Abstract
Objective
The aim of this study was to evaluate whether computed tomography (CT) attenuation, as a measure of fat quality, is associated with cardiometabolic risk factors above and beyond fat quantity.
Background
Visceral (VAT) and subcutaneous adipose tissue (SAT) are pathogenic fat depots associated with cardiometabolic risk. Adipose tissue attenuation in CT images is variable, similar to adipose tissue volume. However, whether the quality of abdominal fat attenuation is associated to cardiometabolic risk independent of the quantity is uncertain.
Methods
Participants were drawn from the Framingham Heart Study CT sub-study. VAT and SAT volumes were acquired by semi-quantitative assessment. Fat quality was measured by CT attenuation and recorded as mean Hounsfield Units (HU) within each fat depot. Sex-specific linear and logistic multivariable regression models were used to assess the association between standard deviation (SD) decrease in HU and each risk factor.
Results
Lower CT attenuation of VAT and SAT was correlated with higher BMI levels in both sexes. Risk factors were generally more adverse with decreasing HU values. For example, in women, per 1-SD decrease in VAT HU, the odds ratio (OR) was increased for hypertension (OR 1.80), impaired fasting glucose (OR 2.10), metabolic syndrome (OR 3.65) and insulin resistance (OR 3.36) (all p<0.0001). In models that further adjusted for VAT volume, impaired fasting glucose, metabolic syndrome and insulin resistance remained significant. Trends were similar but less pronounced in SAT and in men. There was evidence of an interaction between HU and fat volume among both women and men.
Conclusion
Lower CT attenuation of VAT and SAT is associated with adverse cardiometabolic risk above and beyond total adipose tissue volume. Qualitative indices of abdominal fat depots may provide insight regarding cardiometabolic risk independent of fat quantity.
Keywords: Obesity, Epidemiology, CT Imaging, Risk Factors
Introduction
Obesity is a heterogeneous condition with individual variability in both fat deposition(1) and associated metabolic complications (2). In addition, different fat depots are associated with differential metabolic risk (1,3,4). Visceral adipose tissue (VAT) in particular is considered to be a unique pathogenic fat depot (1,3). Most fat distribution studies thus far have focused on the absolute volume of adipose tissue in any given depot (1,3,5). However, several lines of experimental evidence suggest that measures of fat quality may also be important. These studies have predominantly focused on cellular characteristics of fat depots including adipocyte size (6–10), macrophage accumulation (8, 11–14), arteriolar dysfunction (12,14), angiogenesis (15), and cellular hypoxia (10) as related to metabolic risk. However, the majority of these fat quality parameters are only directly measurable via biopsy and invasive procedures.
Radiographic imaging can be exploited to provide information about adipose tissue quality in addition to volume. Computed tomography (CT) imaging uses a quantitative scale for describing radiodensity, referred to as Hounsfield units (HU). HU are based on radiographic pixels and are used to differentiate tissue subtypes with negative HU in the range of −195 to −45, the range typically attributed to fat (16). Small, experimental studies in animal models have demonstrated that lower HU is associated with adipose tissue that contains higher levels of lipid content (17), a radiologic finding that has also been clinically assessed in a small pediatric population (18). Fat depots with higher lipid content and lipolytic activity can increase systemic free fatty acids (19), which can induce muscle (20) and hepatic insulin resistance (21) in addition to endothelial dysfunction (22).
Given this framework, we hypothesized that the quality of adipose tissue characterized by HU would be associated with metabolic risk independent of overall adipose tissue volume. More specifically, we hypothesized that lower CT attenuation would be associated with an adverse metabolic profile. If so, these findings may provide a unique, novel framework by which to interpret CT imaging of fat depots and may potentially add to our understanding of the heterogeneity of clinical outcomes associated with obesity.
Methods
Study Participants
Participants for the present study were drawn from the Framingham Heart Study, which began enrollment of the Original cohort in 1948 (23). In 1971, offspring of the Original cohort and their spouses were enrolled in the Offspring cohort (24) and in 2002, children of the Offspring cohort participants were recruited for the Third Generation cohort (25). Data for the present study was drawn from Offspring and Third Generation cohort participants who have previously undergone multidetector computed tomography (MDCT) as a sub-study in the Framingham Heart Study. A total of 3394 participants (1400 from Offspring and 1994 from Third Generation) underwent MDCT scanning from 2002–2005. Participants with missing covariate and outcomes data were excluded, resulting in a final sample size of 3198 individuals in our analysis. The average amount of time that passed between MDCT completion and the visit exam at which the risk factors were assessed was 1.7 years.
Measurement of SAT and VAT
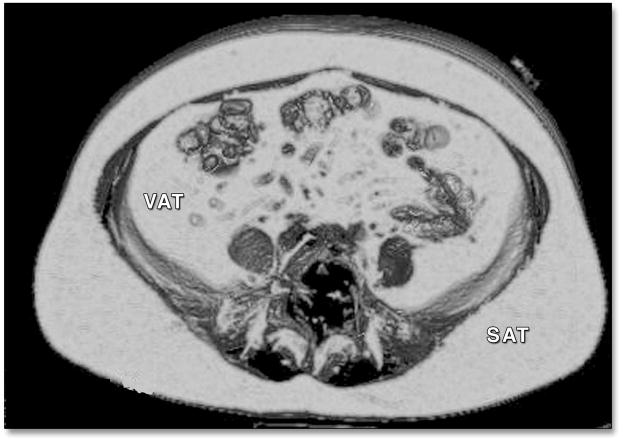
Participants underwent eight-slice MDCT scanning of the abdomen in a supine position. Twenty-five contiguous five mm slices were obtained with an average radiation dose of 3–5 mSv. Subcutaneous and visceral adipose tissue volumes were acquired by manually outlining the abdominal muscular wall separating the visceral from the subcutaneous fat depots. Fat was defined as any voxel between −195 to −45 HU. We recorded the average HU of each fat depot (Figure 1). In previous work by our group, evaluation of this technique has produced high inter-reader and intra-reader correlation (0.997 for SAT, 0.992 for VAT) (16). In order to evaluate the variability within each depot we manually traced 1 cm2 region of interests in 12 anatomically distinct regions within the subcutaneous and visceral adipose tissue in 25 randomly selected participants in our dataset. We evaluated these regions in 3 slices per individual scan. The average standard deviation within SAT was 7.6 HU and within VAT was 5.5 HU suggesting little variability of the Hounsfield unit measurement within each fat depot.
