Background: Ser/Thr phosphorylation of P450c17 increases 17,20 lyase activity and androgenic capacity.
Results: Drug inhibition and siRNA knockdowns in adrenal cells implicate p38α, which phosphorylated bacterially expressed P450c17, doubling 17,20 lyase activity.
Conclusion: Phosphorylation of P450c17 by p38α provides a post-translational mechanism distinguishing glucocorticoid from sex steroid synthesis.
Significance: p38α pathways may participate in hyperandrogenic states and provide targets for glucocorticoid-sparing inhibition of androgen synthesis.
Keywords: Androgen, Cytochrome P450, Metabolic Syndrome, Serine/threonine Protein Kinase, Steroid, Adrenarche, Polycystic Ovary Syndrome
Abstract
Cytochrome P450c17, a steroidogenic enzyme encoded by the CYP17A1 gene, catalyzes the steroid 17α-hydroxylation needed for glucocorticoid synthesis, which may or may not be followed by 17,20 lyase activity needed for sex steroid synthesis. Whether or not P450c17 catalyzes 17,20 lyase activity is determined by three post-translational mechanisms influencing availability of reducing equivalents donated by P450 oxidoreductase (POR). These are increased amounts of POR, the allosteric action of cytochrome b5 to promote POR-P450c17 interaction, and Ser/Thr phosphorylation of P450c17, which also appears to promote POR-P450c17 interaction. The kinase(s) that phosphorylates P450c17 is unknown. In a series of kinase inhibition experiments, the pyridinyl imidazole drugs SB202190 and SB203580 inhibited 17,20 lyase but not 17α-hydroxylase activity in human adrenocortical HCI-H295A cells, suggesting an action on p38α or p38β. Co-transfection of non-steroidogenic COS-1 cells with P450c17 and p38 expression vectors showed that p38α, but not p38β, conferred 17,20 lyase activity on P450c17. Antiserum to P450c17 co-immunoprecipitated P450c17 and both p38 isoforms; however, knockdown of p38α, but not knockdown of p38β, inhibited 17,20 lyase activity in NCI-H295A cells. Bacterially expressed human P450c17 was phosphorylated by p38α in vitro at a non-canonical site, conferring increased 17,20 lyase activity. This phosphorylation increased the maximum velocity, but not the Michaelis constant, of the 17,20 lyase reaction. p38α phosphorylates P450c17 in a fashion that confers increased 17,20 lyase activity, implying that the production of adrenal androgens (adrenarche) is a regulated event.
Introduction
Three classes of steroid hormones are required for mammalian life: mineralocorticoids regulate renal sodium retention, thus regulating intravascular volume and blood pressure; glucocorticoids regulate carbohydrate metabolism and responses to stress; and sex steroids (androgens and estrogens) are required for reproduction of the species. In most mammals, mineralocorticoids are C21 (21-carbon) 17-deoxy steroids, glucocorticoids are C21 17-hydroxysteroids, and sex steroids are produced from C19 steroids. A single steroidogenic enzyme, cytochrome P450c17, encoded by the CYP17A1 gene determines which class of steroid is produced. P450c17 catalyzes both the 17α-hydroxylase activity needed to convert C21 17-deoxysteroids to their 17-hydroxy counterparts and the 17,20 lyase activity that cleaves the bond between carbon atoms 17 and 20 to convert C21 steroids to C19 steroids (for a review, see Ref. (1). In the absence of P450c17, the human adrenal zona glomerulosa produces the mineralocorticoids deoxycorticosterone and aldosterone; in the adrenal zona fasciculata, P450c17 catalyzes 17α-hydroxylase activity but not 17,20 lyase activity to produce the glucocorticoid cortisol; and in the adrenal zona reticularis, testicular Leydig cells, and ovarian theca cells, P450c17 produces dehydroepiandrosterone (DHEA)2 and androstenedione, the principal C19 steroids that are converted to testosterone and estradiol (see Fig. 1A). P450c17 is an evolutionarily ancient enzyme essential for sex steroid synthesis in all vertebrates. Thus, P450c17 is the qualitative regulator of steroidogenesis, determining the class of steroid produced. The 17,20 lyase activity of P450c17 varies considerably among species: pig (2–4), trout (5), and frog (6) P450c17 catalyze 17,20 lyase activity with both 17-OH-pregnenolone (17-OH-Preg; a Δ5-steroid) and 17-OH-progesterone (a Δ4-steroid); bovine P450c17 also catalyzes 17,20 lyase activity with both substrates but with a preference for 17-OH-Preg (7); rat (8) (and presumably mouse, which only differs by two residues), hamster (9), and guinea pig (10) P450c17 strongly prefer 17-OH-progesterone; and human P450c17 catalyzes 17,20 lyase activity almost exclusively with 17-OH-Preg (11–13).
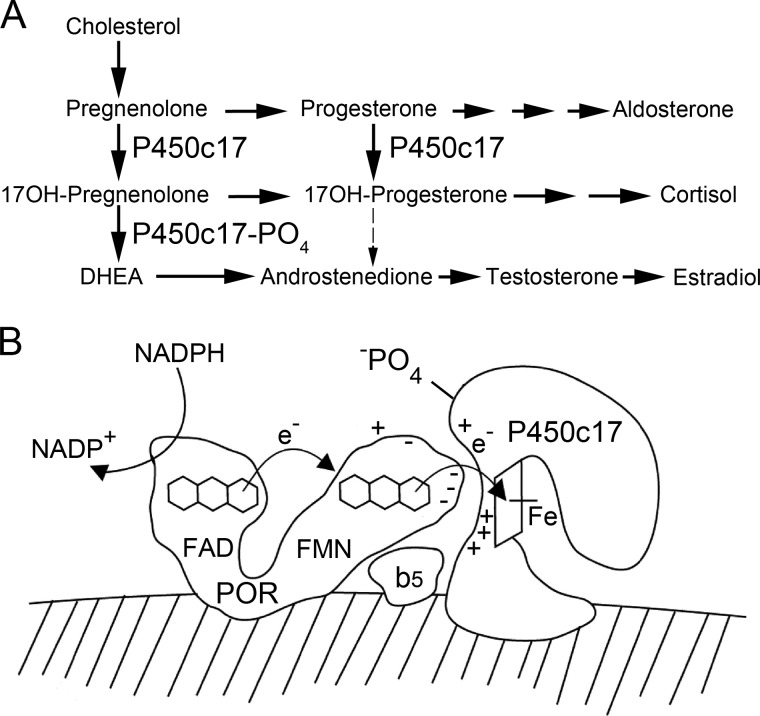
FIGURE 1.
