Abstract
While it is now established that sensory neurons in both the main olfactory epithelium and the vomeronasal organ may be activated by both general and pheromonal odorants, it remains unclear what initiates sampling by the VNO. Anterograde transport of wheat germ agglutinin-horseradish peroxidase was used to determine that adequate intranasal syringing with zinc sulfate interrupted all inputs to the main olfactory bulb but left intact those to the accessory olfactory bulb. Adult male treated mice were frankly anosmic when tested with pheromonal and non-pheromonal odors and failed to engage in aggressive behavior. Treated juvenile females failed to show puberty acceleration subsequent to exposure to bedding from adult males. Activation of the immediate early gene c-Fos and electro-vomeronasogram recording confirmed the integrity of the vomeronasal system in zinc sulfate treated mice. These results support the hypothesis that odor detection by the main olfactory epithelium is required to initiate sampling by the vomeronasal system.
Keywords: vomeronasal organ, accessory olfactory bulb, main olfactory bulb, odor detection
Introduction
The rodent peripheral olfactory system has two well-defined components, a primary or main olfactory epithelium (MOE) and the vomeronasal organ (VNO). Detection of odorous vapors by the main olfactory epithelium occurs during normal respiration but exposure of sensory neurons in the VNO to odorous stimuli requires activation of a pumping mechanism produced by alternate vasodilation and vasoconstriction of blood vessels lateral to the lumen of the VNO (Brennan, 2001; Halpern & Martinez-Marcos, 2003; Meredith & O’Connell, 1979; Meredith et al., 1980).
It had long been assumed that the MOE responded to ‘general’ odors but that sensory neurons of the VNO responded largely or only to pheromonal stimuli (Sam et al., 2001; Baxi et al., 2006; Brennan & Zufall, 2006; Spehr et al., 2006). It is now clear that MOE sensory neurons are activated by many if not all odorous stimuli that are effective in stimulating VNO neurons and that VNO neurons, in turn, can respond to both non-volatile and volatile odorants that are not obviously pheromonal stimuli (Baxi et al., 2006; Brennan & Zufall, 2006; Jakupovic et al., 2008; Spehr et al., 2006; Xu et al., 2005). Less clear is what initiates activation of the pumping mechanism required for vomeronasal sampling in rodents. The most likely hypotheses are that (1) VNO sampling occurs spontaneously, i.e., independent of input from the MOE and of behavioral signals from conspecifics, (2) that sampling is initiated by either olfactory and/or non-olfactory cues from conspecifics, or (3) that sampling requires and is initiated by odorous stimuli detected by the MOE.
In support of the first hypothesis are the findings of Trinh & Storm (2003) that type-3 adenylyl cyclase knockout mice (AC3−/−) like wild type mice whose MOE was treated with zinc sulfate were able to detect both pheromonal and presumed non-pheromonal odors. In support of the possibility that VNO sampling can be initiated in response to conspecifics are the findings of Ma et al. (2002) that, after inactivation of the MOE but not the VNO by a bacterial nitroreductase (ntr) gene linked to the expression of olfactory marker protein, female mice performed poorly in an odor habituation task but still responded to the pregnancy blocking effect of male pheromones.
In contrast, several reports indicate that, in the absence of input from the MOE, mice do not respond to normally effective pheromonal cues. Thus, sexual behavior (Keller et al., 2006a) and activation of neurons in the accessory olfactory bulb (AOB) in response to urinary vapor (Martel & Baum, 2007) were abolished by lesioning the MOE with intranasal zinc sulfate. Also, male mice lacking a functional cyclic nucleotide-gated channel alpha2 (CNGA2) channel fail to mate or fight (Mandiyan et al., 2005).
In this study we reassessed this issue using precision olfactometry, male mouse aggression, puberty acceleration in juvenile female mice, odor-induced c-Fos activation, and electro-vomeronasogram (EVG) recordings in control mice and those treated with a zinc sulfate nasal lavage sufficient to eliminate all input to the MOB but leave intact connections between the VNO and the AOB.
MATERIALS AND METHODS
1. Behavioral Tests
Animals
Thirty-three male CF-1 strain albino mice 3–5 months old served. Mice were purchased from Carworth Farms (New City, NY) and housed on sawdust in colony plastic cages in a temperature and humidity controlled vivarium. Experimental protocols were approved by the University of South Florida Institutional Animal Care & Use Committee.
Olfactometry
Odorants. Aqueous dilutions of ethyl acetate, 2-heptanone, methyl benzoate and urine collected and pooled from three adult female C57-BL6 strain mice were used. Ethyl acetate, 2-heptanone and methyl benzoate were purchased from Sigma and were the highest purities available. Urine and diluted odorants were kept in a freezer until shortly before use.
Olfactometer. Four identical Knosys 8-channel liquid dilution olfactometers (Knosys Olfactometers, Tampa, FL), similar to those described by McBride et al. (2003) were used. Odors were generated by passing 50 cc/min of charcoal filtered air above the surface of 20 ml of the deionized water diluted odorant maintained in 200 ml saturator bottles. This odor stream was then mixed with a 1950 cc/min stream of clean air before being presented to the animal odor sampling port. Odor concentrations are defined as the percent v/v dilution of the odorant in the odor saturator bottle although it should be noted that the headspace of liquid odorant was diluted 1:40 with clean air before being introduced into the odor sampling tube.
Training Procedures. Mice were placed on a restricted water schedule 6–8 days prior to training and for the duration of training. They were given sufficient water to maintain their body weight at 80 – 85% of their pre-deprivation baseline weight. Training procedures followed those described by McBride et al. (2003). Briefly, on each trial, initiated by the mouse inserting its head into the odor sampling port, either the positive (S+) or negative (S-) stimulus was presented for 2.5 sec. Responding (licking a liquid delivery tube within the odor sampling tube) on S+ trials was rewarded by delivery of 3 ul of water and scored as a Hit. Responding on S- trials was not rewarded and was scored as a False Alarm. Not responding on S+ trials was scored as a Miss and not responding on S- trials was scored as a Correct Rejection. There was an enforced 5 sec intertrial interval and mice were given a minimum of 60 trials on each odor detection task.
Twenty-one mice were trained to detect 5%, 1% and 0.1% ethyl acetate, 1% and 0.1% 2-heptanone, 10% and 1% urine, and 1% and 0.1% methyl benzoate in that order. In each task the odor served as the S+ stimulus and the water solvent as the S- stimulus. Training on each detection task was continued for a minimum of 60 trials and until the mouse achieved a criterion accuracy score of at least 80% correct responding in 2 consecutive blocks of 20 trials.
Two – three days after initial training, 16 mice were treated with zinc sulfate and 5 mice were treated with saline as described below. After a rest period of 2–3 days, mice were retested on each of the odor detection tasks. Each mouse was given a minimum of 60 trials on each of the odor detection task. Mice that did not reach criterion were given at least 100 additional trials and some mice were tested multiple times in separate daily sessions. Most zinc sulfate treated mice were tested only on the highest concentration of each odorant. Mice were treated with WGA-HRP as described below within 18 hr of termination of training.
Zinc Sulfate Treatment. Zinc sulfate heptahydrate (Sigma) was diluted in deionized water to a concentration of 5% (v/v). Awake mice were confined in a funnel shaped holder that exposed their snout and the right naris was injected with 50 ul (n = 13) or 30 ul (n = 3) of solution using a 50 ul Hamilton syringe with a blunted smooth needle. The needle was inserted 3–4 mm past the external naris and the solution was forcefully expressed into the nasal vault. The mouse was allowed to rest for 5–10 min and the procedure was repeated in the left naris. Control mice (n = 5) were treated identically except that 50 ul of saline was injected into each naris.
