Abstract
Background
The strength and direction of the associations between inflammation and coagulation biomarkers with kidney disease onset and progression remains unclear, especially in a population-based setting.
Study Design
Prospective observational study.
Setting & Participants
4,966 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) with a cystatin C-based estimate of glomerular filtration rate (eGFRcys) > 60 ml/min/1.73m2 and least one follow-up measure of kidney function. All participants were free of cardiovascular disease (CVD) at entry.
Predictor
We evaluated the associations of C-reactive protein (CRP), interleukin-6 (IL-6), fibrinogen, factor VIII, and D-dimer with kidney function decline.
Outcomes and Measurements
Kidney function decline was assessed primarily by repeated measures of eGFRcys over 5 years. Rapid decline of kidney function was defined as an eGFR decrease of more than 3 ml/min/1.73m2 per year. Incident low eGFR was defined as the onset of eGFRcys<60 ml/min/1.73m2 at any follow up exam and eGFRcys decline ≥1 ml/min/1.73m2 per year.
Results
Mean age was 60 years, 39% were white, 52% were women, and 11% had diabetes. Mean eGFRcys was 96 mL/min/1.73 m2 and 7% had albuminuria. Median follow up time was 4.77 years. Higher Factor VIII levels (per 1-standard deviation [SD] of biomarker) had the strongest association with kidney function decline (β= −0.25; 95% CI, −0.38 to −0.12; p<0.001), followed by IL-6 (β= −0.16; 95% CI, −0.29 to −0.03; p=0.01), CRP (β= −0.09; 95% CI, −0.22 to 0.03; p=0.1), and fibrinogen (β= −0.09; 95% CI, −0.22 to 0.04; p=0.2). Each 1-SD higher concentration of IL-6 (OR, 1.15; 95% CI, 1.07–1.23), Factor VIII (OR, 1.11; 95% CI, 1.03–1.18), and CRP (OR, 1.09; 95% CI, 1.02–1.16) at baseline was significantly associated with rapid kidney function decline. Only IL-6 was significantly associated with incident low eGFR (OR, 1.09; 95% CI, 1.00–1.19).
Limitations
Observational study design and absence of measured GFR.
Conclusions
Inflammation and coagulation biomarkers are associated with declining kidney function in ambulatory adults without established CVD or CKD.
Markers of systemic inflammation, such as C-reactive protein (CRP) and interleukin-6 (IL-6), are consistently and independently associated with increased cardiovascular disease (CVD) risk in the general population1–4. Chronic kidney disease (CKD) also appears to be associated with an inflammatory process, as evidenced by elevated CRP, interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α) concentrations in patients with advanced kidney disease5–8. Disordered inflammation, coagulation, and neutrophil-endothelial interactions may factor considerably in the pathogenesis of kidney injury, which may ultimately contribute to chronically impaired kidney function9–11.
Despite the cross-sectional association of CKD with higher inflammation levels12,13, there is much weaker and conflicting evidence that inflammation and prothrombotic markers are linked longitudinally to kidney function decline. In addition, most previous studies have addressed this research question within cohorts with either underlying CVD or CKD. In the Cardiovascular Health Study (CHS), higher levels of CRP, white blood cell count (WCC), fibrinogen, and Factor VII were associated with increasing creatinine levels14. However, a subsequent study using cystatin C found no association between these biomarkers and longitudinal decline in kidney function in the same cohort15. In subjects with CKD and concomitant coronary disease, Tonelli and colleagues found higher CRP and soluble TNF receptor II concentrations to be associated with loss of kidney function16. In contrast, in the Modification of Diet in Renal Disease (MDRD) Study, CRP had no association with kidney decline in a middle-aged cohort with established CKD17.
The association of inflammation and hemostatic markers with kidney decline has not been thoroughly studied among adults without established CVD or CKD. It is possible that the presence of underlying CVD or CKD confounds the relationship between inflammation and kidney function decline and potentially accounts for the discrepant results from previous studies. Our current study investigated the association of inflammation and coagulation markers with kidney function decline using both creatinine- and cystatin C-based estimates of GFR in the Multi-Ethnic Study of Atherosclerosis (MESA), a large cohort with four racial/ethnic groups without evidence of baseline clinical CVD. We hypothesized that in participants without CKD at baseline, both inflammation and coagulation biomarkers would be associated with faster rates of kidney function decline and incident low eGFR in this setting.
Methods
Study Population
Participants in the MESA cohort consist of 6,814 men and women self-identified as white, African-American, Hispanic, or Chinese. Participants were enrolled from July 2000 to August 2002, recruited from six U.S. communities (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles, CA; New York, NY; and St. Paul, MN), and were 45–84 years of age and free of clinical CVD at the time of enrollment. Details of the MESA study design and recruitment procedures have been described in detail18. Participants have returned for 3 visits, in 2002–2004 (exam 2), 2004–2005 (exam 3) and 2005–2007 (exam 4). Repeat measures of kidney function were conducted at visits 3 and 4. This analysis included persons without CKD and with at least one follow-up measure (n=4,966). All study participants gave informed consent, and the study was approved by the institutional review boards at each center.