Figure 1. Visceral and Subcutaneous Adipose Tissue on Computed Tomography.
VAT = Visceral Adipose Tissue, SAT = Subcutaneous Adipose Tissue. Computer generated Hounsfield units for VAT was −99.8 and for SAT was −109.4 for this scan.
Metabolic Risk Factors
BMI was calculated by weight (kilograms) divided by the square of height (meters). Weight was assessed using either a Detecto scale in clinic or a SECA portable scale off site. Both scales were calibrated on a routine basis. Waist circumference was completed at the level of the umbilicus and measured to the nearest 0.25 inch. Blood pressure was measured at rest twice using a mercury column sphygmomanometer; hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg or the use of hypertensive medications. Fasting plasma glucose (FPG) was collected; impaired fasting glucose was defined by a FPG level of 100 to 125 mg/dL in participants not treated for diabetes, and diabetes was defined as a FPG ≥ 126 mg/dL or treatment with insulin or an oral hypoglycemic agent. Metabolic syndrome was defined based on the modified ATP III criteria (26). Insulin was measured using radioimmunoassay in the Offspring Cohort and enzyme-linked immunosorbent assay in the Third Generation cohort. For the Offspring cohort, the intra-assay coefficient of variation for insulin was 3.9% and the inter-assay coefficient of variation had a range of 4.7–6.1% (27). For the Third Generation cohort, the intra-assay coefficient of variation for insulin was 2.7% and the inter-assay coefficient of variation was 8.1% (27). Due to the differing methods used to measure insulin in the two cohorts, all values for the Third Generation cohort were standardized to those for the Offspring cohort as previously described (27). Total cholesterol, HDL cholesterol and triglycerides were also measured on the fasting plasma sample. HOMA-IR was calculated based on FPG and insulin levels as previously described (28). Insulin resistance was defined by a HOMA-IR ≥ 75th percentile in those free of diabetes.
Measurement of Covariates
Participants were considered current smokers if they had smoked at least 1 cigarette per day during the previous year. A series of physician-administered questions assessed alcohol use and dichotomized participants on the basis of consumption of > 14 drinks per week (in men) or > 7 drinks per week (in women). Physical activity was assessed based on a questionnaire capturing average daily number of hours of sleep and sedentary, slight, moderate, and heavy activity of the participant. Women were classified as menopausal if they were without menstrual bleeding for ≥ 1 year.
Statistical analysis
Mean visceral HU and subcutaneous HU were approximately normally distributed and untransformed data was used for analysis. Triglycerides were log transformed to improve the normality of the distribution. Age-adjusted, sex specific Pearson correlation coefficients were calculated to assess the correlations between mean HU and continuous cardiometabolic risk factors.
Multivariable-adjusted linear regression models were constructed to assess the association per 1 standard deviation (SD) decrement in visceral and subcutaneous HU and continuous risk factors; multivariable-adjusted logistic regression models were performed for dichotomous outcomes. As previous research has identified sex differences (3) among results, all analyses were stratified by sex. For each outcome, 3 models were run. The first model adjusted for age, current smoking status, alcohol use, physical activity, lipid and blood pressure lowering medications. Menopausal status and hormone replacement therapy were also included as covariates in models among women. The second model included the same covariates from model 1 as well as BMI. In model 3, we adjusted for the covariates in model 1 as well as adipose tissue volume (VAT or SAT, respectively).
Additional analyses investigated interactions between mean HU and VAT or SAT. Further analyses tested interactions between HU and both age and sex. We evaluated the association between cardiometabolic risk factors and tertiles of HU across tertiles of VAT volume.
All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). P-values<0.05 were considered statistically significant for all analyses.
Results
Characteristics of the 3198 study participants are presented in Table 1. The sample was comprised of 47% women. The mean age was 51.9 years among women and 49.6 years among men. The mean SAT HU was −102.3 (SD 5.1) for women and −99.6 (SD 4.5) for men. The mean VAT HU was −92.4 (SD 4.4) for women and −95.2 (SD 4.5) for men. Both SAT HU and VAT HU were statistically different between men and women (p<0.0001).
Table 1.
Study Sample Characteristics. Data presented as mean (SD) for continuous characteristics and mean (SD) or median (Q1, Q3) for categorical characteristics
| Women (n= 1518) | Men (n = 1680) | |
|---|---|---|
| Continuous Characteristics, mean (SD) | ||
| Age (yr) | 51.9 (9.8) | 49.6 (10.6) |
| BMI (kg/m2) | 27.0 (5.8) | 28.4 (4.5) |
| Waist circumference (cm) | 93.0 (15.5) | 100.8(11.8) |
| VAT (cm3) | 1353 (832) | 2226 (1020) |
| SAT (cm3) | 3134 (1510) | 2633 (1207) |
| Visceral HU | −92.4 (4.4) | −95.2 (4.5) |
| Subcutaneous HU | −102.3 (5.1) | −99.6 (4.5) |
| Fasting glucose (mg/dl) | 95.8 (18.1) | 102.1 (23.7) |
| HOMA-IR1 | 2.7 (1.3) | 3.1 (1.5) |
| SBP (mm Hg) | 120.1 (17.6) | 123.3 (14.6) |
| DBP (mm Hg) | 73.6 (9.2) | 78.0 (9.0) |
| HDL cholesterol (mg/dl) | 61.3 (16.9) | 45.9 (12.4) |
| Triglycerides (mg/dl)(median (Q1, Q3)) | 93 (66,139) | 115 (77,174) |
| Categorical Characteristics, n (%) | ||
| Hypertension | 401 (26.4) | 523 (31.1) |
| Diabetes | 82 (5.4) | 121 (7.2) |
| Hypertension treatment | 282 (18.6) | 317 (18.9) |
| Diabetes treatment | 44 (2.9) | 59 (3.5) |
| Lipid treatment | 155 (10.2) | 295 (17.6) |
| Metabolic syndrome | 410 (27.0) | 631 (37.6) |
| Insulin resistance1,2 | 334 (25.4) | 402 (27.4) |
| Current Smoking | 184 (12.1) | 225 (13.4) |
| Alcohol Use2 | 226 (14.9) | 269 (16.0) |
| Postmenopausal | 760 (50.1) | NA |
| Current Hormone Replacement Therapy | 292 (19.2) | NA |
Among non-diabetic participants with available HOMA-IR data
Defined as a HOMA-IR ≥ 75th percentile
Defined as >14 drinks/week for men or >7 drinks/week for women.