Role of P450c17 in steroidogenesis. A, simplified human steroidogenic pathways. In the absence of P450c17, steroidal C21 17-deoxy precursors are directed toward the production of the mineralocorticoid aldosterone. In the presence of the 17α-hydroxylase activity of P450c17, C21 17-deoxysteroids are converted to C21 17-hydroxysteroid precursors of the glucocorticoid, cortisol. In the presence of the 17,20 lyase activity of P450c17, C21 17-hydroxysteroids are converted to C19 17-hydroxysteroid precursors of sex steroids. Note that human P450c17 does not convert significant amounts of 17-OH-progesterone to androstenedione, but this reaction occurs in most non-primate mammals. B, mechanism of P450c17 action. Electrons from NADPH are accepted by the FAD group of POR, which then undergoes a conformational change that permits the electrons to be transferred to the FMN group. POR then returns to its initial conformation, and its FMN domain interacts electrostatically with P450c17, permitting electron transfer to the heme iron of the P450, which mediates catalysis. The 17,20 lyase activity of P450c17 can be facilitated by cytochrome b5, apparently acting via an allosteric mechanism, and by the Ser/Thr phosphorylation of P450c17. ©Walter L. Miller.
The single human CYP17A1 gene (14) produces a single species of mRNA (15) and protein, which catalyzes both the hydroxylase and lyase activities on a single active site (16). Nevertheless, the 17,20 lyase activity of human adrenal P450c17 is developmentally regulated. Concentrations of cortisol, an index of 17α-hydroxylase activity, remain essentially constant as a function of age, whereas concentrations of DHEA and its sulfate rise 100-fold at adrenarche (17), which is an event in the primate adrenal that is approximately contemporaneous with but independent of puberty (18–20). Thus, the mechanisms separately regulating these two activities of P450c17 are of substantial enzymological interest.
As with other microsomal (type 2) cytochrome P450 enzymes, catalysis by P450c17 begins with transfer of two electrons from NADPH to the flavin adenine dinucleotide (FAD) moiety of the two-flavin protein P450 oxidoreductase (POR). Electron acceptance by the FAD moiety elicits a conformational change in POR, bringing the FAD close to the flavin mononucleotide (FMN) moiety, which then accepts the electrons from the FAD; the POR molecule then reverts to its original, more open conformation, permitting the FMN domain to dock by charge-charge interactions with the redox partner binding site of the P450 acceptor molecule (21–24). The electrons then flow to the heme iron of P450c17, which mediates catalysis of both the 17-hydroxylase and 17,20 lyase reactions apparently by a ferryl oxene mechanism (16) (Fig. 1B).
Because of its key role in the production of sex steroids, its potential role in hyperandrogenic disorders, and its potential as a therapeutic target for sex steroid-dependent cancers, the 17,20 lyase activity of human P450c17 has received increased attention (25, 26). Studies with pure recombinant proteins and with transfected cells indicate that 17,20 lyase activity is controlled by factors that influence the efficiency of electron transfer from P450 oxidoreductase to P450c17 (27, 28). Thus, 17,20 lyase activity can be augmented by three post-translational mechanisms. First, an increased molar ratio of POR to P450c17 in vitro increases the ratio of C19 to C21 steroidal products (4, 11). Second, cytochrome b5 increases 17,20 lyase activity (29–33). Although some P450 enzymes may receive the second electron in the P450 cycle from cytochrome b5, and cytochrome b5 can receive electrons from POR, the redox potentials of cytochrome b5 and one electron-reduced P450 are unfavorable for cytochrome b5-to-P450 electron transfer. Both apocytochrome b5 (which lacks heme) and Mn2+-b5 (which cannot transfer electrons) can stimulate 17,20 lyase activity of human P450c17 (12, 34, 35), indicating that cytochrome b5 does not act as an electron donor but rather functions by an allosteric mechanism. Functional and modeling studies suggest that cytochrome b5 promotes the interaction of P450c17 and POR (34), but direct evidence for a heterotrimeric complex is lacking. NMR studies indicate that cytochrome b5 and POR compete for binding to P450c17 and that binding of P450c17 to cytochrome b5 is stronger in the presence of pregnenolone than in the presence of 17-OH-Preg (36). The weaker interaction between cytochrome b5 and P450c17 when 17-OH-Preg is the substrate promotes 17,20 lyase activity by enhancing the interaction of P450c17 with POR (36). Third, serine/threonine (Ser/Thr) phosphorylation, but not tyrosine phosphorylation, of P450c17 will increase 17,20 lyase activity (34, 37–40). Either maximal Ser/Thr phosphorylation of P450c17 or maximal concentrations of b5 will maximize 17,20 lyase activity so that adding b5 to phosphorylated P450c17 elicits no further increase in lyase activity (34), indicating that both factors operate via the same mechanism, presumably optimizing interactions with POR.
