Abstract
Butyric acid (BA) is a major extracellular metabolite produced by anaerobic periodontopathic bacteria and is commonly deposited in the gingival tissue. BA induces mitochondrial oxidative stress in vitro; however, its effects in vivo were never elucidated. Here, we determined the effects of butyric acid retention in the gingival tissues on oxidative stress induction in the jugular blood mitochondria. We established that BA injected in the rat gingival tissue has prolonged retention in gingival tissues. Blood taken at 0, 60, and 180 min after BA injection was used for further analysis. We isolated blood mitochondria, verified its purity, and measured hydrogen peroxide (H2O2), heme, superoxide (SOD), and catalase (CAT) to determine BA effects. We found that H2O2, heme, SOD, and CAT levels all increased after BA injection. This would insinuate that mitochondrial oxidative stress was induced ascribable to BA.
Keywords: Butyric acid, Gingival tissue, Mitochondria, Rat blood, Reactive oxygen species homeostasis
Introduction
Butyric acid (BA) is one of the short-chain fatty acids produced by anaerobic bacteria and is present in the normal flora of gut, mouth, and vagina produced through either the butyrate kinase or butyryl-CoA: acetate CoA-transferase pathways (Louis et al. 2010). BA is an extracellular metabolite that helps the intestine maintain colonic health, serves as an energy source for colorectal cells, and positively influence immune responses (Hu et al. 2011; Maslowski et al. 2009; Wong et al. 2006). However, high BA concentrations have been reported to be involved in reactivation of latent viral infection (Imai et al. 2012, 2009) and induction of periodontal pathogenesis (Chen and Zychlinsky 1994; Kurita-Ochiai et al. 2003; Kurita-Ochiai and Ochiai 2010).
Periodontopathic bacterial pathogens, such as Porphyromonas gingivalis and Fusobacterium nucleatum, play a significant role in producing a variety of virulence factors, like BA, which in high amounts lead to the development of periodontal diseases (Socransky and Haffajee 1991; Soder et al. 1993; Teng et al. 2002). Previous published works related to our group have shown that BA induces apoptosis in inflamed fibroblasts (Kurita-Ochiai et al. 2008), Jurkat T cells (Kurita-Ochiai et al. 1997; Kurita-Ochiai and Ochiai 2010), human peripheral blood mononuclear cells (Kurita-Ochiai et al. 1999), WEHI 231 and RAJI B-lymphoma cells (Kurita-Ochiai et al. 1998), splenic T cells and B cells (Kurita-Ochiai et al. 1997, 1998), and murine thymocytes (Kurita-Ochiai et al. 1997).
Apoptosis occurs concurrently with reactive oxygen species (ROS) generation and mainly involves the mitochondria (Chandra et al. 2000). The mitochondria is the site of heme biosynthesis (Ponka 1997) and its product, heme, serves as a prosthetic group in many essential enzymes involved in electron transport, detoxification, antioxidant activity, nitrogen monoxide synthesis, oxygen transport, and apoptosis (Ajioka et al. 2006). In addition, the mitochondria play an important role in redox signaling (Daiber 2010) and utilize several mechanisms to amplify ROS formation needed for ROS-dependent signaling (Brandes 2005; MacMillan-Crow et al. 1998; Radi et al. 2002). It is important, however, to maintain ROS homeostasis in order to avoid excessive ROS accumulation which would eventually lead to oxidative stress and, subsequently, apoptosis.
ROS homeostasis is established when there is a balance between ROS amounts and the antioxidant activity (Mittler et al. 2004; Takada et al. 2002). If there is a disruption in ROS homeostasis, then oxidative stress is achieved. Oxidative stress represents an imbalance between excessive ROS and low antioxidant activity (Arora et al. 2002; Scandalios 2002; Torres et al. 2006). Previous works done in vitro have shown that BA-induced apoptosis is ascribable to mitochondrial oxidative stress induction (Kurita-Ochiai et al. 2003; Kurita-Ochiai and Ochiai 2010), however, BA effects in vivo were never fully elucidated in blood mitochondria.