Inflammation and coagulation markers
Studied biomarkers included CRP, IL-6, fibrinogen, factor VIII, and D-dimer. All assays were performed on fasting plasma or serum stored at −70° C. CRP was measured using a BNII nephelometer (N high sensitivity CRP; Dade Behring Inc., www.dadebehring.com). The intra-assay coefficients of variation (CVs) were 2.3%–4.4% and the inter-assay CVs were 2.1%–5.7% with a detection level of 0.18 mg/L. IL-6 was measured using ultra-sensitive ELISA (Quantikine HS Human IL-6 Immunoassay; R&D Systems, www.rndsystems.com) with an analytical CV of 6.3% and a detection level of 0.04pg/mL. Fibrinogen was measured using a BNII nephelometer (N Antiserum to Human Fibrinogen; Dade Behring Inc., www.dadebehring.com) with intra-assay analytical CV of 2.7% and inter-assay CV of 2.6%. Factor VIII coagulant activity was determined by measurement of the clotting time of a sample in factor VIII deficient plasma in the presence of activators using the Sta-R analyzer (STA-Deficient VIII; Diagnostica Stago, www.stago-us.com). D-dimer was measured using an immunoturbidimetric assay on a Sta-R analyzer (Liatest D-DI; Diagnostica Stago, Parsippany, NJ). The lower limit of detection was 0.01µg/mL.
Kidney Function Outcomes
All MESA participants with at least the baseline measure and one follow-up measure of creatinine and cystatin C were included in the analysis. Cystatin C was measured using a BNII nephelometer on fasting plasma specimens (N Latex Cystatin C; Dade Behring Inc., www.dadebehring.com) stored at −70° C. Intra-assay CVs range from 2.0–2.8% and inter-assay CVs range from 2.3–3.1%. Later cystatin C measures were calibrated for drift, as previously described19. Serum creatinine was measured using a colorimetric method (Johnson & Johnson Clinical Diagnostics Inc., www.orthoclinical.com) with a CV of 2.2%. We estimated the GFR with the creatinine-based CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation (eGFRCKD-EPI)20 and with the cystatin C-based CKD-EPI equation without demographic coefficients (eGFRcys)21. Participants with eGFRcys<60 ml/min/1.73m2 or eGFRCKD-EPI <60 ml/min/1.73m2 were excluded from the analyses. We truncated at estimates of eGFR>120 ml/min/1.73m2 for each marker. Kidney function decline was assessed by each equation in separate models using repeated measures of eGFR. Rapid progression of kidney function decline was defined as a loss of eGFR of more than 3 ml/min/1.73m2 per year, based on its concurrence with the highest quartile of eGFRcys decline in a large cohort22,23. This cutoff also represents a magnitude of change that is 3 times the rate previously described in studies of normal aging24,25, and has been associated with an increased risk of cardiovascular and all-cause mortality22. Incident low eGFR was defined as the onset of eGFRcys<60 ml/min/1.73m2 at any follow up exam and eGFRcys decline ≥1 ml/min/1.73m2 per year. This composite outcome definition was chosen in order to reduce misclassification due to small eGFR changes close to the low eGFR threshold. Our primary analyses were based on cystatin C, as it appears to provide a better assessment of GFR compared to creatinine, especially in persons with higher GFR levels26,27.
Covariates
Participant characteristics were obtained from data collected at the enrollment visit from physical measures, standardized questionnaire, and laboratory tests. These included demographic information (age, sex, race/ethnicity), medical history, medications, and alcohol and tobacco use. Diabetes was defined as a fasting glucose ≥126mg/dL or by the use of insulin or oral hypoglycemic medications. Resting blood pressure was determined by taking three measurements with the participant in the seated position. Systolic and diastolic blood pressures were recorded as the average value of the last two measurements from both the first and second study examinations. Hypertension was defined as an average systolic blood pressure ≥140 mmHg, an average diastolic blood pressure of ≥90 mm Hg, or by the use of any antihypertensive medications (these included angiotensin converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, beta-blockers, and diuretics). High-density lipoprotein cholesterol (HDL) was measured using the cholesterol oxidase cholesterol method (Roche Diagnostics, www.roche.com). Low-density lipoprotein cholesterol (LDL) was calculated using the Friedewald equation. Urine albumin (in micrograms/mL) to creatinine (milligrams/mL) ratio (ACR) was measured on a single spot sample. We used sex-specific cutoffs to define microalbuminuria (≥17 µg/mg in men and ≥25 µg/mg in women) and macroalbuminuria (≥250 µg/mg in men ≥355 µg/mg in women). Baseline serum albumin was not measured in this cohort.