The polycystic ovary syndrome (PCOS) is a hyperandrogenic disorder affecting about 6% of women of reproductive age (41, 42). There are multiple forms of PCOS: some women appear to have an autosomal dominant disorder characterized by both hyperandrogenemia and insulin resistance (43–45). Because Ser/Thr phosphorylation of P450c17 will increase androgen biosynthesis and Ser/Thr phosphorylation of the β chain of the insulin receptor causes postreceptor insulin resistance (46–49), it has been suggested that a gain-of-function mutation affecting the Ser/Thr phosphorylation of both P450c17 and the β chain of the insulin receptor, either directly affecting the kinase or affecting an upstream signal transduction factor, may explain both the hyperandrogenism and insulin resistance of some autosomal dominant forms of PCOS by a single mechanism (37, 50, 51). The mechanism of the insulin resistance in PCOS remains controversial, but Ser/Thr phosphorylation of the β chain of the insulin receptor (52, 53) or its substrate IRS-1 (54) appears to be involved. However, the Ser/Thr kinase(s) that phosphorylates P450c17 and/or the β chain of the insulin receptor has not been identified.
The phosphorylation of P450c17 in human NCI-H295 adrenocortical carcinoma cells appears to be inducible by cAMP (37) and can be reversed by protein phosphatase 2A (PP2A) but not by PP4 or PP6 (39). In an initial search for such a kinase, we performed microarray and inhibitor studies in NCI-H295A cells (55). Of the 518 kinases in the human “kinome,” 278 are Ser/Thr kinases (56), and microarrays identified only 145 of these Ser/Thr kinases in NCI-H295A cells. Several kinases that are implicated in insulin action were absent, including protein kinase A (PKA), mammalian target of rapamycin, phosphatidylinositol 3-kinase (PI3K), mitogen-activated kinase 3 (MAPK3)/extracellular signal-regulated kinase 1 (ERK1), MAPK1/ERK2, MAP2K1/mitogen-activated protein kinase kinase 1 (MEK1), and MAP2K2/MEK2. Thus, the pathways involving ERK1/2 and MEK1/2 that have been implicated in the insulin resistance of PCOS muscle cells (57, 58) cannot be involved in P450c17 phosphorylation in NCI-H295A cells. Incubations with a panel of kinase inhibitors appeared to exclude the PKA/PI3K/Akt and calcium/calmodulin/MEK pathways and suggested a role for Rho-associated, coiled coil-containing protein kinase 1 (ROCK1). However, although recombinant ROCK1 could phosphorylate P450c17 in vitro, this phosphorylation did not affect 17,20 lyase activity; hence ROCK1 may act upstream in a pathway leading to the unidentified kinase (55). Of the 145 Ser/Thr kinases found in NCI-H295A cells, only six were induced more than 2-fold by 8-Br-cAMP, and knockdown of each of these by RNA interference had no effect on 17,20 lyase activity (55). One of the cAMP-inducible kinases was MAPK13, also known as p38δ, which is a major okadaic acid-responsive MAPK and can inactivate ERK1/2 activity directly (59). Therefore, we considered the potential roles of other p38 isoforms. This family of Ser/Thr kinases consists of p38α (MAPK14), p38β (MAPK11), p38δ (MAPK13), and p38γ (MAPK12); p38α and p38β are expressed ubiquitously, whereas p38δ is confined to muscle, and p38γ is found in lung, kidney, gut, and some endocrine tissues (60). Both p38α and p38β have been implicated in regulating steroidogenesis (61–63).
EXPERIMENTAL PROCEDURES
Materials
Activated recombinant human p38α and recombinant cytochrome b5 were from Invitrogen. Pyridinyl imidazole drugs SB202190 and SB203580 were from EMD Millipore (Billerica, MA). Our anti-P450c17 antiserum has been described (11), and anti-FLAG antibody was from Sigma-Aldrich. p38α and p38β expression vectors were kindly provided by Dr. Jiahuai Han (The Scripps Research Institute) (64).
Cell Cultures and Transfections
NCI-H295A cells are an adherent subline (65) of human adrenocortical carcinoma NCI-H295 cells (66) that express all adrenal steroidogenic enzymes in a physiologically appropriate, hormonally responsive fashion (67). Cells were cultured in plastic dishes at 37 °C and 5% CO2 in RPMI 1640 medium supplemented with 2% fetal calf serum (FCS), 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml selenium, and 50 μg/ml gentamycin. Cells were transfected using Effectene (Qiagen, Valencia, CA) according to the manufacturer's protocol.
Bacterial Expression of P450c17 and POR