Here, we showed that BA has prolonged retention in the gingival tissues. Moreover, we found that BA induced an increase in mitochondrial hydrogen peroxide (H2O2), heme, superoxide dismutase (SOD), and catalase (CAT) levels which we suspect would insinuate that BA causes oxidative stress in the jugular blood mitochondria.
Results and discussion
BA has prolonged retention in the rat gingival tissue
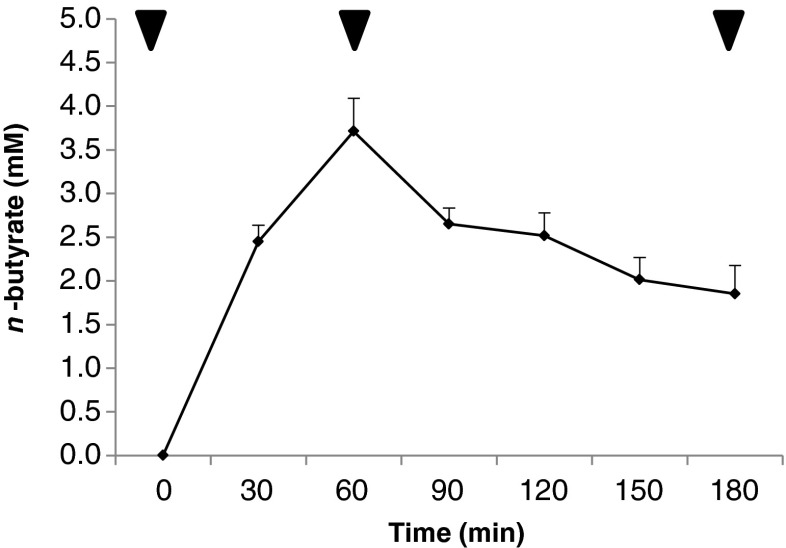
To establish BA retention in the gingival tissues, we injected BA into rat gingival tissues and collected blood from the jugular in 30-min intervals for 180 min. Blood funnels and exit the head and neck through the jugular vein which makes this the ideal site for blood extraction. As seen in Fig. 1, BA was detected in the isolated blood where BA concentrations peak at 60 min after BA injection and gradually decreased from 90 to 180 min after BA injection. We attributed this observation to the gradual diffusion of BA into the blood stream. Nevertheless, we would like to emphasize that even though BA levels were gradually decreased, BA was still detected 180 min after BA injection which would suggest that BA has a longer retention time in the gingival tissues. This would insinuate that high BA amounts could accumulate in the gingival tissues (Kurita-Ochiai and Ochiai 2010; Maslowski et al. 2009). Gingival tissues are composed of stratified squamous epithelial cells which have keratinized regions that exhibit low permeability (Sasaki et al. 2011; Squier 1991). We suspect that due to the low permeability found within the gingival tissues, this would consequentially result into prolonged BA retention and, subsequently, allow high BA levels to accumulate.
Fig. 1.
Butyric acid has prolonged retention in rat gingival tissues. Male Wistar rats (10 weeks old, Japan SLC, Shizuoka, Japan) were housed in individual stainless steel cages with wire-mesh bottoms. The cages were placed in a room under controlled temperature (23–25 °C), relative humidity (40–60 %), and lighting (12 h). The rats had free access to water and a semi-purified diet based on the AIN93G formulation for an acclimation period of 7 days. They were handled in accordance with the guidelines for animal studies of the Kyoto Institute of Nutrition and Pathology. Six acclimated rats were implanted with jugular cannulae under sodium pentobarbital anesthesia (40 mg kg−1 body weight). Lowest possible nonlethal butyric acid concentration was determined based on the rat body weight. Ten microliters of 13C n-butyrate solution (1 M) was injected in several batches into gingival tissues. The jugular blood was collected at 0, 30, 60, 90, 120, 150, and 180 min after injection, and blood serum was analyzed using liquid chromatography–mass spectrometry. Arrows indicated blood samples used for further analysis
High BA levels have been correlated with diseases (Imai et al. 2012; Imai et al. 2009; Margolis et al. 1988) and apoptosis induction (Kurita-Ochiai and Ochiai 2010), whereas, low BA levels have been correlated with colonic health (Maslowski et al. 2009; Wong et al. 2006). BA levels detected in this study were relatively low as compared to those that are related to diseases (Imai et al. 2012, 2009; Margolis et al. 1988), however, we hypothesize that cells exposed to low BA levels but with prolonged exposure time could still affect cellular function, in particular, mitochondrial function.
