Abstract
Despite the strong rationale for combining cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal carcinomatosis, thermotolerance and chemoresistance might result from heat shock protein overexpression. The aim of the present study was thus to determine whether the heat shock protein 27 (Hsp27), a potential factor in resistance to treatment, could have a higher level in serum from patients under this combined therapy. Patients receiving CRS plus HIPEC for peritoneal carcinomatosis (group 1), patients with cancer or a history of cancer undergoing abdominal surgery (group 2), and patients without malignancies undergoing abdominal surgery (group 3) were included. Hsp27 serum levels were determined before and at different times following CRS and HIPEC using enzyme-linked immunosorbent assay. In group 1 (n = 25), the high Hsp27 levels, observed at the end of surgery compared with before (p < 0.0001), decreased during HIPEC, but remained significantly higher than before surgery (p < 0.0005). In groups 2 (n = 11) and 3 (n = 15), surgery did not significantly increase Hsp27 levels. A targeted molecular strategy, inhibiting Hsp27 expression in tumor tissue, could significantly reduce resistance to the combined CRS plus HIPEC treatment. This approach should be further assessed in a clinical phase I trial.
Keywords: Colorectal cancer, Heat shock protein 27 (Hsp7), Hyperthermic intraperitoneal chemotherapy (HIPEC), Ovarian cancer, Peritoneal carcinomatosis
Introduction
Peritoneal tumor dissemination arising from colorectal carcinomatosis, appendiceal carcinomatosis, gastric cancer, gynecologic malignancies, or peritoneal mesothelioma is a common sign of advanced tumor stage or disease recurrence and is usually associated with a poor prognosis (Glehen et al. 2010; Elias et al. 2010a, b; Yan et al. 2009). Patients with peritoneal carcinomatosis (PC) are commonly treated with systemic chemotherapy alone or cytoreductive surgery (CRS). Nevertheless, median survival remains low and prognosis poor in response to this therapeutic strategy (Frank et al. 2012; Wagner et al. 2010; Koizumi et al. 2008). For these reasons, CRS with hyperthermic intraperitoneal chemotherapy (HIPEC) was developed for the management of patients with surface peritoneal malignancies. This combined locoregional treatment enables microscopic or macroscopic sterilization to be carried out and has provided encouraging survival results (Elias et al. 2010b; Glehen et al. 2004).
HIPEC adds several advantages to intraperitoneal chemotherapy: a direct cytotoxic effect of hyperthermia demonstrated in vitro (Hildebrant et al. 2002) and a synergistic effect with some anticancer agents by improving drug cytotoxicity or by increasing their penetration into tissues (Urano and Ling 2002). Despite this strong rationale, many peritoneal surface malignancies still progress or recur following this therapeutic strategy. Indeed, exposure of cancer cells to a transient elevation in temperature can result in the activation of cellular stress responses and induce a state of thermotolerance involving heat shock proteins (Hsps) that renders the cancer cells resistant to subsequent lethal insults such as chemotherapy (Takhashi et al. 2008; Li et al. 1995).
Human heat shock protein 27 (Hsp27) belongs to a family of small Hsps that have cytoprotective roles, including acting as chaperones to protein folding, anti-apoptotic properties, and an antioxidant activity dependent upon reduced glutathione. It is one of the main inducible Hsps, and its increased production was reported to protect cells against stress stimuli including hyperthermia, oxidative stress, death receptor agonists, and chemotherapy (Gabai and Sherman 2002; Arrigo 2001; Charette et al. 2000). High constitutive expression levels of Hsp27 protein have been reported in numerous cancers (Arrigo et al. 2007; Calderwood et al. 2006) and are correlated with poor prognosis in patients with gastric, liver, and prostate carcinoma and with osteocarcinoma (Romani et al. 2007; Ciocca and Calderwood 2005). Furthermore, increased Hsp27 levels have been proposed as a discriminating biomarker between patients with and without cancer for pancreatic carcinoma (Liao et al. 2009) and breast, ovarian, and endometrial cancers (De and Roach 2004).
The aim of this study was to evaluate the kinetics of Hsp27 serum levels during surgical procedures with or without HIPEC in the context of PC, other cancers, or benign illness. We conducted this study in two stages. First, we measured patient serum Hsp27 levels at different times—before surgery, at completion of HIPEC, and then at 24 h following a HIPEC procedure with PC for malignant diseases—and compared these results with those for healthy controls. Second, we evaluated the kinetics of Hsp27 expression before and at different times after the HIPEC procedure to compare the results with those obtained for patients with cancer or a history of cancer undergoing abdominal surgery and noncancerous patients undergoing abdominal surgery. This is the first human study including a biological marker that might offer a potential explanation for HIPEC resistance and possibly represent a target therapy in HIPEC procedures.
