Background: cAMP response element-binding protein (CREB) is a transcriptional regulator that undergoes complex phosphoregulation in response many physiologic stimuli.
Results: Ser-270/Ser-271 are identified as mitotically regulated phosphorylation sites that diminish CREB DNA binding activity.
Conclusion: Carboxyl-terminal phosphorylation of CREB promotes its chromatin eviction during mitosis.
Significance: CDK1-mediated chromatin eviction may serve as a global mechanism to mediate transcriptional inhibition observed during mitosis.
Keywords: CDK (Cyclin-dependent Kinase), Chromatin, CREB, Mitosis, Phosphorylation, Transcription Factors, Homeodomain-interacting Protein Kinase 2
Abstract
The cyclic AMP response element-binding protein (CREB) initiates transcriptional responses to a wide variety of stimuli. CREB activation involves its phosphorylation on Ser-133, which promotes interaction between the CREB kinase-inducible domain (KID) and the KID-interacting domain of the transcriptional coactivator, CREB-binding protein (CBP). The KID also contains a highly conserved phosphorylation cluster, termed the ATM/CK cluster, which is processively phosphorylated in response to DNA damage by the coordinated actions of ataxia-telangiectasia-mutated (ATM) and casein kinases (CKs) 1 and 2. The ATM/CK cluster phosphorylation attenuates CBP binding and CREB transcriptional activity. Paradoxically, it was recently reported that DNA damage activates CREB through homeodomain-interacting protein kinase 2-dependent phosphorylation of Ser-271 near the CREB bZIP DNA binding domain. In this study we sought to further clarify DNA damage-dependent CREB phosphorylation as well as to explore the possibility that the ATM/CK cluster and Ser-271 synergistically or antagonistically modulate CREB activity. We show that, rather than being induced by DNA damage, Ser-270 and Ser-271 of CREB cophosphorylated in a CDK1-dependent manner during G2/M phase. Functionally, we show that phosphorylation of CREB on Ser-270/Ser-271 during mitosis correlated with reduced CREB chromatin occupancy. Furthermore, CDK1-dependent phosphorylation of CREB in vitro inhibited its DNA binding activity. The combined results suggest that CDK1-dependent phosphorylation of CREB on Ser-270/Ser-271 facilitates its dissociation from chromatin during mitosis by reducing its intrinsic DNA binding potential.
Introduction
The cyclic AMP response element-binding protein (CREB)2 is a ubiquitously expressed and highly conserved basic leucine zipper (bZIP) family transcription factor involved in a diverse array of cellular processes. Members of this family include activating transcription factor 1 (ATF1) and cAMP response element modulator (CREM), which share high homology in their kinase-inducible transactivation (KID) and bZIP DNA-binding/dimerization domains. CREB function has been shown to be important in cell growth, neuronal development and function, hematopoiesis, and metabolism (1–3). CREB facilitates the transcription of thousands genes harboring palindromic (TGACGTCA) or half-site cyclic AMP response elements (CREs) (4, 5). The canonical CREB activation pathway involves its phosphorylation on Ser-133 by cAMP-activated protein kinase (PKA) (3). Ser-133 phosphorylation stimulates recruitment of CREB-binding protein (CBP) and transactivation of CREB target genes through CBP histone acetyltransferase-dependent mechanisms (6–8). Many other stimuli trigger activating Ser-133 phosphorylation. For example, calcium (Ca2+) induces Ser-133 phosphorylation through Ca2+ and calmodulin-dependent kinases, and this is critical for the participation of CREB in learning and memory (2, 9–12).
Although it was initially believed that CREB binds to its cognate promoters constitutively, it is now clear that CREB chromatin occupancy is regulated by a family of cAMP/Ca2+-regulated transcriptional coactivators (CRTCs). CRTCs are maintained as latent cytoplasmic factors that rapidly translocate to the nucleus in response to cAMP and Ca2+ (3). Nuclear CRTCs bind to the CREB bZIP domain, increasing its chromatin occupancy over CREB target genes in vivo and enhancing its DNA binding affinity in vitro (13–16). The model that emerged from these studies is that many CREB target genes require both phosphoserine 133-dependent and CRTC-dependent signals for full transcriptional activation (3, 17). These findings raise the possibility that other signals modulate CREB DNA binding activity through CRTC-dependent or CRTC-independent mechanisms.
CREB is also subjected to inhibitory regulation, principally through phosphorylation. Ca2+/calmodulin-dependent protein kinase II-mediated phosphorylation of Ser-142 within the KID diminished CBP/p300 binding, and mutation of Ser-142 led to circadian rhythm defects in vivo (7, 8, 18–23). Work from our laboratory has focused on a cluster of conserved phosphorylation sites (termed the ATM/CK cluster) that are coordinately phosphorylated by the apical DNA damage signaling kinase ataxia-telangiectasia-mutated (ATM), casein kinase 1 (CK1), and casein kinase 2 (CK2) in response to genotoxic stress. DNA damage triggers ATM-dependent phosphorylation of Ser-111, which primes the phosphorylation of Ser-108 by CK2 and Ser-114, and Ser-117 by CK1. The phosphorylation of all four upstream sites is required for additional ATM-dependent phosphorylation on Ser-121 (24–26). The end result of ATM/CK cluster phosphorylation is a 3–5-fold reduction in CBP binding activity. Mechanistically it is unclear how ATM/CK cluster phosphorylation modulates CBP binding given that these sites do not make direct contact with the KID-interacting domain of CBP domain (27, 28); however, we envision that the density of negative charge alters the KID conformation leading to an effective reduction in its binding to the KID-interacting domain. Thus, the ATM/CK cluster may function as a biochemical attenuator of CREB function.
The ATM/CK cluster is conserved in the closely related CREB paralog ATF1, which is phosphorylated by ATM on Ser-51 (analogous to the Ser-121 in CREB) in response to DNA damage (29). In addition, Drosophila CREB harbors a positionally conserved ATM/CK cluster that is phosphorylated by CK1 in vitro (30), although the DNA damage-dependent regulation of these sites has not been tested. Despite this conservation, the biological consequences of DNA damage-dependent CREB phosphoregulation remain to be defined. Finally, DNA damage-dependent CREB phosphorylation has also been observed outside of the KID. Homeodomain-interacting protein kinase 2 (HIPK2) was reported to phosphorylate CREB on Ser-271 and to stimulate its transcriptional activity in response to the topoisomerase poison, etoposide (31). HIPK2 was previously implicated as a p53 kinase required for p53-induced apoptosis in response to UV light (32, 33). The possibility that HIPK2 activates CREB in response to genotoxic stress is intriguing; however, these findings must be reconciled with inhibitory phosphorylation of the ATM/CK cluster.
In this study we provide evidence that Ser-271 is not a DNA damage-inducible site. Rather, Ser-271 is cophosphorylated with Ser-270 are instead phosphorylated by Cyclin-dependent kinase 1 (CDK1) as cells progress through mitosis. Phosphomimetic mutations at Ser-270/Ser-271 diminished CREB chromatin-binding potential, suggesting that the phosphorylation of these residues facilitates CREB chromatin eviction during mitosis. These findings suggest that CDK1 promotes chromatin eviction of CREB transcriptional complexes during mitosis.
EXPERIMENTAL PROCEDURES
Cell Culture and Drug Treatment
HeLa and HEK 293T cells were obtained from American Type Culture Collection and maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum and 1% penicillin-streptomycin. Nocodazole (Sigma 1404) was used at 100 ng/ml, etoposide (Sigma E1384) at 50 μm, colcemid (generous gift from Dr. Beth Weaver) at 100 ng/ml, dibutyryl cAMP (Sigma D0260) at 20 μm, forskolin (Sigma F6886) at 10 μm, and IBMX (Sigma I5879) at 100 μm. γ-Irradiation treatments were carried out with JL Shepherd model JL-109 irradiator with a 137Cs source at 20 gray. Kinase inhibitors were added 1 h prior to nocodazole treatment at 100 nm for BI 2536 (SelleckChem S1109) and 25 nm for CGP74514A (EMD Millipore 217696). To synchronize cells in mitosis, HeLa were grown in 100 ng/ml nocodazole for 16 h. Following incubation, cells arrested in early mitosis were detached by mechanical shake-off and replated in fresh complete medium without nocodazole.
