Abstract
PURPOSE
Recent microarray and RNA-sequencing studies have uncovered aberrantly expressed microRNAs (miRs)in Barrett’s esophagus (BE)-associated esophageal adenocarcinoma (EAC). The functional significance of these miRs in EAC initiation and progression is largely unknown.
EXPERIMENTAL DESIGN
Expression levels of miR-199a/b-3p, −199a-5p, −199b-5p, −200b, −200c, −223, and −375 were determined in microdissected tissues from cardiac mucosa, BE, dysplastic BE, and EAC using quantitative real time PCR. MiR-223 expression was validated in precursors and EACs from 95 EAC patients by in situ hybridization (ISH). MiR-223 was transfected into two EAC cell lines, and in vitro assays were performed. Target genes were identified using Illumina microarray, and results were validated in cell lines and human specimens.
RESULTS
MiR-199 family members and miR-223 were significantly over-expressed in EAC, however only miR-223 showed a stepwise increase during EAC carcinogenesis. A similar trend was observed by ISH, which additionally showed that miR-223 is exclusively expressed by the epithelial compartment.MiR-223 over-expressing cells had statistically significantly more migratory and invasive potential than scramble sequence transfected cells. PARP1was identified as a direct target gene of miR-223 in EAC cells. Increased sensitivity to chemotherapy was observed in cells with enforced miR-223 expression and reduced PARP1.
CONCLUSIONS
MiR-223 is significantly up-regulated during the BE-dysplasia-EAC sequence. Although high miR-223 levels might contribute to an aggressive phenotype, our results also suggest that EAC patients with high miR-223 levels might benefit from treatment with DNA-damaging agents.
Keywords: miR-223, PARP1, Barrett’s esophagus, adenocarcinoma
INTRODUCTION
Barrett’s esophagus (BE)has become a major public health problem in the U.S. and other Western countries over the past decade(1). BE is a risk factor for the subsequent development of esophageal adenocarcinoma (EAC). EACs arise on the background of metaplastic Barrett’s mucosa in the distal esophagus, following a multistep progression through non-dysplastic BE (BE-ND) to dysplasia, and culminating in invasive adenocarcinoma (2). EAC is one of the few cancers with an increase in incidence, particularly among Caucasian males, and has a dismal five-year survival below 20% due to advanced presentation in most patients (1). Although neo-adjuvant chemo-radiation therapy has shown to improve survival(3), it is not beneficial to all EAC patients. There is an unequivocal need for elucidating the molecular underpinnings of BE and EAC pathogenesis in order to develop more potent therapeutic strategies.
MicroRNAs (miRs) are non-coding, 18–24-nucleotide long, single stranded RNAs that have the ability to negatively regulate the expression of genes involved in several cellular processes, including cell proliferation, apoptosis, migration, invasion, and stress response(4–8). It has been shown that abnormal patterns of miR expression are present in many human carcinomas(6, 9), and are associated with the pathogenesis, progression and natural history of several cancers(4, 10). Based on their functional activity, cancer-related miRs can be divided in two groups; oncogenic miRs (onco-miRs) and miRs with tumor suppressor activity (TS-miRs) (10, 11). Onco-miRs are usually silenced in normal tissues and over-expressed in neoplastic or cancerous lesions, causing a reduced expression of target genes with a tumor suppressor role. In contrast, TS-miRs are down regulated during carcinogenesis(11).
Previous microarray and “next-generation” RNA sequencing studies have uncovered aberrantly expressed miRs in EACs compared to normal squamous epithelium (NSE), BE-ND, low grade dysplasia (LGD), and high grade dysplasia (HGD) (12–21). Although numerous miRs have been identified as misexpressed during the multistep progression of BE, there is scant information vis-à-vis their functional role in BE and EAC pathogenesis and therapy thereof.
In the present study, we examined the expression of a limited panel of miRs in tissue samples of non-dysplastic and dysplastic BE, EACs and matched NSE obtained from a cohort of patients with treatment-naïve EAC. The miR panel was selected based on review of published literature for aberrantly expressed candidates during BE and EAC pathogenesis (12–14, 16, 18, 22, 23). Based on our initial screening, we further explored the expression patterns of miR-223 in an independent series of archival tissues, comprised of NSE, precursor lesions, invasive carcinomas and metastases by in situ hybridization (ISH). The function of deregulated miR-223 expression was then investigated using EAC cell lines. Putative targets of miR-223 were identified by transcriptomic profiling, and rigorously validated using in vitro cell-based systems. Our profiling and functional data led us to propose that miR-223 behaves as an onco-miR in EAC, whose expression is progressively up-regulated in the multistep transition from BE to EAC. In addition, we identified the gene encoding the DNA damage repair protein poly(ADP-ribose) polymerase 1 (PARP1) as a bona fide target of miR-223, and demonstrate that miR-223 up-regulation is also associated with reduced PARP1 transcripts, and an increased sensitivity to cis-diamminedichloroplatinum (II) (Cisplatin), Doxorubicin and Mitomycin C, providing a potential therapeutic vulnerability node for exploitation in the clinic.
MATERIALS AND METHODS
MicroRNA selection
A panel of seven miRs (miR-199a/b-3p, −199a-5p, −199b-5p, −200b, −200c, −223 and −375) that have been reported as misexpressed in EAC were chosen for validation by quantitative real-time PCR (qRT-PCR) in a cohort of chemo-radiation-naïve intramucosal carcinoma (IMC)/EAC patient tissue samples (Supplemental Table S1). These miRs were selected based on several criteria, including their misexpression in multiple independent publications, demonstration of aberrant expression in primary tissue samples and not just cell lines, and functional evidence in other tumor types as either onco-miR or TS-miR.
Patient selection and tissue processing for quantitative real-time PCR
Formalin-fixed paraffin-embedded (FFPE) cardio-oxyntic (cardiac) (n=11), BE-ND (n=13), HGD (n=17), IMC/EAC (n=13) and matched NSE tissues were obtained from treatment-naïve EAC patients that had either undergone endoscopic mucosal resection or surgical resection of EAC and precursor lesions. Haematoxylin and eosin (H&E) staining was reviewed by an expert pathologist (EAM), and all normal tissues and lesions were carefully microdissected. For the initial screening (set 1, supplemental Figure S1), all seven miRs were validated in the IMC/EAC and matched NSE tissues. The miRs that revealed a significantly different expression pattern in the IMC/EAC compared to the paired NSE tissues were subjected to further evaluation in cardiac, BE-ND and HGD lesions, additional EAC (n=8, frozen biopsies), and matched NSE tissues (set 2, supplemental Figure S1). Based on the results of the second screening, we decided to explore only miR-223 in a larger BE-ND (frozen biopsy) cohort (n=10) (set 3, supplemental Figure S1). Reference biopsies taken directly adjacent to the frozen research biopsies were examined by an expert pathologist (AM), and purity and high cellularity of the biopsies were confirmed. An overview of the study design for miR validation using FFPE and frozen specimens is provided in supplemental Figure S1.
Cell culture of esophageal cell lines
Two EAC cell lines, OE33 (American Type Culture Collection (ATCC), Manassas, VA) and JHesoAD1(24), and one primary (non-immortalized) squamous epithelium cell line, HEEpiC, were cultured for this study. OE33 and JHesoAD1 were authenticated using short tandem repeat profiling less than six months ago and the cells have not been in culture for more than 2 months. HEEpiC cells were purchased from Sciencell (Carlsbad, CA). OE33 and JHesoAD1 were cultured in RPMI-1640 medium supplemented with 15–20% fetal bovin serum. HEEpiC cells were grown in Epithelial Cell Medium-2 (Sciencell).