Pearson Correlation Coefficients
Visceral and subcutaneous HU were inversely correlated with all cardiometabolic risk factors in both men and women (Table 2). For example, VAT HU was inversely correlated with BMI in women (r=−0.51, p<0.001) and men (r=−0.42, p<0.001); and inversely correlated with VAT volume in women (r=−0.75, p<0.001) and men (r=−0.72, p<0.001). Similar results were observed for SAT HU.
Table 2.
Age-adjusted Pearson correlation coefficients between VAT and SAT HU and cardiometabolic risk, by sex
| VAT HU | SAT HU | |||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| BMI | −0.51*** | −0.42*** | −0.30*** | −0.34*** |
| Waist circumference | −0.53*** | −0.44*** | −0.36*** | −0.42*** |
| SAT | −0.51*** | −0.40*** | −0.49*** | −0.56*** |
| VAT | −0.75*** | −0.72*** | −0.36*** | −0.42*** |
| Log Insulin | −0.42*** | −0.36*** | −0.15*** | −0.20*** |
| Log HOMA-IR | −0.48*** | −0.42*** | −0.23*** | −0.26*** |
| Fasting plasma glucose | −0.22*** | −0.04 | −0.05* | 0.06* |
| SBP | −0.24*** | −0.18*** | −0.15*** | −0.08*** |
| DBP | −0.26*** | −0.28*** | −0.18*** | −0.17*** |
| HDL cholesterol | 0.34*** | 0.36*** | 0.16*** | 0.17*** |
| Log Triglycerides | −0.40*** | −0.38*** | −0.26*** | −0.19*** |
p-value <0.05
p-value <0.01
p-value < 0.001
Quality of VAT
Table 3 presents the association of risk factors per 1 SD decrement in VAT HU. Among both women and men, VAT HU was associated with all risk factors (p<0.001) after multivariable adjustment. After further adjustment for BMI, associations between VAT HU and systolic blood pressure (p<0.0001), diastolic blood pressure (p<0.0001), fasting glucose (p<0.0001), log HOMA-IR (p<0.0001), log triglycerides (p<0.0001) and HDL (p<0.0001) remained significant in both women and men. After adjustment for VAT volume, VAT HU remained associated with diastolic blood pressure (p<0.0001), log HOMA-IR (p<0.001), log triglycerides (p<0.0001) and HDL (p=0.001) in both men and women. For example, among women, 1 standard deviation decrement in VAT HU was associated with a 2.27 mmHg (p<0.0001) higher diastolic blood pressure. This association was attenuated but still remained significant upon adjustment for BMI (1.52 mmHg per 1 SD HU decrement, p<0.0001) and absolute VAT volume (1.39 mmHg per 1 SD HU decrement, p<0.0001).
Table 3.
Multivariable linear regression of Hounsfield units and continuous cardiometabolic risk factors. Data presented as beta coefficients with 95% confidence intervals per 1 SD decrease in HU
| VAT HU | SAT HU | |||||||
|---|---|---|---|---|---|---|---|---|
| Women β (95% CI) | Women P value | Men β (95% CI) | Men P value | Women β (95% CI) | Women P value | Men β (95% CI) | Men P value | |
| SBP (mm Hg) | ||||||||
| Multivariable | 3.46 (2.66,4.26) | <0.0001 | 2.37(1.72,3.02) | <0.0001 | 2.46 (1.67,3.25) | <0.0001 | 1.15 (0.49,1.81) | 0.0007 |
| + BMI | 1.93 (1.03,2.82) | <0.0001 | 1.43 (0.73,2.14) | <0.0001 | 1.44 (0.64,2.25) | 0.0004 | 0.20 (−0.49,0.89) | 0.57 |
| + Fat volume** | 0.91 (−0.26,2.09) | 0.13 | 0.81 (−0.12,1.74) | 0.09 | 0.90 (0.01,1.78) | <0.05 | −0.03 (−0.82,0.76) | 0.94 |
| DBP (mm Hg) | ||||||||
| Multivariable | 2.27 (1.81,2.73) | <0.0001 | 2.47 (2.05,2.88) | <0.0001 | 1.56 (1.11,2.02) | <0.0001 | 1.48 (1.05,1.91) | <0.0001 |
