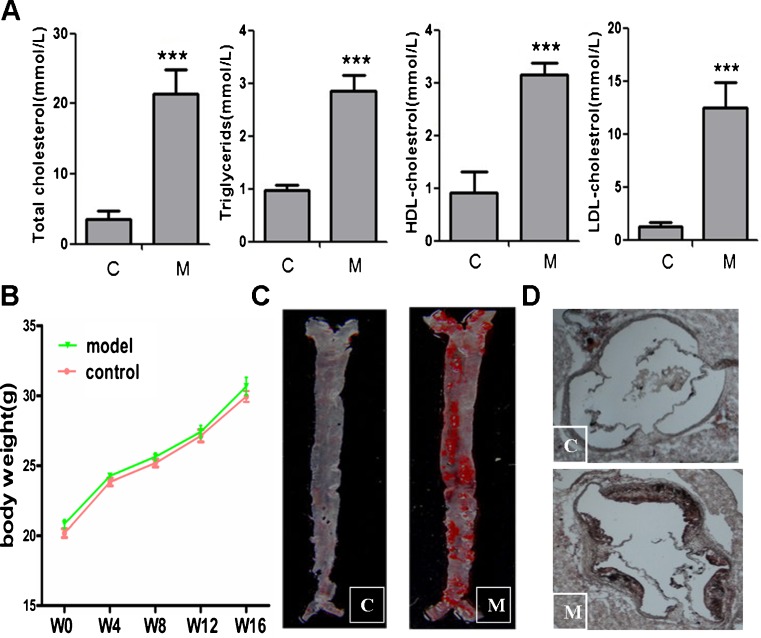
As we know, ApoE KO mouse is a well-established animal model to study atherosclerosis. The mice placed on a western diet gradually develop all the phases of atherosclerotic lesions from fatty streak to atheromatous plaque, which simulates the atherogenesis of humans under the nature state (Plump et al. 1992; Nakashima et al. 1994). After fed with high-fat diet for 16 weeks, the levels of serum lipids in ApoE KO mice, such as TC, TG, HDL-C, and LDL-C, were much higher than that in C57BL/6 J mice (Fig. 1a), while the body weight was not significantly different in these two groups (Fig. 1b). To evaluate the atherosclerotic lesion, whole aorta and aortic sinus were stained with ORO simultaneously and then quantitative analyzed by Image-Pro plus 6.0. The atherosclerotic plaques were much noticeable in both whole aorta and aortic sinus in ApoE KO mice, while there was no obvious lesion in control group (Fig. 1c, d).
Fig. 1.
The serum concentrations of cholesterol and atherosclerotic plaques in ApoE KO mice. a Serum cholesterol including total cholesterol, triglycerides, HDL-C, and LDL-C were determined in both ApoE KO mice with a western diet (M, model) and C57BL/6 J mice with a common diet (C, control) for 16 weeks (n = 6). ***P < 0.0001 vs control group. b Body weight of mice was measured at the beginning on a western diet (W0) and every 4 weeks (W4, W8, W12) till the whole experiment period (W16). The results are the average of eight measurements. P > 0.05 vs control group. c Atherosclerotic plaques in whole aorta both in ApoE KO mice and C57BL/6 J mice after a 16-week experiment. Whole aorta was stained with 0.5 % ORO for 5 min after washing with iced saline. Then the aorta was exposed to 60 % isopropanol for another 5 min and photographed by a digital camera after staining. d Atherosclerotic lesions in mice aorta sinus after a 16-week experiment. Cryosections of aortic sinus was stained with ORO. Representative photographs are shown. Magnification × 200
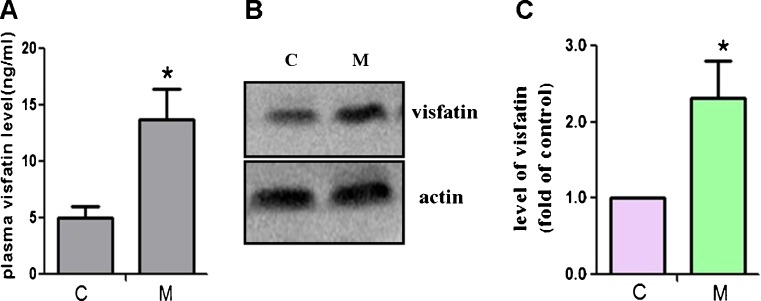
To analyze visfatin expression in mouse atherosclerosis model, we determined its level in both plasma and atherosclerotic lesions, respectively, by ELISA and Western blotting assay. In Fig. 2, we found that both plasma visfatin level and visfatin protein in aorta in the model group were much higher than that in control group. The protein level of visfatin in ApoE KO mice was almost 2.31 times that in C57BL/6 J mice (Fig. 2c). These results indicate that visfatin may play a role in the pathogenesis of atherosclerosis, which is also consistent with the previous research (Dahl et al. 2007).
