Abstract
Raphinus sativus Linn (Cruciferae) commonly known as ‘Radish’ is a multipurpose herb cultivated in different parts of the world for its edible roots and leaves. The present study was aimed to evaluate the antiulcer activity of leaf extracts of R. sativus Linn on acetic acid induced chronic gastric ulcer and pylorus ligation induced gastric ulcer in rats. The acute oral toxicity study revealed that all the extracts were safe up to 2000 mg/kg per oral dose; hence one-tenth of this dose was selected for evaluation of antiulcer activity. In acetic acid induced gastric ulcer models, the ERS, CRS, EARS and AQRS have offered significant protection against acetic acid induced ulcers when compared to control group. While in pylorus ligation induced ulcer model the ERS, EARS and AQRS showed significant protection by decreasing the ulcer index, total acidity and free acidity. In conclusion the leaf extracts of R. sativus Linn are found to possess antiulcer property in the experimental animal models of gastric ulcers, which is consistent with the literature report in the folk medicine.
Keywords: Raphinus sativus, Gastric ulcer, Acetic acid, Regenerated epithelium, Collagen content, Ulcer index
1. Introduction
Peptic ulcer is the most common gastrointestinal disorder in clinical practice. Peptic ulcer disease refers to pathological lesions and ulcers of any portion of gastrointestinal tract exposed to acid activated pepsin (Jain and Santani, 1994). Even though the etiology of gastric ulcer is still debated, it is accepted that ulcers are caused due to net imbalances in mucosal offensive and defensive factors (Goel and Bhattacharya, 1991). Research advances have offered new sights in the therapy and prevention of gastroduodenal ulcerations by measures directed at strengthening the mucosal defense systems rather than by attenuating aggressive acid pepsin factors held responsible for the induction of ulcers. Treatment options available are use of mucoprotective agents, antacids, alginates, motility stimulants, and acid suppressants and antireflux surgery is done in severe cases (Cara, 2001).
In-spite of established antiulcer drugs, a rational therapy for peptic ulcer remains elusive and search for safer potential drugs is being carried out and the use of herbal medicines in gastric ulcer has been reported (Sairam, 2001). Previous reports on the incidence of gastric ulcers in South Asian population indicate that the incidence may be lower due to the type of food consumed by people of this region, one of the food that is speculated to protect against ulcers is leaves of Raphinus sativus Linn (Cruciferae) (Jayaraj et al., 1998). The leaves and roots of R. sativus L. are used as vegetables in different parts of the world. Apart from this, the roots and leaves of the plant have been reported to possess a wide range of pharmacological activities, like gut stimulatory effect (Gilani and Ghayur, 2004), hepatoprotective activity (Zaman and Ahmad, 2004), cardioprotective effect (Zaman, 2004), antioxidant activity (Barillari et al., 2006) and antiurolithiatic activity (Vargas et al., 1999). Furthermore, the freshly squeezed root juice of R. sativus L. has been reported to possess antiulcer activity (Alqasoumi et al., 2008). However, no study has been conducted to scientifically prove that leaves of R. sativus L. possess any effect on gastric ulcers. Hence, the present study was undertaken to evaluate the protective effect of leaves of R. sativus L. on experimentally induced gastric ulcers in rats.
2. Materials and methods
2.1. Drugs and chemicals
Ranitidine (Torrent Pharmaceuticals, Ahmedabad, India), Omeprezole (Torrent Pharmaceutical, Ahmedabad, India) and the solvents used for extraction process and chemicals used for phytochemical analysis were of analytical grade and procured from local firms.
2.2. Collection and identification of the plant material
The Leaves of R. sativus L. were collected from the Madiwala area of Bangalore, India in the month of December. Botanical identification was done by Prof. Balakrishna gowda, GKVK, Bangalore.