| + BMI | 1.52 (1.01,2.04) | <0.0001 | 1.93 (1.48,2.38) | <0.0001 | 1.02 (0.56,1.49) | <0.0001 | 0.87 (0.42,1.31) | 0.0002 |
| + Fat volume** | 1.39 (0.71,2.07) | <0.0001 | 1.87 (1.27,2.47) | <0.0001 | 0.67 (0.16,1.18) | <0.01 | 0.74 (0.23,1.25) | 0.005 |
| Fasting plasma glucose (mg/dl) | ||||||||
| Multivariable | 3.64 (2.94,4.35) | <0.0001 | 1.51 (0.50,2.51) | 0.003 | 1.81 (1.09,2.53) | <0.0001 | −0.23 (−1.25,0.79) | 0.66 |
| + BMI | 1.94 (1.15,2.74) | <0.0001 | −0.39 (−1.49,0.71) | 0.49 | 0.59 (−0.14,1.31) | 0.11 | −2.04 (−3.11,−0.97) | 0.0002 |
| + Fat volume** | −0.11 (−1.16,0.94) | 0.84 | −2.06 (−3.52,− 0.59) | 0.006 | 0.03 (−0.77,0.84) | 0.94 | −2.25 (−3.48,−1.01) | 0.0004 |
| Log HOMA-IR*** | ||||||||
| Multivariable | 0.19 (0.17,0.21) | <0.0001 | 0.18 (0.16,0.19) | <0.0001 | 0.09 (0.07,0.11) | <0.0001 | 0.11 (0.09,0.13) | <0.0001 |
| + BMI | 0.11 (0.09,0.14) | <0.0001 | 0.10 (0.08,0.12) | <0.0001 | 0.03 (0.01,0.05) | 0.005 | 0.03 (0.01,0.05) | 0.0053 |
| + Fat volume** | 0.06 (0.03,0.09) | <0.001 | 0.04 (0.01,0.06) | 0.01 | 0.00 (−0.02,0,03) | 0.70 | 0.01 (−0.01,0.03) | 0.43 |
| Log triglycerides (mg/dl) | ||||||||
| Multivariable | 0.20 (0.17,0.22) | <0.0001 | 0.22 (0.19,0.24) | <0.0001 | 0.12 (0.10,0.15) | <0.0001 | 0.11 (0.08,0.14) | <0.0001 |
| + BMI | 0.14 (0.11,0.17) | <0.0001 | 0.19 (0.16,0.21) | <0.0001 | 0.08 (0.06,0.10) | <0.0001 | 0.06 (0.04,0.09) | <0.0001 |
| + Fat volume** | 0.07 (0.04,0.10) | <0.0001 | 0.15 (0.12,0.19) | <0.0001 | 0.06 (0.04,0.09) | <0.0001 | 0.08 (0.05,0.11) | <0.0001 |
| HDL cholesterol (mg/dl) | ||||||||
| Multivariable | −5.15 (−5.95,−4.36) | <0.0001 | −4.36 (−4.90,−3.82) | <0.0001 | −2.56 (−3.37,−1.74) | <0.0001 | −2.01 (−2.58,−1.43) | <0.0001 |
| + BMI | −3.50 (−4.40,−2.59) | <0.0001 | −3.59 (−4.17,−3.01) | <0.0001 | −1.27 (−2.08,−0.45) | 0.002 | −0.98 (−1.57,−0.39) | 0.001 |
| + Fat volume** | −1.91 (−3.09,−0.74) | 0.001 | −3.15 (−3.92,−2.38) | <0.0001 | −0.77 (−1.67,0.14) | 0.1 | −1.22 (−1.90,−0.54) | 0.0005 |
P-value for sex interaction for VAT HU: SBP (p=0.002), DBP (p=0.86), FPG (p=0.0006), HOMA-IR (p=0.23), TG (p=0.59), HDL (p=0.03)
P-value for sex interaction for SAT HU: SBP (p=0.04), DBP (p=0.90), FPG (p=0.004), HOMA-IR (p=0.06), TG (p=0.93), HDL (p=0.47)
VAT-HU regressions adjusted for VAT volume; SAT-HU regressions adjusted for SAT volume.
Among non-diabetic participants with available HOMA-IR data.
Blood pressure indices were also adjusted for hypertension treatment. Glycemic measures were also adjusted for diabetes treatment. Lipid indices were also adjusted for hyperlipidemia treatment.
Table 4 presents the odds ratios (OR) for cardiometabolic outcomes per 1 SD decrement in VAT HU. Lower VAT HU was associated with an increased odds of hypertension (p<0.0001), impaired fasting glucose (p<0.0001), metabolic syndrome (p<0.0001) and insulin resistance (p<0.0001) among both women and men. In general, consistent associations were observed between VAT HU and outcomes even after adjustment for BMI or VAT volumes, although the results were somewhat attenuated. Results were generally consistent with our a priori hypothesis of more adverse associations with lower HU values, with the exception of diabetes, where lower VAT HU levels were associated with lower odds of diabetes.
Table 4.