Statistical Analysis
Baseline characteristics were compared among those with and without incident low eGFR and were evaluated for statistical significance using a t-test or Chi-Square test where appropriate. To evaluate the association between biomarkers and kidney function decline, we used linear mixed models with random intercepts and slopes to estimate and compare linear trends in mean eGFR. To allow comparisons across biomarkers, each was modeled continuously per standard deviation (SD). This approach takes into account the correlation of observations by subject. The beta coefficients for this model represent the rate of eGFR decline in ml/min/1.73m2 per year per SD increase in biomarker. Multivariate logistic regression models were used to evaluate the association between each individual biomarker and rapid kidney function decline. Model 1 adjusted for age, gender, race/ethnicity, hypertension, diabetes mellitus, HDL, LDL, and baseline eGFR. Model 2 adjusted for Model 1 plus albuminuria (ACR). We used Poisson (log-link) regression with robust variance estimation and an offset for follow-up time to study the association of the biomarkers and incident low eGFR. We stratified by baseline eGFRcys (>90 ml/min/1.73m2 and 60–90 ml/min/1.73m2) and adjusted for baseline eGFR due to the strong cross-sectional association of inflammation markers with eGFR. Each biomarker was assessed individually in all of the analyses. All analyses were performed using S-Plus (release 8.0, Insightful Inc., Seattle, WA) and SPSS statistical software (release 16.0.1, SPSS Inc., Chicago, IL).
Results
Demographics/Participant characteristics
Among the 4,966 participants, the mean age was 60 years, 39% were white, 52% were women, and 11% had diabetes. Mean eGFRcys was 96 mL/min/1.73 m2 and 7% had macro- or microalbuminuria. Participants with incident low eGFR during follow-up were older and more likely to have diabetes, hypertension, and a higher body mass index (BMI) compared to those who did not develop incident low eGFR. Those who developed incident low eGFR also had significantly higher baseline levels of CRP, IL-6, fibrinogen, factor VIII, and D-dimer compared to those who did not develop incident low eGFR (Table 1). Among the participants, 4,055 (82%) had cystatin C measures at exams 1, 3 and 4, while the remaining had cystatin C at baseline and only one subsequent visit. For creatinine, 4,520 (91%) had measurements at exams 1, 3, and 4, while the remaining had creatinine measurement at baseline and only one additional visit. Median follow up time was 4.77 (range, 2.24 – 6.65) years.
Table 1.
Baseline characteristics of MESA participants with and without the subsequent development of incident low eGFR*
| Incident low eGFR | P Value | ||
|---|---|---|---|
| No (n=4,763) |
Yes (n=203) |
||
| Age | 60 ± 10 | 68 ± 9 | <0.001 |
| Male | 2383 (48%) | 97 (48%) | 0.9 |
| Race/Ethnicity | 0.1 | ||
| White | 1838 (39%) | 75 (37%) | |
| Chinese | 596 (13%) | 11 (5%) | |
| African-American | 1273 (27%) | 64 (32%) | |
| Hispanic | 1056 (22%) | 53 (26%) | |
| Smoking | 0.006 | ||
| Never | 2414 (52%) | 80 (39%) | |
| Former | 1723 (36%) | 91 (45%) | |
| Current | 615 (13%) | 32 (16%) | |
| Body Mass Index (kg/m2) | 28.2 ± 5.4 | 30.0 ± 5.9 | <0.001 |
| Diabetes | 501 (11%) | 62 (31%) | <0.001 |
| Hypertension | 1844 (39%) | 142 (70%) | <0.001 |
| LDL cholesterol (mg/dL) | 118 ± 31 | 114 ± 34 | 0.1 |
| HDL cholesterol (mg/dL) | 51 ± 15 | 50 ± 15 | 0.2 |
| Triglycerides (mg/dL) | 109 [76, 158] | 121 [92, 183] | 0.2 |
| Glucose (mg/dL) | 96 ± 28 | 109 ± 41 | 0.001 |
| Biomarkers | |||
| C-reactive protein (mg/L) | 1.78 [0.78, 4.04] | 2.35 [1.05, 5.07] | 0.001 |
| Interleukin-6 (pg/mL) | 1.11 [0.72, 1.75] | 1.68 [1.13, 2.51] | <0.001 |
| Fibrinogen (mg/dL) | 340 ± 70 | 365 ± 78 | <0.001 |
| Factor VIII (%) | 158 ± 63 | 189 ± 7 | <0.001 |
| D-Dimer (ug/mL) | 0.20 [0.11, 0.32] | 0.28 [0.15, 0.48] | <0.001 |
| eGFR | |||
| eGFRcys (mL/min/1.73m2) | 97 ± 15 | 77 ± 13 | <0.001 |
| eGFRCKD-EPI (mL/min/1.73m2) | 82 ± 13 | 74 ± 11 | <0.001 |
Incident low eGFR defined as eGFRcys<60 mL/min/1.73m2 and eGFRcys decline ≥ 1ml/min/1.73m2 per year at follow-up.
Categorical variables given as number (percentage); continuous variables, as mean ± standard deviation or median [25th, 75th percentile]. Conversion factors for units: LDL and HDL cholesterol in mg/dL to mmol/L, x0.02586; triglycerides in mg/dL to mmol/L, x0.01129; glucose in mg/dL to mmol/L, x0.05551; fibrinogen in mg/dL to µmol/L, x0.0294; eGFR in mL/min/1.73m2 to mL/s/1.73 m2, x0.01667.