Materials and methods
Study protocol
Preliminary study
Between February and April 2009, 12 patients were enrolled in the first preliminary study with the goal of assessing variations in Hsp27 protein expression before, at the end of, and at 24 h after the HIPEC procedure. Four patients had primary ovarian carcinomatosis, four had colorectal carcinomatosis, three had pseudomyxoma peritonei, and one had a peritoneal mesothelioma. The group comprised four men and eight women, with a mean age of 51.9 ± 10.2 years. In addition, a group of healthy volunteers (20 employees of the Lyon Sud General Public Hospital) accepted to be tested to determine the basal level of Hsp27 expression. This population comprised 6 men and 14 women with a mean age of 31.3 ± 9.1 years.
Main study
The main study, conducted from April 2009 to February 2011, included patients receiving CRS plus HIPEC for PC (group 1), patients with colorectal cancer or a history of cancer undergoing abdominal surgery (group 2), and patients without cancer undergoing abdominal surgery (group 3). A standard data form was created to collect information on the origin of PC and the status of the patient before undergoing the combined procedure, including age, sex, extent of the PC, and any previous treatment with systemic chemotherapy regimens. The Sugarbaker Peritoneal Cancer Index (PCI) was used to assess the extent of PC (1, 2). Information recorded about the combined procedure included the date; a score for the extent of cytoreduction (CC-0, no disease left; CC-1, nodule(s) size <2.5 mm; CC-2, nodule size 2.5 mm to 2.5 cm; CC-3, nodule size >2.5 cm; Jacquet and Sugarbaker 1996); the simultaneous resection of primary tumor or of liver metastases; the presence or absence of lymph node metastases; the modalities of HIPEC (drugs used, temperatures, duration); and any treatment with adjuvant systemic chemotherapy. Information on the modality open/closed procedure was not collected because the “closed” HIPEC procedure was always used. All indications were discussed in a multidisciplinary oncology meeting. Informed signed consent was obtained from patients and healthy volunteers according to our research ethics requirements. All human investigations were performed after approval by a local Human Investigation Committee and in accordance with an assurance filed with and approved by the Department of Health and Human Services. Our protocols for human experimentation were reviewed in accordance with the precepts established by the Helsinki Declaration.
HIPEC procedures
With the patient under general anesthesia and subject to complete hemodynamic monitoring, careful abdominal exploration was conducted and cytological and pathology samples taken through a median laparotomy (from the xyphoid to the symphysis pubis). Surgical resection of the primary tumor was performed according to oncological surgical rules (lymphadenectomy, acceptable margins). CRS was undertaken to obtain a complete macroscopic cytoreduction with curative intent in the absence of contraindications. After exhaustive surgical exploration, if the extent of the PC did not enable us to perform cytoreduction, patients did not undergo the HIPEC procedure and were placed in group 2. At the end of each surgical procedure after complete cytoreduction, a HIPEC infusion was carried out with the patient under general anesthesia and general hypothermia. HIPEC was performed using the closed abdomen technique at a temperature of 42 °C (Glehen et al. 2003), with the choice of cytotoxic agent and duration of hyperthermia depending on the site of carcinomatosis: oxaliplatin for colorectal carcinomatosis, cisplatin plus mitomycin C or mitomycin C plus irinotecan for pseudomyxoma peritonei, cisplatin for primary ovarian carcinomatosis, or mitomycin C plus cisplatin for peritoneal mesothelioma. This combined procedure enables the delivery of high concentrations of cytotoxic agents to the abdomen and provides the combined cytotoxicity of chemotherapy and heat to destroy microscopic residual tumor cells.
Serum sampling and Hsp27 measurement
Blood was collected before surgery, at the end of surgery before HIPEC, at the end of HIPEC, and at 4 and 24 h after HIPEC or surgery. Serum samples were recovered after blood centrifugation at 1,200×g for 10 min at 4 °C. Serum samples were stored at −80 °C until analysis. Hsp27 levels were measured using an ELISA with Hsp27 ImmunoSet high-sensitivity kit from Enzo Life Science (Villeurbanne, France). Briefly, a monoclonal antibody specific for Hsp27 was precoated onto a microtiter plate. Samples and standards were incubated along with a biotinylated anti-Hsp27 polyclonal antibody in the microplate. After incubation and wash steps, streptavidin conjugated to horseradish peroxidase was added. After another incubation and wash, an enzyme substrate was added. Color development was then monitored using a Multiskan Spectrum (Thermo Electron Corporation, Waltham, MA). The color generated was proportionate to the amount of Hsp27 present in the sample. Analyses were performed in duplicate on two different dilutions in each assay. The lower limit of detection of this kit is 0.097 ng/mL.