RNA extraction, cDNA preparation and quantitative real-time PCR
Total RNA was extracted from FFPE tissues using the Recover All™ Total Nucleic Acid Isolation Kit for FFPE according to the manufacturer’s handbook (Life technologies, Grand Island, NY). Total RNA was recovered from frozen biopsies and cell lines using the mirVana™ miRNA Isolation kit (Life technologies).
cDNA was prepared with the TaqMan® MicroRNA Reverse Transcription kit (Life Technologies) following the manufacturer’s protocol. qRT-PCR was carried out using TaqMan® Universal Master Mix II no UNG (Life Technologies) and TaqMan assays hsa-miR-199a/b-3p, hsa-miR-199a-5p, hsa-miR-199b-5p, hsa-miR-200b, hsa-miR-200c, hsa-miR-223, and hsa-miR-375 (Life Technologies). RNU6B functioned as the endogenous control. MiR expression levels in the lesion tissues were directly compared to the expression levels in the matched NSE tissues and relative expression levels were calculated using the 2-ΔΔCT method.
In situ hybridization
ISH was performed on tissue microarrays (TMAs), which have previously been described (25), to investigate the clinical and biological relevance of miR-223 up-regulation in EAC carcinogenesis. Briefly, the TMAs consisted of 74 NSE, 37 BE-ND, 27 LGD, 43 HGD, 96 EAC, and 29 lymph node metastases (LNM) tissues obtained from a chemo-radiation-naïve EAC cohort. The staining was performed using the tyramide signal amplification system for ISH with biotinylated probes (DAKO North America, Carpinteria, CA) according to the manufacturer’s instructions. The slides were hybridized with 10 nM hsa-miR-223 5’-biotinylated miRCURA LNA™ probes (Exiqon, Woburn, MA) for 16 hours at 56 °C. Staining patterns were reviewed by three reviewers (EAM, AM, MMS), semi-quantified and categorized into four groups; absent, focal (<25% of epithelial cells exhibit miR-223), moderate (25–50%), and diffuse (>50%).
Transfection of miR-223 mimics inEAC cells
In vitro functional studies were performed using transiently enforced expression of miR-223 in the two EAC cell lines. Specifically, hsa-miR-223 miRNA precursor (pre-miR) or pLV-[hsa-miR-223] plasmid (Biosettia, San Diego, CA) were introduced into OE33 (miR-223 OE33) and JHesoAD1 (miR-223 JHesoAD1) cells. Cells transfected with a non-targeting, negative control mimic (scramble transfected EAC cells) and empty LV vector, respectively, were utilized as controls in the functional analyses. In order to identify candidate gene targets of miR-223, we performed Illumina microarray analysis on OE33 cells transiently transfected with a miR-223 mimic. Specifically, 10 nM of hsa-miR-223 miRNA precursor (Life technologies) was transfected into OE33 cells using Lipofectamine® 2000 (Life Technologies), and cells were harvested 24 hours after transfection. Transfection efficiencies of the cells were estimated by transfection experiments with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) siRNA (Thermo Scientific, Pittsburgh, PA).
In vitro assays (cell growth, migration, invasion)
Cell growth, migration, and invasion assays were performed as previously described (26). Briefly, miR-223 mimic and scramble transfected OE33 and JHesoAD1 cells were harvested 24 hours after transfection and assays were conducted for an additional 48 hours. Experiments were performed in triplicates and repeated at least twice. For the migration and invasion assays, parallel plates were quantified with Cell Titer 96® AQueous One Solution Cell Proliferation Assay (MTS)(Promega, Madison, WI) to correct for viability and cell growth variation, and five randomly selected 20x fields per chamber were counted. The Mann-Whitney U test was used to determine differences in cell growth, and migratory and invasion potential between miR-223 and scramble transfected EAC cells.
Microarray and validation experiments
The HT12 v4 Illumina microarray platform (Illumina Inc, San Diego, CA) was utilized for transcriptomic analysis following enforced miR-223 expression in two biologically replicated experiments (GEO accession link: GSE44120). All genes that were down-regulated at least 1.5 fold in miR-223 compared to scramble transfected OE33 cells were considered as potential target genes of interest. Putative target genes of miR-223 were validated using qRT-PCR. The following criteria were used for this selection:
The gene expression was down-regulated by at least 1.5 fold in miR-223 transfectedOE33 cells compared to the scramble transfected OE33 cells.
Binding of miR-223 to the 3’UTR of the down-regulated gene was predicted by MicroCosm (http://www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/) and/or TargetScan(27).
Candidate target genes were validated in OE33 and JHesoAD1 cells by performing qRT-PCR on EAC cells with enforced miR-223 expression. Both pre-miR hsa-miR-223 miRNA precursor and pLV-[hsa-miR-223] were used for validation to exclude off-target effects. qRT-PCR was carried out using Fast SYBR green master mix (Life technologies) and customized primers (sequences are listed in supplemental Table S2). The top candidate target gene PARP1 was further validated at the protein level by Western Blot analysis (clone A6.4.12 (mouse), 1:1,000 dilution, Abcam, Cambridge, MA). In order to demonstrate the binding of miR-223 to the 3’UTR seed sequence of PARP1, luciferase expression was measured following simultaneously transfecting OE33 cells with pLV-hsa-miR-223, the 3’UTR of PARP1fused to a firefly luciferase gene (Origene, Rockville, MD), and renilla luciferase. Furthermore, expression levels of selected target genes (PARP1 and SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily d, member 1 (SMARCD1)) were measured in eight frozen EAC and matched NSE biopsies using qRT-PCR. Patients were divided into groups, higher (>17 fold miR-223 up-regulation) in EAC compared to matched NSE sample) and lower (<4 fold up-regulation), and correlations between miR-223 and target gene expression was tested using the Mann-Whitney U test. A P-value <0.05 was considered statistically significant.
Sensitivity of miR-223 EAC cells to chemotherapeutics
OE33 and JHesoAD1 cells were used to investigate the potential effects of miR-223 misexpression on chemotherapy sensitivity. Cells were harvested 24 hours after transfection with miR-223 mimic or scrambled miR, and plated in 96-well plates. Twenty-four hours later, cells were treated with Cisplatin (range 0–20 µM), Mitomycin C (range: 0–15 µM) or Doxorubicin (DOX) (range: 0–15µM) for 24–48 hours. MTS assays were performed and statistical tests (Mann-Whitney U test and two-way ANOVA) were run to determine differences in viability between miR-223 and scramble transfected OE33 cells. Experiments were repeated at least three times in triplicates, and a P-value <0.05 was considered statistically significant.
Clonogenic assay
Anchorage independent clonogenic assays were performed to determine the effect of miR-223 over-expression on Cisplatin sensitivity. MiR-223 and scramble transfected OE33 cells in 0.5% agarose in normal growth media were plated in triplicates on top of a base layer of 1% agarose in normal growth media. The next day, 1 ml of 0–6µM Cisplatin was added on top of the cell layer, and colonies were treated for 10 days. The colonies were stained with 0.005% crystal violet and manually counted. Statistical significant differences were tested using the Mann-Whitney U test. A P-value <0.05 was considered as statistically significant.
Immunofluorescence for phospho-H2A.X and 53BP1
We further investigated whether DNA breaks are more often induced by treatment with Doxorubicin in miR-223 compared to scramble transfected OE33 cells. Immunofluorescence experiments were performed to detect the phosphorylated histone H2A.X (pH2A.X, a.k.a. γH2AX) and tumor suppressor protein 53 binding protein 1 (53BP1), DNA-damaging response proteins that are recruited to DNA breaks and therefore can be used to visualize DNA breaks. MiR-223 and scramble transfected OE33 cells were plated on chamber slides, and treated with 0–1.25µM Doxorubicin for 24 hours. Primary antibodies against pH2A.X (clone JBW301, dilution 1:500, Millipore, Billerica, MA) and 53BP1 (NB100-304, dilution 1:250, Littleton, CO) and Alexa fluor secondary antibodies (dilutions 1:250, Invitrogen), respectively, were used. Reference photomicrographs were taken from five randomly selected fields. Cells were manually counted and categorized into cells with and without co-staining. The Mann-Whitney U test was used to determine statistically significant differences between miR-223 and scramble transfected OE33 cells. A P-value <0.05 was considered as statistically significant.