Plasmids and siRNA
The expression construct pcDNA3.1-Zeo encoding the 341-amino acid CREB protein with an amino-terminal FLAG tag was previously generated by our laboratory (25). This construct was used as the template to generate the FLAG-CREBS270A/S271A and FLAG-CREBS270D/S271D constructs through the QuikChange site-directed mutagenesis method (Stratagene) with the following oligonucleotide primer pairs: CREBS270A/S271A (5′-GTT ATG GCA GCC GCC CCA GCA CTT CC-3′ (forward) and (5′-GGA AGT GCT GGG GCG GCT GCC ATA AC-3′ (reverse); CREBS270D/S271D (5′-GTT ATG GCA GAC GAC CCA GCA CTT C-3′ (forward) and (5′-GAA GTG CTG CGT CGT CTG CCA TAA C-3′ (reverse). All oligonucleotide primers were purchased from Integrated DNA Technologies (Coralville, IA). Smart pool siRNA against GFP (P-002048-01-20) and HIPK2 (M-003266-03-0005) were purchased from Dharmacon. Cells were transfected with 20 μm siGFP or siHIPK2 for 48 h before drug treatments.
Real-time PCR Analysis
Real-time PCR analysis of HIPK2 mRNA was performed using standard procedures described previously (34). Briefly, RNA extraction was performed by the Qiagen RNA Extraction Kit, per the manufacturer's instructions, followed by real-time PCR analysis using a Bio-Rad MyIQ single-color real-time PCR detection system with SYBR Green. The following HIPK2 primers were used: 5′-ACAGATTTGGAAGGGAGCG-3′ (forward) and 5′-TGGTGACAAAGGGATGGTTC-3′ (reverse).
Transfection and Protein Analysis
Transient transfections were performed using X-tremeGene9 (Roche Applied Science 06365779001) at a ratio of 3:1 according to the manufacturer's protocol. Plasmids expressing FLAG-tagged CREBWT, CREBS270A/S271A, and CREBS270D/S271D were transfected at 3 μg into 60-mm dishes. Whole cell extracts were prepared by incubating cells in high salt lysis buffer (25 mm HEPES, pH 7.4, 300 mm NaCl, 1.5 mm MgCl2, 1 mm EGTA, 0.1% Triton X-100) supplemented with protease and phosphatase inhibitors for 10 min on ice as described previously by Shanware et al. (25). Briefly, total protein concentrations were determined by Bradford assay following the manufacturer's protocol (Bio-Rad 500-0006). Thirty μg of total protein was separated on 10% SDS-polyacrylamide gels and transferred to Immobilon polyvinylidene difluoride membranes (Millipore). Membranes were blocked in Tris-buffered saline (TBS) containing 0.2% Tween 20 (TBS-T) and 5% dried milk for 20 min. Membranes were incubated overnight at 4 °C with the indicated primary antibodies diluted in blocking solution. After washing, the blots were incubated with horseradish peroxidase-conjugated sheep anti-mouse or goat anti-rabbit secondary antibodies (Jackson ImmunoResearch) and developed using SuperSignal chemilluminescent substrate (Pierce 34079). To observe subtle changes in CREB electrophoretic mobility, we resolved whole cell extracts on 10% SDS-polyacrylamide resolving gels 13 cm in length. Each gel was electrophoresed at 40 mA for 4.5 h. Antibodies used in this study were used at a concentration of 1 μg/ml. They included anti-CREB (Millipore NL-904), anti-FLAG (Sigma F7425), anti-β-tubulin (Millipore AA2), anti-HSP90 (Cell Signaling C45G5), anti-pH3 S10 (Millipore 06-570), anti-pCREB-Ser-108/Ser-111/Ser-114 (25), anti-pCREB-Ser-121 (24), and anti-Cyclin B1 (Santa Cruz GNS1).
Luciferase Assays
HEK 293T cells were cotransfected with 3 μg of FLAG-tagged CREBWT, CREBS270A/S271A, or CREBS270D/S271D and 1 μg of 5×-CRE-luciferase (generous gift from Dr. Jerry Yin). Twsenty-four hours later, the cells were exposed to nocodazole or DMSO. Luciferase activity was measured 8 h later by using a Moonlight luminometer (BD Biosciences) as described previously (24). Results were normalized by protein levels and represent the averaged values of at least three independent experiments.
Lentivirus Packaging and Production
The pLKO.1 system was used to package lentiviruses and deliver short hairpin RNA (shRNA) as described previously (35). Briefly, the following shRNA target sequence was cloned into pLKO.1-TRC (Addgene plasmid 10878), according to the manufacturer's suggestions: shRNA targeting the 3′-untranslated region of CREB (shCREB) was directed using the following sequence: 5′-GCTCGATAAATCTAACAGTTA-3′. Lentiviral particles were produced by transient transfection of HEK 293T cells with pLKO.1 shCREB generated from above or pLKO.1 scramble (Addgene plasmid 1864), psPAX2 (Addgene plasmid 12260), and pMD2.G (Addgene plasmid 12259) in a ratio of 4:3:1 following the manufacturer's protocol.
Immune Complex Kinase Assays and EMSA
CDK1-CCNB1 protein complex kinase assays were performed as described (36). Briefly, extracts were immunoprecipitated with 1 μg of control IgG or anti-CCNB1 at 4 °C for 16 h. The immunoprecipitates were washed and incubated with 2 μg of CDC25C, His-CREBWT, His-CREBS271A, or His-CREBS270A/S271A per reaction for 30 min at 30 °C in the presence of [γ-32P]dATP. Kinase reactions were terminated by the addition of 2× SDS sample buffer, and reaction products were resolved by SDS-PAGE. Incorporation of [γ-32P]dATP into the His-CREB substrate was imaged by autoradiography. For unlabeled in vitro immune complex kinase assays, control IgG or CDK1-B1 protein complex was incubated with 0.5 μg of His-CREBWT or His-CREBS270A/S271A in the presence of 100 μm ATP as described above. Binding reaction mixtures (20 μl), containing in vitro labeled His-CREBWT or CREBS270A/S271A, 2 μg of poly(dI-dC), and 32P-labeled probe in binding buffer (4 mm HEPES, pH 7.9, 1 mm MgCl2, 0.5 mm dithiothreitol, 2% glycerol, and 20 mm NaCl), were incubated for 30 min at room temperature (37). The protein-DNA complexes were separated on 4% nondenaturing polyacrylamide gels in 0.25× Tris borate/EDTA buffer and were autoradiographed as described in Ref. 38. 32P-Labeled probe was generated with oligonucleotides purchased from IDT corresponding to the CRE site lying at −115/−93 relative to the transcription site in human TNF-α gene (5′-GTCGACCTCCAGATGACGTCATGGGT-3′). Oligonucleotides were annealed and end-labeled with [γ-32P]dATP using T4 polynucleotide kinase (37).
Chromatin Fractionation Assay
Chromatin fractionation of HeLa cells was carried out essentially as described (39). HeLa cell pellets derived from 2 × 106 cells were extracted with CSK buffer (10 mm PIPES, 100 mm NaCl, 300 mm sucrose, 1 mm MgCl2, 1 mm dithiothreitol, 1 mm EGTA, and 0.1% Triton X-100) with protease and phosphatase inhibitors and centrifuged at 3000 × g for 10 min. The supernatant was collected as the soluble protein fraction. The remaining cell pellets, chromatin fraction, were washed twice with CSK buffer and then boiled in 2× sample loading buffer prior to analysis by SDS-PAGE. Densitometry analysis was done on seven independent experiments to determine whether solubility of the FLAG mutants was affected following nocodazole treatment. Soluble FLAG levels were divided by the summation of the soluble and chromatin levels to correct for differences in expression for each mutant. Correction for chromatin FLAG levels was carried out in the same manner. For each experiment the ratio of corrected soluble to corrected chromatin was normalized to FLAG-CREBWT.