CRP measurement
The levels of C-reactive protein (CRP), a marker of inflammation, were determined using a turbidimetric assay (AU 2700 Olympus Analyzer, Beckman Coulter, Inc., Palo Alto, CA) in each group of patients in the main study at baseline before any kind of surgery. The normal concentration in healthy human serum is <2 mg/L.
Statistical analysis
All values are expressed as the mean ± SD. The Mann–Whitney nonparametric U test was performed to compare independent data, and the Wilcoxon signed-rank test was applied to paired data. Spearman’s rank correlation (ρ) was used to assess the correlation among Hsp27 protein expression levels, the CRP values, and the duration of surgery. A two-tailed p < 0.05 was considered statistically significant. Statistical analyses were performed using the SAS software package (version 9.2, SAS Institute, Cary, NC).
Results
Preliminary study
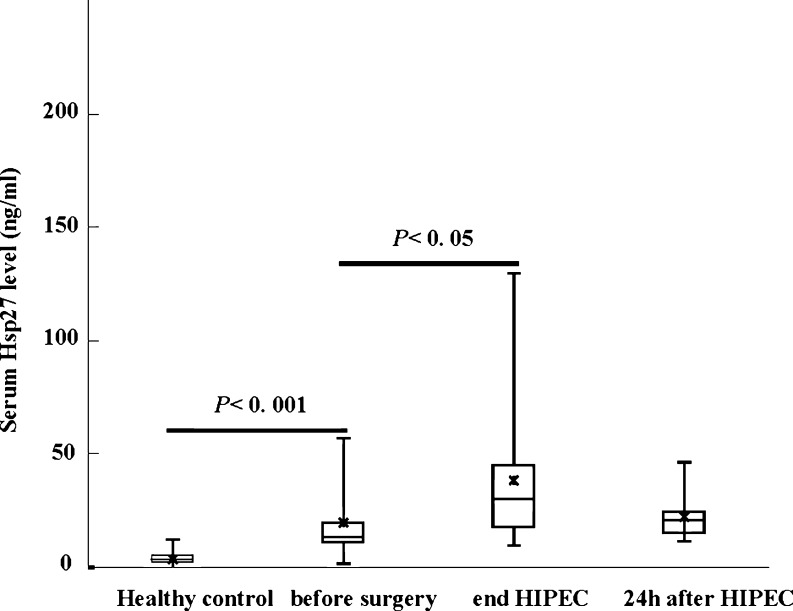
The mean Hsp27 expression levels were 19.9 ± 19.2 ng/mL (range = 1.7–51.2, median = 12.8) before CRS, 38.6 ± 32 ng/mL (range = 9.7–129.2, median = 30.0) at the end of HIPEC, and 22.4 ± 9.8 ng/mL (range = 11.7–45.8, median = 20.6) 24 h afterward. Figure 1 shows a box plot of these data. In healthy volunteer controls, the reference mean Hsp27 value was 4.0 ± 2 ng/mL (range = 0.7–1.7, median = 3.0). Hsp27 levels were significantly higher in patients with PC than in healthy controls (p < 0.001) and increased significantly in response to HIPEC (p = 0.05) compared with before surgery. In contrast, the mean Hsp27 values obtained 24 h after the HIPEC procedure were not significantly different from the values before surgery.
Fig. 1.
Variations in Hsp27 serum levels in the preliminary study. Lines from bottom to top show the minimum, 25th percentile, median, 75th percentile, and maximum. Asterisk indicates the mean value
Main study
To confirm the increase in Hsp27 levels during the combination of CRS and HIPEC and to analyze the influence of each procedure, we followed the kinetics of Hsp27 expression during and following the CRS and HIPEC procedures.
Overall population results
In group 1, nine patients had primary ovarian carcinomatosis, seven had pseudomyxoma peritonei, four had colorectal carcinomatosis, three had a primary serous primitive peritoneal carcinoma, one had a peritoneal mesothelioma, and one had appendiceal carcinomatosis. The mean PCI was 13.5 ± 8.8. Complete cytoreduction (score CC-0) was achieved in 18 patients (72 %). Twenty-one of the 25 patients in group 1 were treated with initial systemic chemotherapy regimens according to the primary malignant disease. In group 2, one patient had pseudomyxoma peritonei, one had gastric carcinomatosis, another had a uterine sarcoma, another had a neuroendocrine carcinoma, and seven had colorectal cancers. In this group, only five patients had PC; three were free of tumor at the time of surgery, but were not considered as being cured. In group 3, no patients had cancer or a history of cancer and all underwent surgery: five patients for an inflammatory and ten for a non-inflammatory gastrointestinal disease. The main characteristics of the three groups of patients are listed in Table 1.