The pCWH17-mod(His)4 expression plasmid containing the cDNA for human P450c17 with N-terminal modifications that facilitate bacterial expression (68) and modified to contain three glycines followed by six histidines (C-terminal G3H6 extension) (69) or a vector expressing human POR carrying a C-terminal G3H6 extension and lacking 27 N-terminal residues (70) was transformed into Escherichia coli strain JM109. A single colony of E. coli strain JM109 transformed with pCWH17mod(G3H6) was first grown to saturation in 10 ml of Luria-Bertani medium containing 100 μg/ml ampicillin at 37 °C with shaking at 220 rpm and then added to 1 liter of terrific broth containing 100 μg/ml carbenicillin, 40 μm FeCl3, 4 μm ZnCl2, 2 μm CoCl2, 2 μm Na2MoO4, 2 μm CaCl2, 2 μm CuCl2, 2 μm H3BO3, 1 mm thiamine and grown at 37 °C with shaking at 220 rpm to an A600 of 0.2; δ-aminolevulinic acid was then added to 0.5 mm. The culture temperature was lowered to 28 °C, and at an A600 of 0.4, isopropyl 1-thio-β-d-galactopyranoside was added to 0.5 mm, and the culture was shaken at 120–150 rpm at 28 °C for 2 days. The culture was iced to 4 °C, and the bacteria were harvested at 5,000 × g for 10 min and resuspended in 20 ml of 0.1 m Tris acetate, pH 7.8, 0.5 mm EDTA, 0.5 m sucrose (TES buffer), and lysozyme was added to 0.2 mg/ml for ≥2 h. Spheroplasts were collected at 12,000 × g for 10 min and homogenized in 50 mm potassium phosphate, pH 7.4, 10 mm MgCl2, 0.1 mm EDTA, 20% glycerol, 1 mm DTT, 40 μm progesterone, 0.2 mm PMSF, 1 μg/ml DNase I (buffer A). Homogenized spheroplasts were sonicated at 4 °C with eight to nine cycles of 25 s on, 30 s off using a 550 Sonic Dismembranator (Fisher Scientific) at 30% power. The lysate was cleared by centrifugation at 12,000 × g for 10 min, and the supernatant was centrifuged at 265,000 × g for 45 min to pellet the membranes. The membrane-associated P450c17 in the pellet was solubilized in 50 mm potassium phosphate, pH 7.4, 20% glycerol, 0.5% Triton X-114, 0.2% sodium cholate, 10 mm imidazole, 40 μm progesterone, 0.1 mm PMSF (buffer B) and then cleared by centrifugation at 27,000 × g for 25 min. The supernatant (20 ml) was loaded slowly onto a Ni-NTA-agarose column (1.6 × 2.5 cm), which had been pre-equilibrated with 25 mm potassium phosphate, pH 7.4, 10% glycerol, 0.1% Triton X-100, 0.1% sodium cholate, 10 mm imidazole, 40 μm progesterone, 0.1 mm PMSF (buffer C). The column was washed with buffer C, then with buffer D (buffer C plus 300 mm NaCl), and finally with 25 mm potassium phosphate, pH 7.4, 10% glycerol, 0.1% Triton X-100, 0.1% sodium cholate, 50 mm imidazole, 40 μm progesterone, 0.1 mm PMSF (buffer E). The P450c17 was eluted with 25 mm potassium phosphate, pH 7.4, 10% glycerol, 0.2% sodium cholate, 300 mm imidazole, 0.1 mm PMSF (buffer F) and desalted into 25 mm potassium phosphate, pH 7.4, 10% glycerol, 0.2 mm DTT, 0.1% sodium cholate (buffer G) by passage through a Sephadex G-25 column (1.6 × 10 cm) or by dialysis against Buffer G. All purification steps were performed at ∼0–4 °C. Protein concentration was determined by the Bradford method, and the purity was examined by SDS-PAGE and Coomassie Brilliant Blue staining. P450 content was determined using a molar extinction difference of 91 cm−1 mm−1 between 450 and 490 nm (71). The base-line spectrum from 400 to 500 nm of 5 mg of protein was measured after the addition of 20 mg of sodium dithionite, and the reduced CO spectrum was measured after the sample was bubbled gently with carbon monoxide for 1 min.
Mutagenesis and Expression
The P450c17 vector with the C-terminal G3H6 extension (69) was modified by site-directed mutagenesis as described (55) to express P450c17 carrying the mutations T341A, S427A, and T341A/S427A. Mutagenic oligonucleotide sequences are given in Table 1.
TABLE 1.
Oligonucleotide sequences
nt, nucleotides.
| Type of assay | Sequences (5′ to 3′) |
|---|---|
| shRNA | |
| MAPK14 (nt 942–960 in NCBI Reference Sequence NM_001315.2) | GTAATCTAGCTGTGAATGA |
| MAPK11 (nt 815–833 in NCBI Reference Sequence NM_002751.5) | GAACACGCCCGGACATATA |
| Scrambled | ACATTGAAGCGAAGAATAA |
| Site-directed mutagenesis | |
| T341A | GTGGGTTTCAGCCGCGCACCAACTATCAGTGAC |
| T341D | GTGGGTTTCAGCCGCGACCCAACTATCAGTGAC |
| T341E | GTGGGTTTCAGCCGCGAACCAACTATCAGTGAC |
| S427A | GGGACCCAGCTCATCGCACCGTCAGTAAGCTAT |
| S427D | GGGACCCAGCTCATCGACCCGTCAGTAAGCTAT |
| S427E | GGGACCCAGCTCATCGAACCGTCAGTAAGCTAT |
Phosphorylation and Dephosphorylation of Human Wild-type and Mutant P450c17
Bacterially expressed wild-type human P450c17 and the mutants T341A, S427A, and T341A/S427A were incubated with catalytically active recombinant human p38α (Invitrogen) in 30 mm HEPES, 30 mm MgCl2, 500 μm [γ-32P]ATP (6000 Ci/mmol; PerkinElmer Life Sciences) for 20 min at 30 °C. P450c17 was captured onto Ni-NTA beads (Qiagen) and washed 10 times with 50 mm Tris-HCl, pH 7.5, 500 mm NaCl. Bound protein was eluted with SDS sample buffer at 95 °C for 2 min, displayed by a 10% polyacrylamide-SDS gel, and detected using a Storm phosphorimaging system (GE Healthcare). Dephosphorylation of 5 pmol of P450c17 that had been phosphorylated by p38α was done by 1-h incubation at 30 °C with 1 or 10 units of recombinant human PP2A (Invitrogen).
Preparation of Short Hairpin RNA (shRNA) Vectors and Knockdown Procedures
We constructed shRNA expression vectors to express short hairpin RNA constructs against p38α, p38β, and a control scrambled sequence in LentiLox PLL 3.7 (72). The constructs were co-transfected with packaging vectors into HEK-293T cells by Effectene (Qiagen) for 2 days. The supernatants containing lentiviral particles were filtered on 0.45-μm polyvinylidene difluoride membranes, used to transduce human adrenal NCI-H295A cells for 2 days, and assayed for 17-hydroxylase and 17,20 lyase activities.