2.3. Extraction
The leaves of R. sativus L. were shade dried and reduced to coarse powder in a mechanical grinder and passed through sieve no. 40. The powdered material obtained was then subjected to extraction by cold maceration using rectified spirit (90%) for a total of seven days. The extract was filtered and concentrated in a rotary evaporator under reduced pressure to yield a thick green ethanolic extract (ERS). The crude extract thus obtained was partition-fractionated with 1:1 of petroleum ether and ethanol (50%), the mixture was shaken vigorously and kept for about 30 min to let the two layers separate. The upper layer consisted of petroleum ether, it was removed and concentrated in a rotary evaporator to obtain petroleum ether fraction (PERS). The same procedure was repeated with the residue using equivalent volumes of chloroform and ethyl acetate to obtain chloroform fraction (CRS) and ethyl acetate fraction (EARS), respectively. The powdered material was subjected to extraction by cold maceration with distilled water for a total of seven days. The extract was filtered and concentrated in rotary evaporator under reduced pressure to yield a thick green aqueous extract. The extracts thus obtained were subjected to phytochemical analysis (Khandelwal, 1996).
2.4. Experimental animals
Male albino wistar rats weighing between 200–230 g were acclimatized for 7 days under standard husbandry conditions, i.e. room temperature 26 ± 1 °C, relative humidity 45–55% and light:dark cycles of 12:12 h. All the experimental protocols were approved by the Institutional Animal Ethics Committee (IAEC) of Bioneeds Preclinical services, Bangalore and conducted according to the Committee for the Purpose of the Control and Supervision of Experiments on Animals (CPCSEA) guidelines.
2.5. Acute toxicity studies
Acute oral toxicity was determined by using female, nulliparous and non-pregnant mice weighing 18–22 g. The animals were fasted for 3 h prior to the experiment. Up and down procedure OECD guideline no. 425 was adopted for toxicity studies (http://www.epa.gov/oppfead1/harmonization/). Animals were administered with a single dose of extract and were observed for their mortality during the 48 h study period (short term) toxicity.
2.6. Antiulcer activity
2.6.1. Acetic acid induced chronic gastric ulcer model
The method described by Okabe et al. was followed (Okabe et al., 1970). The animals were fasted for 24 h prior to the experiment. Under light ether anesthesia, laparotomy was performed on all rats through a midline epigastric incision. After exposing the stomach, glacial acetic acid (0.05 ml) was applied on to the serosal surface of the stomach using a cylindrical mold of 6 mm diameter for 60 s. The acid solution was then removed by rinsing the mold with normal saline twice or thrice to avoid damage to the surrounding tissues. The stomach was placed back carefully and the abdominal wall was closed by interrupted sutures. The animals were treated with ranitidine (50 mg/kg, p.o.) and with different extracts of leaves of R. sativus L. (200 mg/kg p.o.) once daily for 10 days after induction of ulcer.
The animals in the control group received 0.5% Carboxy methyl cellulose (CMC) in water, as vehicle. Rats were sacrificed on the 10th day; stomachs were removed and cut open along the greater curvature, the stomach samples were scanned using a computer scanner and the total mucosal area and total ulcerated area were measured using public domain image processing and analysis program, developed at the National Institute of Health, USA. The scale was set at 6.1 pixels per millimeter. The ulcer index was determined using the formula
where X = Total mucosal area/Total ulcerated area.
Histopathological evaluation: The stomach samples from groups that showed reduction in ulcer index were subsequently processed for histological examination. The tissues were fixed in 10% buffered formalin and were processed using a tissue processor. The processed tissues were embedded in paraffin blocks and about 5-μm thick sections were cut using a rotary microtome. These sections were stained with hematoxylin and eosin, masson’s trichome and periodic acid-Schiff stain using routine procedures. The slides were examined microscopically for four indices namely regenerated glandular epithelial width, capillary density, collagen content and surface epithelium which would reflect the rate and quality of ulcer healing (Wang et al., 1999).
Regenerated lining epithelial width was defined as the average distance from the origin of regenerated lining epithelium to the surface of the ulcer.