HRP Procedures. Each behaviorally tested mouse was treated with a solution of 22 ul of 1% WGA-HRP, 22 ul of 1% DSMO and 2 ul of female mouse urine within 18 hrs after completion of behavioral tests. In awake mice, six ul of this solution was dropped on the external nares and, several minutes later, each naris was injected with 20 ul of solution as described above. On the next day the mouse was deeply anesthetized with ketamine/xylazine (130 mg and 25 mg/kg body weight, respectively) and perfused sequentially with saline, mixed aldehydes and phosphate buffer. The olfactory bulbs were frozen and sectioned in the frontal plane at 60 um and every section or every other section was saved and processed. The perfusion and reaction procedures were as described by McBride et al. (2003). Sections were mounted on gelatin coated slides, counterstained with thionin, dehydrated in cold alcohols, cleared in xylene and cover slipped using Permount. Slides were inspected microscopically using bright field and dark field optics and selected sections were photographed using a Nikon digital camera. All chemicals were purchased from Sigma.
Aggression
Twelve mice were maintained in individual cages for 10 days and then given 6–8 daily aggression tests. In each test the same colony housed mouse (intruder mouse) was introduced into the subject mouse’s home cage for five min. The intruder mouse was submissive in dyadic encounters as a result of being previously introduced into other colony cages where it had been attacked and, eventually, most often showed the typical the typical murine upright submissive posture of the subordinate animal on the approach of another male mouse (Grant & Mackintosh, 1963). The number of attacks in 5 min was recorded. The intruder mouse was allowed to rest for 10 or more minutes between tests. Eight of the resident mice were then treated with 50 ul of zinc sulfate and four resident mice were treated with 50 ul of saline as described above. Beginning on the fourth day after treatment the aggression tests were repeated daily over the next 3 days.
2. Puberty Acceleration
Animals
Sixteen female and 4 male CD-1 strain mice, born in a breeding colony of CD-1 mice at Dalhousie University from adults purchased from Charles River Laboratories, St. Hyacinthe, Quebec were used. Animals were housed in clear plastic cages measuring 29x18x12 cm. with metal wire tops. Bedding consisted of wood shavings and cages were changed weekly. Food (Purina Lab Chow) and water were supplied ad lib and the animal rooms were on a reverse 12:12 light/dark cycle with lights on at 9:30 pm and off at 9:30 am. Temperature and relative humidity were maintained at 21–25°C and 30–60% respectively. Experimental protocols were approved by the Dalhousie University Committee on Animal Care.
Pretreatment Procedures. Following mating, females were housed individually and checked daily until parturition. On the third day following birth, litters were sexed and reduced to five females and two males (2 litters) and 6 females and two males (one litter). The animals were weaned on day 21 and females housed individually. They were pair matched by weight and one animal of each pair was assigned to group ZS (n=8) and one to the control group (n=8).
Zinc Sulfate Administration. When each pair weighed between 15–16.5 g., they received either 5% zinc sulfate (Group ZS) or saline alone (Group C). Because of their small size, only twenty ul of the solution was injected into each naris as described above.
Post Treatment Procedure. Following treatment, all animals were returned to their individual cages and approximately 250 ml of soiled non-parent male bedding was placed in cages of both control and experimental animals. Animals were sacrificed at approximately 20 gr or by the 10th day after treatment. Uteri were removed, excess fat trimmed, fluid drained, and weighed.
3. Odor-evoked Fos Activity
Animals
Twenty three adult CF-1 strain male mice housed in groups of 4 in plastic cages were used. Initial studies were conducted at the University of Colorado and were replicated, in part, at the University of South Florida. Experimental protocols were approved by the University of South Florida and University of Colorado IUCAC committees.
Odor exposure
Detection of odor-evoked activity followed methods of Lin et al. (2004) with modifications indicated below. Urine was collected from five adult female C57BL/6 mice, pooled and kept frozen in 100 µl aliquots until shortly before use. Mice were placed individually in a clean plastic chamber, undisturbed for 1.5 hr and then transferred to a urine direct exposure, urinary vapor only exposure or air only exposure chamber. For the direct stimulation condition, 5 µl of urine was spread onto the walls of the chamber and 5 µl of urine was placed directly on the naris of the subject mouse to maximize the probability of stimulating the vomeronasal organ. For the urinary vapor only stimulation condition, the chamber was fitted with a 5 cm deep glass vial containing 20 µl of urine and covered with a fine mesh screen. For clean air only stimulation condition, a clean glass vial was in place in the exposure cage. There were 4 control mice in the direct and urinary vapor only conditions and 5 controls in the clean air condition. There were 5 experimental mice in the direct condition and 5 experimental mice in the urinary vapor only condition. Experimental and control mice were treated with 50 µl of 5% zinc sulfate heptahydrate or 50 µl of saline, respectively, as described above 3 days prior to use in these studies.
Tissue Preparation and Immunolabeling
After 1.5 hr in the exposure cage, the mouse was removed, immediately anesthetized with ketamine/xylazine, perfused transcardially with 0.1M phosphate buffer (PB) followed by a PB buffered fixative containing 3% paraformaldehyde, 0.019 M L-lysine monohydrochloride, and 0.23% sodium m-periodate. The olfactory bulbs and cerebrum were harvested and post-fixed for 2 h before being transferred into PBS with 25% sucrose overnight. The tissue was frozen and cut sagittally at 35 µm. Sections were rinsed and incubated in blocking solution containing 2% normal donkey serum, 0.3% Triton X-100 and 1% bovine serum albumin in PBS for 1.5 hour. Normal donkey serum and bovine serum albumin were purchased from (Jackson ImmunoResearch Laboratories, Inc. West Grove, PA). All other chemicals used in the tissue preparations were purchased from Sigma (St. Louis, MO).The sections were incubated with polyclonal rabbit antibody against Fos (Oncogen, Boston, MA) at 1:10,000 dilution in the blocking solution 48 to 72h at 4° C. Secondary antibody used was a Alexa fluor 555 donkey anti-rabbit IgG (1:400; Molecular Probes, Eugene, OR) or reacted with the ABC kit (Vector Laboratories, Burlingame, CA) for 1.5 hr before being rinsed and reacted with diaminobenzidine and H2O2. Sections were mounted on slides with Fluoromount-G (Fisher Scientific, Pittsburgh, PA).
Slides were coded and examined blind relative to treatment condition. Sections approximately through the middle of the AOB were examined and two sections, at least 100 um apart, showing the greatest number of Fos positive cells were selected for analysis for each case. Each Fos positive cell in any part of the AOB within the section was counted. Differences in cell counts between the two sections for each case did not differ for any group (t-tests) and, thus, the mean for each case was used for analysis. A one-way ANOVA was used to evaluate differences among groups and between group differences were assessed using Tukey’s HSD tests.