Abbreviations: MESA, Multi-Ethnic Study of Atherosclerosis; LDL, low-density lipoprotein; HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate; eGFRcys, estimated glomerular filtration rate using a serum cystatin C-based equation; eGFRCKD-EPI, estimated glomerular filtration rate using the serum creatinine-based CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation.
Biomarkers and kidney function decline
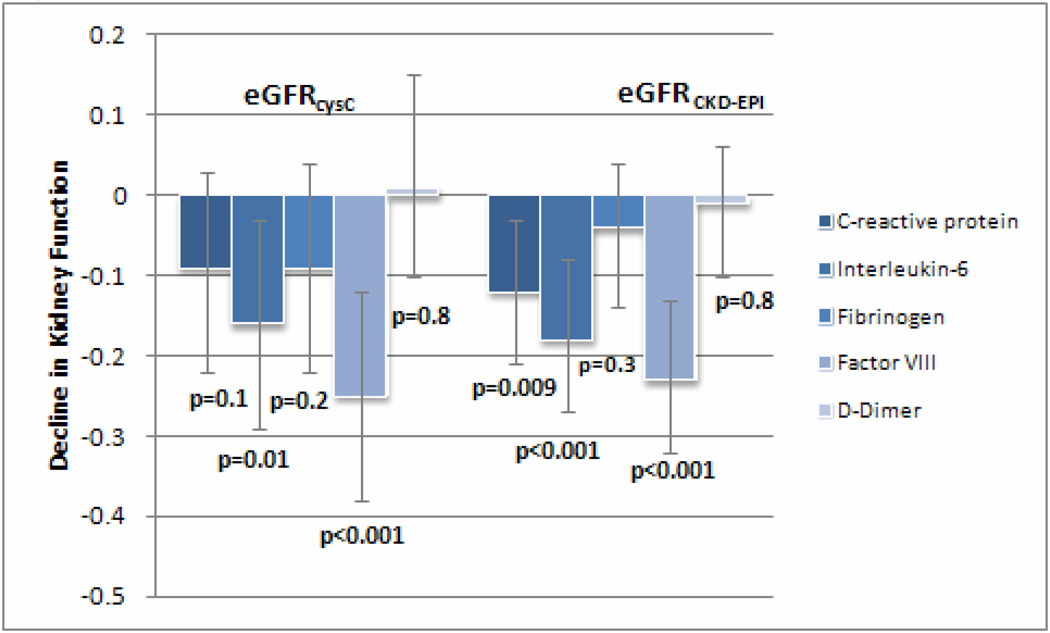
We initially modeled kidney function decline as a linear variable using eGFRcys. In the fully adjusted models, higher baseline Factor VIII levels had the strongest association with subsequent kidney function decline (β= −0.25 per 1-SD higher level of biomarker; 95% CI, −0.38 to −0.12; p<0.001), followed by IL-6 (β= −0.16 per 1-SD higher level of biomarker; 95% CI, −0.29 to −0.03; p=0.01). CRP and fibrinogen had weaker associations that were non-significant (Figure 1). Findings based on eGFRCKD-EPI were similar; higher baseline Factor VIII levels had the strongest association with kidney function decline (β= −0.23; 95% CI, −0.32 to −0.13; p<0.001), followed by IL-6 (β= −0.18; 95%CI, −0.27 to −0.08; p<0.001) and CRP (β= −0.12; 95% CI, −0.21 to −0.03; p=0.009) (Figure 1). D-Dimer levels had no association with decline using either eGFRcys or eGFRCKD-EPI.
Figure 1.
Association of each biomarker with decline in kidney function using adjusted mixed models. Decline in kidney function expressed as β coefficient (95% confidence interval) and adjusted for age, gender, race, hypertension, diabetes, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and albuminuria. The β coefficients for this model represent the rate of eGFR decline in ml/min/1.73m2 per year per 1-SD increase in biomarker.
Abbreviations: eGFRcysC, estimated glomerular filtration rate using a serum cystatin C-based equation; eGFRCKD-EPI, estimated glomerular filtration rate using the serum creatinine-based CKD-EPI equation.
Biomarkers and rapid kidney function decline
The findings from linear models were confirmed in analyses of rapid kidney decline. Factor VIII was significantly associated with rapid decline using both eGFRcys and eGFRCKD-EPI in the fully adjusted logistic regression models (Table 2). Both CRP and IL-6 were also significantly associated with rapid decline, but only when defined by eGFRcys. When the associations of IL-6 and Factor VIII with rapid decline were evaluated in the same model using eGFRcys, each maintained a statistically significant independent association with rapid decline (ORs per 1-SD higher level of each biomarker of 1.12 [95% CI, 1.06–1.20] and 1.07 [95% CI, 1.00–1.14] for IL-6 and Factor VIII, respectively). Because 911/4,966 (18%) participants only had two measures of cystatin C, we performed a sensitivity analysis to evaluate rapid kidney function decline in those with three measures of cystatin C (n=4,055). These results were largely similar to the results for the entire cohort, with the only difference being that Factor VIII was no longer significantly associated with rapid kidney function decline when the analysis was restricted to those with three measures of cystatin C.
Table 2.