Multivariable logistic regression of Hounsfield units and dichotomous cardiometabolic risk factors. Data presented as odds ratio with 95% confidence intervals per 1 SD decrease in HU
| VAT HU | SAT HU | |||||||
|---|---|---|---|---|---|---|---|---|
| Women OR (95% CI) | Women P value | Men OR (95% CI) | Men P value | Women OR (95% CI) | Women P value | Men OR (95% CI) | Men P value | |
| Hypertension | ||||||||
| Multivariable | 1.80 (1.58,2.06) | <0.0001 | 1.70 (1.50,1.93) | <0.0001 | 1.21 (1.05,1.40) | 0.0083 | 1.32 (1.16,1.50) | <0.0001 |
| + BMI | 1.44 (1.24,1.68) | <0.0001 | 1.46 (1.28,1.67) | <0.0001 | 1.07 (0.91,1.25) | 0.40 | 1.13 (0.99,1.29) | 0.08 |
| + Fat volume** | 1.15 (0.93,1.42) | 0.19 | 1.20 (1.01,1.42) | 0.04 | 0.96 (0.82,1.13) | 0.63 | 1.04 (0.90,1.20) | 0.63 |
| Impaired Fasting glucose | ||||||||
| Multivariable | 2.10 (1.80,2.44) | <0.0001 | 1.58 (1.41,1.76) | <0.0001 | 1.57 (1.30,1.89) | <0.0001 | 1.36 (1.21,1.53) | <0.0001 |
| + BMI | 1.74 (1.47,2.05) | <0.0001 | 1.45 (1.29,1.64) | <0.0001 | 1.45 (1.19,1.76) | 0.0003 | 1.24 (1.10, 1.39) | 0.0006 |
| + Fat volume** | 1.48 (1.18,1.85) | 0.0006 | 1.34 (1.15,1.56) | 0.0002 | 1.20 (0.99,1.47) | 0.07 | 1.17 (1.02,1.34) | 0.02 |
| Diabetes | ||||||||
| Multivariable | 1.42 (1.12,1.81) | 0.004 | 0.93 (0.77,1.13) | 0.47 | 0.76 (0.62,0.93) | 0.007 | 0.79 (0.66,0.93) | 0.005 |
| + BMI | 0.99 (0.75,1.29) | 0.91 | 0.69 (0.55,0.85) | 0.0007 | 0.65 (0.52,0.82) | 0.0002 | 0.62 (0.51,0.76) | <0.0001 |
| + Fat volume** | 0.43 (0.29,0.65) | <0.0001 | 0.46 (0.35,0.60) | <0.0001 | 0.55 (0.43,0.69) | <0.0001 | 0.54 (0.44,0.66) | <0.0001 |
| Metabolic syndrome | ||||||||
| Multivariable | 3.65 (3.08,4.32) | <0.0001 | 2.90 (2.52,3.34) | <0.0001 | 1.68 (1.42,1.98) | <0.0001 | 1.80 (1.57,2.06) | <0.0001 |
| + BMI | 2.51 (2.09,3.01) | <0.0001 | 2.21 (1.89,2.58) | <0.0001 | 1.47 (1.19,1.82) | 0.0003 | 1.33 (1.14,1.55) | 0.0003 |
| + Fat volume** | 1.53 (1.20,1.95) | 0.0006 | 1.45 (1.20,1.74) | 0.0001 | 1.06 (0.88,1.29) | 0.53 | 1.08 (0.93,1.26) | 0.32 |
| Insulin resistance*** | ||||||||
| Multivariable | 3.36 (2.82,3.99) | <0.0001 | 3.02 (2.54,3.60) | <0.0001 | 1.87 (1.54,2.28) | <0.0001 | 1.94 (1.64,2.31) | <0.0001 |
| + BMI | 2.27 (1.88,2.74) | <0.0001 | 2.26 (1.87,2.74) | <0.0001 | 1.51 (1.19,1.91) | 0.0008 | 1.33 (1.10,1.61) | 0.004 |
| + Fat volume** | 1.46 (1.13,1.89) | 0.003 | 1.56 (1.24,1.95) | 0.0001 | 1.15 (0.92,1.44) | 0.21 | 1.12 (0.92,1.35) | 0.26 |
P-value for sex interaction for VAT HU: HTN (p=0.43), IFG (p=0.0009), DM (p=0.01), MetS (p=0.06), IR (p=0.29)
P-value for sex interaction for SAT HU: HTN (p=0.34), IFG (p=0.22), DM (p=0.92), MetS (p=0.11), IR (p=0.44)
VAT-HU regressions adjusted for VAT volume; SAT-HU regressions adjusted for SAT volume.
Among non-diabetic participants with available HOMA-IR data.
Blood pressure indices were also adjusted for hypertension treatment. Glycemic measures were also adjusted for diabetes treatment.
Quality of SAT
Results for SAT HU and continuous and dichotomous risk factors generally followed similar patterns as compared to VAT HU, although the magnitude of association was somewhat weaker (Tables 3 and 4).
We observed paradoxical associations between SAT HU and diabetes: a 1 SD SAT HU decrement was associated with a lower odds ratio for diabetes in both women (OR 0.76) and men (OR 0.79). This association persisted after further adjustment for BMI and SAT volumes (all p<0.001). Thus, we performed secondary analyses in which we excluded all individuals with diabetes treated with insulin (n=12). The associations between SAT HU and both diabetes and fasting glucose were not materially different (data not shown).
Secondary Analysis
Because of the possibility for interaction between VAT and SAT HU with sex, age, and/or absolute fat volume, we conducted interaction testing. There was evidence of a sex interaction with VAT HU for systolic blood pressure (p=0.002), fasting plasma glucose (p= 0.0006), HDL (p=0.03), impaired fasting glucose (p=0.0009) and diabetes mellitus (p=0.01). For SAT, there was evidence of an interaction between sex and SAT HU for systolic blood pressure (p=0.04) and fasting plasma glucose (p=0.004). All observed associations were stronger in women than in men. With regards to age, the interactions between age and VAT HU for systolic blood pressure (p=0.01), diastolic blood pressure (p=0.0002), log triglycerides (p<0.0001), hypertension (p<0.0001), metabolic syndrome (p<0.0001) and log HOMA-IR (p=0.002) were significant. For SAT, the interactions between age and SAT HU were significant for fasting plasma glucose (p=0.02), log triglycerides (p=0.0009), HDL (p=0.03) and hypertension (p=0.005). In general, most associations were stronger in younger individuals.
We also tested for an interaction between VAT HU and VAT volume, and SAT HU and SAT volume. The interaction between VAT HU and VAT volume was statistically significant for log triglycerides, HDL, diabetes, metabolic syndrome and insulin resistance (p<0.05 in women and men, data not shown). Fasting plasma glucose was also significant in men (p= 0.002). Similarly, the interaction between SAT HU and SAT volume was significant for diabetes (p<0.0001), metabolic syndrome (p=0.0001) and insulin resistance (p=0.01) in women, and for fasting plasma glucose (p<0.0001), log HOMA-IR (p=0.01), log triglycerides (p=0.002), HDL (p=0.03) and diabetes (p=0.007) in men. For SAT, the overall trend suggested a stronger association with a lower fat volume in both men and women. Conversely, VAT demonstrated a stronger association with higher fat volumes for women, but not men.