4. EVG Recordings
CF-1 strain male mice were sacrificed by CO2 inhalation, followed by decapitation. The head was split along the midline and the vomeronasal vein was removed to expose the sensory epithelium of the VNO by microdissection. The half head was then mounted on a recording chamber using the dental adhesive Impregum F (ESPE, Germany). Ringers saline was perfused continuously over the surface of the VNO. Ringers saline contained, in mM: 145 NaCl, 5 KCl, 20 N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid buffer – HEPES-, 1 MgCl2, 1 CaCl2, 1 Na pyruvate and 5 D-glucose pH 7.2. All chemicals in Ringers were purchased from Sigma (St Louis, MO, USA). Ethyl estate, methyl benzoate, 2-heptanone, and 3,5-dimethypyrazine were purchased from Sigma-Aldrich (St Louis, MO, USA). Ringers and odorants dissolved in Ringers were delivered through a gravity-fed computer-controlled perfusion system with an approximate flow rate of 0.2 ml/s. Each odorant was presented three times and the largest response was used for analysis in order to minimize the effect of perfusion. Following stimulation with an odorant, the VNO was washed with saline for 2 min, or until its response to Ringer’s solution was back to the basal level recorded at the beginning of the experiment (below 0.01 mV). EVG recordings were made using an Axopatch 200 B amplifier controlled by a PC computer with Axon software (Clampex 8, Axon Instruments, Inc. Union City, CA). The recording electrode was filled with 0.9% agar made in Ringers saline with 1% neutral red. The electrode was placed on the apical surface of VNO sensory epithelium, and the reference Ag/AgCl electrode was connected to bath saline through an agar bridge. The recorded signals were low-pass-filtered at 20 Hz, digitized at 500 Hz and analyzed using the Axon software Clampfit.
Statistical Analyses
C-Fos counts among groups were evaluated using the Kruskal-Wallis non parametric analysis of variance and between group differences were evaluated using the Mann Whitney test. Between group differences for the EVG study and uterine weights were evaluated using the Mann Whitney test. Alpha level was set at 0.05 and was adjusted using the Bonferroni correction where multiple contrast tests were used.
RESULTS
Zinc Sulfate Treatment Eliminates Odor Detection and Input to the MOB but Leaves Input to the AOB Intact
Histology. Each of the 21 mice used for olfactometry had moderate to dense HRP reaction product in essentially all glomeruli of the accessory olfactory bulb (Figure 1). All glomeruli of the main olfactory bulb contained moderate or dense HRP reaction product in each of the 5 saline treated mice (Figure 1, A, A1). Of the 16 zinc treated mice, four (each of the three 30 ul and one 50 ul mice) had light to dense reaction product in approximately 20% or more glomeruli in the MOB. These four mice were designated as the Zn+ group. The remaining 12 zinc treated mice had no detectable HRP product in glomeruli of the MOB and were designated as the Zn- group (Figure 1, B, B1, C, C1).
Figure 1.
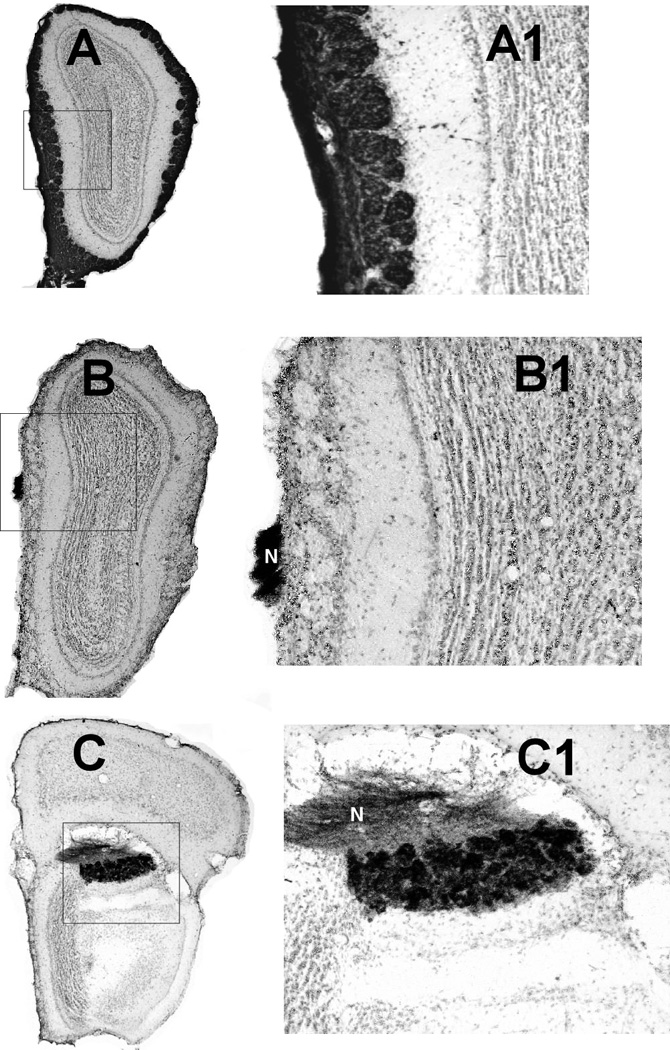
Photomicrographs of frontal sections through the olfactory bulb of a saline treated control (A, A1) and a ZnSO4 treated mouse (B, B1, C, C1) showing anterograde transport of WGA-HRP. Medial is to the left and dorsal is to the top. As shown in A, all glomeruli in saline treated mice were filled with dense WGA-HRP reaction product. In mice of the Zn- group WGA-HRP reaction product was observed only in the vomeronasal nerve (labeled N in B1 and C1) and the AOB (C, C1). A1, B1, and C1 show details of the areas outlined in the A, B, and C figures.
Olfactometry. Prior to treatment, each mouse met the response criterion in 60 – 100 trials on each odor detection task. After treatment, saline treated and Zn+ mice had high accuracy scores on each odorant on which they were tested (Figure 2) and most met the accuracy criterion within the first 40 – 80 trials on each test odorant. In contrast, none of the 12 mice in the Zn- group met this criterion (Figure 2) and scores on all tests ranged from 35% – 70% (mean, 52%). Nine of these mice were tested repeatedly on two or more of the odorants within the first 8 post-treatment days but showed no improvement in accuracy.
Figure 2.
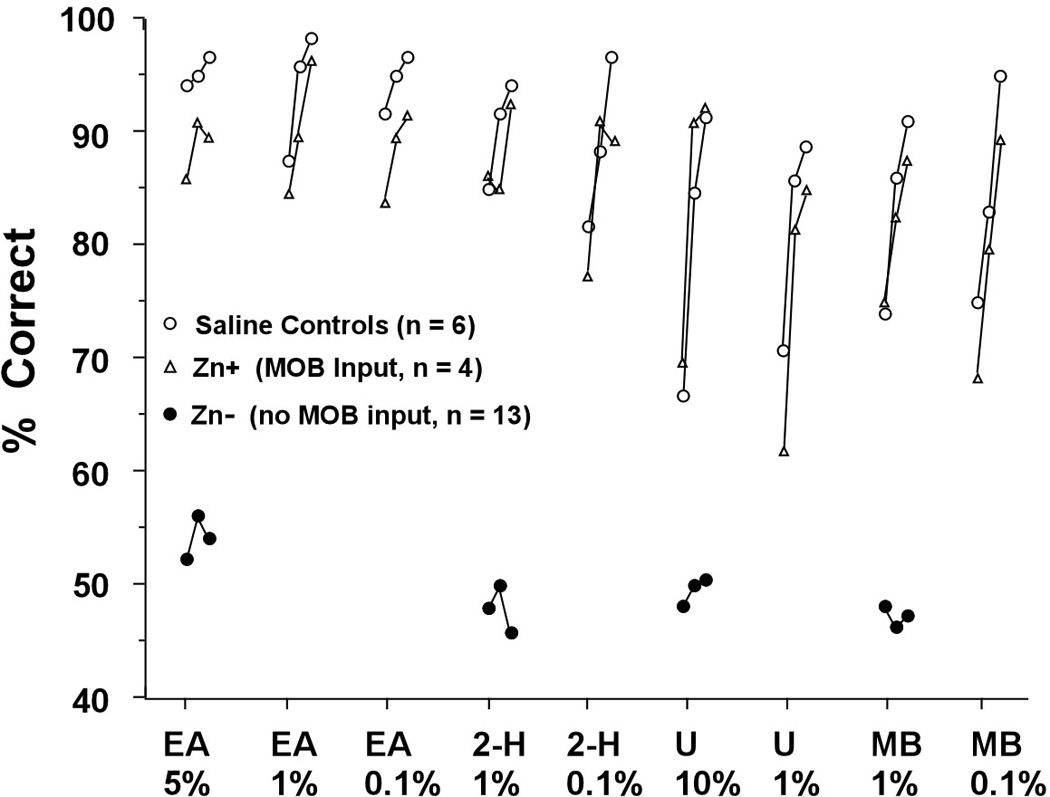
Mean correct responses in the first 60 post-treatment odor detection tasks for saline treated mice, ZnSO4 treated mice that had some remaining input to MOB glomeruli (Group Zn+), and ZnSO4 treated mice that had WGA-HRP reaction product only in the AOB (Group Zn−). EA: ethyl acetate. 2-H: 2-heptanone. U: urine. MB: methyl benzoate. Percent values are the percent dilutions of odorants.