Association of each biomarker with rapid decline in eGFR
| Biomarker | 1 SD unit | Unadjusted | Adjusted† | Further adjusted for Albuminuria |
|---|---|---|---|---|
| eGFRcys (n=1,651 with rapid decline) | ||||
| C-reactive protein | 4.94 mg/dL | 1.07 (1.01– 1.13) | 1.10 (1.04– 1.17) | 1.09 (1.02– 1.16) |
| Interleukin-6 | 1.19 pg/mL | 1.09 (1.03– 1.15) | 1.14 (1.08– 1.22) | 1.15 (1.07– 1.23) |
| Fibrinogen | 71.48 mg/dL | 1.04 (0.99– 1.11) | 1.05 (0.99– 1.14) | 1.04 (0.97– 1.12) |
| Factor VIII | 65.37% | 1.10 (1.04– 1.16) | 1.09 (1.03– 1.16) | 1.11 (1.03– 1.18) |
| D-Dimer | 0.69 µg/mL | 1.05 (0.98– 1.13) | 1.03 (0.95– 1.11) | 1.02 (0.94– 1.11) |
| eGFRCKD-EPI (n=1–446 with rapid decline) | ||||
| C-reactive protein | 4.94 mg/dL | 1.07 (1.01– 1.13) | 1.01 (0.95– 1.07) | 0.99 (0.92– 1.06) |
| Interleukin-6 | 1.19 pg/mL | 1.11 (1.05– 1.18) | 1.06 (0.99– 1.13) | 1.04 (0.97– 1.11) |
| Fibrinogen | 71.48 mg/dL | 1.04 (0.98– 1.10) | 0.98 (0.92– 1.05) | 0.95 (0.88– 1.02) |
| Factor VIII | 65.37% | 1.17 (1.10– 1.24) | 1.13 (1.06– 1.20) | 1.12 (1.05– 1.20) |
| D-Dimer | 0.69 µg/mL | 1.02 (0.97– 1.08) | 1.03 (0.97– 1.09) | 1.01 (0.93– 1.10) |
Except where indicated, values shown are OR (95% CI), and are per 1-SD unit higher biomarker level at baseline, with the value of a 1-SD unit for each biomarker as indicated.
eGFRcys models were adjusted for age, gender, race, hypertension, diabetes, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and baseline eGFRcys. eGFRCKD-EPI models were adjusted for age, gender, race, hypertension, diabetes, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and baseline eGFRCKD-EPI.
Abbreviations: eGFRcys, estimated glomerular filtration rate using a serum cystatin C-based equation; eGFRCKD-EPI, estimated glomerular filtration rate using the serum creatinine-based CKD-EPI EPI (Chronic Kidney Disease Epidemiology Collaboration) equation; eGFR, estimated glomerular filtration rate;
Biomarkers and incident low eGFR
The associations between baseline biomarker levels and incident low eGFR wereevaluated again in an analysis stratified by baseline eGFRcys. There were no associations between any of the biomarkers and incident low eGFR in those participants with a baseline eGFRcys ≥90, but only 30 events occurred within this group. In those with baseline eGFRcys between 60–90, both IL-6 and Factor VIII were associated with a 21% higher likelihood (per 1-SD higher level of biomarker) of incident low eGFR, and CRP was associated with an 11% higher likelihood of incident low eGFR per 1-SD higher level of the biomarker (Table 3). These results were attenuated after adjustment for baseline eGFR and albuminuria, with only IL-6 being significantly associated with incident low eGFR (9% higher likelihood of incident low eGFR per 1-SD higher level of the biomarker). Further adjustment for BMI in all of the above analyses (kidney decline, rapid decline, and incident low eGFR) did not affect the results.
Table 3.
Association of each biomarker with incident low eGFR, stratified by baseline eGFRcys
| 1 SD unit | Adjusted** Risk Ratio (95% CI) | Adjusted† Risk Ratio (95% CI) | |||
|---|---|---|---|---|---|
| eGFRcys 60–90 | eGFRcys ≥90 | eGFRcys 60–90 | eGFRcys ≥90 | ||
| No. of participants*** |
-- | 173/1,868 | 30/3,098 | 173/1,868 | 30/3,098 |
| Biomarker | |||||
| C-Reactive Protein |
4.94 mg/dL | 1.11 (1.00, 1.25) | 0.87 (0.60, 1.27) | 1.06 (0.94, 1.18) | 0.89 (0.60, 1.31) |
| Interleukin-6 | 1.19 pg/mL | 1.21 (1.11, 1.32) | 0.93 (0.57, 1.50) | 1.09 (1.00, 1.19) | 0.89 (0.52, 1.53) |
| Fibrinogen | 71.48 mg/dL | 1.05 (0.91, 1.22) | 0.94 (0.62, 1.44) | 0.89 (0.78, 1.03) | 0.84 (0.53, 1.32) |
| Factor VIII | 65.37% | 1.21 (1.07, 1.37) | 0.91 (0.63, 1.32) | 1.08 (0.95, 1.22) | 0.85 (0.60, 1.19) |
| D-Dimer | 0.69 µg/mL | 1.00 (0.93, 1.07) | 1.15 (0.67, 1.96) | 0.99 (0.92, 1.06) | 1.09 (0.64, 1.84) |
eGFRcys given in mL/min/1.73 m2; Incident low eGFR defined as eGFRcys<60 mL/min/1.73m2 and eGFRcys decline ≥ 1ml/min/1.73m2 per year at follow-up.