Table 1.
Characteristics of the study population
| Group 1: Patients with peritoneal carcinomatosis receiving CRS+HIPEC | Group 2: Patients with cancer or a history of cancer undergoing abdominal surgery | Group 3: Patients without malignancies undergoing abdominal surgery | |
|---|---|---|---|
| N | 25 | 11 | 15 |
| Gender (M/F) | 7/18 | 6/5 | 8/7 |
| Age in years: mean ± SD (median) | 57.5 ± 11.2 (60.1) | 66.8 ± 9.7 (67.4) | 52.7 ± 18.7 (55.4) |
| Pathology | |||
| Colorectal carcinomatosis | 4 | 0 | NA |
| Colorectal cancer | 0 | 7 | NA |
| Primary ovarian carcinomatosis | 9 | 0 | NA |
| Pseudomyxoma peritonei | 7 | 1 | NA |
| Serous primitive peritoneal carcinoma | 3 | 0 | NA |
| Peritoneal mesothelioma | 1 | 0 | NA |
| Gastric cancer | 0 | 1 | NA |
| Uterine sarcoma | 0 | 1 | NA |
| Endocrine carcinoma | 0 | 1 | NA |
| Appendiceal carcinomatosis | 1 | 0 | NA |
| Inflammatory bowel disease | NA | NA | 5 |
| Non-inflammatory benign disease | NA | NA | 10 |
| PCI score: mean ± SD (minimum–maximum) | 13.5 ± 8.8 (1–35) | NA | NA |
| Duration of surgery: mean ± SD (median) | 292.3 ± 84.0 (300) | 129.1 ± 67.7 (100) | 145.7 ± 72.3 (140) |
| CRP level (mg/L): mean ± SD (median) | 15.1 ± 38.7 (1.6) | 14.2 ± 34.1 (3.1) | 4.9 ± 4.3 (4.4) |
CRS cytoreductive surgery, HIPEC hyperthermic intraperitoneal chemotherapy, M/F male/female ratio, NA not applicable, PCI Sugarbaker Peritoneal Cancer Index, CRP C-reactive protein
Levels of Hsp27 in the three groups
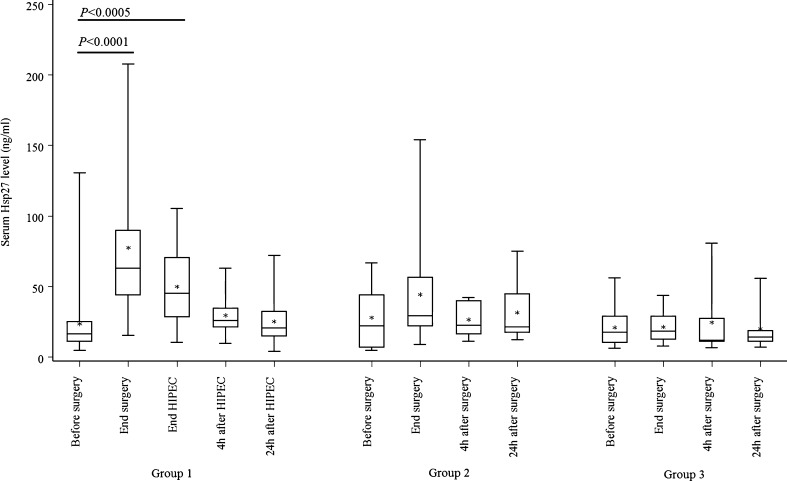
In group 1, the mean Hsp27 expression level before CRS was 23.5 ± 24.7 ng/mL. At the end of CRS and HIPEC, the Hsp27 levels increased respectively to 77.5 ± 52.3 and 49.9 ± 25.4 ng/mL. Then, it decreased to 29.6 ± 13.5 ng/mL at 4 h after HIPEC and 25.3 ± 16.6 ng/mL at 24 h after HIPEC (Fig. 2). The high Hsp27 levels observed at the end of surgery compared with before surgery (p < 0.0001) decreased during the HIPEC procedure, but remained significantly higher than the levels measured before surgery (p < 0.0005). Hsp27 levels continued to decrease following HIPEC and returned to baseline levels at 24 h.
Fig. 2.