Immunofluorescence Microscopy
Cells grown on 15-mm coverslips were fixed with 4% paraformaldehyde in PBS for 15 min and then permeabilized with PBS containing 0.2% Triton X-100 for 15 min similar to the protocol described in Ref. 40. Fixed cells were incubated with primary antibodies specific for CREB (see above) diluted in TBS-T containing 3% bovine serum albumin. After washing, coverslips were incubated with secondary antibody conjugated to goat anti-rabbit Alexa Fluor 594 (Invitrogen). Cells were stained with 0.1 μg/ml 4′, 6-diamidino-2-phenylindole (DAPI) in TBS-T and mounted using Vectashield (Vector Laboratories H-1000). Images were gathered using Carl Zeiss Axiovert 200 inverted fluorescence microscope as previously described (40).
RESULTS
Distinct Patterns of CREB Phosphorylation in Response to DNA Damage and Mitotic Spindle Poisons
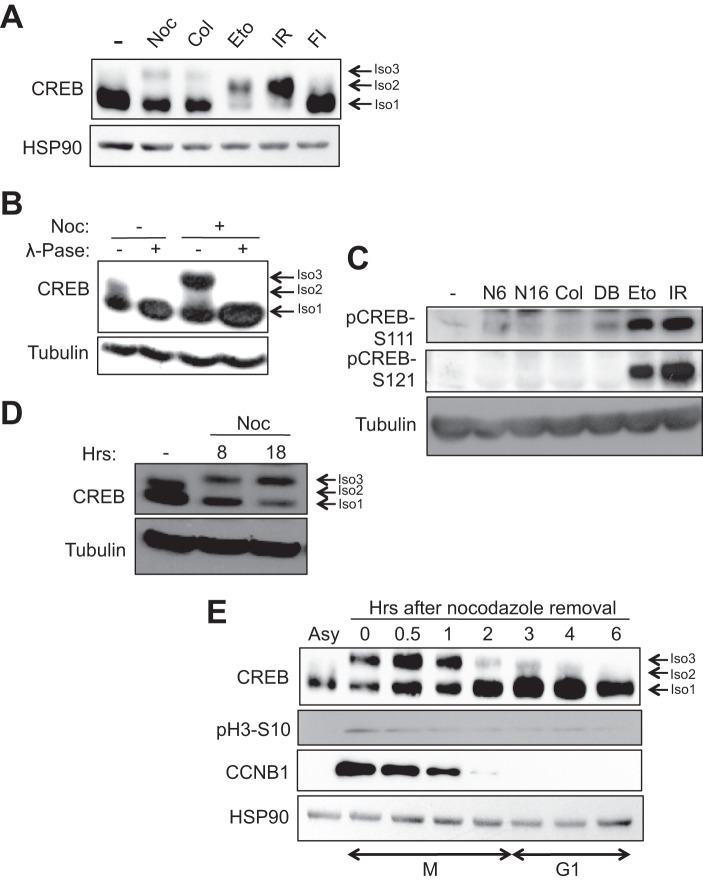
DNA damage-induced phosphorylation of CREB on the ATM/CK cluster results in a highly characteristic electrophoretic mobility shift on SDS-PAGE gels (Fig. 1A, isoform 2). During the course of cell cycle synchronization experiments we found that the anti-mitotic drugs nocodazole and colcemid also induced a CREB electrophoretic mobility shift; however, the magnitude of this shift exceeded that caused by γ-irradiation or exposure to the DNA-damaging agent, etoposide (Fig. 1A, isoform 3). λ-Phosphatase treatment of extracts prepared from nocodazole-treated cells abolished the CREB electrophoretic mobility shift, suggesting that it is a result of phosphorylation (Fig. 1B). Finally, the nocodazole-induced appearance of CREB isoform 3 in HeLa cells occurred independent of phosphorylation on Ser-108/Ser-111/Ser-114 or Ser-121, which comprise the major DNA damage-inducible phosphorylation sites in CREB (Fig. 1C). These findings indicate that the nocodazole/colcemid-induced electrophoretic mobility shift occurs independent of ATM/CK cluster phosphorylation.
FIGURE 1.
DNA-damaging agents and anti-mitotic drugs elicit distinct changes in CREB electrophoretic mobility. A and B, HeLa cells were treated with DMSO, nocodazole (Noc), or colcemid (Col) for 16 h, etoposide (Eto) for 6 h, irradiation (IR) for 2 h, or forskolin and isobutylmethylxanthine (FI) for 90 min. Cell extracts were immunoblotted with α-CREB or α-HSP90. Isoform 1 (Iso1, bottom arrow) denotes unphosphorylated CREB, isoform 2 (Iso2, middle arrow) denotes CREB phosphorylated on the ATM/CK cluster (Ser-108, Ser-111, Ser-114, Ser-117, and Ser-121) (24, 25), and isoform 3 (Iso3, top arrow) denotes the CREB phospho-species selectively induced by microtubule poisons. B, cells were treated with DMSO or nocodazole for 8 h. Afterward, 30 μg of cell extracts were mock or λ-phosphatase (λ-Pase)-treated for 20 min at 37 °C. C, nocodazole does not induce CREB phosphorylation on DNA damage-inducible sites. HeLa cells were treated with DMSO, nocodazole for 6 h (N6) or 16 h (N16), colcemid for 16 h, dibutyryl cAMP (DB) (20 μm) for 90 min, etoposide for 6 h, or exposed to 10 Gy of γ-irradiation and harvested 2 h later. Cell extracts were run on a SDS-polyacrylamide gel and immunoblotted with α-pCREB-Ser-108/Ser-111/Ser-114 (pCREB-S111), α-pCREB-Ser-121, or α-β-tubulin. D, cells were treated with nocodazole for the indicated times. Cell extracts were immunoblotted with α-CREB or α-β-tubulin. E, kinetics of CREB phosphorylation during mitotic arrest and release. Cells were arrested in early mitosis by treatment with nocodazole for 16 h. Mitotic cells were isolated by mechanical shake-off and released into fresh medium. Cells were collected at the indicated times following nocodazole release. Cell extracts were immunoblotted with α-CREB, α-pH3-Ser-10, α-CCNB1, or α-HSP90.
Nocodazole traps cells in prometaphase by preventing β-tubulin polymerization and mitotic spindly assembly (41). Given that prolonged nocodazole exposures caused further accumulation of hyperphosphorylated CREB species (Fig. 1D, isoform 3), we wished to examine CREB phosphorylation status in a mitotically pure cell population. To this end, we performed a mitotic shake-off procedure and then replated the cells in nocodazole-free medium for varying lengths of time. This procedure substantially enriched the hyperphosphorylated CREB species (Fig. 1E, Asy versus t = 0 lanes), which persisted for up to 1 h after replating and correlated with Cyclin B1 expression. By 2 h after replating both CREB hyperphosphorylation and CCNB1 expression were largely extinguished. These results strongly suggest that CREB is phosphorylated in early mitosis and becomes dephosphorylated concomitant with Cyclin B1 degradation as cells enter anaphase.