Assays of Steroidogenic Activities
[14C]Progesterone (114.4 Ci/mmol) and [3H]pregnenolone (22.9 Ci/mmol) were purchased from PerkinElmer Life Sciences. 17-OH-[7-3H]Preg was produced from [3H]pregnenolone in collaboration with Professor Richard J. Auchus (University of Michigan, Ann Arbor, MI) as described (73) using bacterially expressed human P450c17 and POR prepared as described above. The [3H]Preg (∼60 μCi) was dried under N2, dissolved in 10 μl of EtOH, and combined with 0.4 ml of 50 mm potassium phosphate buffer, pH 7.4 supplemented with 6 mm potassium acetate, 10 mm MgCl2, 1 mm reduced glutathione, 20% glycerol and also containing 1.8 μg of purified P450c17 (80 nm), 10 μg of purified human POR (320 nm), 30 μm dilaurylphosphatidylcholine, and 1 mm NADPH. The mixture was incubated for 40 min at 37 °C, and the steroid products were extracted with dichloromethane and dried under N2. The extract was dissolved in 25 μl of dichloromethane and loaded onto a 2-ml column of silica gel (Dynamic Adsorbants, Norcross, GA) previously washed with hexane, and the steroids were eluted with a stepwise gradient of ethyl acetate (10–40%) in hexane. The eluate was collected in 2-ml fractions and assayed by HPLC, and the fractions containing 17-OH-Preg were pooled and concentrated. The resulting product was assayed by thin layer chromatography (TLC) in a 3:1 chloroform:ethyl acetate solvent system as described (74); >98% of the radioactivity was in 17-OH-Preg.
Enzyme Kinetics
Purified bacterially expressed wild-type or mutant P450c17 (10 pmol) with or without phosphorylation by p38α was assayed for 17,20 lyase activity with 20 pmol of POR in 100 mm potassium phosphate, 6 mm potassium acetate, 20 μg of 1,2-dioleoyl-sn-glycero-3-phosphocholine, 10 mm MgCl2, 1 mm reduced glutathione, 20% glycerol, 3 units of glucose-6-phosphate dehydrogenase, 0.1 mm glucose 6-phosphate and incubated for 3 h at 37 °C with 17-OH-[3H]Preg in a total volume of 200 μl. Steroids were extracted, analyzed by TLC, and quantitated. Lineweaver-Burk plots were made in GraphPad Prism (GraphPad Software, San Diego, CA). Statistical analyses were performed using two-tailed unpaired t tests, and significance was accepted for tests where the p value was <0.05. P450c17 kinetic parameters (Km and Vmax) were calculated as mean ± S.E. (three independent experiments, each performed in triplicate), whereas all other comparisons were calculated as mean ± S.D.
RESULTS
p38α Increases 17,20 Lyase Activity in Adrenal Cells
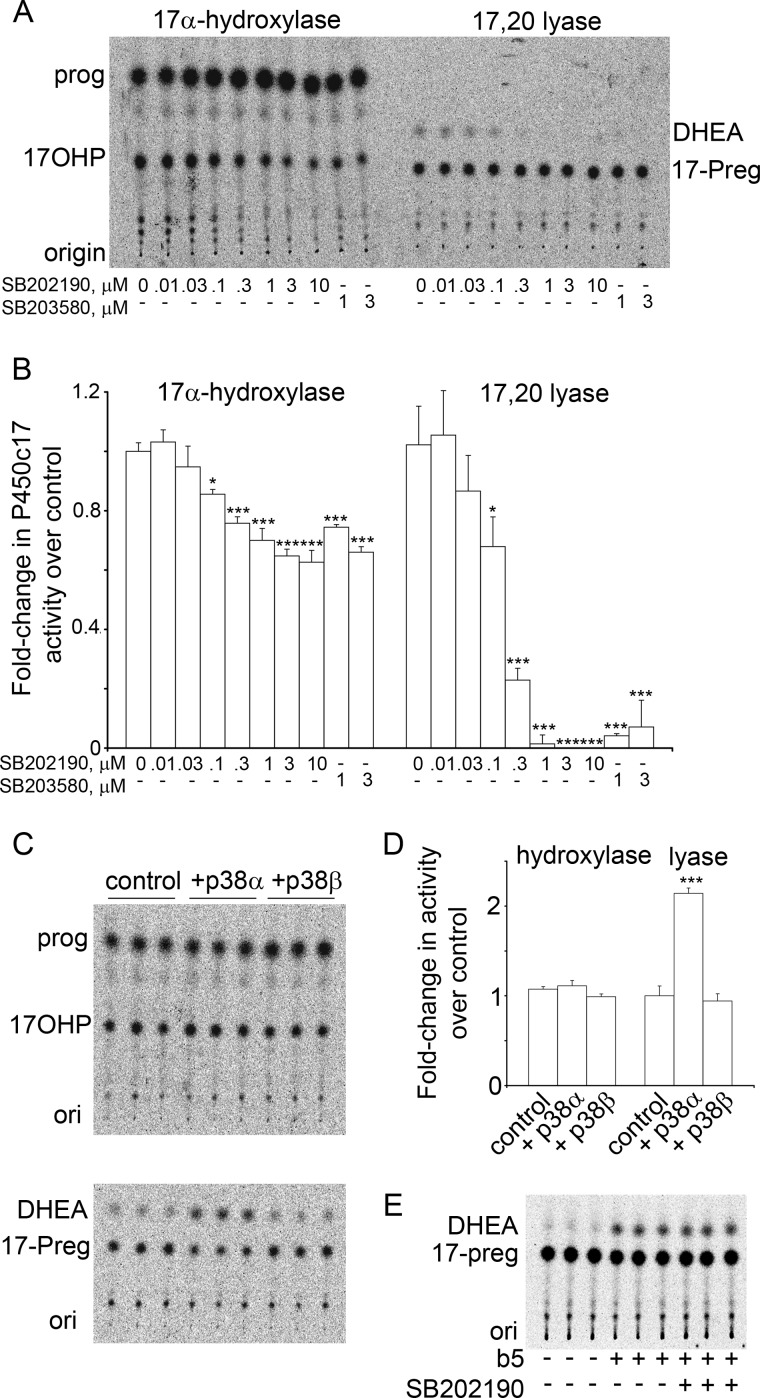
Among the kinases that were induced by cAMP in NCI-H295A cells was MAPK13 (55), also known as p38δ, which is a major okadaic acid-responsive MAPK and can inactivate ERK1/2 activity directly (59). Therefore, we considered the potential roles of other p38 isoforms. This family of Ser/Thr kinases consists of p38α (MAPK14), p38β (MAPK11), p38δ (MAPK13), and p38γ (MAPK12); p38α and p38β are expressed ubiquitously, whereas p38δ is confined to muscle, and p38γ is found in lung, kidney, gut, and some endocrine tissues (60). Both p38α and p38β have previously been implicated in regulating steroidogenesis (61–63). To determine whether p38α and p38β might influence 17,20 lyase activity, we first tested the activities of the pyridinyl imidazole drugs SB202190 and SB203580, which selectively inhibit p38α and p38β but not p38δ or p38γ (60), using human adrenocortical NCI-H295A cells (67) in which the phosphorylation of P450c17 and its action on 17,20 lyase activity were first described (37). We used NCI-H295A cells deprived of serum for 4 h because serum starvation, but not insulin starvation, augments 17,20 lyase activity in these cells (55). The 17α-hydroxylase activity of human P450c17 is approximately the same with either pregnenolone or progesterone as the substrate; therefore, we assessed 17α-hydroxylase activity by the conversion of [14C]progesterone to 17-OH-progesterone because human P450c17 has minimal 17,20 lyase activity with 17-OH-progesterone and does not convert it to androstenedione (11–13). Similarly, 17,20 lyase activity was assessed by conversion of 17-OH-[3H]Preg to DHEA. Steroids were separated by TLC and quantitated by phosphorimage analysis. Neither drug affected 17α-hydroxylase activity, whereas 1 μm SB202190 or SB203580 eliminated detectable 17,20 lyase activity (Fig. 2, A and B).