Risk Ratios are per 1-SD unit higher level of biomarker at baseline, with the value of a 1-SD unit for each biomarker as indicated.
Adjusted for age, gender, race, hypertension, diabetes, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol.
indicates no. of participants with low incident eGFR as a fraction of number of participants with baseline in eGFRcys the indicated range.
Adjusted further for baseline eGFRcys and albuminuria.
Abbreviations: eGFRcys, estimated glomerular filtration rate using a serum cystatin C-based equation; eGFR, estimated glomerular filtration rate; CI, confidence interval
Discussion
Although systemic inflammation is well established as a risk factor for CVD, the association between inflammation and kidney function decline has been less clear, possibly because of the inclusion of individuals with concomitant CVD in many studies. Our analysis demonstrated that an inflammation marker (IL-6) and a coagulation marker (Factor VIII) were both independently associated with decline in kidney function, even after adjustment for age, gender, race/ethnicity, and medical co-morbidities known to be associated with CKD. Our findings were consistent when decline in kidney function was defined using either cystatin C- or creatinine-based estimates of GFR. Although the magnitude of change in kidney function was small, it is important to note that both IL-6 and Factor VIII were also independently associated with rapid decline in kidney function. Few studies have evaluated inflammation and coagulation pathways as risk factors for declining kidney function in populations free of clinical CVD or CKD. One study of a middle-aged, population-based cohort, The ARIC (Atherosclerosis Risk in Communities) Study, reported that higher quartiles of WCC, fibrinogen, von Willebrand Factor, and factor VIII were associated with an increased risk of incident low eGFR28. Another population-based cohort study of predominantly white participants found higher TNF-α receptor 2, WCC, and IL-6 levels to be positively associated with incident low eGFR29. Our findings are consistent with these prior studies, and further expand on these by examining both longitudinal kidney function decline and rapid decline, in addition to incident low eGFR in a multi-ethnic cohort.
Most previous studies have examined the role of inflammation and coagulation biomarkers in cohorts comprised partly or completely of persons with CVD or CKD, with varied results. In the CHS, inflammation and coagulation biomarkers were associated with kidney function decline when evaluated using creatinine-based estimates of GFR, but not cystatin C; only low albumin levels were associated with decline using cystatin C14,15. One study in persons with CVD demonstrated an association of CRP with kidney function decline16; an analysis from a cohort with moderate-to-advanced CKD found no association of CRP with kidney decline17. It is possible that individuals with established CVD or CKD already have higher baseline levels of these biomarkers, making it more difficult to detect an association with kidney disease progression. Alternatively, these pathways may be less important for kidney function decline once the disease has been initiated.
Our findings build on data from these previous studies. In a cohort free of underlying CVD or CKD, we found strong associations with both an inflammation and a coagulation biomarker. Although a number of pro-inflammatory cytokines play key roles in the inflammatory response, IL-6 may be the central regulator of the inflammatory process30. The IL-6 pathway upregulates inflammation via the activation and proliferation of lymphocytes, differentiation of B cells, and the stimulation of the acute-phase response in the liver31. Barreto et al demonstrated rising IL-6 levels as CKD progressed, and an inverse linear relationship between IL-6 levels and eGFR32. IL-6 was the only biomarker associated with incident low eGFR in our study, and only in those with eGFRcys between 60–90 mL/min/1.73 m2. The lack of associations with other biomarkers and incident low eGFR in our cohort may have been due to lack of adequate power.
In healthy cohorts, levels of IL-6 tend to be quite low (< 2 pg/mL)33,34. This was also seen in our cohort, where those without incident low eGFR had a median IL-6 level of 1.11 pg/mL. Even though the IL-6 values in our cohort do not reach levels that are typically associated with acute illness, we did observe a positive relationship between higher IL-6 levels and kidney function decline. This suggests that there is a dose-dependent association even in this lower range of values. In a previous study of 340 apparently healthy women enrolled in the Women’s Health Study, a positive relationship between IL-6 levels and clinical risk factors (hypertension, diabetes, smoking, and sedentary status) was also found in this lower range of IL-6 values, suggesting biological meaning for these low levels33.
We also found elevated baseline levels of Factor VIII to be associated with longitudinal decline in kidney function and rapid kidney function decline. Factor VIII is synthesized by the vascular, glomerular, and tubular endothelium, as well as the sinusoidal cells of the liver. Elevated factor VIII levels could be a marker of endothelial cell dysfunction and vascular damage, and increased factor VIII levels may be a risk factor for venous thromboembolism35–37. Interestingly, transcription of the factor VIII gene has been shown to be promoted by IL-638. In liver cell lines stimulated with IL-6, levels of factor VIII messenger RNA increased six-to nine-fold above baseline values, but not after stimulation with other cytokines (IL-1 and IL-2)38. There may be a complex interplay between the inflammation and coagulation response, and specifically between IL-6 and Factor VIII.