RESULTS
MicroRNA expression in the BE-dysplasia-EAC sequence
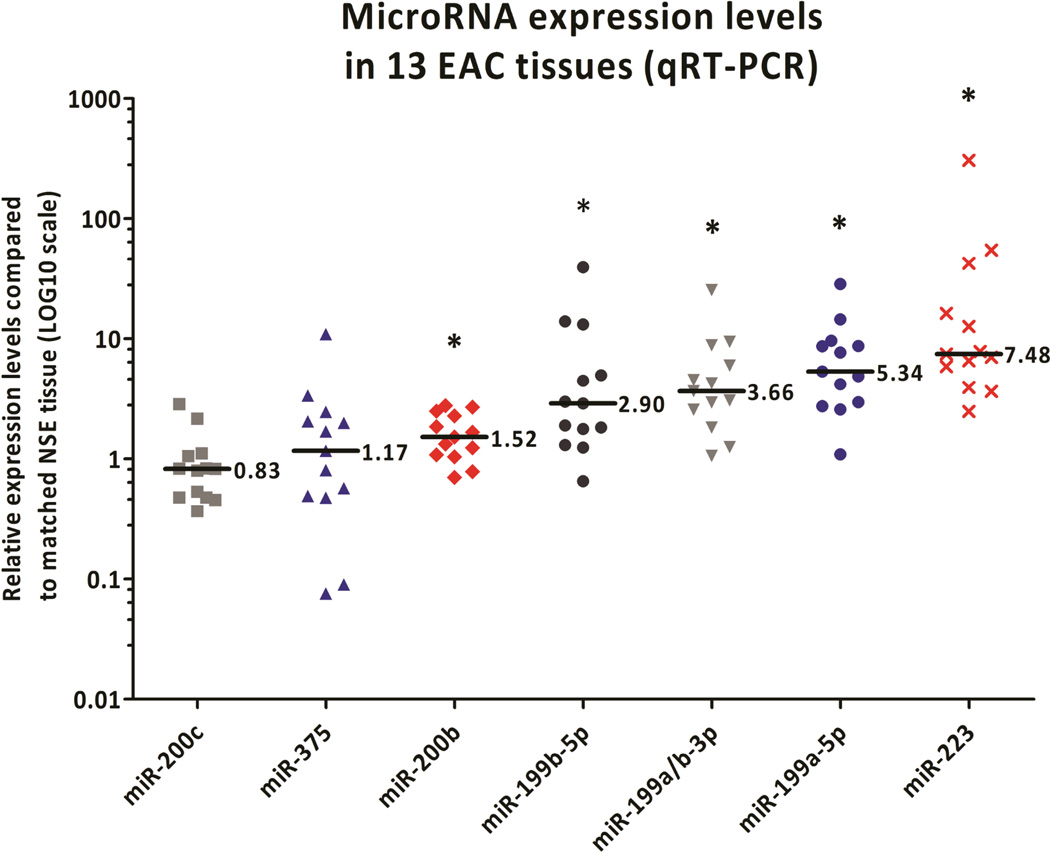
A panel of seven miRs was selected for profiling, based on their recurrent misexpression in BE and EAC across multiple independent studies (Supplemental Table S1). All transcript profiling was performed on chemo-radiation-naïve EAC patient samples. Five miRs, miR-199a/b-3p, −199a-5p, −199b-5p, −200b, and −223, were significantly differentially expressed between EAC and paired NSE tissues (Figure1). The median relative expression values of the miR-199 family members and miR-223 were greater than 2-fold higher in EAC than matched NSE, and these miRs were subjected to further investigation (Figure 2).
Figure 1.
MicroRNA validation in set 1. The miR-199 family members, miR-200b, and miR-223 were significantly up-regulated in EAC tissues compared to matched NSE tissues. The bar indicates the median expression levels, whereas the asterisk (*) depicts that the median significantly differs (P<0.05) from the median in the matched NSE tissue.
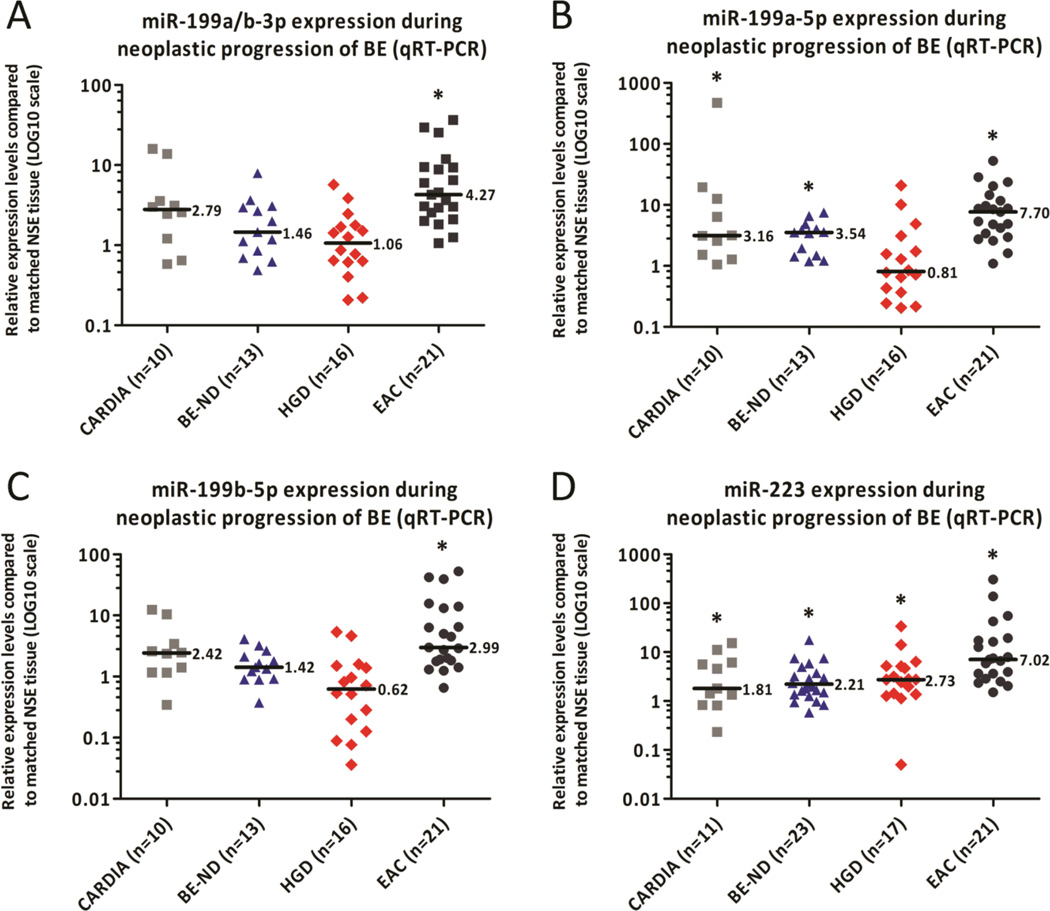
Figure 2.
Validation of four microRNAs in set 2. MiR-199a/b-3p (A), −199a-5p (B), −199b-5p (C), and −223 (D) expression patterns were further analyzed in cardiac, BE-ND, HGD, and additional EAC specimens and matched NSE specimens. The highest median expression level in EAC was noted for miR-199a-5p (B), whereas miR-223 was the only miR showing stepwise up-regulation during the multistep progression towards EAC. Significantly higher miR-223 levels were detected in cardiac, BE-ND, HGD, and EAC compared to the paired NSE tissues (D). Notably, expression levels in cardiac mucosa and BE-ND showed great similarities across the four miRs. In these figures each dot represents a patient. The bar indicates the median expression level, whereas the asterisk (*) depicts that the median significantly differs (P<0.05) from the median in the matched NSE tissue.
Expression of miR-199 family members and miR-223 was examined in gastric cardia, BE-ND, HGD, and an expanded group of treatment-naïve EAC tissues, and compared to the expression of these miRs in matched NSE tissues from the same patient. The expression levels of gastric cardia and BE-ND tissues were remarkably similar for all four miRs, suggesting ontogenic similarities between the two differentiated epithelial subtypes. Significantly higher expression of the miR-199 family members was found in EAC tissues compared to HGD, BE-ND and paired NSE tissues (all P<0.006), and among those, miR-199a-5p showed the highest median expression in EACs (7.70) (Figure 2A–C).