Capillary density within the granulation tissues of the ulcers was determined using eyepiece reticule (×100 magnification) on the hematoxylin and eosin stained sections in the ulcer center, each field was 140 μm × 140 μm. At least four fields were examined on each section. Capillary density within the granulation tissues of the ulcers was expressed as the average capillary numbers in the field.
Collagen content within the scar tissues of ulcers displayed blue color in sections stained with Masson’s stain and was determined by point count using 1 square eye piece reticule (×100 magnification). Collagen content was expressed as
Regenerated glandular epithelium is the total length of the epithelium found in the secretory gland.
2.6.2. Pyloric ligation induced ulcer model
The animals were fasted for 36 h with water ad libitum before pylorus ligation (Shay et al., 1945). Normal saline (1 ml/rat, p.o.) was administered twice daily to all the animals during the fasting period. Under light ether anesthesia, the pyloric portion of the stomach was ligated. The leaf extracts (200 mg/kg) were administered intraduodenally immediately after pylorus ligation. Four hours after pylorus ligation, animals were sacrificed, stomachs were isolated and the contents were collected, measured and centrifuged. The free and total acidity were estimated (Hawk et al., 1947). The stomachs were cut open along the greater curvature and the surface was examined for ulceration. The ulcer index was calculated by using the equation
where X = Total mucosal area/Total ulcerated area.
2.7. Statistical analysis
The statistical significance was assessed using ANOVA followed by Dunnett multiple comparison test. The values are expressed as mean ± SEM and p < 0.05 was considered significant.
3. Results
3.1. Phytochemical screening
Preliminary phytochemical screening of the leaf extracts revealed the presence of steroids in PERS, CRS found to possess saponins and alkaloids, EARS contains proteins, saponins, glycosides and flavonoids; AQRS showed the presence of carbohydrates, proteins, alkaloids, saponins, flavonoids and glycosides, while the ERS showed presence of tannins along with all the constituents present in aqueous extract.
3.2. Acute oral toxicity
In acute oral toxicity study, no mortality was observed after treatment with the highest tested dose (2000 mg/kg p.o.) of all the extracts of leaves.
3.3. Antiulcer activity
3.3.1. Acetic acid-induced chronic gastric ulcer model
The ERS, CRS, EARS and AQRS showed a significant reduction in ulcer index when compared with control (p < 0.01). The ERS was most potent than other extracts; it has shown 80% decrease in ulcer index when compared to control, While the PERS did not show any significant effect on ulcer index (Fig. 1).
Figure 1.
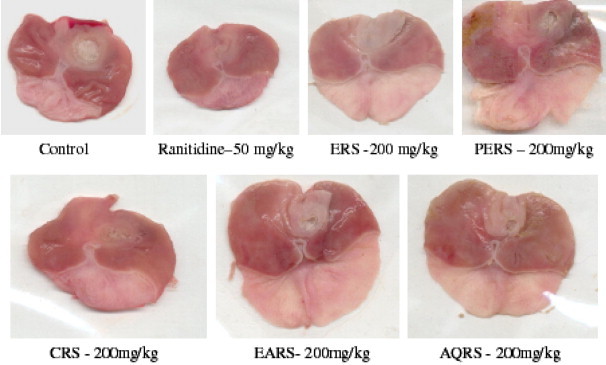
Effect of R. sativus L. on Acetic acid induced chronic gastric ulcers in rats.
Histological examination of the ulcerated area revealed that there was a significant increase in regenerated glandular epithelium width after treatment with ranitidine, ERS, CRS and AQRS (p < 0.01) when compared with control.
A significant increase in capillary density in scar tissue was observed after treatment with ERS, CRS, AQRS and ranitidine. The collagen content in the ulcerated tissue was significantly increased by all the four extracts of the leaves and ranitidine, maximum effect was observed with ERS. No significant difference in surface epithelium in scar tissue was observed in any of the treated groups, including ranitidine treated group (Table 1).
Table 1.