Variations in Hsp27 serum levels in group 1 (patients receiving cytoreductive surgery plus HIPEC for PC); in group 2 (patients with cancer or a history of cancer undergoing abdominal surgery); and group 3 (patients with no malignancies undergoing abdominal surgery). Lines from bottom to top show the minimum, 25th percentile, median, 75th percentile, and maximum. Asterisk indicates the mean value
The mean Hsp27 expression levels in group 2 were 28.1 ± 22.2 ng/mL before abdominal surgery, 44.4 ± 40.5 ng/mL at the end of surgery, 26.7 ± 12.5 ng/mL at 4 h after surgery, and 31.7 ± 22.0 ng/mL at 24 h after surgery (Fig. 2). There were no statistically significant variations in Hsp27 expression levels during and after abdominal surgery among these patients The mean Hsp27 expression levels in group 3 were 21.1 ± 13.7 ng/mL before abdominal surgery, 21.5 ± 10.9 ng/mL at the end of surgery, 24.6 ± 24.4 ng/mL at 4 h after surgery, and 20.3 ± 15.1 ng/mL at 24 h after surgery (Fig. 2). There were no statistically significant variations in Hsp27 expression levels throughout the course of abdominal surgery in these patients.
CRP levels in the three groups
CRP serum levels before surgery were usually higher than the reference value (<2 mg/mL) in all three groups. However, no statistically significant correlations were found between CRP and Hsp27 levels.
Specific data for group 1
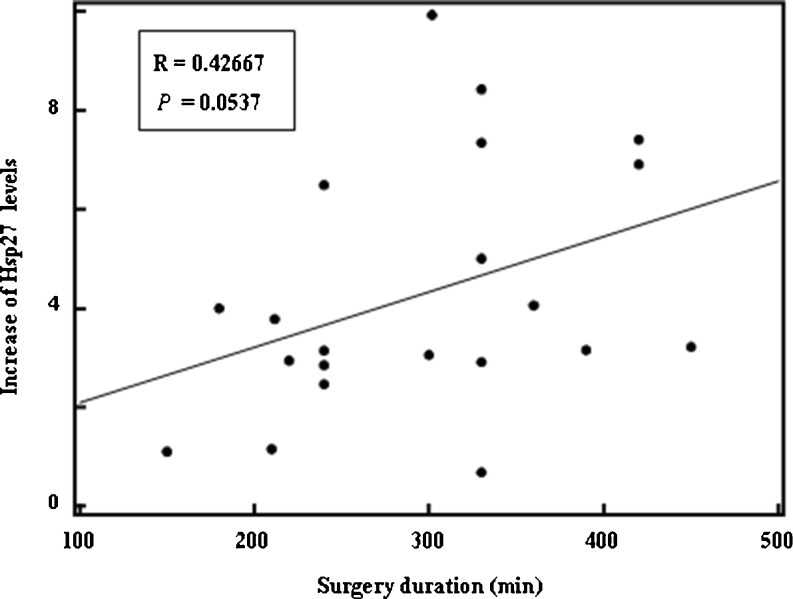
A slightly positive correlation was obtained between the increase in Hsp27 levels and surgery duration (ρ = 0.43, p = 0.05; shown in Fig. 3, with one extreme value omitted). The Hsp27 levels were not significantly different between patients scoring CC-0 and those scoring CC-1 or CC-2 before surgery (p = 0.06), at the end of surgery (p = 0.15), at the end of HIPEC (p = 0.07), or at 4 h after HIPEC (p = 0.25). However, for patients scoring CC-1 or CC-2, Hsp27 was significantly higher at 24 h after HIPEC (36.8 ± 18.8 vs. 18.4 ± 11.1 ng/mL, p < 0.05; Table 2).
Fig. 3.
Correlation between the increase in Hsp27 levels and surgery duration in group 1, comprising patients receiving CRS plus HIPEC for PC (one extreme value has been omitted)
Table 2.
Variation of serum Hsp27 values with CC-scores in group 1 (patients receiving CRS plus HIPEC for PC)
| Hsp27 (ng/mL) | CC-0 | CC-1 and CC-2 | p | |
|---|---|---|---|---|
| Before surgery | n | 18 | 7 | 0.065 |
| Median (range) | 15.0 (4.6–130.4) | 22.6 (14.3–51.1) | ||
| Mean ± SD | 21.8 ± 27.9) | 27.9 ± 14.3 | ||
| After surgery | n | 18 | 7 | 0.146 |
| Median (range) | 57.8 (15.3–180.7) | 89.8 (43.8–207.6) | ||
| Mean ± SD | 66.1 ± 42.1 | 107 ± 67.1 | ||
| After HIPEC | n | 17 | 5 | 0.072 |
| Median (range) | 39.7 (10.2–86.6) | 71.75 (34.2–105.1) | ||
| Mean ± SD | 44.8 ± 23.1 | 67.2 ± 27.8 | ||
| 4 h after HIPEC | n | 10 | 6 | 0.255 |
| Median (range) | 23.3 (9.4–52.0) | 28.2 (21.5–62.7) | ||
| Mean ± SD | 27.5 ± 12.8 | 33.0 ± 15.0 | ||
| 24 h after HIPEC | n | 10 | 6 | 0.026 |
| Median (range) | 16.3 (4.0–42.8) | 32.0 (19.5–71.9) | ||
| Mean ± SD | 18.4 ± 11.1 | 36.8 ± 18.8 |
Discussion
HIPEC has shown promise in the treatment of PC. Nevertheless, despite its curative intent and a strong rationale for treating digestive tract and ovarian carcinomatosis, the patient survival and oncologic results could still be improved (Yan et al. 2009; Frank et al. 2012; Cioppa et al. 2008; Vaira et al. 2010). Thus, it is essential to understand the mechanisms underlying the resistance to HIPEC. To this end, clinical prognostic factors have been researched extensively in the past few years with the aim of identifying patients who might be sensitive to HIPEC treatment (Glehen et al. 2010; Elias et al. 2010a, b). Unfortunately, the biological processes underlying resistance remain unknown. Hyperthermia improves the effectiveness of chemotherapy by creating oxygen free radicals, which activate signaling pathways leading to cell death, by oxidizing DNA, membrane lipids, and proteins (Katschinski et al. 2000; Wang et al. 2007).