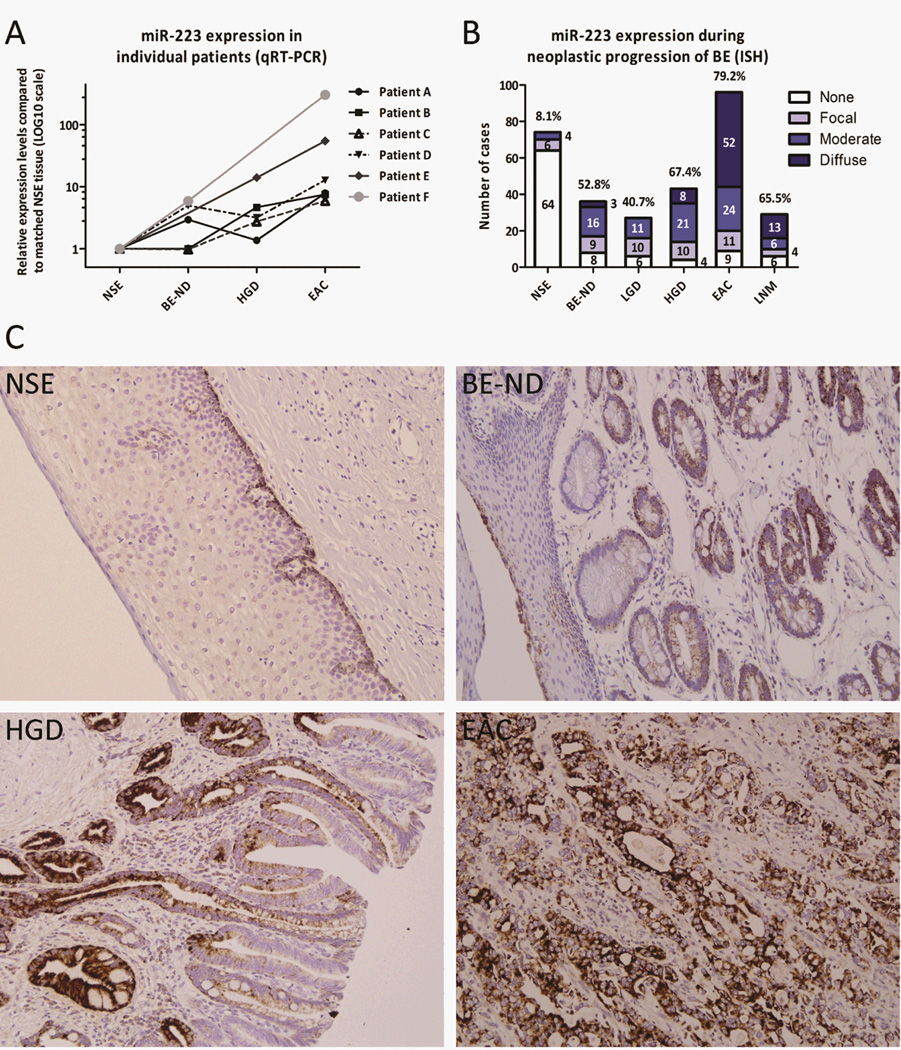
Significantly increased miR-223 levels were found in BE-ND (median: 2.21, P=0.003), HGD (P=0.001), and EAC (P<0.001) compared to their matched NSE tissue (Figure 2D). MiR-223 was up-regulated in all EAC tissues, and the median expression level in the EAC group (7.02) was significantly higher than in the HGD (2.73), BE (2.21)and cardiac group (1.81) (all P<0.014, Figure 2D). Up-regulation in a stepwise manner was revealed during EAC carcinogenesis (P=0.002, Figure 2D). From six patients two or more neoplastic lesions were available and analysis of miR-223 expression in these lesions confirmed that miR-223 is also up-regulated in a stepwise manner during the multistep progression of BE in any given individual (Figure 3A). MiR-223 expression was validated in a larger cohort of lesions obtained from treatment-naïve EAC patients using ISH. MiR-223 transcripts were present in 8.1%, 52.8%, 40.7%, 67.4%, 79.2%, 65.5% of the NSE, BE, LGD, HGD, EAC, and LNM tissues, respectively (Figure 3B). Diffuse expression was more often detected in EACs and LNMs. ISH furthermore showed that miR-223 is exclusively expressed by metaplastic, dysplastic and neoplastic epithelial cells, and not by the inflammatory or stromal cells adjacent to the lesion (Figure 3C).
Figure 3.
miR-223 in esophageal adenocarcinoma carcinogenesis. Panel (A) shows that miR-223 expression is up-regulated in a stepwise manner in six individuals (qRT-PCR). MiR-223 expression analysis in a large EAC cohort using ISH revealed miR-223 presence in 8.1%, 52.8%, 40.7%, 67.4%, 79.2%, and 65.5% of the NSE, BE-ND, LGD, HGD, EAC, and LNM tissues, respectively (B). The percentages above the columns in graph B indicate the total percentage of tissues exhibiting either moderate or diffuse miR-223 expression. Representative ISH photos in panel (C) illustrate the stepwise increased miR-223 expression from NSE, to BE-ND, to HGD, and ultimately EAC. MiR-223 expression was limited to the (non-)dysplastic/neoplastic epithelial cells, and completely undetectable in inflammatory and stromal cells.
The role of miR-223 in cell growth, invasion, and migration
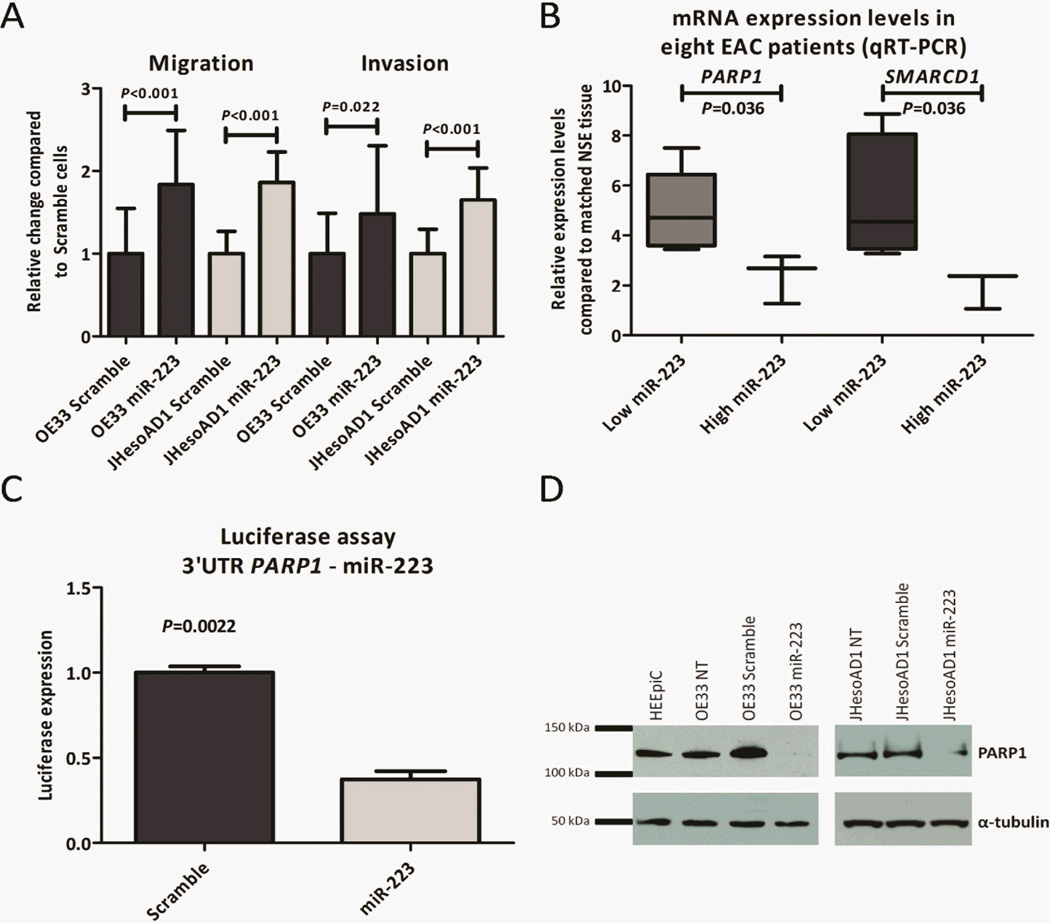
Low endogenous levels of miR-223 were detected in the EAC cell lines OE33 and JHesoAD1 compared to the normal esophageal cell line HEEpiC (data not shown). In order to explore the function of miR-223 in the esophagus, we ectopically expressed miR-223 in OE33 and JHesoAD1 cells. In short-term viability assays, miR-223 transfected EAC cells had lower cell numbers than scramble transfected EAC cells (P<0.001, data not shown). In contrast, additional in vitro assays showed a more aggressive phenotype in miR-223 than scramble transfected EAC cells; miR-223 transfected cells exhibited statistically significantly more migratory and invasive potential (P<0.022, Figure 4A).