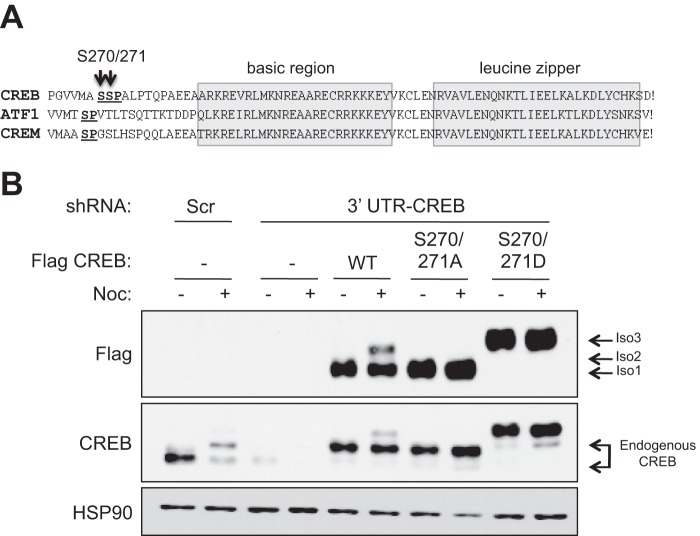
Ser-270 and Ser-271 Are Required for Nocodazole-induced Electrophoretic Mobility Shifts
The above findings suggested that the major mitotic Cyclin-dependent kinase, CDK1, might be involved in nocodazole induced CREB phosphorylation. Among several consensus serine-proline CDK1 phosphorylation sites, Ser-271, which lies proximal to the bZIP DNA binding domain, emerged as a strong candidate. This Ser-Pro motif is also found in ATF1 and CREM, suggesting functional importance (Fig. 2A). Further enhancing our interest in Ser-271, Dephoure et al. identified the adjacent Ser-270 residue as a likely phosphorylation site during a proteomic analysis of mitotic phosphoproteins (42). To test whether Ser-270/Ser-271 are responsible for nocodazole-induced CREB electrophoretic mobility changes, we mutated both residues to either alanines (S270A/S271A) to block phosphorylation or aspartic acids (S270D/S271D) to mimic phosphorylation, and examined the electrophoretic mobilities of the recombinant proteins relative to wild-type CREB (CREBWT). For these experiments we utilized a HeLa cell line rendered deficient for CREB through RNAi (shCREB:HeLa cells; see “Experimental Procedures”). We transduced shCREB:HeLa cells with retroviruses encoding FLAG-tagged, shRNAi-resistant CREBWT, CREBS270A/S271A, and CREBS270D/S271D proteins (Fig. 2B). Results using these cell lines clearly revealed that CREBS270A/S271A was defective for the nocodazole-induced electrophoretic mobility shift (Fig. 2B), whereas CREBS270D/S271D showed a constitutive electrophoretic mobility shift. These findings strongly suggest that Ser-270/Ser-271 are phosphorylated during mitosis. We further attempted to generate antibodies that detect Ser-270/Ser-271-phosphorylated CREB; however, these attempts were not successful.
FIGURE 2.
Identification of Ser-270 and Ser-271 as likely sites of CREB phosphorylation in response to nocodazole. A, alignment of CREB, ATF1, and CREM demonstrating the relative positions of conserved SP sites relative to the bipartite bZIP domain. B, impact of Ser-270/Ser-271 mutations on CREB electrophoretic mobility. HeLa cells stably expressing an shCREB or control lentiviral vector (see “Experimental Procedures”) were stably transduced with CREBWT, CREBS270A/S271A, or CREBS270D/S271D vectors. Cells were treated with DMSO or 100 ng/ml nocodazole (Noc) for 16 h, and extracts were resolved by SDS-PAGE and then analyzed by immunoblotting with α-FLAG, α-CREB, or α-HSP90. The extent of CREB knockdown in shCREB:HeLa cells can be seen by comparing the first and third lanes 1.
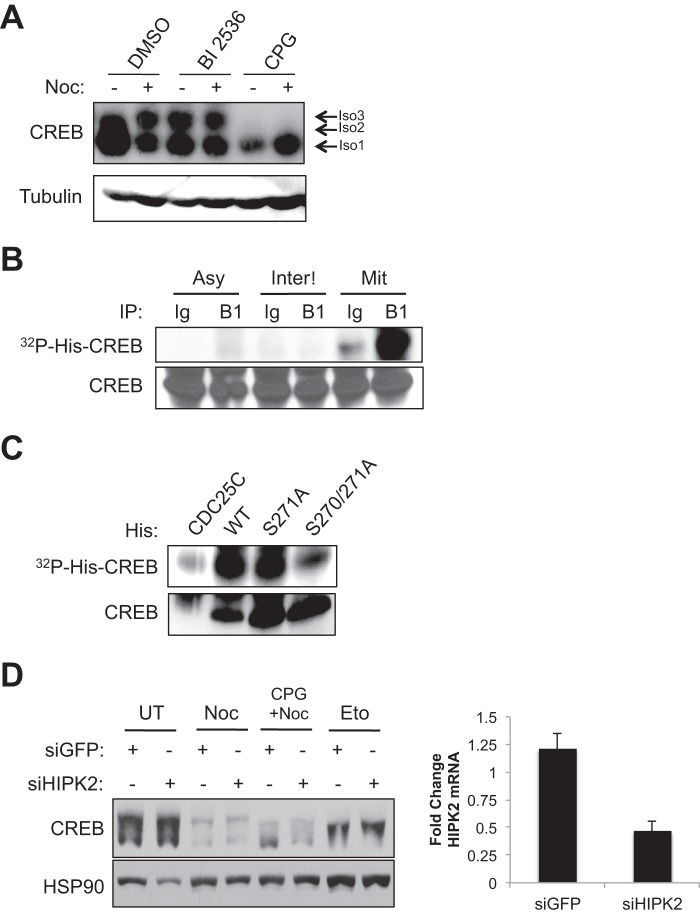
CDK1 Phosphorylates Ser-270/Ser-271 in Vivo and in Vitro
To test whether CDK1 is required for CREB Ser-270/Ser-271 phosphorylation we incubated HeLa cells with the CDK1 inhibitor CPG74514A prior to nocodazole treatment. As shown in Fig. 3A, CPG74514A ablated nocodazole-induced CREB phosphorylation. By contrast, the well characterized polo-like kinase 1 (PLK1) inhibitor BI 2536 actually induced Ser-270/Ser-271 phosphorylation independent of nocodazole treatment, which is consistent with the fact that this drug causes mitotic arrest in early prometaphase (Fig. 3A) (43). To test whether CDK1 directly phosphorylates CREB on Ser-270/Ser-271 we immunoprecipitated CDK1-Cyclin B1 complexes from mitotic HeLa extracts using a Cyclin-B1 antibody and incubated the immune complexes with a purified His-tagged CREB fusion protein substrate in the presence of [γ-32P]ATP. Specific phosphorylation of His-CREB was observed using CDK1-Cyclin B1 complexes from mitotic, but not interphase cell extracts (Fig. 3B). Additionally, the combined S270A/S271A mutation diminished His-CREB phosphorylation whereas an individual S271A mutation had little effect (Fig. 3C). The combined cell-based and in vitro findings support a model whereby CDK1 is a CREB Ser-270/Ser-271 kinase.
FIGURE 3.
CDK1 phosphorylates Ser-270 and Ser-271 in vivo and in vitro. A, CDK1 inhibitor disrupted nocodazole (Noc)-induced phosphorylation. HeLa cells were incubated with DMSO, BI 2536, or CPG74514A (CPG) for 1 h. Following inhibitor treatment, cells were DMSO- or nocodazole-treated for 16 h. Cell extracts were immunoblotted with α-CREB or α-β-tubulin. B, CDK1 phosphorylates CREB in vitro. HeLa cells were treated with DMSO (Asy) or treated with nocodazole for 16 h. Mitotic cells were enriched by mechanical shake-off (Mit). The remaining cells on the dish were designated as interphase cells (Inter). B and C, cell extracts were immunoprecipitated (IP) with control immunoglobulin (Ig) or CCNB1 (B1) antibody. Control or CCNB1 beads were incubated with His-CREBWT (B) or His-CDC25C, His-CREBWT, His-CREBS271A, or His-CREBS270A/S271A (C) and [γ-32P]ATP for 30 min. Samples were resolved on SDS-polyacrylamide gel and immunoblotted with α-CREB or imaged by autoradiography to detect phosphorylated His-CREB. D, knockdown of HIPK2 did not impact CREB Ser-270/Ser-271 phosphorylation. HeLa cells were transfected with SmartPool (Dharmacon) siGFP or siHIPK2 for 48 h. Afterward, cells were treated with nocodazole for 16 h or etoposide for 6 h. Cell extracts were immunoblotted with α-CREB or α-HSP90. The bar graph displays -fold change in HIPK2 mRNA levels normalized to GAPDH mRNA levels from four independent transfections. Error bars, S.E.