Field Potential Responses of the Vomeronasal Epithelium are Intact After Zinc Sulfate Treatment
To assess the responsiveness of the vomeronasal epithelium, we measured the changes in field potential measured on the surface of the vomeronasal epithelium (the electrovomeronasalogram) evoked by odorants, putative pheromones and their Ringer’s solution solvent. Figure 3 shows that, while there was a small decrease in response to some of the stimuli, the vomeronasal epithelium of zinc sulfate-treated mice responded to all stimuli tested and the between group difference for each odorant was not significant (p > 0.2, each case). Each odorant elicited responses in both control and experimental cases that were considerably greater than to Ringer’s solution. After Treatment with Zinc Sulfate there is Upregulation of c-Fos in the AOB in Response to Nasal Inhalation of Urine but Not to Urinary Volatiles.
Figure 3.
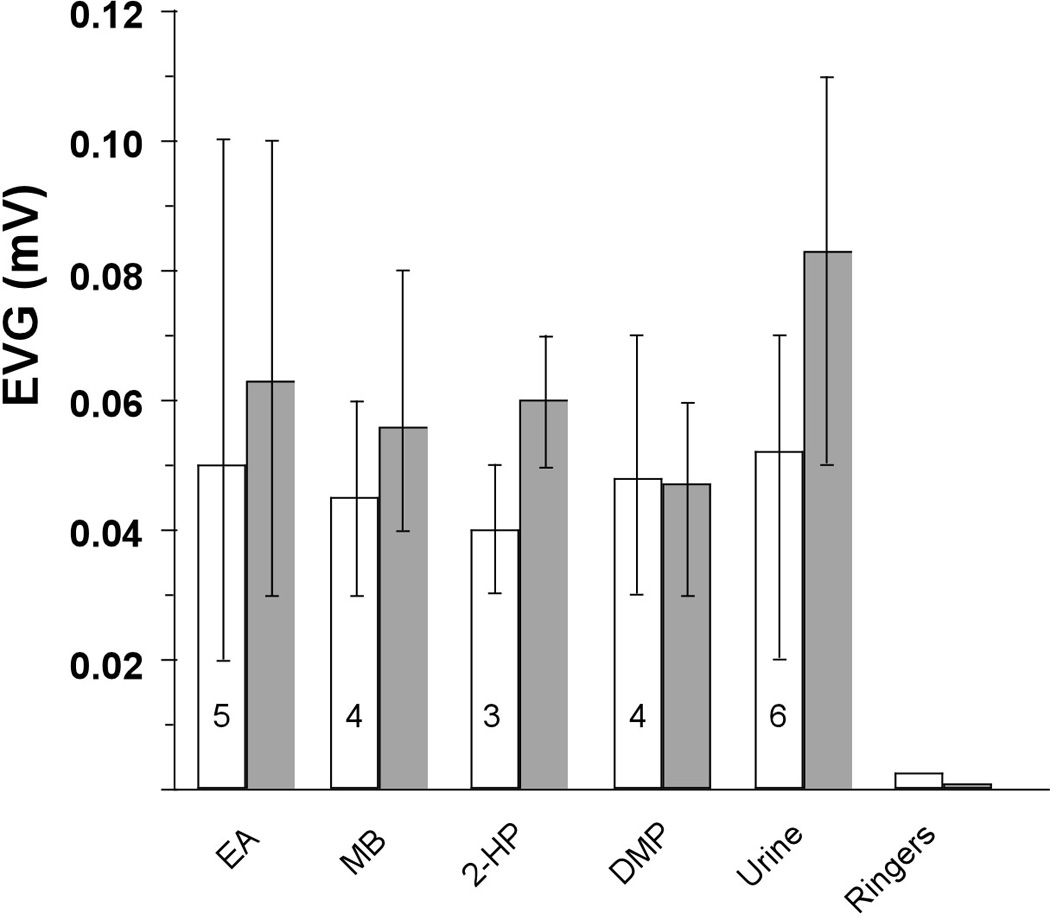
Peak mean EVG response and range of responses to different stimuli in experimental (open bars) and control mice (shaded bars). Urine was C57BL/6 female urine diluted 1:200 in Ringers. All other odorants were dissolved in Ringers at 100 µM. EA: ethyl acetate. MB: methyl benzoate. 2-Hep: 2-heptanone. DMP: 3,5-dimethylpyrazine. Control scores based on 3 mice and numbers in open bars represent the number of experimental mice used.
In the MOB of saline treated controls, both liquid and vapor only exposure to female urine elicited robust up-regulation in Fos in periglomerular cells of a large number of glomeruli, particularly those in the ventrolateral and ventromedial areas but there was essentially no response in the MOB of zinc sulfate-treated mice (Figure 4).
Figure 4.