BA influences mitochondrial function by increasing both mitochondrial H2O2 and heme amounts
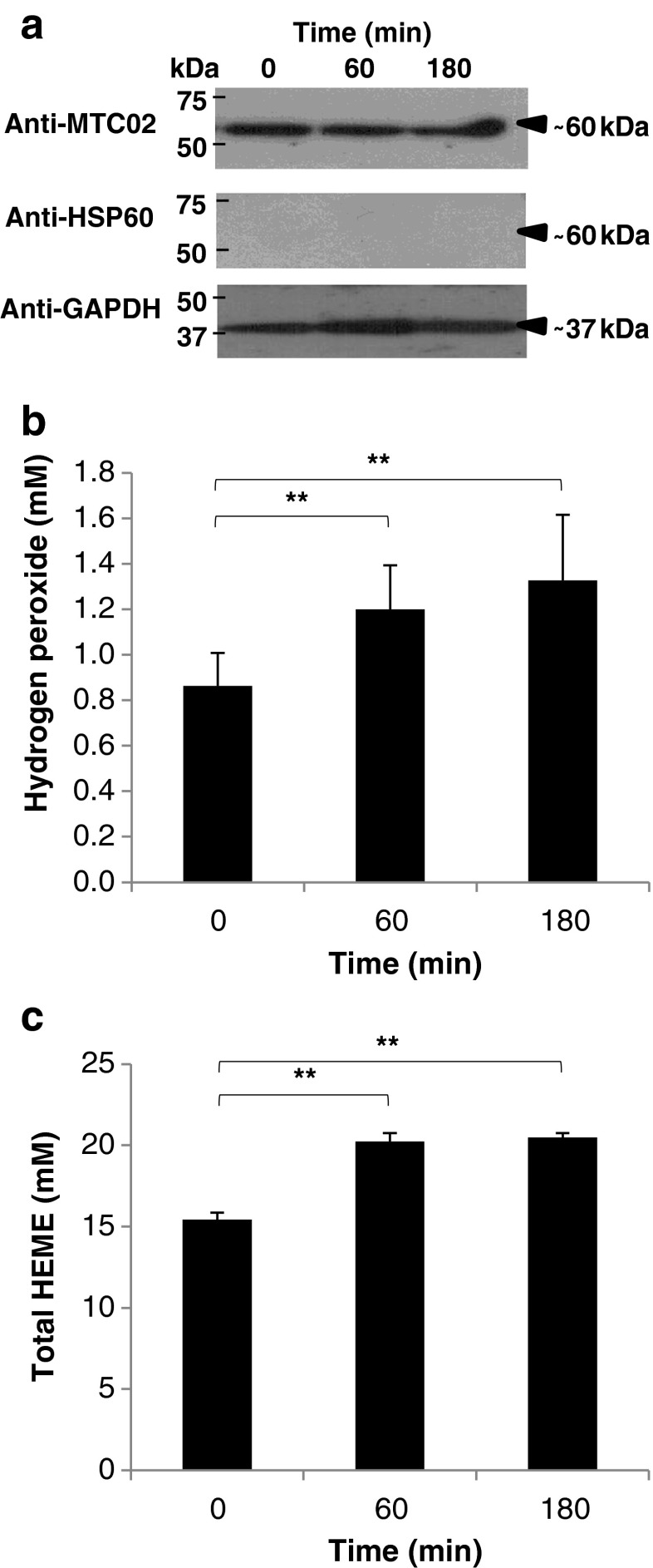
To confirm BA effects on mitochondrial function, H2O2 amounts were measured. We used blood obtained from 0, 60, and 180 min after BA injection and isolated blood mitochondria. Purity of the mitochondria was confirmed using Western blotting (Fig. 2a). We found that in mitochondrial H2O2, the amounts gradually increased 60 and 180 min after BA injection (Fig. 2b), and we correlated the gradual increase in mitochondrial H2O2 amounts to BA. Moreover, this would further imply that prolonged BA retention in the gingival tissues could cause mitochondrial H2O2 buildup leading to oxidative stress. Moreover, our results would imply that BA effects in vitro and in vivo are consistent (Kurita-Ochiai and Ochiai 2010).