In contrast to normal tissues, malignantly transformed cells have been found to overexpress Hsps in the cytosol, which might cause their translocation into the plasma membranes and into the extracellular milieu (serum). Members of the Hsp70 and Hsp90 families are present on the plasma membrane of a number of different tumor entities (Shin et al. 2003), where they act as danger signals for the innate and adaptive cellular immune system (Schmitt et al. 2007). The literature fails to report information on the interaction of Hsp27, hsp70 and 90 proteins with the immune response, such as their association with immunogenic peptides or immunoregularity activities which could contribute to immunopathology. By regulating the metabolism of glutathione (a major intracellular antioxidant) through the activation of glucose-6-phosphate dehydrogenase, Hsp27 protects cells from oxidative stress. Hsp27 can inhibit apoptosis through direct interaction with cytochrome c and inhibition of caspase activation and is also involved in the regulation of the Akt serine/threonine protein kinase pathway involved in cell survival (Aloy et al. 2008; Havasi et al. 2008). Because the overexpression of Hsp27 is frequently associated with thermotolerance and chemoresistance (Takhashi et al. 2008; Li et al. 1995), it appears to be a good candidate for explaining resistance to HIPEC. It should be noted that overexpression of Hsp27 in serum could be related to its release following stress-induced necrotic cell death.
To test this hypothesis, we followed the variations in Hsp27 expression in the serum of patients throughout the treatment. At the end of CRS, i.e., at the beginning of HIPEC, Hsp27 levels significantly increased in patients with PC, but not in those with cancer or a history of cancer who underwent abdominal surgery. Hsp27 expression has been described as an independent prognostic factor for patients with ovarian carcinomas, constituting 36 % of those in groups 1, and in patients with rectal cancer (Geisler et al. 2000; Tweedle et al. 2010). Moreover, in patients without cancer with inflammatory disease, abdominal surgery did not increase Hsp27 levels. As suggested by the absence of correlation with the CRP levels, Hsp27 expression is independent from inflammatory processes. Indeed, the Spearman correlations found between Hsp27 levels and either the CCR score or the duration of surgery suggest that Hsp27 could be incorporated as a biological marker in the calculation of the score index.
As hyperthermia frequently induces heat tolerance in cancer cells, the response to HIPEC becomes limited. Therefore, the inhibition of Hsp27 accumulation is a key issue in promoting the effectiveness of hyperthermia. Antisense oligonucleotides (ASOs) are powerful tools to inhibit target gene expression in a sequence-specific manner. Thus, OGX-427 (Oncogenex), a second-generation ASO drug that in preclinical experiments significantly decreased the levels of Hsp27 and induced apoptosis, acted as a chemosensitizer in combination with gemcitabine (Baylot et al. 2011) and as a radiation sensitizer in patients with radioresistant head and neck squamous cell carcinomas (Hadchity et al. 2009). Phase I and II clinical trials on patients with bladder and prostate cancers that are underway have already demonstrated the potential benefit of OGX-427 when administered to patients in combination with chemotherapy (Chi et al. 2012). The administration of OGX-427 or compounds targeting Hsp27 would be appropriate in the context of HIPEC for patients with PC regardless of the primary cancer location, and selection of patients by their Hsp27 serum level expression might represent a further refinement of this therapeutic strategy.
Acknowledgments
We thank Isabelle Bonnefoy for her excellent technical assistance.
Conflict of interest
The authors have declared that they have no competing interests.
Footnotes
Vahan Kepenekian and Marie-Thérèse Aloy should be considered as first co-authors.