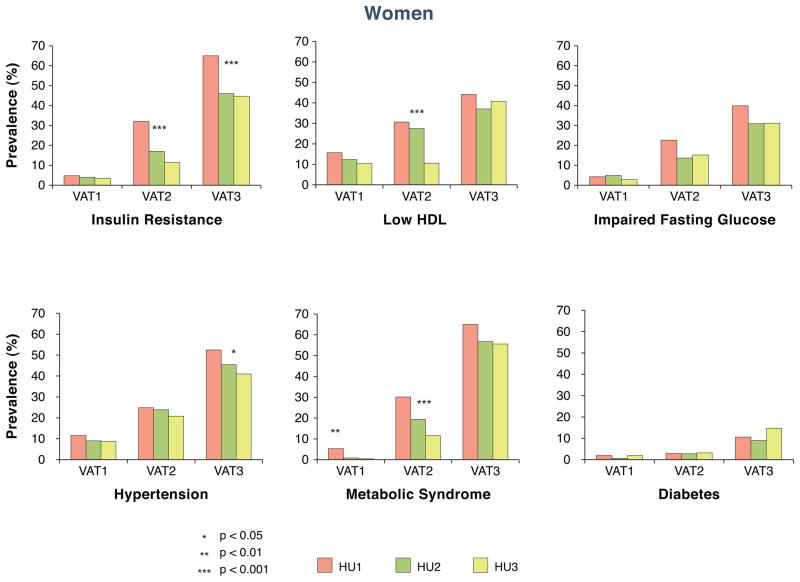
Finally, we examined the associations of VAT and SAT HU within tertiles of absolute levels of VAT or SAT (Figure 2 and Supplemental Figure 1). Among women, each lower tertile of VAT HU was associated with more adverse levels of risk factors, particularly for insulin resistance, low HDL and metabolic syndrome. Similar results were observed in men. Results for SAT HU are shown in Supplemental Figure 1. Whereas similar trends were observed in SAT compared to VAT for impaired fasting glucose, we observed paradoxical associations, with decreasing risk associated with decreased prevalence of insulin resistance, low HDL, metabolic syndrome and diabetes in women. General trends were similar but less pronounced in men.
Figure 2. Associations of HU (fat dens ity) within tertiles of absolute levels of VAT for women (Panel A) and men (Panel B).
In women, the cutpoint between tertile HU 1 and tertile HU 2 was −94.7. The cutpoint between tertile HU 2 and tertile HU 3 was −90.1. In men, the cutpoint between tertile HU 1 and tertile HU 2 was −97.7. The cutpoint between tertile HU 2 and tertile HU 3 was −93.9. VAT1 represented the lowest tertile of VAT volume and VAT3 represented the highest tertile of VAT volume.
Discussion
Our principal findings are three-fold. First, lower CT attenuation as measured in HU is associated with adverse cardiometabolic risk. Second, these findings persisted even after adjustment for generalized adiposity (BMI) and absolute VAT or SAT volume. Finally, we observed interactions between quality and quantity of VAT and SAT for several cardiometabolic risk factors, where individuals with high volumes of VAT and low HU had the most adverse risk profiles. Taken together, these findings suggest that quality of VAT and SAT is associated with cardiometabolic risk above and beyond absolute levels of fat volumes.
Overall, our results were consistent with our a priori hypothesis. Lower CT attenuation was associated with a more adverse metabolic profile with the notable exception of diabetes. Diabetes, but not impaired fasting glucose, demonstrated a paradoxical association in that lower HU was associated with a decreased risk of diabetes. This association was particularly pronounced in association with SAT HU. A secondary analysis showed that this association was unlikely to be driven by a focal disruption of the SAT by insulin use, as the exclusion of these individuals from analysis did not significantly alter this trend. In addition, as shown in Supplemental Figure 1 lower SAT HU was associated with a decreased risk of diabetes within narrow ranges of SAT volume. Taken together, these findings suggest that there may be characteristic differences in fat quality of SAT depots contributing to variable CT attenuation when compared to VAT depots. In addition, these findings appear unique in diabetes per se and are not associated with impaired fasting glucose alone. Recent findings in the area of extracellular matrix remodeling in adipose tissue may provide insights into our results, as differences in collagen fibrosis between VAT and SAT have revealed more abundant levels of fibrosis in SAT (29). In addition, fibrosis in VAT appears to limit adipocyte hypertrophy and is associated with a more favorable lipid profile, whereas SAT fibrosis appears metabolically maladaptive and hampers efforts at weight loss (29). Higher level of fibrosis would be theoretically manifested by less negative HUs and may in part help to explain this paradoxical association with diabetes risk in SAT.
In the context of the current literature
Numerous studies in the current literature have focused on the quantification of fat volumes in regards to cardiometabolic risk (1,3,5). Many of these studies have used CT imaging to determine the quantity of fat as VAT or SAT volumes. The majority of these studies, including work by our group (3), have found that VAT volume in particular is associated with an adverse cardiometabolic risk profile when compared to SAT (1,3,5,30–32).
Few studies have utilized qualitative approaches to study the association between ectopic fat depots and cardiometabolic risk. The notion that fat quality, in addition to quantity, can be an important determinant of metabolic risk have typically been performed using invasive fat biopsy techniques and have thus been limited to small sample sizes and animal models. For example, fat biopsy studies evaluating adipocyte size have shown an inverse correlation between mean adipocyte size and indicators of systemic insulin resistance (7,9). Other studies in human fat tissue have focused on the vasculature and the angiogenic potential of varying fat depots (10,15). In a recent study of subcutaneous and visceral human fat tissue, it was shown that the angiogenic potential of fat tissue decreased with increasing BMI and morbid obesity (15). Finally, there is an extensive body of literature describing cellular mechanisms of inflammation in fat tissue and the role of macrophage accumulation (8,11–14) in worsening cardiometabolic risk. Our study advances the literature in several important ways. First, we present a non-invasive, qualitative approach to the study of fat distribution. Second, we present an association between a qualitative fat measure and metabolic risk in a large, well defined human cohort. Finally, this novel approach to the study of fat distribution is associated with cardiometabolic risk above and beyond quantification of fat volumes alone.