Fig. 2.
Butyric acid-induced increase in mitochondrial H2O2 amounts is correlated to increased heme levels. a Isolated blood mitochondria from 0, 60, and 180 min after butyric acid injection. QproteomeTM Mitochondria Isolation Kit (Qiagen) was used to isolate blood mitochondria. Pierce® Detergent Removal Spin Columns (Thermo Scientific) was used to purify samples from traces of detergents. Pierce® Microplate BCA Protein Assay Kit-Reducing Agent Compatible Kit (Thermo Scientific) was used to standardize the protein concentration in all samples used. All kits were used according to manufacturer’s recommendation. Anti-MTC02 (Novus Biologicals) is a mitochondria-specific antibody used to verify the purity of the isolated blood mitochondria. Anti-HSP60 (StressMarq Biosciences Inc., Canada) is used to detect cytoplasmic heat-shock protein 60 in the blood mitochondria to further confirm the purity of the mitochondrial samples. Anti-GAPDH (GeneTex) is used to detect the glyceraldehydes-3-phosphate in the blood mitochondria to serve as control. b H2O2 amounts in blood mitochondria samples. Red Hydrogen Peroxide Assay Kit (Enzo Life Sciences) was used to measure mitochondrial H2O2 amounts according to manufacturer’s recommendation. c Total heme levels in blood mitochondria samples. QuantiChromTM Heme Assay Kit was used to measure total mitochondrial heme levels (free heme and heme-proteins) according to manufacturer’s recommendation. In all assays performed, results shown are mean ± SE, n = 5 replicates of six independent samples. Statistical analyses were performed using Student’s t test (**represents p value < 0.01compared to normal)
ROS (like H2O2) are generated as either as cellular products or by-products (Liu et al. 2008; Valko et al. 2006). ROS is commonly related to pathophysiological processes and has long been proposed to function in cell signaling (Suzuki et al. 1997; Valko et al. 2006; Zitomer and Lowry 1992). One primary source of ROS is the mitochondria wherein several mechanisms including oxidative damage (Radi et al. 2002), manganese SOD inactivation (MacMillan-Crow et al. 1998), and changes in mitochondrial membrane potential (Brandes 2005; Kurita-Ochiai and Ochiai 2010) signal ROS formation. Interestingly, heme which is synthesized in the mitochondria has also been associated with ROS formation.
Heme is a biomolecule that plays an essential role in various biological reactions and interacts with various apoproteins giving rise to functional heme-proteins (Ponka 1999). However, free heme and heme-proteins have been associated with ROS formation and, subsequently, toxicity (Balla et al. 2000; Hasan and Schafer 2008). This would suggest that heme concentration is likewise affected by BA. To determine BA effects on mitochondrial heme concentrations, total heme levels in the blood mitochondria were measured. As seen in Fig. 2c, we found that total heme levels increased at 60 and 180 min after BA injection in blood mitochondria. This would imply that BA could affect mitochondrial heme concentrations. We hypothesize that BA-associated increase in mitochondrial H2O2 amounts is somehow associated to an increase in mitochondrial heme levels and, similarly, may influence the mitochondria-related section of the heme biosynthesis pathway. Further studies are recommended to elucidate this observation.