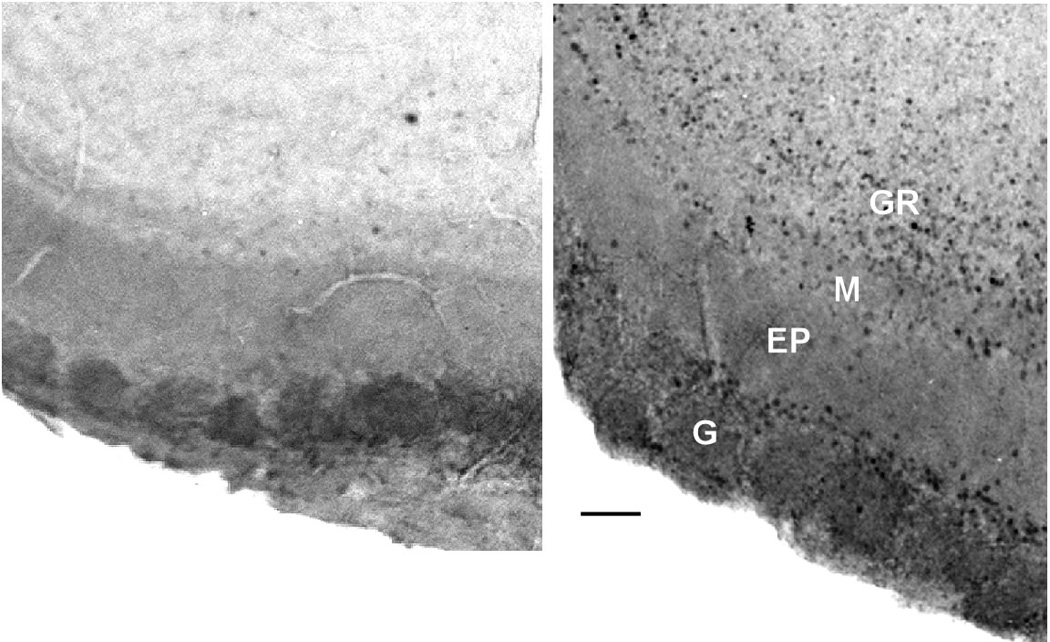
Photomicrographs showing c-Fos expression in the glomerular region from the ventromedial area of the MOB in a ZnSO4 treated (A) and saline treated mouse (B). Little or no Fos expression was found in the MOB in Zn- mice. G: glomerular layer. EP: external plexiform layer. M: mitral cell layer. GR: granule cell layer. Calibration bar: 50 microns.
Fos positive cells were found in the AOB in all cases (Figure 5) and the difference among groups was significant (H=16.54, p = .002). The highest mean counts were obtained for controls exposed to urinary vapor (269.5), controls treated with liquid urine (256.9) and experimental mice treated with liquid urine (240.2). These 3 groups did not differ from one another (H=1.32, p = 0.94) but each had significantly higher counts than controls exposed to only to air (mean 47.3), or experimental mice exposed to urinary vapor (mean 43, p <0.008, each case). In brief, stimulation with urinary vapor elicited up-regulation of Fos only in saline-treated mice.
Figure 5.
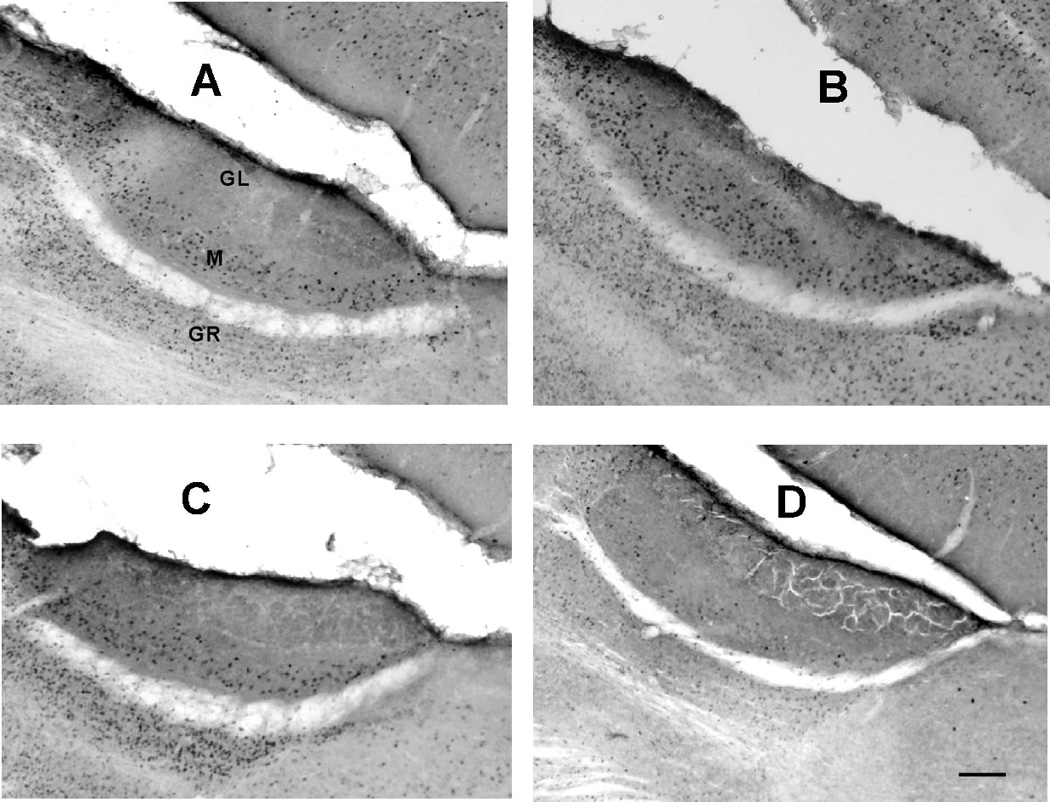
Representative sagittal sections showing c-Fos expression in mitral and granule cells of the accessory olfactory bulb. A and B, saline control mice exposed to female urine placed on external nares (A) or only to urinary vapor (B). C and D, ZnSO4 treated mice exposed to female urine placed on external nares (C) or only to urinary vapor (D). GR: granule cell layer. M: mitral cell layer. GL: glomerular cell layer. Calibration bar: 100 microns.
Zinc Sulfate Treatment Blocks Intermale Aggression
Prior to treatment, each of the 12 mice attacked the intruder mouse at least once (mean 7, range 1–12 attacks) in each of the daily 5 min tests. After treatment, the 4 saline treated controls continued to attack the intruder at least once (mean 3, range 1–7 attacks) in each test. In post-treatment tests the intruder showed the defensive upright posture typical of a subordinate mouse when it was approached by the control mouse (Eisenberg, 1968). The 8 zinc sulfate treated mice did not initiate an attack on the intruder in any post-treatment test but, on several occasions, engaged in a short bout of fighting when attacked by the intruder mouse.
Zinc Sulfate Treatment Retards Puberty Acceleration
Mean uterine weights of ZnSO4-treated mice and saline controls were 31.8 mg and 81.9 mg, respectively (U15 = 8.7, p <0.005). The uteri of all eight of the saline treated mice were fluid filled with a well developed vascularity and typical of pubertal onset. The uteri of seven of the eight ZnSO4 treated mice were threadlike with little or no fluid or vascular development (Figure 6). The uteri of the one exceptional ZnSO4-treated mouse were similar to that of controls and it is likely that zinc treatment of this mouse was inadequate. Mean body weight of all mice at sacrifice was 20.3 gr and the difference in weight between groups was not significant (p >0.1).
Figure 6.