Fig. 2.
Visfatin expression is markedly increased in ApoE KO mice. a Plasma level of visfatin in ApoE KO mice with a western diet (M, model) and C57BL/6 J mice with a common diet (C, control) for 16 weeks was determined by ELISA assay (n = 6). *P < 0.05 vs control group. b The protein expression of visfatin in mice aorta was determined by Western blot analysis. c Protein bands of visfatin and actin as a control were quantified by Molecular Imaging Software (n = 3). *P < 0.05 vs control group
Effect of ox-LDL on visfatin expression in RAW264.7 cells
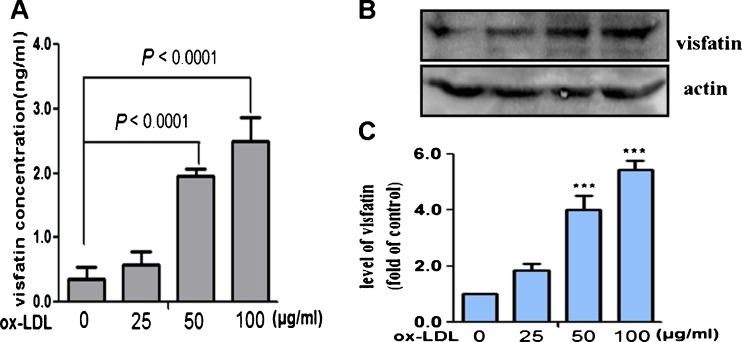
It is well known that modified LDL is pro-atherogenic in the development of atherosclerosis, particularly the important role of ox-LDL in cytokine secretion (Stemme et al. 1995). In this study, we examined for the first time whether ox-LDL could regulate visfatin expression in RAW264.7 cells. Mouse RAW264.7 cells are a macrophage-like cell line and also can be induced to form foam cells by ox-LDL (Li et al. 2009). Moreover, this cell line is more feasible for mechanistic studies compared with primary macrophages. We, thus, used RAW264.7 cells to form foam cells for further studies here. After pretreatment with ox-LDL (25, 50, or 100 μg/ml) for 24 h, the cell supernatant and protein were subjected to ELISA and Western blotting, respectively. In Fig. 3a, we saw that ox-LDL could dose-dependently increase visfatin release from cellular into supernatant culture. Furthermore, Western blotting of the protein samples also revealed a significant increase in visfatin expression by ox-LDL in a dose-dependent manner (Fig. 3b, c).
Fig. 3.