It was recently reported that HIPK2 phosphorylates CREB on Ser-271 in response to etoposide (31). However, this conclusion is counter to results in Fig. 1 showing that etoposide and nocodazole induce distinct CREB electrophoretic mobility shifts. To further investigate the role of HIPK2 in CREB Ser-270/Ser-271 phosphorylation we performed HIPK2 knockdown experiments. Knockdown of HIPK2 did not reduce CREB electrophoretic mobility shifts in response to either etoposide or nocodazole (Fig. 3D). Although contributions of residual HIPK2 cannot be ruled out, HIPK2 does not appear to be a major CREB Ser-270/Ser-271 kinase in response to etoposide or nocodazole.
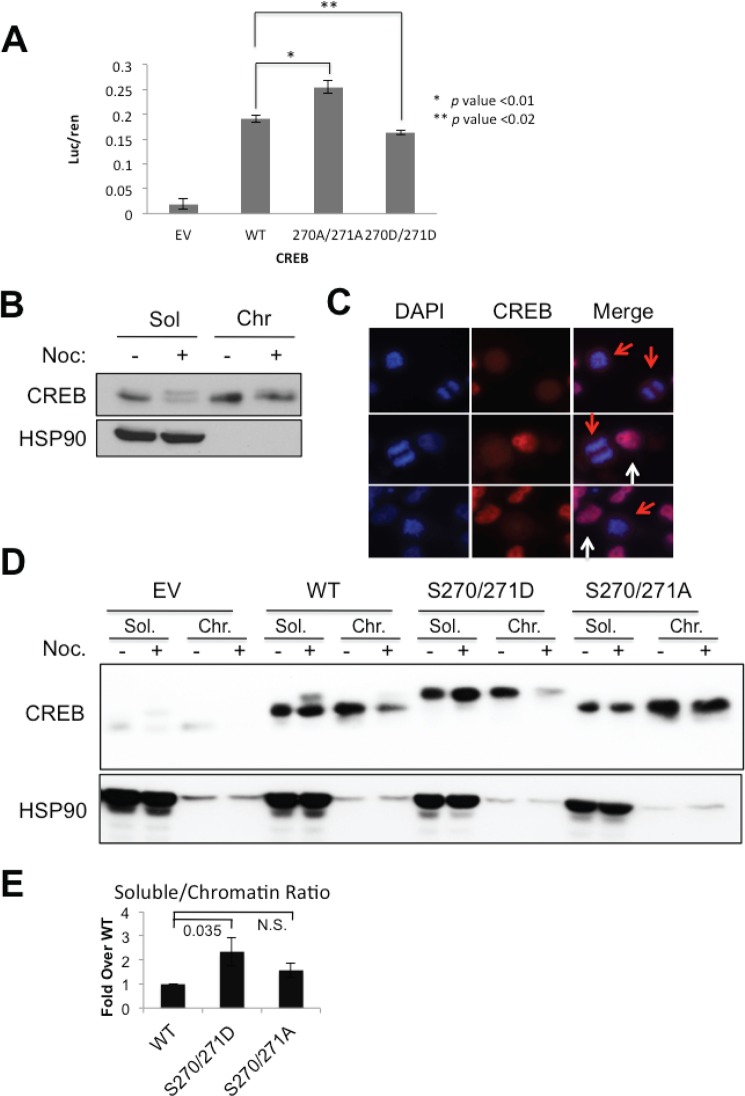
Ser-270/Ser-271 Phosphorylation Decreases CREB DNA Binding Affinity
Ser-270/Ser-271 lie near the junction of the Q2/CAD and bZIP domains which influences both transactivation potential and DNA binding activity (44, 45). To assess the functional impacts of Ser-270/Ser-271 phosphorylation on CREB transactivation potential we transfected HeLa cells stably expressing CREBWT, CREBS270A/S271A, or CREBS270D/S271D with CRE-luciferase reporter plasmid. The transactivation potential of CREBS270A/S271A was slightly higher than CREBWT, whereas CREBS270D/S271D showed slightly reduced activity versus CREBWT (Fig. 4A). Nocadazole did not alter CREB activity in these assays (data not shown). These findings suggested that Ser-270/Ser-271 phosphorylation antagonizes CREB transcriptional activity.
FIGURE 4.
CREB Ser-270/Ser-271 phosphorylation decreases chromatin occupancy. A, transactivation potential of CREB Ser-270/Ser-271 phosphosite mutants is shown. shCREB:HeLa cells stably expressing empty vector (EV) or the indicated CREB alleles were cotransfected with 5×-CRE-luciferase reporter and Renilla luciferase. Forty-eight hours after transfection cells were harvested for luciferase assays. The luciferase data were normalized to Renilla; n = 3. B, nocodazole (Noc) impacts CREB chromatin occupancy. HeLa cells were DMSO- or nocodazole-treated for 16 h. Cells were fractionated into soluble (Sol) or chromatin (Chr) fractions (see “Experimental Procedures”). Samples were run on a SDS-polyacrylamide mini gel and immunoblotted with α-CREB or α-HSP90. Isoform 2 is not resolved from isoform 1 under these conditions. C, asynchronous HeLa cells were mounted on glass coverslips and stained with DAPI and α-CREB. CREB is excluded from mitotic chromatin. D, Ser-270/Ser-271 phosphorylation modulates CREB chromatin association. shCREB:HeLa cells reconstituted with either CREBWT, CREBS270D/S271D, or CREBS270A/S271A were treated with nocodazole and fractionated into soluble and chromatin fractions. Extracts were immunoblotted for α-FLAG or α-HSP90. Cells were treated with the CDK1 inhibitor CPG where indicated. E, densitometric quantification of chromatin fractionation data. The chromatin/soluble ratio for CREBWT after nocodazole treatment was normalized to 1 (n = 7).
Given the proximity of Ser-270/Ser-271 to the bZIP domain, we tested the impact of Ser-270/Ser-271 phosphorylation on CREB DNA binding activity. Specifically, we hypothesized that CDK1-mediated phosphorylation of CREB may facilitate its dissociation from chromatin during prophase, as has been shown for several other transcription factors (46–48). To explore this possibility, we measured the chromatin association of CREB in HeLa cells before and after nocodazole treatment. The hypophosphorylated form of CREB partitioned roughly equally between soluble and chromatin fractions prior to nocodazole treatment (Fig. 4B). Upon nocodazole treatment, the Ser-270/Ser-271-phosphorylated form of CREB partitioned almost exclusively with the soluble fraction, whereas chromatin-associated CREB existed predominantly in a dephosphorylated state (Fig. 4B). Consistent with this finding, endogenous CREB was excluded from condensed chromatin in mitotic HeLa cells versus cells in interphase (Fig. 4C, red arrows versus white arrows). Thus, phosphorylation of endogenous CREB on Ser-270/Ser-271 correlated with its reduced association with chromatin.
We next tested the impacts of S270A/S271A and S270D/S271D mutations on CREB chromatin occupancy using the CREB knockdown/reconstituted HeLa cell lines. As expected, CREBWT was phosphorylated upon nocodazole treatment, with the phosphorylated species partitioning with the soluble, nucleoplasmic fraction (Fig. 4D). CREBS270D/S271D exhibited a significant reduction in chromatin binding versus CREBWT upon nocodazole treatment, suggesting that the phosphomimetic substitutions decrease CREB chromatin occupancy (Fig. 4, D and E). Although the S270A/S271A mutation was predicted to abolish nocodazole-induced CREB chromatin dissociation, this was not the case under the fractionation conditions we employed (Fig. 4, D and E). The fractionation findings suggest that Ser-270/Ser-271 phosphorylation promotes CREB chromatin dissociation during mitosis, but that phosphorylation of these sites is not absolutely required for CREB to dissociate from chromatin following nocodazole treatment.