References
- Aloy MT, Hadchity E, Bionda C, Diaz-Latoud C, Claude L, Rousson R, Arrigo AP, Rodriguez-Lafrasse C. Protective role of Hsp27 protein against gamma radiation-induced apoptosis and radiosensitization effects of Hsp27 gene silencing in different human tumor cells. Int J Radiat Oncol Biol Phys. 2008;70:543–553. doi: 10.1016/j.ijrobp.2007.08.061. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. Hsp27. Novel regulator of intracellular redox state. IUBMB Life. 2001;52:303–307. doi: 10.1080/152165401317291156. [DOI] [PubMed] [Google Scholar]
- Arrigo AP, Simon S, Gibert B, Kretz-Remy C, Nivon M, Czekalla A, Guillet D, Moulin M, Diaz-Latoud C, Vicart P. Hsp27 (HspB1) and αB-crystalin (HspB5) as therapeutic targets. FEBS Lett. 2007;581:3665–3674. doi: 10.1016/j.febslet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- Baylot V, Andrieu C, Katsogiannou M, Taieb D, Garcia S, Giusiano S, Acunzo J, Iovanna J, Gleave M, Garrido C, Rocchi P. OGX 427 inhibits tumor progression and enhances gemcitabine chemotherapy in pancreatic cancer. Cell Death Dis. 2011;2:e221. doi: 10.1038/cddis.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Charette SJ, Lavoie JN, Lambert H, Landry J. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol. 2000;20:7602–7612. doi: 10.1128/MCB.20.20.7602-7612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi KN, Hotte SJ, Ellard S, Yu EY, Gingerich JR, Joshua AM, Gleave ME (2012) A randomized phase II study of OGX427 plus prednisolone versus prednisolone alone in patients with chemotherapy-naive metastatic castration resistant prostate cancer. J Clin Oncol 30 (Supp l5; abstr 4514)
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioppa T, Vaira M, Bing C, D’Amico S, Bruscino A, De Simone M. Cytoreduction and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal carcinomatosis from pseudomyxoma peritonei. World J Gastroenterol. 2008;14:6817–6823. doi: 10.3748/wjg.14.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De AK, Roach SE. Detection of the soluble heat shock protein (Hsp27) in human serum by an ELISA. J Immunoassay Immunochem. 2004;25:159–170. doi: 10.1081/IAS-120030525. [DOI] [PubMed] [Google Scholar]
- Elias D, Gilly FN, Boutitie F, Quenet F, Bereder JM, Mansvelt B, Lorimier G, Dubè P, Glehen O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- Elias D, Glehen O, Pocard M, Quenet F, Goéré D, Arvieux C, Rat P, Gilly F, Association Française de Chirurgie A comparative study of complete cytoreductive surgery plus intraperitoneal chemotherapy to treat peritoneal dissemination from colon, rectum, small bowel, and nonpseudomyxoma appendix. Ann Surg. 2010;251:896–901. doi: 10.1097/SLA.0b013e3181d9765d. [DOI] [PubMed] [Google Scholar]
- Frank J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, Pitot HC, Grothey A, Alberts SR, Sargent DJ. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of North Central Cancer Treatment Group phase III trials N9741 and N9841. J Clin Oncol. 2012;30(3):263–267. doi: 10.1200/JCO.2011.37.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabai VL, Sherman MY. Invited review: Interplay between molecular chaperones and signaling pathways in survival of heat shock. J Appl Physiol. 2002;92:1743–1748. doi: 10.1152/japplphysiol.01101.2001. [DOI] [PubMed] [Google Scholar]
- Geisler JP, Tammela JE, Manahan KJ, Geisler HE, Miller GA, Zhou Z, Wiemann MC. Hsp27 in patients with ovarian carcinoma; still an independent prognostic indicator at 60 months follow-up. Eur J Gynaecol Oncol. 2000;25:165–168. [PubMed] [Google Scholar]
- Glehen O, Mithieux F, Osinky D, Beaujard AC, Freyer G, Guertsch P, Francois Y, Peyrat P, Panteix G, Gilly FN VJ. Surgery combined with peritonectomy procedures and intraperitoneal chemohyperthermia in abdominal cancers with peritoneal carcinomatosis: a phase II study. J Clin Oncol. 2003;21(5):799–806. doi: 10.1200/JCO.2003.06.139. [DOI] [PubMed] [Google Scholar]
- Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004;5:219–228. doi: 10.1016/S1470-2045(04)01425-1. [DOI] [PubMed] [Google Scholar]
- Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, Mansvelt B, Lorimier G, Msika S, Elias D, The French Surgical Association Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116:5608–5618. doi: 10.1002/cncr.25356. [DOI] [PubMed] [Google Scholar]