ox-LDL could dose-dependently increase visfatin expression in RAW264.7 cell line. RAW264.7 cells were exposed to different concentrations of ox-LDL (25, 50, or 100 μg/ml) for 24 h. a The level of visfatin secretion into the culture medium was measured by ELISA assay (n = 3), P < 0.0001 vs untreated group. b and c Western blot analysis of visfatin expression in RAW264.7 cells incubated with ox-LDL. n = 3, ***P < 0.0001 vs untreated group
Visfatin induces cholesterol accumulation in macrophages both in vivo and in vitro
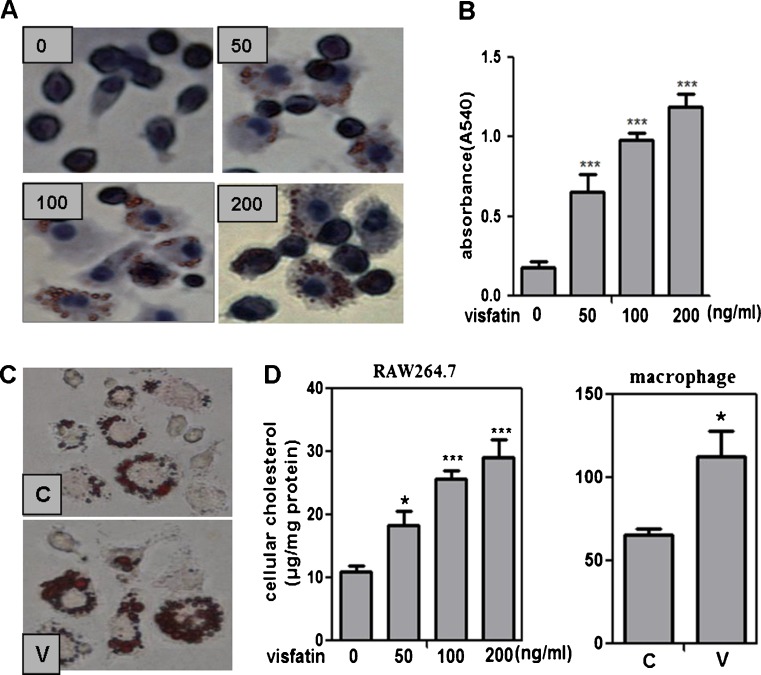
Though the study has indicated that visfatin may play a role in plaque destabilization, whether visfatin involved in the early foamy cell formation is largely unknown. As we have showed above, visfatin expression was markedly enhanced both in ApoE KO mice and ox-LDL-treated RAW264.7 cells. The molecular mechanism of visfatin in the formation of foam cells remains elusive. In our study, we found that visfatin could induce cholesterol accumulation in RAW264.7 cells (Fig. 4a, b). To evaluate the effect of visfatin in vivo foamy cells, six ApoE KO mice were treated with visfatin (15 μg day−1 mouse−1 sc for 8 days) after feeding with high-fat diet for 4 weeks. Notably, in accordance with excessive cholesterol accumulation induced by visfatin in RAW264.7 cells, we found that pretreatment with visfatin showed more lipids loaded in peritoneal macrophages than that in mice without visfatin treatment (Fig. 4c). Moreover, the content of TC in visfatin-treated mice was higher than that in the control mice, which is consistent with the result in RAW264.7 cells (Fig. 4d). All of these results suggest that visfatin promotes the formation of foam cells both in vivo and in vitro, which is mainly due to increasing cholesterol uptake in macrophages.
Fig. 4.
Visfatin induces lipid accumulation in macrophages both in vivo and in vitro. a and b RAW264.7 cells were incubated with different doses of visfatin (50, 100, or 200 ng/ml) for 24 h. After fixation by 4 % paraformaldehyde, cells were stained with 0.5 % ORO to detect lipid accumulation. Cellular nuclei were stained with hematoxylin. n = 6, ***P < 0.0001 vs untreated group. Magnification × 400. c Visfatin accelerates lipid accumulation in peritoneal macrophages in visfatin-treated mice. After feeding with high-fat diet for 4 weeks, ApoE KO mice were treated with visfatin at the concentration of 15 μg day−1 (V) or not (C) for another 8 days, and then peritoneal macrophages were collected to detect lipid accumulation by staining with ORO (n = 6). d Cellular cholesterol content was determined by an enzymatic method in RAW264.7 cells and peritoneal macrophages. The results are the average of six mice. *P < 0.05, ***P < 0.0001 vs untreated group
Cholesterol uptake was reduced in RAW264.7 cells after visfatin downregulation by RNAi
To study whether cholesterol accumulation in the treatment of ox-LDL is visfatin-dependent, we downregulated visfatin expression in RAW264.7 cells using RNA interference. After pretreatment with ox-LDL (50 μg/ml) for 24 h, we found that RAW264.7 cells with downregulated visfatin expression exhibited significantly lower levels of cholesterol uptake (Fig. 5a–c). The efficiency of silencing of visfatin gene expression by RNAi was evaluated at the protein level by Western blot analysis. Levels of visfatin protein was significantly downregulated after RNAi treatment compared to cells transfected with a nonsense sequence as negative control (Fig. 5d, e). These results indicate that cholesterol uptake of RAW264.7 cells in ox-LDL treatment was largely regulated by the effects of visfatin.