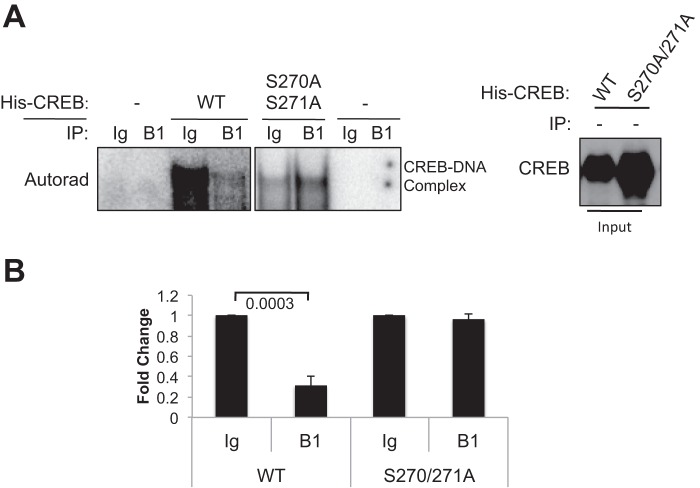
To further probe the relationship between Ser-270/Ser-271 phosphorylation and CREB DNA binding activity we tested whether in vitro phosphorylation of CREB by CDK1 diminished binding to CRE-containing oligonucleotides. Purified His6-tagged CREBWT and CREBS270A/S271A were incubated with either IgG or CDK1-Cyclin B1 immune complexes prepared from nocodazole-treated HeLa cell extracts in the presence of unlabeled ATP. The CDK1- or mock-phosphorylated CREB proteins were then incubated with a 32P-labeled, double-stranded oligonucleotide corresponding to a CRE in the TNF-α gene (37) and the protein-DNA complexes analyzed using an EMSA. Preincubation of CREBWT with CDK1-Cyclin B1 caused a clear decrease in its CRE binding affinity relative to CREBWT that had been incubated with IgG control immunoprecipitation (Fig. 5A). On the other hand, CDK1-Cycllin B1 did not inhibit the interaction between CREBS270A/S271A and DNA relative to CREBS270A/S271A incubated with IgG control (Fig. 5B). These data provide direct evidence that CDK1-mediated phosphorylation of CREB on Ser-270/Ser-271 reduces its affinity for DNA in vitro and support the chromatin findings.
FIGURE 5.
Phosphorylation of CREB by CDK1 reduced its DNA binding activity in vitro. A, mitotic HeLa extracts (see “Experimental Procedures”) were immunoprecipitated (IP) with α-CCNB1 or IgG antibodies, and the resulting immune complexes were incubated with His-CREBWT or His-CREBS270A/S271A in the presence of unlabeled ATP. The in vitro phosphorylated His-CREB proteins were then incubated with a 32P-labeled CRE oligonucleotides and an EMSA performed as described under “Experimental Procedures.” Input of His-CREB proteins was resolved on SDS-polyacrylamide gel and immunoblotted with α-CREB. B, densitometric quantification of the EMSA data (n = 4) was performed.
DISCUSSION
In this study we have demonstrated that CREB is phosphorylated at Ser-270/Ser-271 in a CDK1-dependent manner during mitosis. Our findings are consistent with a report by Dephoure et al. that identified Ser-270 as a phosphorylation site during a proteomic screen for mitotic phosphoproteins (42). Although Ser-271 conforms to the serine-proline CDK1 consensus, a S271A mutation did not abolish CDK1-mediated CREB phosphorylation in vitro (Fig. 3C), and neither S271A nor S270A individual mutations abolished the nocodazole-induced electrophoretic mobility shift (data not shown). These findings suggest that both Ser-270 and Ser-271 are phosphorylated during mitosis, which is strongly supported by results using the CREB S270A/S271A double mutant (Fig. 2B). Our findings suggest that CDK1 is a major Ser-270/Ser-271 kinase, but do not rule out a role for other kinases that are active during mitosis.
Sakamoto et al. previously reported that HIPK2 phosphorylates CREB on Ser-271 (31). This conclusion was based in part on the observation that HIPK2 overexpression caused an Ser-271-dependent retardation in CREB electrophoretic mobility. However, given that HIPK2 plays a role in cytokinesis and its overexpression causes G2/M delay (33, 49–51), it is possible that the observed CREB electrophoretic mobility shift was a secondary consequence of mitotic arrest and CDK1-dependent phosphorylation. Additionally, we find no evidence that etoposide or other DNA-damaging agents induce CREB Ser-271 phosphorylation. Indeed, genotoxic agents (e.g. etoposide) and mitotic spindle poisons (e.g. nocodazole) cause characteristically different reductions in CREB electrophoretic mobility that can be distinguished using high resolution SDS-PAGE (Fig. 1A). Altogether, we feel that current evidence argues against a major role for HIPK2 in the DNA damage-induced phosphorylation of CREB; however, its contributions to Ser-270/Ser-271 phosphorylation under other conditions cannot be ruled out.
What are the functional implications of CREB phosphorylation during mitosis? Mutation of Ser-270/Ser-271 to Ala or Asp did not alter CREB subcellular localization, but did modestly impact CREB transactivation potential in luciferase assays. Specifically, CREBS270D/S271D showed slightly reduced transcriptional activity relative to CREBWT and CREBS270A/S271A in reporter assays. Reduced activity of CREBS270D/S271D correlated with its reduced association with chromatin, suggesting that Ser-270/Ser-271 phosphorylation antagonizes CREB DNA binding activity (Fig. 4D). A direct role for Ser-270/Ser-271 in modulating CREB DNA binding activity is strongly suggested by EMSA experiments, where incubation of CREB with Cyclin B-CDK1 reduced its in vitro DNA-binding potential in an Ser-270/Ser-271-dependent manner (Fig. 5). Although other functional consequences are not ruled out, these findings support a model whereby CDK1 promotes CREB chromatin eviction during mitosis. It is unclear whether all CREB binding sites are equally evacuated during mitosis or whether CREB dissociates hierarchically from low affinity and high affinity CREB binding sites. However, the fact that some CREB remains in the chromatin fractionation even after prolonged nocodazole treatment implies that some CREB binding sites remain occupied in condensed chromosomes.
How Ser-270/Ser-271 phosphorylation modulates CREB DNA binding affinity is unknown; however, modification of these sites may alter the availability or conformation of the adjacent bZIP DNA binding domain or may disrupt binding of CREB cofactors such as CRTC2 that enhance its DNA binding potential. Interestingly, Mémin et al. recently showed that CDK1 phosphorylated the ICER (inducible cyclic AMP early repressor) splice variant of CREM on Ser-35, which is analogous to CREB Ser-271 (52). Thus, mitotic phosphorylation of positionally conserved CDK1 sites appears to be common to all members of the CREB/ATF family of transcription factors. Mémin et al. concluded that Ser-35 phosphorylation promoted CREM/ICER ubiquitylation and nuclear export; however, this does not appear to be the case for CREB, as both CREBS270A/S271A and CREBS270D/S271D mutants showed normal nuclear distribution, and we did not detect ubiquitylated forms of CREB following nocodazole treatment (data not shown). Nevertheless, we cannot rule out that ubiquitylation or other modifications contribute to reduce CREB chromatin occupancy in mitotic cells.
CDK1-dependent phosphorylation of CREB/ATF subfamily of bZIP transcription factors may reflect broader inhibition of transcription factors by CDK1 during mitosis. We note that CDK1-dependent phosphorylation of AB1 and TFII-I promoted their chromatin eviction during mitosis (47, 48). Additionally, the C2H2 zinc finger DNA binding domain transcription factors were phosphorylated and inactivated during mitosis (46). The fact that many site-specific transcription factors were identified as mitotic phosphoproteins by Dephoure et al. is compatible with the general idea that many transcription factors are evicted from chromatin during mitosis, although in most instances the functional consequences of these phosphorylations are unknown (42, 46).