Figure 4.
MiR-223 functional studies in esophageal adenocarcinoma cell lines. MiR-223 EAC cells showed significantly increased migratory and invasive potential compared to scramble EAC cells (A). Validation of the Illumina microarray results in OE33 and JHesoAD1 cells by qRT-PCR confirmed that PARP1 and SMARCD1 are prone to be direct target genes of miR-223 in the esophagus. Correlation of miR-223 and target gene expression in eight EAC patients provided additional evidence that PARP1 and SMARCD1 are targeted by miR-223. PARP1 and SMARCD1 levels were significantly lower among patients with high miR-223 expression levels (B). We next focused on PARP1, and established that firefly luciferase activity is significantly decreased when miR-223 and plasmid DNA consisting of PARP1 3’UTR and a firefly luciferase gene are co-transfected in OE33 cells (C). Complete knockdown of PARP1 and significant reduction of PARP1 activity at protein level was detected upon miR-223 transfection in OE33 and JHesoAD1 cells, respectively (D). The error bars in panels A–C depict the standard error of the mean.
PARP1 and SMARCD1 are target genes of miR-223 in the esophagus
In order to discover direct target genes of miR-223 that could explain the phenotype of miR-223 over-expressing cells in the esophagus, microarray experiments were performed in replicate on OE33 cells. The expression profiles of miR-223 transfected OE33 cells were compared with those of scramble transfected OE33 cells, and fifty-eight genes showed an average down-regulation of more than 1.5 fold in miR-223 relative to scramble transfected OE33 cells. The miR target gene prediction platforms, MicroCosm and Target Scan(27), were queried to determine whether binding of miR-223 to the 3 prime untranslated region (3’UTR) was predicted by the respective in silico algorithms. A match between miR-223 and 3’UTR of the putative target gene was found in 51.7% (30/58) of the >1.5 fold down-regulated genes (Supplemental Table S3).
Ten potential target genes were validated in replicate experiments in OE33 and JHesoAD1 cells using qRT-PCR. Nine genes were significantly down-regulated in miR-223 compared to scramble transfected OE33 cells, with PARP1, CYB5A, and SMARCD1as top candidates. The three top candidates were also found to be down-regulated upon miR-223 over-expression in JHesoAD1 cells (Table 1). We observed that the effect of miR-223 over-expression on target gene expression was more modest in JHesoAD1 than OE33 cells due to poorer transfection efficiency. Nonetheless, in both cell lines, we established that miR-223 expression leads to reduction in PARP1, CYB5A and SMARCD1 transcripts (Table 1).
Table 1.
Putative target genes of miR-223. Illumina microarray on OE33 cells identified candidate target genes. Publicly available databases were queried to search for binding sites of miR-223 in the 3’UTR of the putative targets. Ten potential target genes were selected for further validation in OE33 and JHesoAD1 cells using qRT-PCR. All putative targets except LMO2 were significantly down-regulated in OE33 cells upon miR-223 transfection. Expression of PARP1, CYB5A, and SMARCD1 was significantly inhibited in miR-223 compared to scramble JHesoAD1 cells.
| Binding of miR- 223 to 3’UTR gene predicted by: |
Fold change qRT- PCR validation |
||||||
|---|---|---|---|---|---|---|---|
| Gene | Full name | Average fold change miR223 vs Mock OE33 cells |
Micro Cosm |
Target Scan |
Match miR-223 and 3’UTR gene |
OE33* | JHesoAD1* |
| PARP1 | Poly (ADP-ribose) polymerase 1 | −2.42 | Yes | Yes | 8mer | −2.71** | −1.42** |
| MT1E | Metallothionein 1E | −2.27 | Yes | Yes | 7mer-1A | −1.90** | −1.09 |
| CYB5A | Cytochrome b5 type A (microsomal) |
−1.91 | Yes | Yes | 8mer | −2.43** | −1.55** |
| SLC11A2 | Solute carrier family 11, member 2 | −1.84 | Yes | Yes | 8mer | −1.82** | −1.17 |
| SLC39A1 | solute carrier family 39 , member 1 | −1.84 | Yes | Yes | 7mer-m8 | −1.79** | −1.21 |
| SMARCD1 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily d, member 1 |
−1.79 | No | Yes | 8mer | −1.98** | −1.52** |
| STMN1 | Stathmin 1 | −1.75 | Yes | Yes | 7mer-m8 | −1.75** | 1.15 |
| GFPT1 | Glutamine-fructose-6-phosphate transaminase 1 |
−1.65 | Yes | Yes | 8mer | −1.73** | −1.13 |
| LMO2 | LIM domain only 2 (rhombotin-like 1) |
−1.60 | No | Yes | 8mer | −1.36 | 1.38** |
| KIAA1279 | KIAA1279 | −1.56 | Yes | Yes | 7mer-m8 | −1.77** | −1.22 |
Key notes and abbreviations:
8mer: an exact match to positions 2–8 of miR-223 (the seed plus position 8) followed by an ‘A’;
7mer-m8: an exact match to positions 2–8 of miR-223 (the seed plus position 8);
7mer-1A: an exact match to positions 2–7 of miR-223 followed by an ‘A;’
average of two biological replicated experiments;
indicates P<0.05;
3’UTR: 3’ untranslated region.
The expression levels of PARP1and SMARCD1 were examined in eight treatment-naïve EAC and matched NSE frozen biopsies. The patients were divided into two groups based on the miR-223 level in EAC: “low” (n=5, range: 1.5–3.6) and “high” (n=3, range: 17.1–137.8). PARP1 and SMARCD1 were significantly lower expressed in the “high miR-223” group (P=0.036, Figure 4B).
MiR-223 regulates chemotherapy sensitivity via PARP1
Direct binding of miR-223 to the 3’UTR of PARP1 was further confirmed by a firefly luciferase assay. Co-transfection of the miR-223 mimic, plasmid DNA consisting of a 3’UTR clone of PARP1fused to the firefly luciferase gene, and the Renilla luciferase vector as transfection control resulted in a 2.7 fold decreased firefly luciferase activity compared to co-transfection with a scramble miR (Figure 4C). In addition, functional down-regulation of PARP1 protein was confirmed by Western blot analysis in OE33 and JHesoAD1 cells (Figure 4D).