Fig. 5.
Cholesterol uptake was reduced in RAW264.7 cells after visfatin downregulation by RNAi. Cells were divided into four groups as follows: control group (C), ox-LDL group (O), transfection of siRNA group (T), and negative control group (NC). Cells in the latter three groups were pretreated with ox-LDL (50 μg/ml) for 24 h, then were subjected to transfection or not. a and b Lipid accumulation in RAW264.7 cells were analyzed by ORO staining after transfection of siRNA. Hematoxylin was used as counterstaining. n = 6, *P < 0.05, ***P < 0.0001 vs control group, #P < 0.05 vs ox-LDL group. Magnification × 400. c Cellular cholesterol content was determined after visfatin downregulation in RAW264.7 cells. ***P < 0.0001 vs control group, #P < 0.05 vs ox-LDL group. d and e The efficiency of visfatin silencing was analyzed 48 h after transfection of siRNA by Western blotting. The actin was used as the quantitative standard. n = 3, **P < 0.01 vs control group, #P < 0.05 vs ox-LDL group
Visfatin induces lipid uptake by activation of SR-A and CD36
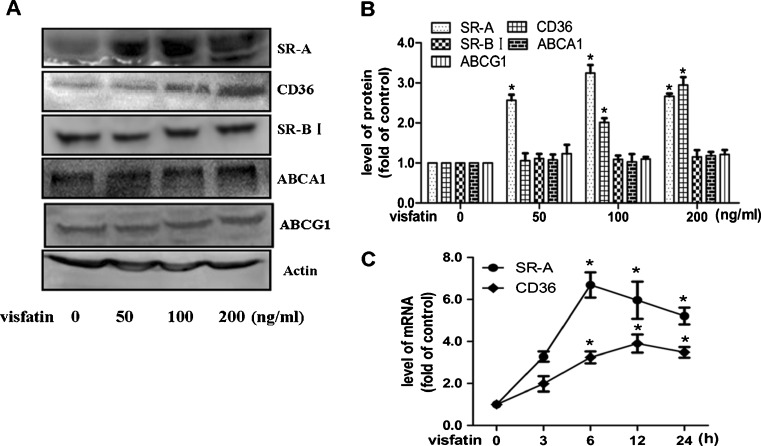
To further clarify the underlying mechanism of visfatin in promoting foamy cell formation, the macrophages SR and RCT were evaluated after treatment with visfatin. As we all know, SR-A, CD36, SR-BI, ABCA1, and ABCG1 have been established to play crucial keys in cholesterol homeostasis during formation of foam cells (Kunjathoor et al. 2002; Van et al. 2004; Wang et al. 2001; Out et al. 2006 ). As shown in Fig. 5a, visfatin could induce the activation of SR-A and CD36, which are responsible for the lipid uptake by macrophages. On the other hand, visfatin showed no effect in expression of SR-BI, ABCA1, or ABCG1, which mainly maintain the course of reverse cholesterol transport (Fig. 6a, b). Furthermore, to better understand the effect of visfatin on the expression of SR-A and CD36, RAW264.7 cells were exposed to visfatin (100 ng/ml) for 3, 6, 12, and 24 h. In accordance with the promotion in protein level, we found that visfatin also markedly increased mRNA levels of SR-A and CD36, reaching a maximum at 6 and 12 h, respectively (Fig. 6c). These data indicate that visfatin accelerates foamy cell formation through modulating expression of SR-A and CD36.