BA similarly induces an increase in mitochondrial SOD and CAT activities
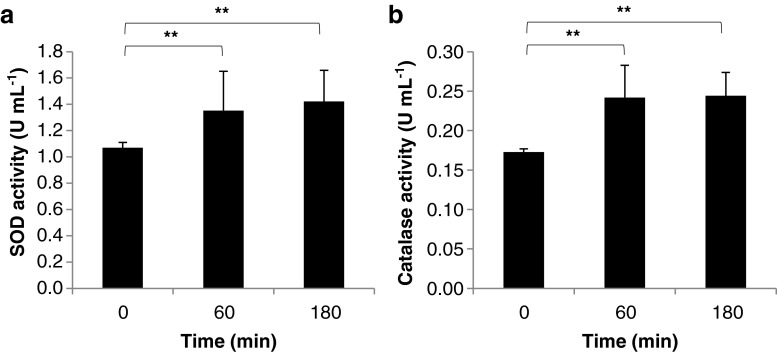
An increase in mitochondrial H2O2 amounts would insinuate that mitochondrial oxidative stress was attained. Consequentially, an increase in antioxidant activities is a common occurrence during oxidative stress (Bhandari et al. 2000). To verify whether mitochondrial antioxidant activities were similarly increased, SOD and CAT activities were measured. SODs are metalloenzymes involved in catalyzing dismutation of superoxide radicals to H2O2 (Alscher et al. 2002), whereas, CATs are ubiquitous antioxidant enzymes that catalyze the breakdown of H2O2 to water and oxygen (Du et al. 2008). Both enzymes serve as antioxidants that help maintain and protect intracellular redox homeostasis (Valko et al. 2007; Valko et al. 2006). Any shift in the balance between pro-oxidants and antioxidants toward oxidation leads to oxidative stress (Arora et al. 2002) which may cause DNA mutations, protein oxidation, and lipid peroxidation which eventually may lead to loss of molecular function (Giorgio et al. 2007; Liu et al. 2008; Valko et al. 2006). We found that SOD and CAT activities in blood mitochondria were increased 60 and 180 min after BA injection (Fig. 3a, b, respectively). This would imply that BA-induced mitochondrial oxidative stress is accompanied by an increase in antioxidant activities (SOD and CAT).
Fig. 3.
Increase is SOD and CAT activities help maintain mitochondrial ROS homeostasis. a SOD activity in blood mitochondria samples. Superoxide Dismutase Assay Kit (Cayman Chemical Company) was used to measure mitochondrial SOD activity according to manufacturer’s recommendation. b CAT activity in blood mitochondria samples. EnzyChromTM Catalase Assay Kit (BioAssay Systems) was used to measure mitochondrial CAT activity according to manufacturer’s recommendation. In all assay performed, results shown are mean ± SE, n = 5 replicates of six independent samples. Statistical analyses were performed using Student’s t test (**represents p value < 0.01compared to normal)
In conclusion, we established that BA has prolonged retention in gingival tissues. We found that BA retention in the blood mitochondria affected mitochondrial function by increasing both mitochondrial H2O2 and heme levels. Moreover, the increase in mitochondrial H2O2 amounts would suggest that mitochondrial oxidative stress was achieved. Furthermore, we observed that BA-induced mitochondrial oxidative stress is accompanied by an increase in mitochondrial SOD and CAT activities.
Acknowledgments
This work was supported by the Dental Research Center-Nihon University School of Dentistry and funded by the Sato Fund-Nihon University School of Dentistry and both the Ministry of Health, Labor and Welfare and the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan through the Strategic Research Base Development Program for Private Universities 2010–2014 (S1001024).