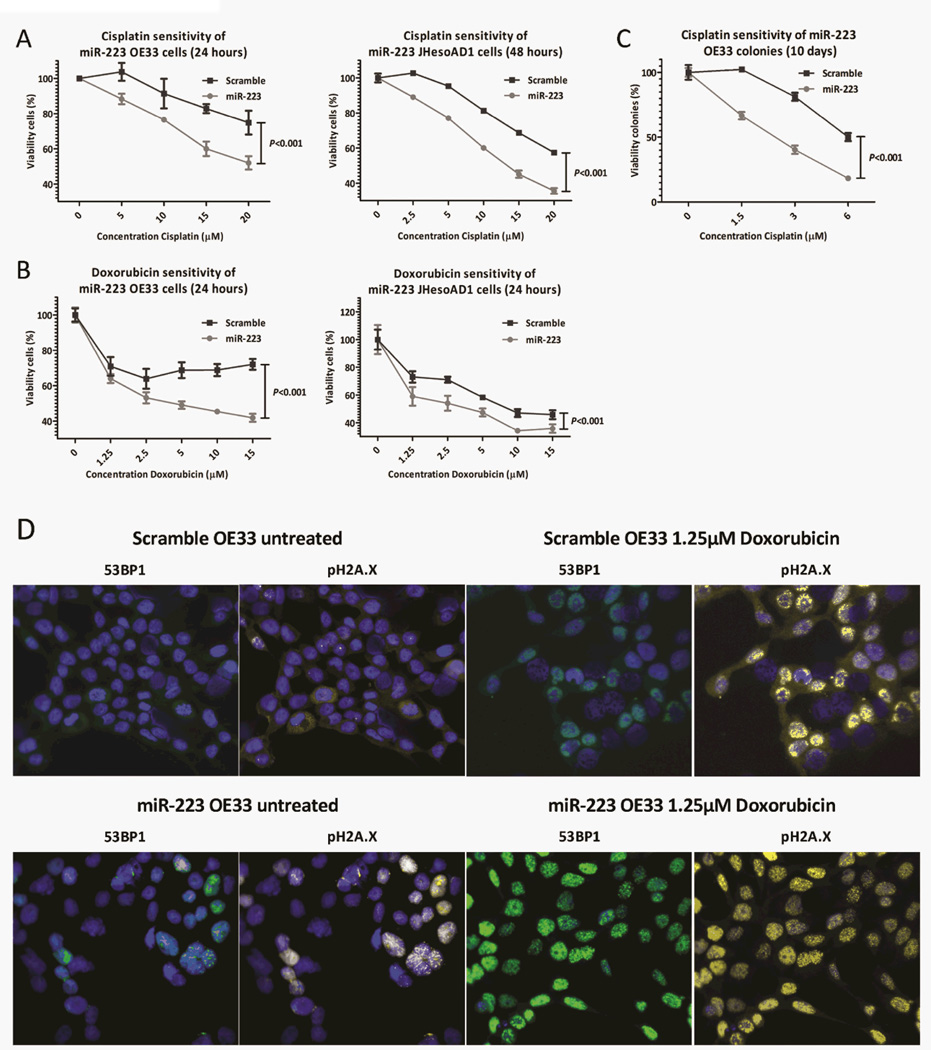
PARP1 is involved in the repair of single stranded DNA breaks, and studies have shown that PARP1 inhibitors improve the sensitivity of carcinomas to chemotherapeutics (28). We investigated whether ectopic miR-223 expression, and thus PARP1 down-regulation, can increase the sensitivity of OE33 and JHesoAD1 cells to the DNA-damaging agents Cisplatin, Mitomycin C and Doxorubicin. In comparison with scramble transfected cells, miR-223 transfected OE33 and JHesoAD1 cells were significantly more sensitive to Cisplatin and Doxorubicinat all tested concentrations, as assessed by in vitro viability assays (P<0.001, Figure 5A and B). MiR-223 transfected JHesoAD1 were also significantly less viable after Mitomycin C treatment at all tested concentrations (P<0.001, data not shown), while miR-223 transfected OE33 cells were only more sensitive to 7.5–10µM Mitomycin C (P=0.009, data not shown) than scramble transfected cells. In longer term (T = 10 days) clonogenic survival assays, we observed that miR-223 colonies were significantly more sensitive to all tested Cisplatin treatments relative to scramble transfected OE33 colonies (P<0.001, Figure 5C). The IC50 was reached at concentrations of 2.45µM and 6.00µM for miR-223 and scramble colonies, respectively, suggesting that miR-223 colonies are approximately 2.45 times more sensitive to Cisplatin than scramble sequence expressing colonies. We further determined that significantly more DNA breaks, as visualized by co-staining of pH2A.X and 53BP1, are encountered in untreated (P=0.032) as well as with 1.25µM Doxorubicin treated (P=0.008) miR-223 expressing compared to scramble transfected OE33 cells(Figure 5D) indicating that the DNA repair mechanism is impaired in miR-223OE33 cells. Together, these data suggest that EAC patients with high miR-223 levels in their EACs might benefit more from treatment with DNA-damaging agents than patients with lower miR-223 levels in their tumors.
Figure 5.
Since PARP1 inhibition has been previously linked to increased chemotherapy sensitivity, we tested Cisplatin (A) and Doxorubicin(B) sensitivity of miR-223 OE33 and JHesoAD1 cells. MiR-223 OE33 and JHesoAD1 cells were significantly more sensitive to both chemotherapeutics compared to scramble cells in short-term MTS assays (B, C). Long-term (10 days) sensitivity to Cisplatin was evaluated in miR-223 and scramble transfected OE33 colonies using a drug clonogenic survival assay (C). The concentration on the X-axis depicts the concentration of Cisplatin in de media (1 ml) that was added on top of the cell-containing agarose layer. MiR-223 transfected OE33 cells were significantly more sensitive to Cisplatin after 10 days than scramble transfected cells at all concentrations (C). Panel (D) shows example photomicrographs of the immunofluorescence experiment. Co-localization of pH2A.X and 53BP1 indicates the presence of a DNA break. Significantly more DNA breaks were detected in miR-223 transfected untreated and treated OE33 cells compared to scramble transfected OE33 cells (P<0.05).
DISCUSSION
Recent microarray and “next-generation” RNA sequencing studies have uncovered numerous miRs that are differentially expressed in EAC and BE; however, surprisingly little is known about the functional roles of these miRs in the multistep progression of BE. In this study we validated a panel of miRs that have previously been reported in the literature, on an independent cohort of treatment-naïve EAC patients. Only four miRs, miR-199a/b-3p, −199a-5p, −199b-5p, −223, showed a significant aberrant expression pattern in EAC compared to NSE, and these miRs were subjected to further analysis. For miR-223 a stepwise increase in expression was observed in the NSE-BE-dysplasia-EAC sequence. A similar trend was observed on a larger cohort using ISH hybridization.
In the context of upper gastrointestinal tract malignancies, miR-223 has been extensively studied in gastric cancer pathogenesis, where it is significantly up-regulated in gastric cancer tissues compared to normal gastric mucosae(29–31). Furthermore, it has been reported that miR-223 can be detected in serum of gastric cancer patients and implicates that miR-223 might function as a diagnostic biomarker for gastric cancer(32).In agreement with these studies, we showed that miR-223 is already over-expressed in the non-dysplastic precursors and HGD lesions in the esophagus, suggesting that we might be able to predict EAC development in an early, curable stage using miR-223 detection.
Our in vitro data suggest that miR-223 plays a role in the regulation of cell proliferation and viability. Paradoxically, over-expression of miR-223 in EAC cells resulted in significantly reduced cell growth. These results are in agreement with miR-223 expression studies in other cancer cell lines: significantly less proliferative potential was revealed in miR-223 over-expressing osteosarcoma, hepatoma, hepatocellular cancer, colorectal cancer, cervical cancer, and gastric cancer cells (31, 33–35). This seemingly tumor suppressive role of miR-223 is greatly opposed to the aggressive behavior, characterized by enhanced migratory and invasive capacity, of miR-223 over-expressing EAC cells. Other groups have previously also shown in esophageal squamous cell carcinoma and gastric cancer that miR-223 is able to promote migration, invasion, and metastasis formation(30, 31, 36). These studies have furthermore reported that high miR-223 levels are correlated with advanced disease and poor outcome in these cancers(31, 37, 38).To further explore how miR-223 regulates cancer-promoting cellular processes, we profiled EAC cells in the setting of ectopic miR-223 expression.