Fig. 6.
Effects of visfatin on the expression of SR-A, CD36, SR-BI, ABCA1, and ABCG1. a RAW264.7 cells were incubated with visfatin (50, 100, or 200 ng/ml) for 24 h. Cell samples were then subjected to Western blotting to detect the protein levels of SR-A, CD36, SR-BI, ABCA1, ABCG1, and actin. b Protein bands of SR-A, CD36, SR-BI, ABCA1, ABCG1, and actin as a control were analyzed by quantified Software. Data are mean ± SEM from three independent experiments. *P < 0.05 vs untreated group. c Effects of visfatin on mRNA level of SR-A and CD36. RAW264.7 cells were exposed to visfatin (100 ng/ml) at different time points (0, 3, 6, 12, or 24 h). Total cellular RNA was collected and then subjected to qRT-PCR to determine mRNA level of SR-A and CD36 (n = 3). *P < 0.05 vs untreated group
Discussion
Visfatin (also known as PBEF) was first reported to be secreted by activated lymphocytes in bone marrow stromal cells (Samal et al. 1994). Over the past several years, a series of evidence has indicated that it is also ubiquitously present in most other tissues, such as adipose tissue, skeletal muscle, spleen, and liver (Sethi and Vidal-Puig 2005). Visfatin could stimulate the release of cytokines and is induced by inflammatory stimuli such as TNF-α, LPS, and IL-6 (Jia et al. 2004; Nowell et al. 2006). As a multifunctional adipokine, visfatin has also been implicated in the pathogenesis of obesity, diabetes mellitus, hyperlipidemia, and such atherosclerosis-related diseases. Due to the close association between visfatin and inflammation, Dahl and his colleagues studied the effect of visfatin in macrophages of humans with unstable carotid and coronary atherosclerosis. They have identified that visfatin is a potential inflammatory mediator in plaque destabilization. Another study has further demonstrated that the regulation of visfatin in macrophages is related to pro-atherogenic stimuli, including hypoxia, TNF-α and ox-LDL (Dahl et al. 2011). The pro-inflammatory effect of visfatin in macrophages has been well established. However, the effect of visfatin and its possible mechanism involved in cholesterol metabolism of macrophage-derived foam cells remained elusive.
In the present study, we revealed the novel molecular mechanisms underlying the pro-atherogenic action of visfatin in foamy cell formation during development of atherosclerosis. Firstly, we found that plasma visfatin concentrations were significantly increased in atherosclerotic mice with the elevation of serum lipid level (Figs. 1 and 2). This finding indicates that circulating levels of visfatin are increased during hyperlipidemia state. Western blotting of protein samples in mouse aorta also showed a similar increase in visfatin expression, which is consistent with previous studies in patients with carotid atherosclerosis. In this study, to our best knowledge, we demonstrate for the first time that both circulating and protein levels of visfatin are markedly promoted in atherosclerotic mice. Our finding further implies that visfatin may play an important role in the pathogenesis of atherosclerosis.
Accumulation of modified LDL such as ox-LDL in macrophages is a key event in all stages of atherogenesis. Previous studies have reported that cholesterol accumulation in macrophages could be regulated by mediating expression of SRs and RCTs or inflammatory responses by cytokine induction (Glass and Witztum 2001; Berliner and Heinecke 1996). Here, we found that ox-LDL pretreatment increased visfatin expression both in a supernatant culture and within RAW264.7 cells in a dose-dependent manner (Fig. 3). This finding is consistent with the previous report in THP-1 cells (Dahl et al. 2007, 2011). On the basis of our findings in vitro, such a pro-atherogenic factor could be a potent stimulus for visfatin expression, potentially explaining its enhanced expression in plasma and atherosclerotic plaques. In addition, to clarify the effect of visfatin on lipid metabolism in macrophages, we determined the level of cellular cholesterol induced by visfatin both in cultured cells and in mice (Fig. 4). Lipid accumulation in RAW264.7 cells was induced by visfatin in a dose-dependent manner. Furthermore, RNA interference against visfatin significantly reduced the lipid uptake of RAW264.7 cells (Fig. 5). Peritoneal macrophages from ApoE KO mice treated with visfatin for 8 days were more lipid-loaded than did that from the control mice. Although some of our findings were rather modest, our data may suggest that exogenous visfatin promotes accumulation of unwanted self-lipids in macrophages and accelerates foamy cell formation induced by ox-LDL and other pro-atherogenic stimuli in vivo, further supporting a link between visfatin and atherogenesis.