Acknowledgments
We thank Dr. Jerry Yin and Dr. Beth Weaver for expert technical advice and generous gifts of reagents and advice; and Eric Britigan, Lihong Zhan, and Bennett Fox for helpful advice and discussion.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 CA 124722-01 (to R. S. T.). This work was also supported by an American Heart Association predoctoral fellowship (to A. T. T.) and the Science and Medicine Graduate Research Scholars Fellowship program at the University of Wisconsin-Madison.
- CREB
- cyclic AMP response element-binding protein
- ATF1
- activating transcription factor 1
- ATM
- ataxia-telangiectasia-mutated
- bZIP
- basic leucine zipper
- CBP
- CREB-binding protein
- CDK1
- Cyclin-dependent kinase 1
- CK
- casein kinase
- CRE
- cyclic AMP response element
- CREM
- cAMP response element modulator
- CRTC
- CREB-regulated transcriptional coactivator
- DMSO
- dimethyl sulfoxide
- HIPK2
- homeodomain-interacting protein kinase 2
- KID
- CREB kinase-inducible domain.
REFERENCES
- 1. Mayr B., Montminy M. (2001) Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2, 599–609 [DOI] [PubMed] [Google Scholar]
- 2. Lonze B. E., Ginty D. D. (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35, 605–623 [DOI] [PubMed] [Google Scholar]
- 3. Altarejos J. Y., Montminy M. (2011) CREB and the CRTC coactivators: sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 12, 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang X., Odom D. T., Koo S. H., Conkright M. D., Canettieri G., Best J., Chen H., Jenner R., Herbolsheimer E., Jacobsen E., Kadam S., Ecker J. R., Emerson B., Hogenesch J. B., Unterman T., Young R. A., Montminy M. (2005) Genome-wide analysis of cAMP-response element-binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc. Natl. Acad. Sci. U.S.A. 102, 4459–4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Impey S., Fong A. L., Wang Y., Cardinaux J. R., Fass D. M., Obrietan K., Wayman G. A., Storm D. R., Soderling T. R., Goodman R. H. (2002) Phosphorylation of CBP mediates transcriptional activation by neural activity and CaM kinase IV. Neuron 34, 235–244 [DOI] [PubMed] [Google Scholar]
- 6. Chrivia J. C., Kwok R. P., Lamb N., Hagiwara M., Montminy M. R., Goodman R. H. (1993) Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature 365, 855–859 [DOI] [PubMed] [Google Scholar]
- 7. Parker D., Ferreri K., Nakajima T., LaMorte V. J., Evans R., Koerber S. C., Hoeger C., Montminy M. R. (1996) Phosphorylation of CREB at Ser-133 induces complex formation with CREB-binding protein via a direct mechanism. Mol. Cell. Biol. 16, 694–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parker D., Jhala U. S., Radhakrishnan I., Yaffe M. B., Reyes C., Shulman A. I., Cantley L. C., Wright P. E., Montminy M. (1998) Analysis of an activator:coactivator complex reveals an essential role for secondary structure in transcriptional activation. Mol. Cell 2, 353–359 [DOI] [PubMed] [Google Scholar]
- 9. Yin J. C., Del Vecchio M., Zhou H., Tully T. (1995) CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell 81, 107–115 [DOI] [PubMed] [Google Scholar]
- 10. Schacher S., Castellucci V. F., Kandel E. (1988) cAMP evokes long-term facilitation in Aplysia sensory neurons that requires new protein synthesis. Science 240, 1667–1669 [DOI] [PubMed] [Google Scholar]
- 11. Dash P. K., Hochner B., Kandel E. R. (1990) Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature 345, 718–721 [DOI] [PubMed] [Google Scholar]
- 12. Kandel E. R. (2012) The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol. Brain 5, 5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ravnskjaer K., Kester H., Liu Y., Zhang X., Lee D., Yates J. R., 3rd, Montminy M. (2007) Cooperative interactions between CBP and TORC2 confer selectivity to CREB target gene expression. EMBO J. 26, 2880–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bittinger M. A., McWhinnie E., Meltzer J., Iourgenko V., Latario B., Liu X., Chen C. H., Song C., Garza D., Labow M. (2004) Activation of cAMP response element-mediated gene expression by regulated nuclear transport of TORC proteins. Curr. Biol. 14, 2156–2161 [DOI] [PubMed] [Google Scholar]
- 15. Iourgenko V., Zhang W., Mickanin C., Daly I., Jiang C., Hexham J. M., Orth A. P., Miraglia L., Meltzer J., Garza D., Chirn G. W., McWhinnie E., Cohen D., Skelton J., Terry R., Yu Y., Bodian D., Buxton F. P., Zhu J., Song C., Labow M. A. (2003) Identification of a family of cAMP response element-binding protein coactivators by genome-scale functional analysis in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 100, 12147–12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luo Q., Viste K., Urday-Zaa J. C., Senthil Kumar G., Tsai W. W., Talai A., Mayo K. E., Montminy M., Radhakrishnan I. (2012) Mechanism of CREB recognition and coactivation by the CREB-regulated transcriptional coactivator CRTC2. Proc. Natl. Acad. Sci. U.S.A. 109, 20865–20870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Screaton R. A., Conkright M. D., Katoh Y., Best J. L., Canettieri G., Jeffries S., Guzman E., Niessen S., Yates J. R., 3rd, Takemori H., Okamoto M., Montminy M. (2004) The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell 119, 61–74 [DOI] [PubMed] [Google Scholar]
- 18. Gau D., Lemberger T., von Gall C., Kretz O., Le Minh N., Gass P., Schmid W., Schibler U., Korf H. W., Schütz G. (2002) Phosphorylation of CREB Ser-142 regulates light-induced phase shifts of the circadian clock. Neuron 34, 245–253 [DOI] [PubMed] [Google Scholar]
- 19. Wu X., McMurray C. T. (2001) Calmodulin kinase II attenuation of gene transcription by preventing cAMP response element-binding protein (CREB) dimerization and binding of the CREB-binding protein. J. Biol. Chem. 276, 1735–1741 [DOI] [PubMed] [Google Scholar]
- 20. Kornhauser J. M., Cowan C. W., Shaywitz A. J., Dolmetsch R. E., Griffith E. C., Hu L. S., Haddad C., Xia Z., Greenberg M. E. (2002) CREB transcriptional activity in neurons is regulated by multiple, calcium-specific phosphorylation events. Neuron 34, 221–233 [DOI] [PubMed] [Google Scholar]
- 21. Sheng M., Thompson M. A., Greenberg M. E. (1991) CREB: a Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science 252, 1427–1430 [DOI] [PubMed] [Google Scholar]
- 22. Dash P. K., Karl K. A., Colicos M. A., Prywes R., Kandel E. R. (1991) cAMP response element-binding protein is activated by Ca2+/calmodulin- as well as cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. U.S.A. 88, 5061–5065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun P., Enslen H., Myung P. S., Maurer R. A. (1994) Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 8, 2527–2539 [DOI] [PubMed] [Google Scholar]
- 24. Shi Y., Venkataraman S. L., Dodson G. E., Mabb A. M., LeBlanc S., Tibbetts R. S. (2004) Direct regulation of CREB transcriptional activity by ATM in response to genotoxic stress. Proc. Natl. Acad. Sci. U.S.A. 101, 5898–5903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shanware N. P., Trinh A. T., Williams L. M., Tibbetts R. S. (2007) Coregulated ataxia telangiectasia-mutated and casein kinase sites modulate cAMP-response element-binding protein-coactivator interactions in response to DNA damage. J. Biol. Chem. 282, 6283–6291 [DOI] [PubMed] [Google Scholar]