References
- Ajioka RS, Phillips JD, Kushner JP. Biosynthesis of heme in mammals. Biochim Biophys Acta. 2006;1763:723–736. doi: 10.1016/j.bbamcr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot. 2002;53:1331–1341. doi: 10.1093/jexbot/53.372.1331. [DOI] [PubMed] [Google Scholar]
- Arora A, Sairam RK, Srivastava GC. Oxidative stress and antioxidative system in plants. Curr Sci. 2002;82:1227–1238. [Google Scholar]
- Balla J, Balla G, Jeney V, Kakuk G, Jacob HS, Vercellotti GM. Ferriporphyrins and endothelium: a 2-edged sword-promotion of oxidation and induction of cytoprotectants. Blood. 2000;95:3442–3450. [PubMed] [Google Scholar]
- Bhandari V, Maulik N, Kresch M. Hyperoxia causes an increase in antioxidant enzyme activity in adult and fetal rat type II pneumocytes. Lung. 2000;178:53–60. doi: 10.1007/s004080000006. [DOI] [PubMed] [Google Scholar]
- Brandes RP. Triggering mitochondrial radical release: a new function for NADPH oxidases. Hypertension. 2005;45:847–848. doi: 10.1161/01.HYP.0000165019.32059.b2. [DOI] [PubMed] [Google Scholar]
- Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2000;29:323–333. doi: 10.1016/S0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zychlinsky A. Apoptosis induced by bacterial pathogens. Microb Pathog. 1994;17:203–212. doi: 10.1006/mpat.1994.1066. [DOI] [PubMed] [Google Scholar]
- Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta. 2010;1797:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- Du YY, Wang PC, Chen J, Song CP. Comprehensive functional analysis of the catalase gene family in Arabidopsis thaliana. J Integr Plant Biol. 2008;50:1318–1326. doi: 10.1111/j.1744-7909.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- Hasan RN, Schafer AI. Hemin upregulates Egr-1 expression in vascular smooth muscle cells via reactive oxygen species ERK-1/2-Elk-1 and NF-kappaB. Circ Res. 2008;102:42–50. doi: 10.1161/CIRCRESAHA.107.155143. [DOI] [PubMed] [Google Scholar]
- Hu S, Dong TS, Dalal SR, Wu F, Bissonnette M, Kwon JH, Chang EB. The microbe-derived short chain fatty acid butyrate targets miRNA-dependent p21 gene expression in human colon cancer. PLoS One. 2011;6:e16221. doi: 10.1371/journal.pone.0016221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Ochiai K, Okamoto T. Reactivation of latent HIV-1 infection by the periodontopathic bacterium Porphyromonas gingivalis involves histone modification. J Immunol. 2009;182:3688–3695. doi: 10.4049/jimmunol.0802906. [DOI] [PubMed] [Google Scholar]
- Imai K, Inoue H, Tamura M, Cueno ME, Takeichi O, Kusama K, Saito I, Ochiai K. The periodontal pathogen Porphyromonas gingivalis induces the Epstein-Barr virus lytic switch transactivator ZEBRA by histone modification. Biochimie. 2012;94:839–846. doi: 10.1016/j.biochi.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Kurita-Ochiai T, Ochiai K. Butyric acid induces apoptosis via oxidative stress in Jurkat T-cells. J Dent Res. 2010;89:689–694. doi: 10.1177/0022034510365456. [DOI] [PubMed] [Google Scholar]
- Kurita-Ochiai T, Fukushima K, Ochiai K. Butyric acid-induced apoptosis of murine thymocytes, splenic T cells, and human Jurkat T cells. Infect Immun. 1997;65:35–41. doi: 10.1128/iai.65.1.35-41.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita-Ochiai T, Ochiai K, Fukushima K. Volatile fatty acid, metabolic by-product of periodontopathic bacteria, induces apoptosis in WEHI 231 and RAJI B lymphoma cells and splenic B cells. Infect Immun. 1998;66:2587–2594. doi: 10.1128/iai.66.6.2587-2594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita-Ochiai T, Fukushima K, Ochiai K. Lipopolysaccharide stimulates butyric acid-induced apoptosis in human peripheral blood mononuclear cells. Infect Immun. 1999;67:22–29. doi: 10.1128/iai.67.1.22-29.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita-Ochiai T, Amano S, Fukushima K, Ochiai K. Cellular events involved in butyric acid-induced T cell apoptosis. J Immunol. 2003;171:3576–3584. doi: 10.4049/jimmunol.171.7.3576. [DOI] [PubMed] [Google Scholar]