SR-modulated ox-LDL internalization and RCT-dependent cholesterol efflux have been demonstrated to be crucial events in the maintenance of intracellular cholesterol homeostasis of macrophages (Kleemann et al. 2008; Cheng et al. 2011; Kunjathoor et al. 2002; Ji et al. 2011). Therefore, we examined the regulatory effect of visfatin on SRs and RCTs. Our data show that visfatin induced a marked increase in SR-A and CD36, but not SR-BI, ABCA1, and ABCG1 (Fig. 6). Here, we may be the first to report that acceleration of cholesterol accumulation by visfatin during formation of foam cell is mainly associated with ox-LDL uptake. Both SR-A and CD36 play a key role in the uptake of ox-LDL during the development of atherosclerosis (Kunjathoor et al. 2002). It was reported recently that antiatherogenic antioxidants could ameliorate the formation of foam cells through transcriptional regulation of SR-A (Tsai et al. 2010). In the current study, our observations suggest that both transcriptional and posttranscriptional regulation may work in concert to mediate the expression of SR-A and CD36 in visfatin-treated cells, indicating that the pro-atherogenic action of visfatin is mainly due to regulation of the expression of these two receptors.
Accumulating evidences have shown that some signaling pathway may be implicated in the uptake of ox-LDL by SR-A and CD36, including MAPK, PPAR, PI3K, and so on (Rahaman et al. 2006; Xie et al. 2011; Li et al. 2009). CD36 was found to be mediated by Lyn and MEKK2 signaling pathway that were activated by ox-LDL specifically in macrophages. In view of the function of these two receptors, the increase in their expression in visfatin-induced macrophages likely contributes to elevating ox-LDL uptake and subsequently promoting the development of foam cells. Although the current study has some limitations (e.g., some of the findings were rather modest, we did not test visfatin inhibitor in all experiments), our findings may suggest that visfatin promotes cholesterol accumulation in macrophages and atherogenesis through modulating expression of SR-A and CD36. The signaling pathway implicated in the activation of SR-A and CD36 induced by visfatin is under investigation.
In summary, we propose new insights into the pro-atherogenic effect of visfatin in promoting cholesterol accumulation during the development of foam cells by up-regulating the expression of SR-A and CD36 in macrophages. We are willing to provide some new information for better understanding on the potential effect of visfatin in accelerating foamy cell formation in atherosclerosis. However, whether visfatin will eventually become a therapeutic target warrants further investigation.
Acknowledgments
The authors thank Yu Xie for excellent technical assistance. This project was supported by grants from the National Natural Sciences Foundation of China (no. 81072776) and Guangdong 211 key disciplines construction project.
Conflict of interests
The authors declare that there is no conflict of interests.
Footnotes
Fenghua Zhou and Yunyun Pan equally contributed to this work.