- Hadchity E, Aloy MT, Paulin C, Armandy E, Watkin E, Rousson R, Gleave M, Chapet O, Rodriguez-Lafrasse C. Heat shock protein 27 as a new therapeutic target for radiation sensitization of head and neck squamous cell carcinoma. Mol Ther. 2009;17:1387–1394. doi: 10.1038/mt.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havasi A, Li Z, Wang Z, Martin JL, Botla V, Ruchalski K, Schwartz JH, Borkan SC. Hsp27 inhibits Bax activation and apoptosis via PI3K kinase-dependent mechanism. J Biol Chel. 2008;283:12305–12313. doi: 10.1074/jbc.M801291200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrant B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner FR, Riess H. The cellular and molecular basis of hyperthermia. Crit Rev Hematol. 2002;43:33–56. doi: 10.1016/S1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- Jacquet P, Sugarbaker PH. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res. 1996;15:49–58. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- Katschinski DM, Boos K, Schindler SG, Fandrey J. Pivotal role of reactive oxygen species as intracellular mediators of hyperthermia-induced apoptosis. J Biol Chem. 2000;275:21094–21098. doi: 10.1074/jbc.M001629200. [DOI] [PubMed] [Google Scholar]
- Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Ml T. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9(3):215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- Li GC, Michevi NF, Weitzel G. Heat shock proteins, thermotolerance, and their relevance to clinical hyperthermia. Int J Hyperthermia. 1995;11:459–488. doi: 10.3109/02656739509022483. [DOI] [PubMed] [Google Scholar]
- Liao WC, Wu MS, Wang HP, Tien YW, Lin JT. Serum heat shock protein 27 is increased in chronic pancreatitis and pancreatic carcinoma. Pancreas. 2009;38:422–426. doi: 10.1097/MPA.0b013e318198281d. [DOI] [PubMed] [Google Scholar]
- Romani AA, Crafa P, Dezenzanis S, Graiani G, Lagrasta C, Sianesi M, Soliani P, Borghetti AF. The expression of Hsp27 is associated with poor clinical outcome in intrahepatic cholangiocarcinoma. BMC Cancer. 2007;7:232. doi: 10.1186/1471-2407-7-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol. 2007;81:15–27. doi: 10.1189/jlb.0306167. [DOI] [PubMed] [Google Scholar]
- Shin BK, Wang H, Yim AM, Le Naour F, Brichory F, Jang JH, Zhao R, Puravs E, Tra J, Michael CW, Misek DE, Hanash SM. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J Biol Chem. 2003;278:7607–7616. doi: 10.1074/jbc.M210455200. [DOI] [PubMed] [Google Scholar]
- Takhashi A, Yamakawa N, Mori E, Ohnishi K, Yokota S, Sugo N, Aratani Y, Koyama H, Ohnishi T. Development of thermotolerance requires interaction between polymerase β and heat shock proteins. Cancer Sci. 2008;99:973–978. doi: 10.1111/j.1349-7006.2008.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedle EM, Khattak I, Ang CW, Nedjadi T, Jenkins R, Park BK, Kalirai H, Dodson A, Azadeh B, Terlizzo M, Grabsch H, Mueller W, Myint S, Clark P, Wong H, Greenhalf W, Neoptolemos JP, Rooney PS, Costello E. Low molecular weight heat shock protein HSP27 is a prognostic indicator in rectal cancer but not colon cancer. Gut. 2010;59:1501–1510. doi: 10.1136/gut.2009.196626. [DOI] [PubMed] [Google Scholar]
- Urano M, Ling CC. Thermal enhancement of melphalan and oxaliplatin cytotoxicity in vitro. Int J Hyperthermia. 2002;18:307–315. doi: 10.1080/02656730210123534. [DOI] [PubMed] [Google Scholar]
- Vaira M, Cioppa T, D’Amico S, de Marco G, D’Alessandro M, Fiorentini G, De Simone M. Treatment of peritoneal carcinomatosis from colonic cancer by cytoreduction, peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC). Experience of ten years. In Vivo. 2010;24:79–84. [PubMed] [Google Scholar]
- Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE (2010) Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev (3):CD004064 [DOI] [PubMed]
- Wang CC, Chen F, Kim E, Harrison LE. Thermal sensitization through ROS modulation: a strategy to improve the efficacy of hyperthermic intraperitoneal chemotherapy. Surgery. 2007;142:384–392. doi: 10.1016/j.surg.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, Gilly FN, Levine EA, Shen P, Mohamed F, Moran BJ, Morris DL, Chua TC, Piso P, Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27:6237–6242. doi: 10.1200/JCO.2009.23.9640. [DOI] [PubMed] [Google Scholar]