Effect of R. sativus L. on regenerated glandular epithelium width, capillary density, volume of collagen content and surface epithelium in acetic acid induced chronic gastric ulcers.
| Treatment | Regenerated glandular epithelium width (μm) | Capillary density (no.) in 19,600 μm2 | Volume of collagen content | Surface epithelium (μm) |
|---|---|---|---|---|
| Vehicle control (1 ml/kg p.o.) | 257 ± 4.91 | 3.05 ± 0.15 | 0.07 ± 0.001 | 106.80 ± 20.49 |
| Ranitidine (50 mg/kg p.o.) | 332 ± 0.01∗∗ | 6.01 ± 0.30∗∗ | 0.12 ± 0.002∗∗ | 99.67 ± 16.67 |
| ERS (200 mg/kg p.o.) | 375 ± 8.66∗∗ | 6.55 ± 0.25∗∗ | 0.13 ± 0.005∗∗ | 120.33 ± 12.78 |
| PERS (200 mg/kg p.o.) | 298 ± 19.34 | 3.01 ± 0.30 | 0.06 ± 0.004 | 42.93 ± 5.002 |
| CRS (200 mg/kg p.o.) | 382 ± 4.04∗∗ | 7.01 ± 0.02∗∗ | 0.15 ± 0.001∗∗ | 99.13 ± 0.569 |
| EARS (200 mg/kg p.o.) | 258 ± 4.72 | 2.15 ± 0.05 | 0.09 ± 0.002∗ | 154.01 ± 22.67 |
| AQRS (200 mg/kg p.o.) | 397 ± 10.69∗∗ | 6.90 ± 0.40∗∗ | 0.12 ± 0.002∗∗ | 119.33 ± 8.09 |
All values are expressed as Mean ± SEM, n = 6. ∗p < 0.05, ∗∗p < 0.01 when compared to control group.
3.3.2. Pylorus ligation induced gastric ulcer model
The ERS, EARS and AQRS showed a significant reduction in ulcer index, gastric juice volume, free acidity and total acidity, when compared with control (p < 0.01). Whereas the PERS and CRS did not show any significant effect on ulcer index, gastric juice volume, free acidity and total acidity (Fig. 2 and 3, Table 2).
Figure 2.
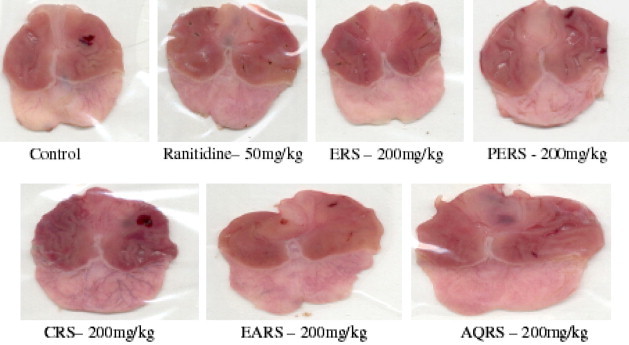
Effect of R. sativus L. on Pylorus ligation induced gastric ulcers in rats.
Figure 3.
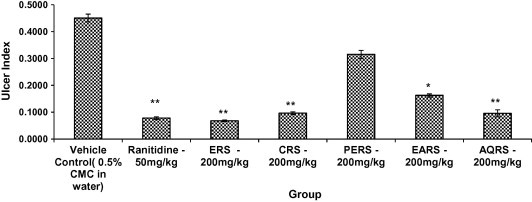
Effect of R. sativus L. on Pylorus ligation gastric ulcers in rats. All values are Mean ± SEM, n = 6. ∗p < 0.05, ∗∗p < 0.01, when compared to control group.
Table 2.