Potential physiological mechanisms
There are several potential mechanisms to explain the associations of lower HU in both VAT and SAT depots with more adverse metabolic risk factor levels. First, lower HU is a marker of more lipid dense fat tissue (17). Furthermore, cellular lipid content helps to determine adipocyte size (33) with large, mature adipoctyes filled almost entirely by large lipid droplets (34). In turn, adipocyte volume is a determinant of the cell’s functionality with larger adipocytes predicting more adverse cardiometabolic risk (7,9). As excess energy accumulates, it is stored in fat depots, which undergo constant remodeling to accommodate for changing fuel stores (35). Several decades ago, fat distribution studies determined that individuals with adipocyte hypertrophy were at risk for adverse cardiometabolic outcomes (6), which has been corroborated with more recent findings that have demonstrated an inverse correlation between mean adipocyte size, insulin sensitivity (7), and adiponectin secretion (9). The reason for this remains uncertain, but adipocyte hypertrophy may be a compensatory mechanism in the overflow of fatty acids (9) and result in the accommodation of fuel surplus. Thus, it is possible that fat tissue with lower attenuation on CT scans represents more lipid-laden fat tissue, which may be a marker of adipocyte hypertrophy.
In addition to lipid content, HU may also reflect tissue vascularity. Highly vascularized tissue appears less negative on computed tomography studies due to the tissue properties of blood (36). This concept has been exploited in the area of brown fat quantification, as brown fat corresponds to less negative Hounsfield units (17,18). It has been hypothesized that the relatively less negative HU that characterizes brown fat is due to the higher vascularity of this tissue bed (18). In addition, several recent studies have highlighted a role of angiogenesis (10,15) and vascular function (12,14) in association with dysfunctional adipose tissue. A recent study of human SAT and VAT demonstrated lower levels of angiogenesis in association with increased adipose tissue volume and increased BMI (15). Studies have also implicated adipocyte hypertrophy in relation to vascularity, demonstrating a decrease in capillary density and angiogenic potential (10) in tissues with increasing adipocyte size. These studies have suggested that ultimately, this decrease in perfusion may lead to hypoxia and inflammation of the adipose tissue (10,15). Taken together, these experimental findings raise the interesting possibility that lower HU may additionally be a marker of adipose tissue vascularity.
Implications for further research
Our findings highlight the importance of applying both qualitative and quantitative methods to the study of body fat distribution, as these complimentary methods identified areas of fat with risk above and beyond that predicted by fat volume alone. It remains uncertain as to the mechanism linking lower HU in both VAT and SAT to more adverse metabolic risk. Thus, future research on this topic, particularly studies that correlate imaging to fat histology and macrophage accumulation, should help to determine the underlying pathophysiology, which may ultimately shed further light on the metabolic sequelae of obesity.
Strengths and Limitations
Strengths include a large, well-defined cohort with rich phenotyping. Some limitations warrant mention. As our sample is predominantly of European ancestry, generalizability to other ethnicities is uncertain. Our study design is observational and cross-sectional, limiting inferences of temporality and causality. In addition, increased BMI has been associated with increased CT photon absorption, which would bias the CT results in individuals with higher BMI towards a higher HU. Thus, our results may actually underestimate the true magnitude of the association between lower HU and cardiometabolic outcomes. There is also the consideration of collinearity because individuals with larger fat volumes have lower CT attenuation. However, after adjustment for fat volume we found that results were attenuated but not abolished, suggesting a residual association of fat attenuation with metabolic risk factors even after accounting for absolute volume. CT reconstruction algorithms incorporate corrections for beam hardening which are based on assumptions on the expected soft tissue composition. As tissue composition varies in individuals, in some cases the measured attenuation could be lower or higher than the actual attenuation. However, if and how much this effected our data remains unknown. The findings of our study, while statistically significant, represent clinically small effect sizes in risk factors and are illustrative of the multifactorial nature of obesity related complications. Finally, the mechanisms underlying the association between HU and metabolic risk remain speculative; further research will be necessary to uncover the underlying fat tissue characteristics captured by the variation in CT attenuation.
Conclusion
Fat quality, as measured by tissue attenuation on computed tomography, is associated with metabolic risk factors above and beyond generalized adiposity and ectopic fat tissue volume. These findings provide a unique, novel framework by which to interpret CT imaging of fat depots and may potentially add to our understanding of the metabolic sequelae of obesity.
Supplementary Material
Associations of HU (fat density) within tertiles of absolute levels of SAT for women (Panel A) and men (Panel B)
Acknowledgments
Funding: This research was conducted in part using data and resources from the FHS of the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health and Boston University School of Medicine. This work was partially supported by the NHLBI’s FHS (Contract No. N01-HC-25195). Dr. Rosenquist is supported through funding from the Whitaker Cardiovascular Institute (T32 HL007224).