FIGURE 2.
p38α increases the 17,20 lyase activity of P450c17. A, TLC of one inhibitor experiment. Serum-starved NCI-H295A cells were treated for 4 h with the indicated doses (μm) of SB202190 and SB203580 and incubated with 1 μm [14C]progesterone (prog) (1 h) or 1 μm 17-OH-[3H]Preg (17-Preg) (1 h), and extracted media were analyzed by TLC. Both drugs inhibit 17,20 lyase activity (DHEA production) at 1 μm. B, pooled phosphorimaging data of three experiments done as in A. Data are compared with untreated control. Error bars represent S.E.; *, p < 0.05; ***, p < 0.001. C, TLC of steroids from COS-1 cells co-transfected with expression vectors for P450c17 and p38α or p38β. 17α-Hydroxylase activity (upper panel) was unchanged; p38α, but not p38β, increased 17,20 lyase activity 2.2-fold (lower panel). D, pooled data from three experiments, performed as in panel C, each performed in triplicate. (Error bars represent S.E.; ***, p < 0.001). E, SB202190 is not an enzymatic inhibitor of P450c17. Recombinant human P450c17 was incubated with cytochrome b5 to confer 17,20 lyase activity; the molar ratio of cytochrome b5:P450c17:POR was 1:1:2. The reaction was initiated with 1 mm NADPH. The presence of 10 μm SB202190, which ablated detectable 17,20 lyase activity in the cell system of A and B, had no effect on the 17,20 lyase activity of purified P450c17. ori, origin; 17OHP, 17-OH-progesterone; 17-Preg, 17-OH-pregnenolone.
These data suggested that p38α or p38β might be involved in the regulation of 17,20 lyase activity. To determine whether only one or both of these kinases affected 17,20 lyase activity, we co-transfected non-steroidogenic COS-1 cells with expression vectors for P450c17 and for either p38α or p38β and measured 17α-hydroxylase and 17,20 lyase activities by conversion of radiolabeled steroids. In co-transfected COS-1 cells, p38α, but not p38β, reproducibly increased 17,20 lyase activity >2-fold, but neither kinase affected 17α-hydroxylase activity (Fig. 2, C and D).
Because some planar hydrocarbons having structures unrelated to steroids may act as competitive inhibitors of P450c17 (75), we determined whether the pyridinyl imidazole kinase inhibitors had a direct action on P450c17. When purified bacterially expressed human P450c17, POR, and cytochrome b5 were incubated with 10 μm SB202190, there was no effect on either 17α-hydroxylase or 17,20 activity (Fig. 2E). Thus, SB202190 affected an intracellular kinase that influences 17,20 lyase activity.
p38α Phosphorylates P450c17 in Vivo and in Vitro
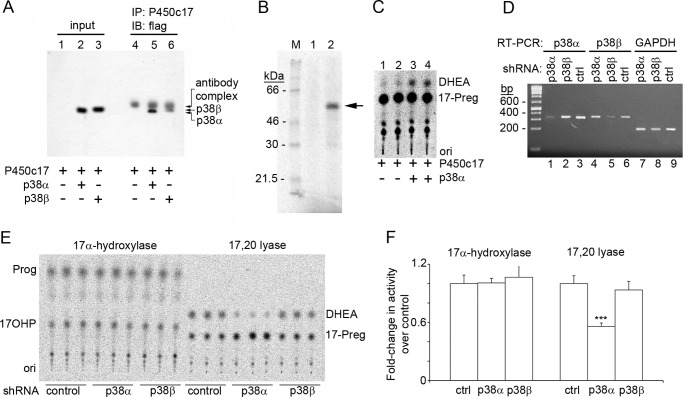
Because the p38α expressed in COS-1 cells might act at any of several levels in a signal transduction pathway that eventually leads to phosphorylation of P450c17, we sought to determine whether p38α could interact with and phosphorylate P450c17 in a cellular context. COS-1 cells were co-transfected with the vector expressing P450c17 and a vector expressing FLAG-tagged p38α or p38β. The P450c17-p38 complexes were immunoprecipitated with our high affinity antiserum to bacterially expressed human P450c17, displayed by SDS-PAGE, and analyzed by Western immunoblotting with anti-FLAG antiserum, which showed that p38α associated with P450c17 more avidly than did p38β in COS-1 cells (Fig. 3A).