Effect of R. sativus L. leaf extracts on gastric juice volume, free acidity, total acidity, and ulcer index in pylorus ligated rats.
| Treatment | Volume of Gastric juice per 100 g rat (ml) | Ulcer index | Free acidity (mEq/4 h) | Total acidity (mEq/4 h) |
|---|---|---|---|---|
| Vehicle Control (1 ml/kg p.o.) | 3.64 ± 0.328 | 0.249 ± 0.03 | 2.68 ± 0.46 | 5.98 ± 0.47 |
| Omeperazole (2 mg/kg p.o.) | 1.71 ± 0.121∗∗ | 0.018 ± 0.02∗∗ | 1.22 ± 0.08∗∗ | 2.43 ± 0.14∗∗ |
| ERS (200 mg/kg p.o.) | 1.80 ± 0.248∗∗ | 0.123 ± 0.01∗∗ | 1.02 ± 0.16∗∗ | 2.42 ± 0.27∗∗ |
| PERS (200 mg/kg p.o.) | 3.58 ± 0.168 | 0.275 ± 0.03 | 2.93 ± 0.24 | 5.52 ± 0.37 |
| CRS (200 mg/kg p.o.) | 3.29 ± 0.202 | 0.249 ± 0.01 | 2.26 ± 0.40 | 4.98 ± 0.17 |
| EARS (200 mg/kg p.o.) | 2.30 ± 0.144∗∗ | 0.165 ± 0.01∗ | 1.85 ± 0.30∗∗ | 3.70 ± 0.16∗∗ |
| AQRS (200 mg/kg p.o.) | 1.65 ± 0.050∗∗ | 0.109 ± 0.01∗∗ | 1.01 ± 0.18∗∗ | 2.21 ± 0.17∗∗ |
All values are Mean ± SEM, n = 5–6. ∗p < 0.05 when compared to vehicle treated group. ∗∗p < 0.01 when compared to control group.
4. Discussion
The etiology of peptic ulcer is unknown in most of the cases and generally it is believed that may be due to an imbalance between the aggressive factors and mucosal integrity through the endogenous defense mechanisms (Piper and Stiel, 1986). To regain the balance, different therapeutic agents including plant extracts are used. The leaves of R. sativus are one such herbal drug undertaken in the present study to evaluate its antiulcerogenic potential.
Acetic acid induced ulcer model resembles clinical ulcers in location, chronicity and severity and serves as the most reliable model to study healing process, although specific mechanisms remain controversial, increase in acid output and subsequent pyloric obstruction may be the cause for ulceration due to acetic acid. Application of glacial acetic acid (0.05 ml) on to the serosal surface of the rat stomach produces deep penetrating gastric ulcers (Okabe and Pfeiffer, 1972; Okabe et al., 1971).
The ERS, CRS, EARS and AQRS have offered significant protection against acetic acid induced gastric ulcers. The histopathological examination was carried out to determine the effect of extracts on regeneration of glandular epithelium, surface epithelium, formation of collagen and capillary density; all of which are essential processes for the healing of ulcers. The ERS, CRS, EARS and AQRS had shown significant increase in tissue collagen content, regeneration of glandular epithelium and capillary density in scar tissue, whereas the PERS did not produce any significant changes.
Pylorus ligation induced gastric ulcer model is generally used to study the effect of test drugs on gastric secretions. The ligation of the pyloric end of the stomach causes accumulation of gastric secretions containing hydrochloric acid; it produces ulcers in the stomach. The agents that decrease gastric acid secretion and/or increase mucus secretion are effective in ulcers induced by this method.
The original Shay rat model involves fasting of rats for 36 h followed by ligation of pyloric end of the stomach; the ulcer index is determined 4 hours after pylorus ligation, the lesions produced by this method are located in the mucosal lining of the stomach. The ERS, EARS and AQRS had significantly reduced the volume of gastric acid secretion, indicating their antisecretory effect.
The ERS and AQRS were effective in both models of peptic ulcer disease, where as the CRS was effective only in healing chronic gastric ulcers. Hence, the healing of gastric ulcers in acetic acid induced gastric ulcers may be due to decreased acid secretion, increased mucus secretion or decreased GI motility in case of ERS and AQRS, where as the CRS may be acting through decreasing the gastric motility.