- 26. Dodson G. E., Tibbetts R. S. (2006) DNA replication stress-induced phosphorylation of cyclic AMP response element-binding protein mediated by ATM. J. Biol. Chem. 281, 1692–1697 [DOI] [PubMed] [Google Scholar]
- 27. Radhakrishnan I., Pérez-Alvarado G. C., Parker D., Dyson H. J., Montminy M. R., Wright P. E. (1997) Solution structure of the KIX domain of CBP bound to the transactivation domain of CREB: a model for activator:coactivator interactions. Cell 91, 741–752 [DOI] [PubMed] [Google Scholar]
- 28. Shaywitz A. J., Dove S. L., Kornhauser J. M., Hochschild A., Greenberg M. E. (2000) Magnitude of the CREB-dependent transcriptional response is determined by the strength of the interaction between the kinase-inducible domain of CREB and the KIX domain of CREB-binding protein. Mol. Cell. Biol. 20, 9409–9422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shanware N. P., Zhan L., Hutchinson J. A., Kim S. H., Williams L. M., Tibbetts R. S. (2010) Conserved and distinct modes of CREB/ATF transcription factor regulation by PP2A/B56γ and genotoxic stress. PloS One 5, e12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horiuchi J., Jiang W., Zhou H., Wu P., Yin J. C. (2004) Phosphorylation of conserved casein kinase sites regulates cAMP-response element-binding protein DNA binding in Drosophila. J. Biol. Chem. 279, 12117–12125 [DOI] [PubMed] [Google Scholar]
- 31. Sakamoto K., Huang B. W., Iwasaki K., Hailemariam K., Ninomiya-Tsuji J., Tsuji Y. (2010) Regulation of genotoxic stress response by homeodomain-interacting protein kinase 2 through phosphorylation of cyclic AMP response element-binding protein at serine 271. Mol. Biol. Cell 21, 2966–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hofmann T. G., Möller A., Sirma H., Zentgraf H., Taya Y., Dröge W., Will H., Schmitz M. L. (2002) Regulation of p53 activity by its interaction with homeodomain-interacting protein kinase-2. Nat. Cell Biol. 4, 1–10 [DOI] [PubMed] [Google Scholar]
- 33. D'Orazi G., Cecchinelli B., Bruno T., Manni I., Higashimoto Y., Saito S., Gostissa M., Coen S., Marchetti A., Del Sal G., Piaggio G., Fanciulli M., Appella E., Soddu S. (2002) Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser-46 and mediates apoptosis. Nat. Cell Biol. 4, 11–19 [DOI] [PubMed] [Google Scholar]
- 34. Kim S. H., Shanware N. P., Bowler M. J., Tibbetts R. S. (2010) Amyotrophic lateral sclerosis-associated proteins TDP-43 and FUS/TLS function in a common biochemical complex to co-regulate HDAC6 mRNA. J. Biol. Chem. 285, 34097–34105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moffat J., Grueneberg D. A., Yang X., Kim S. Y., Kloepfer A. M., Hinkle G., Piqani B., Eisenhaure T. M., Luo B., Grenier J. K., Carpenter A. E., Foo S. Y., Stewart S. A., Stockwell B. R., Hacohen N., Hahn W. C. (2006) A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124, 1283–1298 [DOI] [PubMed] [Google Scholar]
- 36. Sarkaria J. N., Tibbetts R. S., Busby E. C., Kennedy A. P., Hill D. E., Abraham R. T. (1998) Inhibition of phosphoinositide 3-kinase-related kinases by the radiosensitizing agent wortmannin. Cancer Res. 58, 4375–4382 [PubMed] [Google Scholar]
- 37. Chong Y. H., Shin Y. J., Suh Y. H. (2003) Cyclic AMP inhibition of tumor necrosis factor α production induced by amyloidogenic C-terminal peptide of Alzheimer's amyloid precursor protein in macrophages: involvement of multiple intracellular pathways and cyclic AMP response element-binding protein. Mol. Pharmacol. 63, 690–698 [DOI] [PubMed] [Google Scholar]
- 38. Wuerzberger-Davis S. M., Chang P. Y., Berchtold C., Miyamoto S. (2005) Enhanced G2-M arrest by nuclear factor-κB-dependent p21Waf1/Cip1 induction. Mol. Cancer Res. 3, 345–353 [DOI] [PubMed] [Google Scholar]
- 39. Shi Y., Dodson G. E., Mukhopadhyay P. S., Shanware N. P., Trinh A. T., Tibbetts R. S. (2007) Identification of carboxyl-terminal MCM3 phosphorylation sites using polyreactive phosphospecific antibodies. J. Biol. Chem. 282, 9236–9243 [DOI] [PubMed] [Google Scholar]
- 40. Sakasai R., Tibbetts R. (2008) RNF8-dependent and RNF8-independent regulation of 53BP1 in response to DNA damage. J. Biol. Chem. 283, 13549–13555 [DOI] [PubMed] [Google Scholar]
- 41. Jordan M. A., Thrower D., Wilson L. (1992) Effects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles: implications for the role of microtubule dynamics in mitosis. J. Cell Sci. 102, 401–416 [DOI] [PubMed] [Google Scholar]
- 42. Dephoure N., Zhou C., Villén J., Beausoleil S. A., Bakalarski C. E., Elledge S. J., Gygi S. P. (2008) A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 105, 10762–10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Burkard M. E., Maciejowski J., Rodriguez-Bravo V., Repka M., Lowery D. M., Clauser K. R., Zhang C., Shokat K. M., Carr S. A., Yaffe M. B., Jallepalli P. V. (2009) Plk1 self-organization and priming phosphorylation of HsCYK-4 at the spindle midzone regulate the onset of division in human cells. PLoS Biol. 7, e1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mayr B. M., Guzman E., Montminy M. (2005) Glutamine-rich and basic region/leucine zipper (bZIP) domains stabilize cAMP-response element-binding protein (CREB) binding to chromatin. J. Biol. Chem. 280, 15103–15110 [DOI] [PubMed] [Google Scholar]
- 45. Asahara H., Santoso B., Guzman E., Du K., Cole P. A., Davidson I., Montminy M. (2001) Chromatin-dependent cooperativity between constitutive and inducible activation domains in CREB. Mol. Cell. Biol. 21, 7892–7900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dovat S., Ronni T., Russell D., Ferrini R., Cobb B. S., Smale S. T. (2002) A common mechanism for mitotic inactivation of C2H2 zinc finger DNA-binding domains. Genes Dev. 16, 2985–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ferrero M., Ferragud J., Orlando L., Valero L., Sánchez del Pino M., Farràs R., Font de Mora J. (2011) Phosphorylation of AIB1 at mitosis is regulated by CDK1/CYCLIN B. PloS One 6, e28602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ashworth T., Roy A. (2009) Phase-specific functions of the transcription factor TFII-I during cell cycle. Cell Cycle 8, 596–605 [DOI] [PubMed] [Google Scholar]
- 49. Pierantoni G. M., Fedele M., Pentimalli F., Benvenuto G., Pero R., Viglietto G., Santoro M., Chiariotti L., Fusco A. (2001) High mobility group I (Y) proteins bind HIPK2, a serine-threonine kinase protein which inhibits cell growth. Oncogene 20, 6132–6141 [DOI] [PubMed] [Google Scholar]
- 50. Tomasini R., Samir A. A., Carrier A., Isnardon D., Cecchinelli B., Soddu S., Malissen B., Dagorn J0 -C., Iovanna J. L., Dusetti N. J. (2003) TP53INP1s and homeodomain-interacting protein kinase-2 (HIPK2) are partners in regulating p53 activity. J. Biol. Chem. 278, 37722–37729 [DOI] [PubMed] [Google Scholar]
- 51. Rinaldo C., Moncada A., Gradi A., Ciuffini L., D'Eliseo D., Siepi F., Prodosmo A., Giorgi A., Pierantoni G. M., Trapasso F., Guarguaglini G., Bartolazzi A., Cundari E., Schininà M. E., Fusco A., Soddu S. (2012) HIPK2 controls cytokinesis and prevents tetraploidization by phosphorylating histone H2B at the midbody. Mol. Cell 47, 87–98 [DOI] [PubMed] [Google Scholar]
- 52. Mémin E., Genzale M., Crow M., Molina C.A. (2011) Evidence that phosphorylation by the mitotic kinase Cdk1 promotes ICER monoubiquitination and nuclear delocalization. Exp. Cell Res. 317, 2490–2502 [DOI] [PubMed] [Google Scholar]