FIGURE 3.
Interactions between P450c17 and p38α. A, both p38α and p38β interact with P450c17 in vivo. COS-1 cells were co-transfected with P450c17 and FLAG-tagged p38α or p38β and probed with anti-FLAG (lanes 1–3). The signal in lanes 2 and 3 shows expression of p38. COS-1 cell lysate was immunoprecipitated with anti-P450c17. Probing with anti-FLAG detects IgG in lanes 4–6 and co-immunoprecipitated p38α in lane 5 and p38β in lane 6. B, p38α phosphorylates P450c17 in vitro. Purified P450c17 was incubated with [γ-32P]ATP without (lane 1) or with (lane 2) activated recombinant human p38α, captured onto Ni-NTA beads, washed, eluted, and analyzed by SDS-PAGE and autoradiography. Arrow, phospho-P450c17. C, 17,20 lyase activity of P450c17 phosphorylated in vitro with p38α. Purified P450c17 was incubated without (duplicate lanes 1 and 2) or with (duplicate lanes 3 and 4) p38α and “cold” ATP and assayed for 17,20 lyase activity in the presence of NADPH, recombinant POR, and 17-OH-[3H]Preg. D, shRNA constructs specifically knock down p38α and p38β. Constructs directed against p38α, p38β, and a control scrambled sequence were transformed into NCI-H295A cells for 2 days followed by RT-PCR of p38α, p38β, and GAPDH RNA. Lanes 1–3, shRNA against p38α reduces p38α but not p38β; lanes 4–6, shRNA against p38β reduces p38β but not p38α; lanes 7–9, none of the shRNAs affects GAPDH. E, knockdown of p38α inhibits 17,20 lyase activity. Incubation with radiolabeled steroids shows the knockdown of p38α, but not p38β, inhibited 17,20 lyase activity. F, quantitation of three triplicate experiments as in E; error bars represent S.E. Knockdown of p38α inhibited 17,20 lyase activity 1.7-fold (***, p < 0.001). IP, immunoprecipitation; IB, immunoblot; ori, origin; ctrl, control; 17OHP, 17-OH-progesterone; 17-Preg, 17-OH-pregnenolone.
To determine whether increased 17,20 lyase activity associated with p38α resulted from direct P450c17 phosphorylation rather than some other COS-1 cell kinase that had been activated by p38α, we assessed the activity of recombinant, activated p38α on recombinant human P450c17 purified to apparent homogeneity (69). The recombinant human p38α could phosphorylate the highly purified bacterially expressed P450c17 in vitro (Fig. 3B). To assess the impact of phosphorylation by p38α on the activities of P450c17, we assessed the 17α-hydroxylase and 17,20 lyase activities on two aliquots of the same P450c17 preparation, one treated with p38α and the other untreated. Using bacterially expressed human P450 oxidoreductase (70) as the electron donor, the P450c17 phosphorylated by p38α in vitro exhibited 17,20 lyase activity, but the P450c17 that had not been phosphorylated had substantially less 17,20 lyase activity (Fig. 3C).
Knockdown of p38 Inhibits 17,20 Lyase Activity in Adrenal Cells
To determine whether a p38 kinase participates in the physiologic phosphorylation of P450c17 in vivo, we examined the effect of knocking down the expression of p38 isoforms in NCI-H295A cells. Using a lentivirus system (72), we built shRNA constructs designed to yield small inhibiting RNAs (siRNAs) against p38α, p38β, and a control scrambled sequence; propagated the viruses in HEK-293T cells; and then used the virus to transform NCI-H295A cells (Fig. 3D). Assays of the hydroxylase and lyase in the transformed cells showed that 17,20 lyase activity was inhibited 1.7-fold by shRNA knockdown of p38α but not by knockdown of p38β or by the scrambled shRNA control (Fig. 3, E and F). Thus, p38α augments the 17,20 lyase activity of P450c17 in vivo as well as in vitro.
p38α Phosphorylates P450c17 at a Non-canonical Site
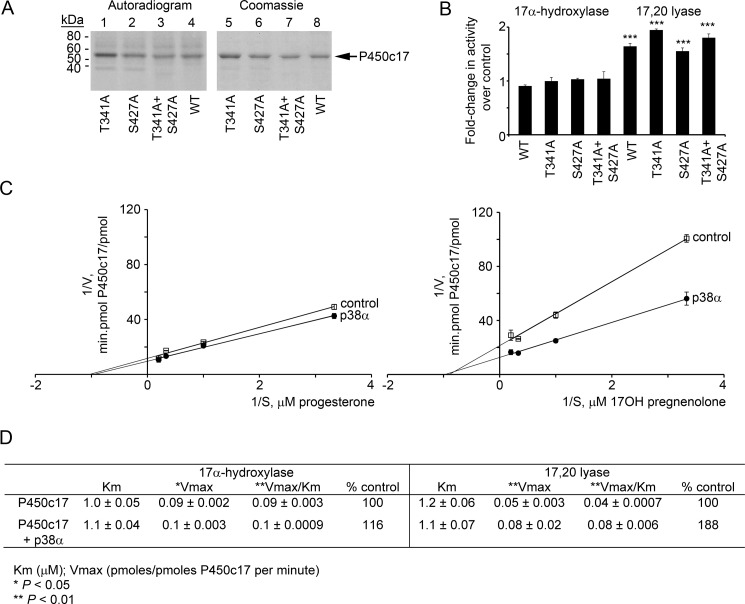
The consensus amino acid recognition sequence for p38α substrates is (Ser/Thr)-Pro (76) with 159 of 191 sites phosphorylated by p38α following this rule; this recognition sequence is typically assisted by docking motifs that may be dozens of residues upstream (77, 78). The Ser/Thr residue(s) of P450c17 phosphorylated by p38α that results in the increased 17,20 lyase activity has not yet been identified. Human P450c17 has 32 Ser residues and 25 Thr residues (15) of which only two, Thr-341 and Ser-427, are immediately followed by a proline. To test whether these consensus phosphorylation sites participate in the p38α-induced increase in the 17,20 lyase activity of P450c17, they were mutagenized to Ala both singly (T341A and S427A) and in combination (T341A/S427A), expressed, and purified to apparent homogeneity. Despite the mutation of the p38α consensus phosphorylation sites, the mutant proteins were phosphorylated in vitro by p38α to an equivalent extent as wild-type P450c17 (Fig. 4A). We then assayed the activities of these bacterially expressed mutants in comparison with the wild-type protein. All three mutants retained their p38α-induced acquisition of 17,20 lyase activity at a level that was indistinguishable from wild-type P450c17 (Fig. 4B). Thus, p38α appears to phosphorylate P450c17 at a non-canonical site(s).