- Kurita-Ochiai T, Seto S, Suzuki N, Yamamoto M, Otsuka K, Abe K, Ochiai K. Butyric acid induces apoptosis in inflamed fibroblasts. J Dent Res. 2008;87:51–55. doi: 10.1177/154405910808700108. [DOI] [PubMed] [Google Scholar]
- Liu B, Chen Y, St Clair DK. ROS and p53: a versatile partnership. Free Radic Biol Med. 2008;44:1529–1535. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol. 2010;12:304–314. doi: 10.1111/j.1462-2920.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- MacMillan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry. 1998;37:1613–1622. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- Margolis HC, Duckworth JH, Moreno EC. Composition and buffer capacity of pooled starved plaque fluid from caries-free and caries-susceptible individuals. J Dent Res. 1988;67:1476–1482. doi: 10.1177/00220345880670120701. [DOI] [PubMed] [Google Scholar]
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Ponka P. Tissue-specific regulation of iron metabolism and heme synthesis: distinct control mechanisms in erythroid cells. Blood. 1997;89:1–25. [PubMed] [Google Scholar]
- Ponka P. Cell biology of heme. Am J Med Sci. 1999;318:241–256. doi: 10.1097/00000441-199910000-00004. [DOI] [PubMed] [Google Scholar]
- Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451–1464. doi: 10.1016/S0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Yamada T, Inoue K, Momoi T, Tokunaga H, Sakiyama K, Kanegae H, Suda N, Amano O. Localization of heat shock protein 27 (hsp27) in the rat gingiva and its changes with tooth eruption. Acta Histochem Cytochem. 2011;44:17–24. doi: 10.1267/ahc.10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios JG. The rise of ROS. Trends Biochem Sci. 2002;27:483–486. doi: 10.1016/S0968-0004(02)02170-9. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. Microbial mechanisms in the pathogenesis of destructive periodontal diseases: a critical assessment. J Periodontal Res. 1991;26:195–212. doi: 10.1111/j.1600-0765.1991.tb01646.x. [DOI] [PubMed] [Google Scholar]
- Soder PO, Jin LJ, Soder B. DNA probe detection of periodontopathogens in advanced periodontitis. Scand J Dent Res. 1993;101:363–370. doi: 10.1111/j.1600-0722.1993.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Squier CA. The permeability of oral mucosa. Crit Rev Oral Biol Med. 1991;2:13–32. doi: 10.1177/10454411910020010301. [DOI] [PubMed] [Google Scholar]
- Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/S0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- Takada Y, Hachiya M, Park SH, Osawa Y, Ozawa T, Akashi M. Role of reactive oxygen species in cells overexpressing manganese superoxide dismutase: mechanism for induction of radioresistance. Mol Cancer Res. 2002;1:137–146. [PubMed] [Google Scholar]
- Teng YT, Taylor GW, Scannapieco F, Kinane DF, Curtis M, Beck JD, Kogon S. Periodontal health and systemic disorders. J Can Dent Assoc. 2002;68:188–192. [PubMed] [Google Scholar]
- Torres MA, Jones JD, Dangl JL. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006;141:373–378. doi: 10.1104/pp.106.079467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- Zitomer RS, Lowry CV. Regulation of gene expression by oxygen in Saccharomyces cerevisiae. Microbiol Rev. 1992;56:1–11. doi: 10.1128/mr.56.1.1-11.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]