The exact mechanisms by which inflammation could lead to kidney function decline are not entirely clear, but cytokines could act on the endothelium and mesangium of the glomerus, leading to chronic renal injury. Oxidative stress (Nrf2 [nuclear factor erythroid 2-related factor 2] pathway) can also lead to dysfunction of the endoplasmic reticulum, creating an imbalance between protein-folding capacity and protein-folding load39. Bardoxolone methyl activates the Keap1 [kelch-like ECH-associated protein 1]-Nrf2 pathway, which plays an important role in maintaining kidney function and structure by regulating inflammation and oxidative stress40–43. In a recent study, treatment with bardoxolone methyl for 52 weeks led to sustained, significant improvements in the eGFR in patients with advanced CKD and type 2 diabetes44. Bardoxolone methyl and other similar agents may be attractive therapies that warrant further study in patients with CKD.
The findings of this study may have public health relevance. CKD continues to grow in prevalence in the U.S. beyond what could be attributed to population aging45. Although there are shared risk factors between CKD and CVD, there are far fewer strong risk factors for kidney disease than for CVD, and the only modifiable ones appear to be the prevention and treatment of diabetes and hypertension. Public health initiatives are needed for CKD prevention, given that there are limited interventions that prevent CKD progression to ESRD, CVD, or death. Our findings suggest that inflammation/coagulation pathways may contribute to CKD onset. Future studies are required to determine whether therapies that inhibit inflammation or coagulation may ultimately prove useful in decreasing CKD incidence.
Our study has several limitations. Most importantly, this is an observational study, and we cannot demonstrate causality. There is also the possibility of residual confounders. As with all other population based studies, we did not have a gold standard for kidney function available, such as iothalamate or iohexol clearance. We also recognize that estimating equations using either cystatin C or creatinine have limitations, especially in the general population, and because of this, we provided analyses for both cystatin C- and creatinine-based estimating equations. In our analysis for rapid kidney function decline, 18% of the participants had only two measures of cystatin C. Finally, our data were obtained from a middle-aged to older population of multi-ethnic subjects; the results from this group may not be generalizable to a younger population or to those living outside the U.S.
In summary, we found inflammation and coagulation markers to be associated with kidney function decline in community-living individuals without CVD. Further studies are warranted to evaluate whether interventions that reduce the inflammatory/prothrombotic state could decrease the incidence and progression of CKD.
Acknowledgements
Support: This publication was supported by NIH/NCRR/OD UCSF-CTSI Grant Number KL2 RR024130 (Dr. Hiramoto), NIH/NIDDK/R01 DK066488-01 (Dr. Shlipak), and AHA Established Investigator Award 0640012N (Dr. Shlipak). Its contents are the responsibility of the authors and do not necessarily represent the official views of the NIH or AHA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000 Mar 23;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000 Apr 18;101(15):1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 3.Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004 Dec 16;351(25):2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006 Dec 21;355(25):2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 5.Herbelin A, Nguyen AT, Zingraff J, Urena P, Descamps-Latscha B. Influence of uremia and hemodialysis on circulating interleukin-1 and tumor necrosis factor alpha. Kidney Int. 1990 Jan;37(1):116–125. doi: 10.1038/ki.1990.16. [DOI] [PubMed] [Google Scholar]
- 6.Herbelin A, Urena P, Nguyen AT, Zingraff J, Descamps-Latscha B. Elevated circulating levels of interleukin-6 in patients with chronic renal failure. Kidney Int. 1991 May;39(5):954–960. doi: 10.1038/ki.1991.120. [DOI] [PubMed] [Google Scholar]
- 7.Landray MJ, Wheeler DC, Lip GY, et al. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the chronic renal impairment in Birmingham (CRIB) study. Am J Kidney Dis. 2004 Feb;43(2):244–253. doi: 10.1053/j.ajkd.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 8.Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003 Jan 7;107(1):87–92. doi: 10.1161/01.cir.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 9.Liu KD, Glidden DV, Eisner MD, et al. Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med. 2007 Dec;35(12):2755–2761. [PMC free article] [PubMed] [Google Scholar]
- 10.Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol. 2009 Jan;130(1):41–50. doi: 10.1016/j.clim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109(4):e102–e107. doi: 10.1159/000142934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuveling EM, Hillege HL, Bakker SJ, Gans RO, De Jong PE, De Zeeuw D. C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int. 2003 Feb;63(2):654–661. doi: 10.1046/j.1523-1755.2003.00762.x. [DOI] [PubMed] [Google Scholar]
- 13.Pecoits-Filho R, Heimburger O, Barany P, et al. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003 Jun;41(6):1212–1218. doi: 10.1016/s0272-6386(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 14.Fried L, Solomon C, Shlipak M, et al. Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J Am Soc Nephrol. 2004 Dec;15(12):3184–3191. doi: 10.1097/01.ASN.0000146422.45434.35. [DOI] [PubMed] [Google Scholar]