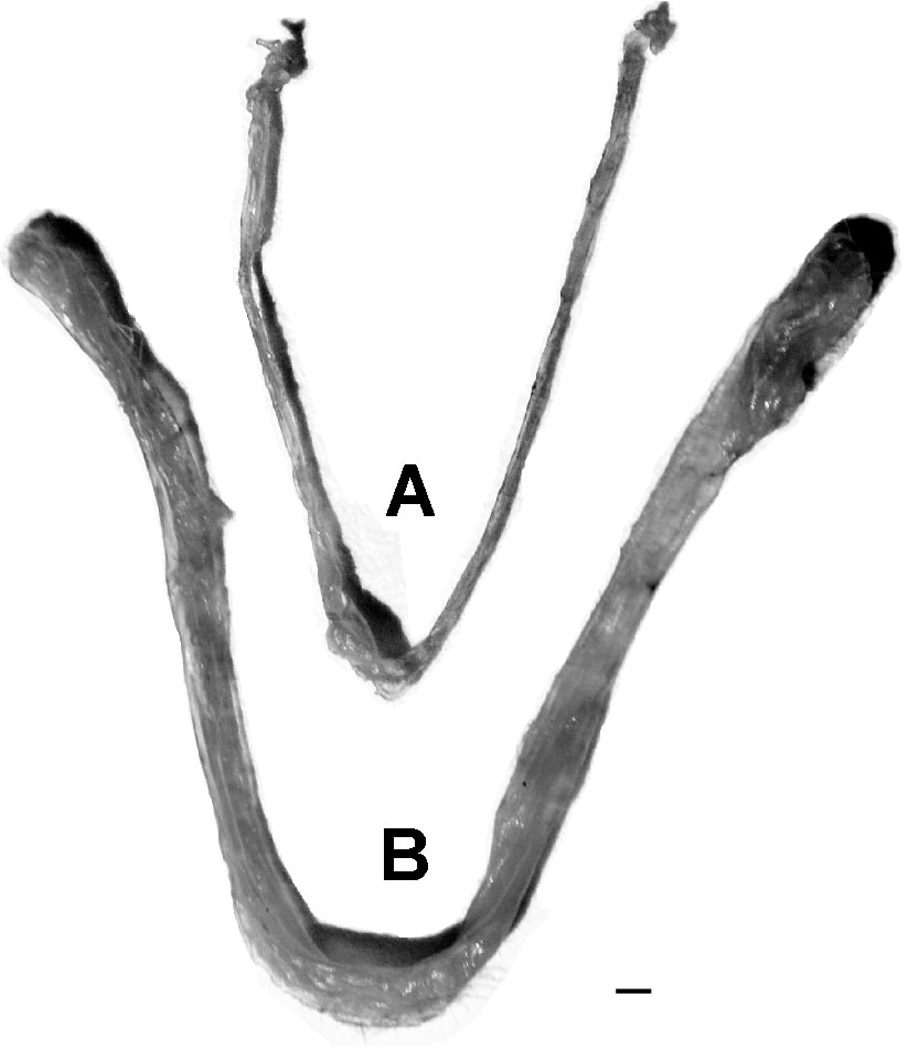
Uteri from zinc sulfate treated (A) and control (B) juvenile mice after exposure to sexually mature male mice. Scale bar: 1mm
DISCUSSION
The present anatomical results using anterograde transport of WGA-HRP demonstrate that a zinc sulfate lavage of the nasal cavity adequate to disrupt essentially all input to the mouse main olfactory bulb still leaves intact connections to the accessory olfactory bulb. This confirms and extends prior similar reports on the selective effects of zinc sulfate of McBride et al. (2003), Keller et al. (2006a), and Martel and Baum (2007). Although the evidence that connections to the AOB in zinc sulfate treated mice are functional is indirect, it is clearly favorable and consistent: anterograde transport of WGA-HRP to the AOB (this study) and expression of soybean agglutinin, which stains intact axons of vomeronasal organ neurons terminating in the AOB (Keller et al., 2006a) is similar in treated mice and controls, there is upregulation of cfos expression in the AOB of experimental mice in response to liquid urine placed on the nose (this study), and EVG recordings demonstrate responses of the VON epithelium to presumed pheromonal and non-pheromonal odorants (this study). In brief, syringing the nasal vault with zinc sulfate appears to be an effective method for eliminating input to the MOB with little or no disruption of inputs to the AOB.
Our findings that, in the absence of MOB input, trained mice were frankly anosmic in olfactometric tests using pheromonal and non-pheromonal stimuli and, as indexed by puberty acceleration and tests of aggression, did not respond appropriately to either species specific social cues or pheromonal stimuli are in agreement with and extend those of Keller et al. (2006b). They found that zinc sulfate treatment in sexually experienced male mice disrupted the detection of vapor cues and the initiation of sexual behavior when exposed to social and pheromonal stimuli of receptive females. In a related study Mandiyan et al. (2005) found that male mice lacking a functional cyclic nucleotide-gated channel alpha2 (CNGA2), used for odor-evoked MOE signaling, failed to mate or fight, and showed little or no sniffing at pads wetted with male or female urine. The demonstration that, in the absence of input from the MOE, garter snakes do not engage in tongue flick odor sampling in the presence of prey odors (Zun & Halpern, 2003) suggests that the dependence of vomeronasal sampling on input from the main olfactory system may be a general feature of vertebrates with dual olfactory systems.
It has generally been assumed that reproductive behaviors initiated by pheromonal stimuli are mediated by the accessory olfactory system and, as such, provide an index of vomeronasal function. However, recent findings indicate that gonadotropin-releasing hormone (LHRH) neurons in the hypothalamus receive projections from the main olfactory bulbs (Bohem et al., 2005; Yoon et al., 2005) and that mating behavior in mice may be unaffected in the absence of vomeronasal input (Yoon et al., 2005). Thus, it appears that at least some reproductive behaviors induced or released by pheromones may not require the vomeronasal system and, hence, such behaviors may not provide an index of vomeronasal function. However, it is evident that the two pheromone dependent measures used in the present study, puberty acceleration and aggressive behavior are mediated by accessory olfactory system. Thus, puberty acceleration (as measured by uterine weight) is blocked by transection of the vomeronasal nerves (Kaneko et al., 1980) or removal of the vomeronasal organ (Lomas & Kaverne, 1982). Likewise, mouse aggressive behavior is sharply reduced or eliminated by vomeronasal nerve transection or removal of the VNO (Maruniak, 1985; Clancy et al., 1984; Bean, 1982) or by selective disruption of vomeronasal function (e.g., Stowers et al., 2002; Zufall et al. 2005; Norlin et al., 2003; Leypold et al., 2003).
These various outcomes clearly favor the hypothesis that the initiation of vomeronasal sampling may be largely dependent upon the detection of odors of interest by the MOE. Although it is possible that, independent of MOE activity, mice engage in spontaneous VN sampling (e.g., Meredith, 1994), both Kervene (2004) and Luo et al. (2003) report that, in social interactions, neurons recorded from the mouse AOB were silent until the mouse was in physical contact with the conspecific.
Of particular interest are the findings of an augmented Fos response in the AOB of male mice when exposed to the odor of an oestrous female but not to that of an intact male or ovariectomized female (Muroi et al., 2006) and an enhanced Fos response to AOB projection targets in female mice when exposed to opposite sex but not same sex odors (Martel & Baum, 2009). Thus, an alternate possibility explaining the absolute requirement for MOB function in AOB responses in our studies could be that MOB gated AOB function on or off through centrifugal fibers from the medial amygdala or that the responses of AOB to urine are directly mediated by the centrifugal input from the medial amygdala.
Additional, albeit indirect, support for the proposed primacy of MOE input for the initiation of VON sampling is the greater access of the main olfactory epithelium to airborne odor molecules. Thus, during odor sampling, sensory neurons in the MOE would probably be activated earlier than those in the VON. Although there appears to be no direct evidence for this, Xu et al. (2005), in an fMRI study of the mouse, found that the maximal response to an odor stimulus in the AOB followed the peek response in the MOB.