We identified two novel target genes of miR-223, namely SMARCD1and PARP1, the inhibition of which may contribute to cancer development and/or progression as well as to chemotherapy sensitivity in the esophagus. SMARCD1, also known as BRG1-associated factor 60a, has binding sites for the glucocorticoid receptor and BRG1, and appears to be essential for chromatin remodeling processes. (39). In addition, it has been shown that interaction of the SNF/SWI complex and p53 is required for p53-mediated transcription and cell cycle regulation (40). SMARCD1 possesses a binding site for p53, and inhibition of SMARCD1 reduces the SWI/SNF complex-mediated transcriptional activity of p53 (41). We showed in this study that miR-223 expression is negatively correlated with SMARCD1 expression. MiR-223 over-expression may lead to reduced chromatin remodeling activity, impaired transcription from the chromatin, and decreased p53 activity by directly inhibiting SMARCD1 transcription.
PARP1 was down-regulated at both the transcript and protein level upon miR-223 over-expression in EAC cells, and we confirmed the direct binding of miR-223 to the 3’UTR of PARP1 using a luciferase assay. Furthermore, we showed that lower PARP1 levels are significantly associated with EACs with high miR-223 levels than lower miR-223 levels, indicating that this regulatory mechanism is intact in vivo in human tissue samples. PARP1 is a poly(ADP-ribose) polymerase, and plays a role in processes involving DNA recognition(42). PARP1 interacts with single and double DNA breaks, which results in automodification, self-activation, and the addition of poly (ADP-ribose) polymers to itself and surrounding histones (28, 43). It recruits DNA repair proteins to the site of DNA breaks by, amongst other mechanisms, changing local chromatin structures to admit DNA repair proteins to the DNA breaks.(28). We showed in the current study that in miR-223 over-expressing EAC cells the DNA damage repair system is significantly impaired through direct down-regulation of PARP1. Induction of DNA damage by Doxorubicin resulted in significantly more DNA breaks in miR-223 over-expressing than scramble transfected OE33 cells.
Interestingly, PARP1 also seems to play an essential role in the maintenance of genetic stability. Reduced PARP1 activity promotes homologous recombination, and it has been extensively reported that homologous deficient cells, for instance BRCA1/2 deficient cells, are sensitive to PARP1 inhibitors since these cells are incapable of repairing recombinogenic lesions induced by PARP1 inhibition (28). A rational therapeutic approach for cancers that are not homologous deficient such as EAC is to combine PARP1 inhibitors with DNA-damaging agents (42), or recruit patients who already exhibit low PARP1 levels for therapy with DNA-damaging agents. To test the latter approach, we investigated the sensitivity of miR-223 EAC cells to Cisplatin, Mitomycin C and Doxorubicin, and found that miR-223 EAC cells were significantly more sensitive to these chemotherapeutics than scramble transfected OE33 cells in short term viability and longer term anchorage independent survival assays. These data suggest that EAC patients with high miR-223 levels might benefit more from treatment with DNA-damaging agents.
Our study has two related limitations. First, the correlation of miR-223 and PARP1 expression could only be confirmed in snap frozen specimens since mRNA levels are greatly degraded in FFPE tissues. Secondly, the specimens must be obtained from treatment-naïve EAC patients, and these specimens are scarce. Therefore, studies in larger cohorts are required before miR-223 can be implemented as predictive biomarker for chemotherapy sensitivity in the clinics.
In conclusion, we showed that miR-223 is up-regulated in the multistep progression of BE-ND to EAC in the majority of patients. MiR-223 over-expressing cells exhibited an aggressive phenotype; however, they were also more sensitive to DNA-damaging agents due to the direct interaction between miR-223 and PARP1. High endogenous levels of miR-223 in EACs were significantly associated with lower PARP1 levels, and those patients might benefit more from treatment with DNA-damaging agents.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
Esophageal adenocarcinomas (EAC) typically present at an advanced stage of disease, and current chemo-radiation therapies have only modest impact on survival. In order to develop more effective therapeutic strategies, there is an urgent need for elucidating the molecular basis underlying EAC. MicroRNAs (miRs) could potentially help in identifying patients who would benefit from existing cancer therapy or represent targets for future therapy. Although prior studies have discovered aberrant miR signatures during EAC carcinogenesis, there is a lack of functional studies that could lead to improvements in therapeutic strategies.
In this study, we showed that miR-223, whose expression is progressively up-regulated during EAC carcinogenesis, behaves as an onco-miR in EAC. We identified the DNA damage repair gene PARP1 as a direct target of miR-223, and demonstrated that miR-223 up-regulation results in increased sensitivity to chemotherapy by down-regulating PARP1, providing a potential therapeutic vulnerability node for exploitation in the clinic.
ACKNOWLEDGEMENTS
The patient’s consent was obtained under an IRB-approved protocol. We would like to acknowledge Conover Talbot Jr. from the Institute for Basic Biomedical Sciences at Johns Hopkins School of Medicine for his help with the bioinformatics analysis of the Illumina microarray data.
Grant support: This project has been funded by the Jerry D’Amato foundation and the NIH (1K23 DK068149). MMS has been sponsored by the Fulbright/Netherland America Foundation and the René Vogels Foundation.
List of abbreviations
- 53BP1
tumor suppressor protein 53 binding protein 1
- BE
Barrett’s esophagus
- BE-ND
non-dysplastic Barrett’s esophagus
- cardiac
cardio-oxyntic
- Cisplatin
cis-diamminedichloroplatinum (II)
- CYB5A
cytochrome b5 type A
- EAC
esophageal adenocarcinoma
- FFPE
formalin-fixed paraffin-embedded
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IMC
intramucosal carcinoma
- ISH
in situ hybridization
- miR
microRNA
- MTS
Cell Titer 96® AQueous One Solution Cell Proliferation Assay (Promega)
- onco-miR
oncogenic microRNA
- PARP1
poly(ADP-ribose) polymerase 1
- pH2A.X
phosphorylated histone H2A.X
- qRT-PCR
quantitative real-time PCR
- SMARCD1
SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily d, member 1
- TMA
tissue microarray
- TS-miR
tumor suppressor microRNA
Footnotes
Conflicts of interest: All authors declare that they have no financial conflicts of interest to disclose.
Author contributions: MMS designed the experiments, acquired, analyzed and interpreted data, and drafted the manuscript. SP, NRC, CH, and SY were involved in the acquisition of data and critically revised the manuscript. MIC and JSW provided materials and critically revised the manuscript. EAC and AM designed the study, developed methodology, analyzed and interpreted data, drafted the manuscript and supervised the study.