Selected Abbreviations
- VAT
visceral adipose tissue
- CT
computed tomography
- HU
Hounsfield unit
- MDCT
multidetector computed tomography
- HOMA IR
homeostatic model assessment insulin resistance
- SAT
subcutaneous adipose tissue
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- FPG
fasting plasma glucose
- SD
standard deviation
- OR
odds ratio
Footnotes
Disclosures: Alison Pedley is an employee of Merck and Co, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. Journal of Clinical Endocrinology & Metabolism. 2011;96:E1756–E1760. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, Massaro JM, Hoffman U, Pou KM, Maurovich-Horvat P, Liu C-Y, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB, O’Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin T, Abbasi F, Lamendola C, Reaven G. Heterogeneity in the prevalence of risk factors for cardiovascular disease and Type 2 diabetes mellitus in obese individuals: effect of differences in insulin sensitivity. Arch Intern Med. 2007;167:642–8. doi: 10.1001/archinte.167.7.642. [DOI] [PubMed] [Google Scholar]
- 5.Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, Nevitt M, Holvoet P, Newman AB. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–83. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 6.Salans LB, Knitel JL, Hirsch J. Role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. Journal of Clinical Investigation. 1968;47:153–65. doi: 10.1172/JCI105705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts Type II diabetes independent of insulin resistance. Diabetologia. 2000;43:1498–506. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- 8.Weisberg S, McCann D, Desai M, Rosenbaum M, Leibel R, Ferrante A. Obesity is associated with machrophage accumulation in adipose tissue. Journal of Clinical Investigation. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anand SS, Tarnopolsky MA, Rashid S, Schulze KM, Desai D, Mente A, Rao S, Yusuf S, Gerstein HC, Sharma AM. Adipocyte hypertrophy, fatty liver and metabolic risk factors in South Asians: the Molecular Study of Health and Risk in Ethnic Groups (mol-SHARE) PLoS ONE. 2011;6:e22112. doi: 10.1371/journal.pone.0022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity. Diabetes. 2009;58:718–25. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu H, Barnes G, Yang Q, Tan G, Yang D, Chou C, Sole J, Nichols A, Ross J, Tartaglia L, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. Journal of Clinical Investigation. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:1654–9. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farb MG, Bigornia S, Mott M, Tanriverdi K, Morin KM, Freedman JE, Joseph L, Hess DT, Apovian CM, Vita JA, Gokce N. Reduced adipose tissue inflammation represents an intermediate cardiometabolic phenotype in obesity. Journal of the American College of Cardiology. 2011;58:232–7. doi: 10.1016/j.jacc.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farb MG, Ganley-Leal L, Mott M, Liang Y, Ercan B, Widlansky ME, Bigornia SJ, Fiscale AJ, Apovian CM, Carmine B, Hess DT, Vita JA, Gokce N. Arteriolar function in visceral adipose tissue is impaired in human obesity. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32:467–73. doi: 10.1161/ATVBAHA.111.235846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran KV, Straubhaar J, Nicoloro S, Czech MP, Thompson M, Perugini RA, Corvera S. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123:186–94. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O’Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. International Journal of Obesity. 2006;31:500–6. doi: 10.1038/sj.ijo.0803454. [DOI] [PubMed] [Google Scholar]
- 17.Baba S, Jacene HA, Engles JM, Honda H, Wahl RL. CT Hounsfield units of brown adipose tissue increase with activation: preclinical and clinical studies. Journal of Nuclear Medicine. 2010;51:246–50. doi: 10.2967/jnumed.109.068775. [DOI] [PubMed] [Google Scholar]
- 18.Hu H, Chung S, Nayak K, Jackson HA, Gilanz V. Differential computed tomographic attenuation of metabolically active and inactive adipose tissues: preliminary findings. Journal of Computer Assisted Tomography. 2011;35:65–71. doi: 10.1097/RCT.0b013e3181fc2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koutsari C, Jensen MD. Thematic review series: patient-oriented research. Free fatty acid metabolism in human obesity. Journal of Lipid Research. 2006;47:1643–50. doi: 10.1194/jlr.R600011-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Kelley D, Mokan J, Simoneau J, Mandarino L. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. Journal of Clinical Investigation. 1993;92:91–8. doi: 10.1172/JCI116603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebrin K, Steil G, Mittelman S, Bergman R. Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. Journal of Clinical Investigation. 1996;98:741–9. doi: 10.1172/JCI118846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinberg H, Tarshboy M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron A. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. Journal of Clinical Investigation. 1997;100:1230–9. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: The Framingham Study. Annals of the New York Academy of Sciences. 1963;107:539–56. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 24.Kannel W, Feinleib M, McNamara P, Garrison R, Castelli W. An investigation of coronary heart disease in families. The Framingham offspring study. American Journal of Epidemiology. 1979;110:281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 25.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D’Agostino RB, Fox CS, Larson MG, Murabito JM, O’Donnell CJ, Vasan RS, Wolf PA, Levy D. The third generation cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. American Journal of Epidemiology. 2007;165:1328–35. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 26.Expert Panel on Detection EaToHBCiA. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA: The Journal of the American Medical Association. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 27.Preis SR, Massaro JM, Robins SJ, Hoffmann U, Vasan RS, Irlbeck T, Meigs JB, Sutherland P, D’Agostino RB, Sr, O’Donnell CJ, Fox CS. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham Heart Study. Obesity. 2010;18:2191–8. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and b-cell function from plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 29.Divoux A, Tordjman J, Lacasa Dl, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo Ml, Poitou C, Zucker JD, Bedossa P, Clement K. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59:2817–25. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Despres JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 1990;10:497–511. doi: 10.1161/01.atv.10.4.497. [DOI] [PubMed] [Google Scholar]
- 31.Ross R, Freeman J, Hudson R, Janssen I. Abdominal obesity, muscle composition, and insulin resistance in premenopausal women. Journal of Clinical Endocrinology & Metabolism. 2002;87:5044–51. doi: 10.1210/jc.2002-020570. [DOI] [PubMed] [Google Scholar]
- 32.Nieves DJ, Cnop M, Retzlaff B, Walden CE, Brunzell JD, Knopp RH, Kahn SE. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes. 2003;52:172–9. doi: 10.2337/diabetes.52.1.172. [DOI] [PubMed] [Google Scholar]
- 33.Wronska A, Kmiec Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol (Oxf) 2012 doi: 10.1111/j.1748-1716.2012.02409.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Ohsaki Y, Cheng J, Suzuki M, Shinohara Y, Fujita A, Fujimoto T. Biogenesis of cytoplasmic lipid droplets: from the lipid ester globule in the membrane to the visible structure. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2009;1791:399–407. doi: 10.1016/j.bbalip.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Lee MJ, Wu Y, Fried SK. Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr Metab Care. 2010;13:371–6. doi: 10.1097/MCO.0b013e32833aabef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furlan A, Fakhran S, Federle MP. Spontaneous abdominal hemorrhage: causes, ct findings, and clinical implications. American Journal of Roentgenology. 2009;193:1077–87. doi: 10.2214/AJR.08.2231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Associations of HU (fat density) within tertiles of absolute levels of SAT for women (Panel A) and men (Panel B)