If VNO sampling is dependent upon signals from the MOE then there must be anatomical connections between them. Although there are no known connections between the two sensory epithelia, Larriva-Sahd (2008) found that main and interstitial neuron of the accessory olfactory bulb receive inputs from fibers arising from the main olfactory bulb but, interestingly, no evidence for a reciprocal connection. In addition, axonal tracing studies have demonstrated centrifugal projections to the AOB from medial amygdala targets of the MOB (Martel & Baum, 2009). The two systems also have convergent inputs at more central olfactory structures, connections that appear to be conserved as they appear in both reptiles (Martínez-Marcos et. al., 2002) and rodents (Pro-Sistiaga et al., 2007). Pro-Sistiaga et al. (2007) suggest that areas of convergence, including the nucleus of the lateral olfactory tract, anterior cortical, anterior ventral, medial and ventral anterior nuclei of the amygdala, and the bed nucleus of the accessory olfactory tract, be considered ‘mixed chemosensory structures’ which could mediate interactions between the two systems.
Evidence contrary to the conclusion that sampling by the VNO requires input from the main olfactory system are the results from two studies suggesting that VN sampling may occur in the absence of input to the MOE. Trinh and Storm (2003) report that type-3 adenylyl cyclase knockout mice (AC3−/−) and those treated with zinc sulfate could detect some but not all odors used and concluded that detection was mediated by the VN system in the absence of input from the MOE. However, Lin et al. (2004) demonstrated that at least some odorants are detected by a cAMP-independent pathway in the MOE. In their study, odors detected by CNGA2 knockout mice produced EOG responses in the MOE, resulted in upregulation of Fos in some glomeruli in the posterior MOB, and that these glomeruli were among the few that expressed tyrosine hydroxylase in these knockouts. Thus, it is unlikely that elimination of a cAMP pathway blocks all input to the MOB.
Also, in contrast to Trinh and Storm (2003), Lin et al. (2004) found that zinc sulfate treated mice were anosmic to all tested odors. The effectiveness of a nasal lavage with zinc sulfate to disrupt connections between the MOE and MOB is critically dependent upon concentration, volume and time after treatment (McBride et al., 2003). Although both studies used the same concentration of zinc sulfate (.17M), Trinh and Storm used only half the volume (25ul) shown by McBride et al. (2003) and Slotnick et al. (2007) to produce virtually complete disruption of input from the MOE for at least several days after treatment. Even at a 50 ul volume, some mice are able to detect odors within 4–5 days (McBride et al., 2003) and, in the present study, one mouse treated with 50 ul and each of three mice given only 30 ul of zinc sulfate were not anosmic when tested 3 days after treatment. Thus, it seems likely that the zinc sulfate treatment used by Trinh and Storm did not block all input to the MOB in their behaviorally tested mice.
The only other evidence in support of odor detection by the VN system in the absence of input from the MOE is that of Ma et al. (2002) who report that OMP-nitroreductase transgenic mice treated with the pro-drug CB1954 appeared anosmic to airborne odors in habituation-dishabituation tests but showed the VN-dependent pregnancy blocking effect when exposed to pheromonal stimuli of males from another strain. However, the experimental treatment in that study did not completely block all input from the MOE to the MOB and the habituation test used is a less sensitive measure of odor detection and discrimination than is reinforcement training (e.g., Linster et al., 2002; Slotnick & Schellnick, 2002).
In general, attempts to demonstrate VNO function in the absence of input from the MOE require not only behavioral tests sufficiently sensitive to demonstrate frank anosmia but also complete interruption of inputs to the MOB. The latter is particularly important because it is now clear that there may be considerable savings in odor detection and discrimination after extensive but still subtotal destruction of the MOE (McBride et al., 2003; Setzer & Slotnick, 1998; Slotnick, 2007; Youngentob et al., 1997) or extensive lesions of the olfactory bulbs themselves (Lu & Slotnick, 1998).
Conclusions
The present results together with the studies of the Baum group, provide strong evidence for the contention that, in mice, activation of the VNO-AOB system by chemosensory signals from conspecifics requires prior detection of such signals via the MOE-MOB system. By extension, these results suggest that, at least in the mouse, activation of the VNO pump is initiated only after detection of appropriate signals by the MOE.
Acknowledgements
We thank Michael Meredith for his many useful comments on earlier drafts of this manuscript.
Grant Support
Burton Slotnick grant support: NIH Grant DC04671
Heather Schellinck grant support: NSERC Discovery Grant
Diego Restrepo grant support: NIH Grants DC006070 and DC004657
Weihong Lin grant support: NIH DC009269
Georgina Archbold grant support: none
Stephen Price grant support: none
Abbreviations
- AC3−/−
type-3 adenylyl cyclase knockout mice
- AOB
accessory olfactory bulb
- CNGA2
cyclic nucleotide-gated channel alpha2
- EVG
electro-vomeronasogram
- MOB
main olfactory bulb
- MOE
main olfactory epithelium
- PB
phosphate buffer
- S+
positive stimulus
- S−
negative stimulus
- VNO
vomeronasal organ
References
- Baxi KN, Dorries KM, Eisthen HL. Is the vomeronasal system really specialized for detecting pheromones? Trends Neurosci. 2006;29:1–7. doi: 10.1016/j.tins.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Bean NJ. Modulation of agonistic behavior by the dual olfactory system in male mice. Physiol Behav. 1982;29:433–437. doi: 10.1016/0031-9384(82)90262-1. [DOI] [PubMed] [Google Scholar]
- Boehm U. The vomeronasal system of mice: from the nose to the hypothalamus- and back! Semin. Cell Dev. Biol. 2006;17:471–479. doi: 10.1016/j.semcdb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Brennan PA. The vomeronasal system. Cell. Mol. Life Sci. 2001;58:546–555. doi: 10.1007/PL00000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature. 2006;444:308–315. doi: 10.1038/nature05404. [DOI] [PubMed] [Google Scholar]
- Clancy AN, Coquelin A, Macrides F, Gorski RA, Noble NP. Sexual behavior and aggression in male mice: involvement of the vomeronasal organ. J. Neurosci. 1984;4:2222–2229. doi: 10.1523/JNEUROSCI.04-09-02222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg JF. Behavior patterns. In: King JA, editor. Biology of Peromyscus (Rodentia) Washington DC: American Society of Mammologists; 1968. pp. 451–495. [Google Scholar]
- Grant EC, Mackintosh JL. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21:246–524. [Google Scholar]
- Halpern M, Martínez-Marcos A. Structure and function of the vomeronasal system: an update. Prog. Neurobiol. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Jakupovic J, Kang N, Baum MJ. Effect of bilateral accessory olfactory bulb lesions on volatile urinary odor discrimination and investigation as well as mating behavior in male mice. Physiol. Behav. 2008;93:467–473. doi: 10.1016/j.physbeh.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N, Debski EA, Wilson MC, Whitten WK. Puberty acceleration in mice. II. Evidence that the vomeronasal organ is a receptor for the primer pheromone in male mouse urine. Biol. Reprod. 1980;22:873–878. doi: 10.1095/biolreprod22.4.873. [DOI] [PubMed] [Google Scholar]