R. sativus: Linn contains flavonoids, saponins, tannins, glycosides, steroids, alkaloids and many other chemical constituents (Ross, 1999; Gutierrez et al., 2004). Some of these bioactive constituents have been associated with gastro-protective and antiulcer effects in previous studies. The non-specific gastro-protective activities of the extracts may be the result of a combined effect of the different phytoconstituents present. In previous studies the flavonoidal compounds were proved to have antisecretory and cytoprotective properties (Guaraldo et al., 2001) also believed to increase capillary resistance and cause an improvement in microcirculation. Further, the leaves contain other flavonoids like medication and kaempferol which are known to have gastric cytoprotective effect (Reyes et al., 1996; Izzo et al., 1994). These findings from the previous studies defend the more potent ulcer healing effect of ERS, EARS and AQRS compared to CRS. Apart from flavonoids, the leaves of the plant are also rich in saponins and tannins, which have been shown to exhibit antiulcer properties (Aguwa and Okunji, 1986; Lewis and Hanson, 1991). Tannins generally have vasoconstrictive and protein precipitating effects, precipitation of protein at ulcer sites forms impervious protective pellicle which renders the lesion less permeable to toxic substances and more resistant to attack of proteolytic enzymes (Nwafor et al., 2000). This probably explains the activity of CRS. However, further study is required to isolate the active components responsible for the antiulcer activity.
The results of the present study suggest that consumption of the leaves of R. sativus Linn may be beneficial in healing of ulcers in patients suffering from peptic ulcer disease.
Acknowledgments
The authors are thankful to Prof. Balakrishna gowda, GKVK, Bangalore (India) for identification of plant species.
References
- Aguwa C.N., Okunji C.O. Gastrointestinal studies of Pyrenacantha staudii leaf extracts. J. Ethnopharmacol. 1986;15:45–55. doi: 10.1016/0378-8741(86)90103-0. [DOI] [PubMed] [Google Scholar]
- Alqasoumi S., Yahya M.A., Al-Howiriny T., Rafatullah S. Gastroprotective effect of radish (Raphanus sativus L.) on experimental gastric ulcer models in rats. Farmacia LVI. 2008;(2):204–214. [Google Scholar]
- Barillari J., Cervellati R., Costa S. Antioxidant and Choleretic Properties of Raphanus sativus L. Sprout (Kaiware Daikon) Extract. J. Agric. Food Chem. 2006;54:9773–9778. doi: 10.1021/jf061838u. [DOI] [PubMed] [Google Scholar]
- Cara K. A gut full of complaints. The Aus. J. Pharm. 2001;82:624–630. [Google Scholar]
- Gilani A.H., Ghayur M.N. Pharmacological basis for the gut stimulatory activity of Raphanus sativus leaves. J. Ethnopharmacol. 2004;95:169–172. doi: 10.1016/j.jep.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Goel R.K., Bhattacharya S.K. Gastroduodenal mucosal defense and mucosal protective agents. Ind. J. Exp. Biol. 1991;29:701–714. [PubMed] [Google Scholar]
- Guaraldo L., Sertie J.A.A., Bachi E.M. Anti-ulcer action of the hydroalchoholic extract and fractions of Davilla rugosa Poiret in rat. J. Ethnopharmacol. 2001;76:191–195. doi: 10.1016/s0378-8741(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Gutierrez R.M., Perez R.L. Raphanus sativus (radish): their chemistry and biology. Sci. World J. 2004;4:811–837. doi: 10.1100/tsw.2004.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk, P.B., Oser, B.L., Summerson, H.W., 1947. Practical Physiological Chemistry. Churchill, London, pp. 347–348.