Reference
- Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- Castagna A, Polati P, Bossi AM, Girelli D. Monocyte/macrophage proteomics: recent findings and biomedical applications. Expert Rev Proteom. 2012;9:201–215. doi: 10.1586/epr.12.11. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhang H, McAfee S, Zhang C. The reciprocal relationship between adiponectin and LOX-1 in the regulation of endothelial dysfunction in ApoE knockout mice. Am J Physiol Heart Circ Physiol. 2010;299:605–612. doi: 10.1152/ajpheart.01096.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Shyue SK, Ching LC, Su KH, Wu YL, Kou YR, et al. Wogonin promotes cholesterol efflux by increasing protein phosphatase 2B-dependent dephosphorylation at ATP-binding cassette transporter-A1 in macrophages. J Nutr Biochem. 2011;22:1015–1021. doi: 10.1016/j.jnutbio.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Cheng LC, Su KH, Kou YR, Shyue SK, Ching LC, Yu YB, et al. α-Lipoic acid ameliorates foam cell formation via liver X receptor α-dependent upregulation of ATP-binding cassette transporters A1 and G1. Free Radic Biol Med. 2011;50:47–54. doi: 10.1016/j.freeradbiomed.2010.10.706. [DOI] [PubMed] [Google Scholar]
- Collot-Teixeira S, Martin J, McDermott-Roe C, Poston R, McGregor JL. CD36 and macrophages in atherosclerosis. Cardiovasc Res. 2007;75:468–477. doi: 10.1016/j.cardiores.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Dahl TB, Yndestad A, Skjelland M, Øie E, Dahl A, Michelsen A, et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation. 2007;115:972–980. doi: 10.1161/CIRCULATIONAHA.106.665893. [DOI] [PubMed] [Google Scholar]
- Dahl T, Ranheim T, Holm S, Berge R, Aukrust P, Halvorsen P. Nicotinamide phosphoribosyltransferase and lipid accumulation in macrophages. Eur J Clin Invest. 2011;41:1098–1104. doi: 10.1111/j.1365-2362.2011.02515.x. [DOI] [PubMed] [Google Scholar]
- De Luis DA, Gonzalez Sagrado M, Conde R, Aller R, Izaola O, Romero E. Effect of a hypocaloric diet on serum visfatin in obese non-diabetic patients. Nutrition. 2008;24:517–521. doi: 10.1016/j.nut.2008.01.052. [DOI] [PubMed] [Google Scholar]
- Glass CK, Witztum JL. Atherosclerosis: the road ahead. Cell. 2001;104:503–516. doi: 10.1016/S0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Ji A, Meyer JM, Cai L, Akinmusire A, Akinmusire A, de Beer MC, et al. Scavenger receptor SR-BI in macrophage lipid metabolism. Atherosclerosis. 2011;217:106–112. doi: 10.1016/j.atherosclerosis.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental experimental inflammation and clinical sepsis. J Clin Invest. 2004;113:1318–1327. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamari Y, Shaish A, Shemesh S, Vax E, Grosskopfl I, Dotan S, et al. Reduced atherosclerosis and inflammatory cytokines in apolipoprotein-e-deficient mice lacking bone marrow-derived interleukin-1alpha. Biochem Biophys Res Commun. 2011;405:197–203. doi: 10.1016/j.bbrc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Kjerrulf M, Berke Z, Aspegen A, Umaerus M, Nilsson T, Svensson L, et al. Reduced cholesterol accumulation by leptin deficient (ob/ob) mouse macrophages. Inflamm Res. 2006;55:300–309. doi: 10.1007/s00011-006-0087-8. [DOI] [PubMed] [Google Scholar]
- Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79:360–376. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopff B, Jegier A. Adipokines: adiponectin, leptin, resistin and coronary heart disease risk. Przegl Lek. 2005;62:69–72. [PubMed] [Google Scholar]
- Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- Li K, Yao WQ, Zheng XD, Liao K. Berberine promotes the development of atherosclerosis and foam cell formation by inducing scavenger receptor A expression in macrophage. Cell Res. 2009;19:1006–1017. doi: 10.1038/cr.2009.76. [DOI] [PubMed] [Google Scholar]