- 15.Keller C, Katz R, Sarnak MJ, et al. Inflammatory biomarkers and decline in kidney function in the elderly: the Cardiovascular Health Study. Nephrol Dial Transplant. 2010 Jan;25(1):119–124. doi: 10.1093/ndt/gfp429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005 Jul;68(1):237–245. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 17.Menon V, Shlipak MG, Wang X, et al. Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Intern Med. 2007 Jul 3;147(1):19–27. doi: 10.7326/0003-4819-147-1-200707030-00004. [DOI] [PubMed] [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002 Nov 1;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 19.Peralta CA, Katz R, Deboer I, et al. Racial and Ethnic Differences in Kidney Function Decline among Persons without Chronic Kidney Disease. J Am Soc Nephrol. 2011 Jul;22(7):1327–1334. doi: 10.1681/ASN.2010090960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008 Mar;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rifkin DE, Shlipak MG, Katz R, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008 Nov 10;168(20):2212–2218. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shlipak MG, Katz R, Kestenbaum B, et al. Rate of kidney function decline in older adults: a comparison using creatinine and cystatin C. Am J Nephrol. 2009;30(3):171–178. doi: 10.1159/000212381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006 Jan;69(2):375–382. doi: 10.1038/sj.ki.5000058. [DOI] [PubMed] [Google Scholar]
- 25.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. Journal of the American Geriatrics Society. 1985 Apr;33(4):278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 26.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002 Aug;40(2):221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 27.Perkins BA, Nelson RG, Ostrander BE, et al. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol. 2005 May;16(5):1404–1412. doi: 10.1681/ASN.2004100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bash LD, Erlinger TP, Coresh J, Marsh-Manzi J, Folsom AR, Astor BC. Inflammation, hemostasis, and the risk of kidney function decline in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2009 Apr;53(4):596–605. doi: 10.1053/j.ajkd.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shankar A, Sun L, Klein BE, et al. Markers of inflammation predict the long-term risk of developing chronic kidney disease: a population-based cohort study. Kidney Int. 2011 Dec;80(11):1231–1238. doi: 10.1038/ki.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001 Jan;15(1):43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- 31.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998 Jan 15;128(2):127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 32.Barreto DV, Barreto FC, Liabeuf S, et al. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 2010 Mar;77(6):550–556. doi: 10.1038/ki.2009.503. [DOI] [PubMed] [Google Scholar]
- 33.Bermudez EA, Rifai N, Buring J, Manson JE, Ridker PM. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arteriosclerosis, thrombosis, and vascular biology. 2002 Oct 1;22(10):1668–1673. doi: 10.1161/01.atv.0000029781.31325.66. [DOI] [PubMed] [Google Scholar]
- 34.Czarkowska-Paczek B, Bartlomiejczyk I, Gabrys T, Przybylski J, Nowak M, Paczek L. Lack of relationship between interleukin-6 and CRP levels in healthy male athletes. Immunology letters. 2005 Jun 15;99(1):136–140. doi: 10.1016/j.imlet.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Cristina L, Benilde C, Michela C, Mirella F, Giuliana G, Gualtiero P. High plasma levels of factor VIII and risk of recurrence of venous thromboembolism. Br J Haematol. 2004 Feb;124(4):504–510. doi: 10.1046/j.1365-2141.2003.04795.x. [DOI] [PubMed] [Google Scholar]
- 36.Oger E, Lacut K, Van Dreden P, et al. High plasma concentration of factor VIII coagulant is also a risk factor for venous thromboembolism in the elderly. Haematologica. 2003 Apr;88(4):465–469. [PubMed] [Google Scholar]
- 37.Kyrle PA, Minar E, Hirschl M, et al. High plasma levels of factor VIII and the risk of recurrent venous thromboembolism. N Engl J Med. 2000 Aug 17;343(7):457–462. doi: 10.1056/NEJM200008173430702. [DOI] [PubMed] [Google Scholar]
- 38.Stirling D, Hannant WA, Ludlam CA. Transcriptional activation of the factor VIII gene in liver cell lines by interleukin-6. Thromb Haemost. 1998 Jan;79(1):74–78. [PubMed] [Google Scholar]
- 39.Inagi R. Endoplasmic reticulum stress as a progression factor for kidney injury. Current opinion in pharmacology. 2010 Apr;10(2):156–165. doi: 10.1016/j.coph.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Yoh K, Itoh K, Enomoto A, et al. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001 Oct;60(4):1343–1353. doi: 10.1046/j.1523-1755.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- 41.Ma Q, Battelli L, Hubbs AF. Multiorgan autoimmune inflammation, enhanced lymphoproliferation, and impaired homeostasis of reactive oxygen species in mice lacking the antioxidant-activated transcription factor Nrf2. The American journal of pathology. 2006 Jun;168(6):1960–1974. doi: 10.2353/ajpath.2006.051113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dinkova-Kostova AT, Liby KT, Stephenson KK, et al. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci U S A. 2005 Mar 22;102(12):4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yates MS, Tauchi M, Katsuoka F, et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Molecular cancer therapeutics. 2007 Jan;6(1):154–162. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]
- 44.Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011 Jul 28;365(4):327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 45.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007 Nov 7;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]