- Keller M, Douhard Q, Baum MJ, Bakker J. Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem. Senses. 2006a;31:315–323. doi: 10.1093/chemse/bjj035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Douhard Q, Baum MJ, Bakker J. Sexual experience does not compensate for the disruptive effects of zinc sulfate-lesioning of the main olfactory epithelium on sexual behavior in male mice. Chem. Senses. 2006b;31:753–762. doi: 10.1093/chemse/bjl018. [DOI] [PubMed] [Google Scholar]
- Keverne EB. Importance of olfactory and vomeronasal systems for male sexual function. Physiol. Behav. 2004;83:177–187. doi: 10.1016/j.physbeh.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Larriva-Sahd J. The accessory olfactory bulb in the adult rat: a cytological study of its cell types, neuropil, neuronal modules, and interactions with the main olfactory system. J. Comp. Neurol. 2008;510:309–350. doi: 10.1002/cne.21790. [DOI] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mice. Proc. Natl. Acad. Sci. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Arellano J, Slotnick B, Restrepo D. Odors detected by mice deficient in cyclic nucleotide-gated channel subunit A2 stimulate the main olfactory system. J. Neurosci. 2004;24:3703–3710. doi: 10.1523/JNEUROSCI.0188-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Morse A, Yue E, Leon M. Spontaneous versus reinforced olfactory discriminations. J. Neurosci. 2002;22:6842–6845. doi: 10.1523/JNEUROSCI.22-16-06842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomas DE, Keverne EB. Role of the vomeronasal organ and prolactin in the acceleration of puberty in female mice. J. Reprod. Fertil. 1982;66:101–107. doi: 10.1530/jrf.0.0660101. [DOI] [PubMed] [Google Scholar]
- Lu XM, Slotnick BM. Olfaction in rats with extensive lesions of the olfactory bulbs: Implications for odor coding. Neuroscience. 1998;84:849–866. doi: 10.1016/s0306-4522(97)00520-4. [DOI] [PubMed] [Google Scholar]
- Luo MM, Fee MS, Katz LC. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science. 2003;299:1196–1201. doi: 10.1126/science.1082133. [DOI] [PubMed] [Google Scholar]
- Ma D, Allen ND, Van Bergen YC, Jones CM, Baum MJ, Keverne EB, Brennan PA. Selective ablation of olfactory receptor neurons without functional impairment of vomeronasal receptor neurons in OMP-ntr transgenic mice. Eur. J. Neurosci. 2002;16:2317–2323. doi: 10.1046/j.1460-9568.2002.02303.x. [DOI] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat. Neurosci. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. Sexually dimorphic activation of the accessory, but not the main, olfactory bulb in mice by urinary volatiles. Eur. J. Neurosci. 2007;29:368–376. doi: 10.1111/j.1460-9568.2007.05651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. A centrifugal pathway to the mouse accessory olfactory bulb from the medial amygdala conveys gender-specific volatile pheromonal signals. Eur. J. Neurosci. 2009;26:463–475. doi: 10.1111/j.1460-9568.2008.06564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Marcos A, Lanuza E, Halpern M. Neural substrates for processing chemosensory information in snakes. Brain Res. Bull. 2002;57:543–546. doi: 10.1016/s0361-9230(01)00686-4. [DOI] [PubMed] [Google Scholar]
- Maruniak JA, Wysocki CJ, Taylor JA. Mediation of male mouse urine marking and aggression by the vomeronasal organ. 1985 doi: 10.1016/0031-9384(86)90300-8. [DOI] [PubMed] [Google Scholar]
- McBride K, Slotnick B, Margolis F. Does intranasal application of zinc sulfate produce anosmia in the mouse? Chem. Senses. 2003;28:659–670. doi: 10.1093/chemse/bjg053. [DOI] [PubMed] [Google Scholar]
- Meredith M. Chronic recording of vomeronasal pump activation in awake behaving hamsters. Physiol. Behav. 1994;56:345–354. doi: 10.1016/0031-9384(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Meredith M, Marques DM, O’Connell RO, Stern FL. Vomeronasal pump: significance for male hamster sexual behavior. Science. 1980;207:1224–1226. doi: 10.1126/science.7355286. [DOI] [PubMed] [Google Scholar]
- Meredith M, O’Connell RJ. Efferent control of stimulus access to the hamster vomeronasal organ. J. Physiol. 1979;286:301–316. doi: 10.1113/jphysiol.1979.sp012620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroi Y, Ishii T, Komori S, Kitamura N, Nishimura M. Volatile female odors activate the accessory olfactory system of male mice without physical contact. Neurosci. 2006;141:551–558. doi: 10.1016/j.neuroscience.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Norlin EM, Guessing F, Berghard A. Vomeronasal phenotype and behavioral alterations in G alpha i2 mutant mice. Curr. Biol. 2003;13:1214–1219. doi: 10.1016/s0960-9822(03)00452-4. [DOI] [PubMed] [Google Scholar]
- Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Banon I, Del mar Arroyo-Jimenez M, Marcos P, Artacho-Perula E, Crespo C, Insausti R, Martinez-Marcos A. Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J. Comp. Neurol. 2007;504:346–362. doi: 10.1002/cne.21455. [DOI] [PubMed] [Google Scholar]
- Sam M, Vora S, Malnic B, Ma W, Novotny MV, Buck LB. Neuropharmacology. Odorants may arouse instinctive behaviour. Nature. 2001;412:142. doi: 10.1038/35084137. [DOI] [PubMed] [Google Scholar]
- Setzer AK, Slotnick B. Odor detection in rats with 3-methylindole-induced reduction of sensory input. Physiol. Behav. 1998;65:489–496. doi: 10.1016/s0031-9384(98)00186-3. [DOI] [PubMed] [Google Scholar]
- Slotnick B. Olfactory performance of rats after selective olfactory bulb deafferentation by 3-methyl indole. Chem. Senses. 2007;32:173–181. doi: 10.1093/chemse/bjl046. [DOI] [PubMed] [Google Scholar]
- Slotnick B, Sanguino A, Husband S, Marquino G, Silberberg A. Olfaction and olfactory epithelium in mice treated with zinc gluconate. Laryngoscope. 2007;117:743–749. doi: 10.1097/MLG.0b013e318033006b. [DOI] [PubMed] [Google Scholar]
- Slotnick B, Schellinck H. Methods in olfactory research with rodents. In: Simon SA, Nicolelis M, editors. Frontiers and Methods in Chemosenses. New York: CRC Press; 2002. pp. 21–61. [Google Scholar]
- Spehr M, Spehr J, Ukhanov K, Kelliher KR, Leinders-Zufall T, Zufall F. Parallel processing of social signals by the mammalian main and accessory olfactory systems. Cell. Mol. Life Sci. 2006;63:1476–1484. doi: 10.1007/s00018-006-6109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koenteges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Trinh K, Storm DR. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat. Neurosci. 2003;6:519–525. doi: 10.1038/nn1039. [DOI] [PubMed] [Google Scholar]
- Xu F, Schaefer M, Kida I, Schafer J, Liu N, Rothman DL, Hyder F, Restrepo D, Shepherd GM. Simultaneous activation of mouse main and accessory olfactory bulbs by odors or pheromones. J. Comp. Neurol. 2005;489:491–500. doi: 10.1002/cne.20652. [DOI] [PubMed] [Google Scholar]
- Yoon, et al. Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123:669–682. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Schwob JE, Sheehe PR, Youngentob LM. Odorant threshold following methyl bromide-induced lesions of the olfactory epithelium. Physiol. Behav. 1997;62:1241–1252. doi: 10.1016/s0031-9384(97)00301-6. [DOI] [PubMed] [Google Scholar]
- Zufall Ukhanov K, Lucas P, Liman ER, Leinders-Zufall T, et al. Neurobiology of TRPC2: from gene to behavior. Pflugers Arch. 2005;451:61–71. doi: 10.1007/s00424-005-1432-4. [DOI] [PubMed] [Google Scholar]
- Zun I, Halpern M. Differential effects of lesions of the vomeronasal and olfactory nerves on garter snake (Thamnophis sirtalis) responses to airborne chemical stimuli. Behav. Neurosci. 2003;117:169–183. doi: 10.1037//0735-7044.117.1.169. [DOI] [PubMed] [Google Scholar]