- Liu SW, Qiao SB, Yuan JS, Liu DQ. Association of plasma visfatin levels with inflammation, atherosclerosis and acute coronary syndromes (ACS) in humans. Clin Endocrinol (Oxf) 2009;71:202–207. doi: 10.1111/j.1365-2265.2008.03453.x. [DOI] [PubMed] [Google Scholar]
- Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.ATV.14.1.133. [DOI] [PubMed] [Google Scholar]
- Nowell MA, Richards PJ, Fielding CA, Ognjanovic S, Topley N, Williams AS, et al. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2006;54:2084–2095. doi: 10.1002/art.21942. [DOI] [PubMed] [Google Scholar]
- Out R, Hoekstra M, Hildebrand RB, Kruit JK, Meurs I, Li ZS, et al. Macrophage ABCG1 deletion disrupts lipid homeostasis in alveolar macrophages and moderately influences atherosclerotic lesion development in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2295–2300. doi: 10.1161/01.ATV.0000237629.29842.4c. [DOI] [PubMed] [Google Scholar]
- Paschou P, Kukuvitis A, Yavropoulou MP, Dritsoula A, Giapoutzidis V, Anastasiou O, et al. Genetic variation in the visfatin (PBEF1/NAMPT) gene and type 2 diabetes in the Greek population. Cytokine. 2010;51:25–27. doi: 10.1016/j.cyto.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Plump AS, Smith JD, Hayek T, Aalto-Setӓlӓ K, Walsh A, Verstuyft JG, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-G. [DOI] [PubMed] [Google Scholar]
- Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis-an inflammatory disease. N Eng J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi JK, Vidal-Puig A. Visfatin: the missing link between intra-abdominal obesity and diabetes? Trends Mol Med. 2005;11:344–347. doi: 10.1016/j.molmed.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH. Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb. 2010;17:115–130. doi: 10.5551/jat.1735. [DOI] [PubMed] [Google Scholar]
- Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kawada T, Goto T, Kim CS, Taimatsu A, Eqawa K, et al. Abietic acid activates peroxisome proliferator-activated receptor-gamma (PPARgamma) in RAW264.7 cells and 3 T3-L1 adipocytes to regulate gene expression involved in inflammation and lipid metabolism. FEBS Lett. 2003;550:190–194. doi: 10.1016/S0014-5793(03)00859-7. [DOI] [PubMed] [Google Scholar]
- Tsai JY, Su KH, Shyue SK, Kou YR, Yu YB, Hsiao SH, et al. EGb761 ameliorates the formation of foam cells by regulating the expression of SR-A and ABCA1: role of haem oxygenase-1. Cardiovasc Res. 2010;88:415–423. doi: 10.1093/cvr/cvq226. [DOI] [PubMed] [Google Scholar]
- Van Eck M, De Winther MP, Herijgers N, Havekes LM, Hofker MH, Groot PH, et al. Effect of human scavenger receptor class A overexpression in bone marrow-derived cells on cholesterol levels and atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:2600–2606. doi: 10.1161/01.ATV.20.12.2600. [DOI] [PubMed] [Google Scholar]
- Van Eck M, Bos IST, Hildebrand RB, Van Rij BT, Van Berkel TJC. Dual role for scavenger receptor class B, type I on bone marrow-derived cells in atherosclerotic lesion development. Am J Pathol. 2004;165:785–794. doi: 10.1016/S0002-9440(10)63341-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Silver DL, Thiele C, Tall AR. ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem. 2001;276:23742–23747. doi: 10.1074/jbc.M102348200. [DOI] [PubMed] [Google Scholar]
- Wu H, Moulton KS, Glass CK. Macrophage scavenger receptors and atherosclerosis. Trends Cardiovasc Med. 1992;2:220–225. doi: 10.1016/1050-1738(92)90028-Q. [DOI] [PubMed] [Google Scholar]
- Xie C, Kang J, Chen JR, Lazarenko OP, Ferguson ME, Badger TM, et al. Lowbush blueberries inhibit scavenger receptors CD36 and SR-A expression and attenuate foam cell formation in ApoE-deficient mice. Food Funct. 2011;2:588–594. doi: 10.1039/c1fo10136f. [DOI] [PubMed] [Google Scholar]
- Yan JJ, Tang NP, Tang JJ, Jia EZ, Wang MW, Wang QM, et al. Genetic variant in visfatin gene promoter is associated with decreased risk of coronary artery disease in a Chinese population. Clin Chim Acta. 2010;411:26–30. doi: 10.1016/j.cca.2009.09.033. [DOI] [PubMed] [Google Scholar]