GEO accession number of repository for microarray expression data: GSE44120.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Reid BJ, Li X, Galipeau PC, Vaughan TL. Barrett's oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat Rev Cancer. 2010;10:87–101. doi: 10.1038/nrc2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 4.Mendell JT. MicroRNAs: critical regulators of development, cellular physiology and malignancy. Cell Cycle. 2005;4:1179–1184. doi: 10.4161/cc.4.9.2032. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang TC, Mendell JT. microRNAs in vertebrate physiology and human disease. Annu Rev Genomics Hum Genet. 2007;8:215–239. doi: 10.1146/annurev.genom.8.080706.092351. [DOI] [PubMed] [Google Scholar]
- 7.Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122:6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 8.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kan T, Meltzer SJ. MicroRNAs in Barrett's esophagus and esophageal adenocarcinoma. Curr Opin Pharmacol. 2009;9:727–732. doi: 10.1016/j.coph.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 11.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 12.Feber A, Xi L, Luketich JD, Pennathur A, Landreneau RJ, Wu M, et al. MicroRNA expression profiles of esophageal cancer. J Thorac Cardiovasc Surg. 2008;135:255–260. doi: 10.1016/j.jtcvs.2007.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathe EA, Nguyen GH, Bowman ED, Zhao Y, Budhu A, Schetter AJ, et al. MicroRNA expression in squamous cell carcinoma and adenocarcinoma of the esophagus: associations with survival. Clin Cancer Res. 2009;15:6192–6200. doi: 10.1158/1078-0432.CCR-09-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bansal A, Lee IH, Hong X, Anand V, Mathur SC, Gaddam S, et al. Feasibility of mcroRNAs as biomarkers for Barrett's Esophagus progression: a pilot cross-sectional, phase 2 biomarker study. Am J Gastroenterol. 2011;106:1055–1063. doi: 10.1038/ajg.2011.37. [DOI] [PubMed] [Google Scholar]
- 15.Fassan M, Volinia S, Palatini J, Pizzi M, Baffa R, De Bernard M, et al. MicroRNA expression profiling in human Barrett's carcinogenesis. Int J Cancer. 2011;129:1661–1670. doi: 10.1002/ijc.25823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leidner RS, Ravi L, Leahy P, Chen Y, Bednarchik B, Streppel M, et al. The microRNAs, MiR-31 and MiR-375, as candidate markers in Barrett's esophageal carcinogenesis. Genes Chromosomes Cancer. 2012;51:473–479. doi: 10.1002/gcc.21934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wijnhoven BP, Hussey DJ, Watson DI, Tsykin A, Smith CM, Michael MZ. MicroRNA profiling of Barrett's oesophagus and oesophageal adenocarcinoma. Br J Surg. 2010;97:853–861. doi: 10.1002/bjs.7000. [DOI] [PubMed] [Google Scholar]
- 18.Yang H, Gu J, Wang KK, Zhang W, Xing J, Chen Z, et al. MicroRNA expression signatures in Barrett's esophagus and esophageal adenocarcinoma. Clin Cancer Res. 2009;15:5744–5752. doi: 10.1158/1078-0432.CCR-09-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kan T, Sato F, Ito T, Matsumura N, David S, Cheng Y, et al. The miR-106b-25 polycistron, activated by genomic amplification, functions as an oncogene by suppressing p21 and Bim. Gastroenterology. 2009;136:1689–1700. doi: 10.1053/j.gastro.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maru DM, Singh RR, Hannah C, Albarracin CT, Li YX, Abraham R, et al. MicroRNA-196a is a potential marker of progression during Barrett's metaplasia-dysplasia-invasive adenocarcinoma sequence in esophagus. Am J Pathol. 2009;174:1940–1948. doi: 10.2353/ajpath.2009.080718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith CM, Watson DI, Michael MZ, Hussey DJ. MicroRNAs, development of Barrett's esophagus, and progression to esophageal adenocarcinoma. World J Gastroenterol. 2010;16:531–537. doi: 10.3748/wjg.v16.i5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen GH, Schetter AJ, Chou DB, Bowman ED, Zhao R, Hawkes JE, et al. Inflammatory and microRNA gene expression as prognostic classifier of Barrett's-associated esophageal adenocarcinoma. Clin Cancer Res. 2010;16:5824–5834. doi: 10.1158/1078-0432.CCR-10-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith CM, Watson DI, Leong MP, Mayne GC, Michael MZ, Wijnhoven BP, et al. miR-200 family expression is downregulated upon neoplastic progression of Barrett's esophagus. World J Gastroenterol. 2011;17:1036–1044. doi: 10.3748/wjg.v17.i8.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvarez H, Koorstra JB, Hong SM, Boonstra JJ, Dinjens WN, Foratiere AA, et al. Establishment and characterization of a bona fide Barrett esophagus-associated adenocarcinoma cell line. Cancer Biol Ther. 2008;7:1753–1755. doi: 10.4161/cbt.7.11.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streppel MM, Vincent A, Mukherjee R, Campbell NR, Chen SH, Konstantopoulos K, et al. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol. 2012;43:1755–1763. doi: 10.1016/j.humpath.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streppel MM, Lata S, Delabastide M, Montgomery EA, Wang JS, Canto MI, et al. Next-generation sequencing of endoscopic biopsies identifies ARID1A as a tumor-suppressor gene in Barrett's esophagus. Oncogene. 2013 doi: 10.1038/onc.2012.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 28.Helleday T, Bryant HE, Schultz N. Poly(ADP-ribose) polymerase (PARP-1) in homologous recombination and as a target for cancer therapy. Cell Cycle. 2005;4:1176–1178. doi: 10.4161/cc.4.9.2031. [DOI] [PubMed] [Google Scholar]
- 29.Yao Y, Suo AL, Li ZF, Liu LY, Tian T, Ni L, et al. MicroRNA profiling of human gastric cancer. Mol Med Rep. 2009;2:963–970. doi: 10.3892/mmr_00000199. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Zhang Y, Zhang H, Liu X, Gong T, Li M, et al. miRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3. Mol Cancer Res. 2011;9:824–833. doi: 10.1158/1541-7786.MCR-10-0529. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Guo Y, Liang X, Sun M, Wang G, De W, et al. MicroRNA-223 functions as an oncogene in human gastric cancer by targeting FBXW7/hCdc4. J Cancer Res Clin Oncol. 2012;138:763–774. doi: 10.1007/s00432-012-1154-x. [DOI] [PubMed] [Google Scholar]
- 32.Li BS, Zhao YL, Guo G, Li W, Zhu ED, Luo X, et al. Plasma microRNAs, miR-223, miR-21 and miR-218, as novel potential biomarkers for gastric cancer detection. PLoS One. 2012;7:e41629. doi: 10.1371/journal.pone.0041629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu L, Li H, Jia CY, Cheng W, Yu M, Peng M, et al. MicroRNA-223 regulates FOXO1 expression and cell proliferation. FEBSLett. 2012;586:1038–1043. doi: 10.1016/j.febslet.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 34.Li G, Cai M, Fu D, Chen K, Sun M, Cai Z, et al. Heat Shock Protein 90B1 Plays an Oncogenic Role and is a Target of microRNA-223 in Human Osteosarcoma. Cell Physiol Biochem. 2012;30:1481–1490. doi: 10.1159/000343336. [DOI] [PubMed] [Google Scholar]
- 35.Wong QW, Lung RW, Law PT, Lai PB, Chan KY, To KF, et al. MicroRNA-223 is commonly repressed in hepatocellular carcinoma and potentiates expression of Stathmin1. Gastroenterology. 2008;135:257–269. doi: 10.1053/j.gastro.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Li S, Li Z, Guo F, Qin X, Liu B, Lei Z, et al. miR-223 regulates migration and invasion by targeting Artemin in human esophageal carcinoma. J Biomed Sci. 2011;18:24. doi: 10.1186/1423-0127-18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurashige J, Watanabe M, Iwatsuki M, Kinoshita K, Saito S, Hiyoshi Y, et al. Overexpression of microRNA-223 regulates the ubiquitin ligase FBXW7 in oesophageal squamous cell carcinoma. Br J Cancer. 2012;106:182–188. doi: 10.1038/bjc.2011.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Zhang Y, Ding J, Wu K, Fan D. Survival prediction of gastric cancer by a seven-microRNA signature. Gut. 2010;59:579–585. doi: 10.1136/gut.2008.175497. [DOI] [PubMed] [Google Scholar]
- 39.Hsiao PW, Fryer CJ, Trotter KW, Wang W, Archer TK. BAF60a mediates critical interactions between nuclear receptors and the BRG1 chromatin-remodeling complex for transactivation. Mol Cell Biol. 2003;23:6210–6220. doi: 10.1128/MCB.23.17.6210-6220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee D, Kim JW, Seo T, Hwang SG, Choi EJ, Choe J. SWI/SNF complex interacts with tumor suppressor p53 and is necessary for the activation of p53-mediated transcription. J Biol Chem. 2002;277:22330–22337. doi: 10.1074/jbc.M111987200. [DOI] [PubMed] [Google Scholar]
- 41.Oh J, Sohn DH, Ko M, Chung H, Jeon SH, Seong RH. BAF60a interacts with p53 to recruit the SWI/SNF complex. J Biol Chem. 2008;283:11924–11934. doi: 10.1074/jbc.M705401200. [DOI] [PubMed] [Google Scholar]
- 42.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10:293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat Rev Mol Cell Biol. 2012;13:411–424. doi: 10.1038/nrm3376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.