- Izzo A.A., Dicarlo G., Mascolo N., Autore G., Capasso F. Antiulcer effect of flavonoids Role of endogenous PAF. Phtyother. Res. 1994;6:179–181. [Google Scholar]
- Jain S.G., Santani D.D. Peptic ulcer disease and status of current drug therapy. Ind. Drugs. 1994;31:395–401. [Google Scholar]
- Jayaraj A.P., Tovey F.I., Clark C.G. Possible dietary protective factors in relation to the distribution of duodenal ulcer in India and Bangladesh. Gut. 1998;21:1068–1076. doi: 10.1136/gut.21.12.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal, K.R., 1996. Practical Pharmacognosy techniques and experiments; Nirali Prakashan, Pune, pp. 171–172.
- Lewis D.A., Hanson D. Anti-ulcer drugs of plant origin. Prog. Med. Chem. 1991;28:208–210. doi: 10.1016/s0079-6468(08)70365-5. [DOI] [PubMed] [Google Scholar]
- Nwafor P.A., Okwuasaba F.K., Binda L.G. Antidiarrhoeal and anti-ulcerogenic effects of methanolic extract of Asparagus Pubescens root in rats. J. Ethnopharmacol. 2000;72:421–427. doi: 10.1016/s0378-8741(00)00261-0. [DOI] [PubMed] [Google Scholar]
- OECD, 2001. OECD guidelines for testing of chemicals no. 425. Acute oral toxicity-modified up and down procedure. Paris, France: Organization of Economic Co operation and development <http://www.epa.gov/oppfead1/harmonization/>.
- Okabe S., Pfeiffer C.J., Roth J.L. Experimental production of duodenal and antral ulcers in rats. Federal Proc. 1970;29:255–260. [Google Scholar]
- Okabe S., Pfeiffer C. Chronicity of acetic acid in the rat stomach. Dig. Dis. 1972;7:619–629. doi: 10.1007/BF02231748. [DOI] [PubMed] [Google Scholar]
- Okabe S., Roth J.L., Pfeiffer C.J. Differential healing periods of the acetic acid ulcer model in rats and cats. Experientia. 1971;27:146–148. doi: 10.1007/BF02145860. [DOI] [PubMed] [Google Scholar]
- Piper D.W., Stiel D.D. Pathogenesis of chronic peptic ulcer. Current thinking and clinical implications. Med. Prog. 1986;2:7–10. [Google Scholar]
- Reyes M., Martin C., Alarcon de-L.L.C., Trujillo J., Toro M.V., Ayuso M.J. Anti-ulcerogenicity of the flavonoids fraction from Erica andevalensis Cabezudo Rivera. Z. Naturforsch. 1996;51:563–569. [PubMed] [Google Scholar]
- Ross, I.A., 1999. Medicinal Plants of the World. Humana Press Inc., Totowa, New Jersey, pp. 213–229
- Sairam K. Effect of Centella asiatica L. on physical and chemical factors induced gastric ulceration and secretion in rats. Ind. J. Exp. Biol. 2001;39:137–142. [PubMed] [Google Scholar]
- Shay H., Komarov S.A., Fele S.S., Meranze D., Gruenstein H., Siplet H. A simple method for uniform production of gastric ulceration in rat. Gastroenterology. 1945;5:43–61. [Google Scholar]
- Vargas B.R., Perez G.R.M., Perez G.S., Zavala S.M.A., Perez G.C. Antiurolithiatic activity of Raphinus sativus L. aqueous extract on rats. J. Ethnopharmacol. 1999;68:335–338. doi: 10.1016/s0378-8741(99)00105-1. [DOI] [PubMed] [Google Scholar]
- Wang J.Z., Wuy J., Rao C.M., Gao M.T., Li W.G. Effect of recombinant human basic fibroblast growth factor on stomach ulcers in rats and mice. Acta Pharmacol. Sin. 1999;209:763–768. [PubMed] [Google Scholar]
- Zaman R.U., Ahmad M. Evaluation of Hepatoprotective effects of Raphanus sativus L. J. Biol. Sci. 2004;4:463–469. [Google Scholar]
- Zaman R.U. Study of cardioprotective activity of Raphinus sativus L. in the rabbits. Pak. J. Biol. Sci. 2004;7:843–847. [Google Scholar]