FIGURE 4.
Phosphorylation of P450c17 does not occur at canonical (Ser/Thr)-Pro sites. A, the bacterially expressed human P450c17 mutants T341A, S427A, and T341A/S427A (lanes 1–3) and wild-type human P450c17 (lane 4) were phosphorylated in vitro with p38α, separated by 10% PAGE-SDS, and imaged by autoradiography (left) and by Coomassie staining (right). B, mutation of the canonical p38α sites in P450c17 does not affect the action of p38α. Wild-type (WT), T341A, S427A, and T341A/S427A P450c17 were expressed in COS-1 cells with co-transfection of a vector expressing p38α, and their 17α-hydroxylase and 17,20 lyase activities were compared with the activities of the P450c17 constructs without co-transfected p38α. Radiolabeled steroid products of the assays were separated by TLC, autoradiographed, and quantitated using Scion Image software (Frederick, MD). The 17α-hydroxylase activities of the phospho- and dephospho-P450c17 did not differ (left), but all four phosphorylated forms of P450c17 had significantly increased 17,20 lyase activity as assessed by Vmax/Km (p < 0.001 for all four bars). C, Lineweaver-Burk analysis of enzyme kinetics. Data are from three independent experiments, each performed in triplicate, and are shown ±S.E. (n = 3). D, kinetic data calculated from C. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Phosphorylation of P450c17 Selectively Augments the Velocity of 17,20 Lyase Activity
To understand how phosphorylation of P450c17 augments its 17,20 lyase activity, we assessed the kinetics of the 17,20 lyase reaction as catalyzed by either non-phosphorylated P450c17 or P450c17 phosphorylated in vitro by p38α. Bacterially expressed, purified P450c17 was phosphorylated with p38α and used for kinetic assays of 17,20 lyase activity compared with P450c17 that was not phosphorylated. Phosphorylation had no measurable effect on the maximum velocity (Vmax) or Michaelis constant (Km) of the 17α-hydroxylase reaction. By contrast, whereas both forms of P450c17 had similar Michaelis constants for the 17,20 lyase reaction, the phosphorylated P450c17 had a significantly greater Vmax (Fig. 4, C and D). A change in Km would suggest a conformational change that affects steroid binding or release; however, the only change was an increased Vmax. All available information about the promotion of 17,20 lyase activity converges on increasing the efficiency of electron receipt. Thus, our data suggest that the p38α-mediated phosphorylation of P450c17 affects its interaction with P450 oxidoreductase and hence substrate turnover.
P450c17 Phosphorylation by p38α Is Reversed by PP2A
We have shown previously that P450c17 that has been phosphorylated by endogenous kinase(s) from human adrenal NCI-H295A cells can be dephosphorylated by PP2A in a fashion that ablates kinase-induced 17,20 lyase activity (39). To test whether PP2A exerts a similar effect on P450c17 phosphorylated by p38α, we incubated bacterially expressed human P450c17 with p38α and [γ-32P]ATP and then dephosphorylated the phospho-P450c17 with PP2A. PP2A eliminated all detectable phosphate incorporation and returned the 17,20 lyase activity to the level seen without p38α phosphorylation (Fig. 5).
FIGURE 5.
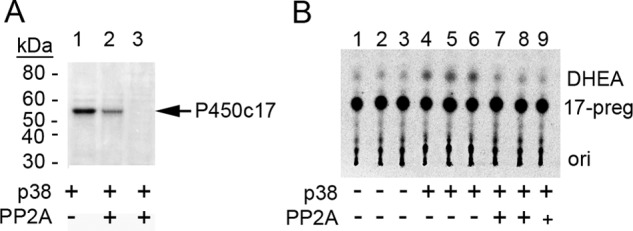
Phosphorylation of recombinant P450c17 by p38α is reversed by PP2A. A, P450c17 was phosphorylated by p38α and [γ-32P]ATP (lane 1) followed by incubation with either 1 (lane 2) or 10 units (lane 3) of PP2A and analyzed by autoradiography. Arrow, phospho-P450c17. B, 17,20 lyase activity of P450c17 phosphorylation by p38α and dephosphorylation by PP2A. ori, origin.
DISCUSSION
Adjusted for body surface area, human secretion of cortisol remains fairly constant across the life spectrum from infancy to old age, whereas the secretion of C19 adrenal steroids is low in childhood, begins to rise just before puberty, reaches maximum levels in the mid 20s (well after the completion of puberty), and then falls slowly to childhood levels in the elderly (17, 18). “Adrenarche” refers to the peripubertal rise in C19 steroids, especially DHEA, DHEA sulfate, and androstenedione (25, 79). These steroids are commonly called “adrenal androgens,” but they are androgen precursors rather than true androgens as they do not effectively activate the androgen receptor. Surveys of multiple mammalian species have shown that primates are unique in having high concentrations of adrenal C19 steroids and that the pattern of human age-related rise and fall in C19 steroids is unique (79–81). Rodents do not express P450c17 in their adrenals (82) and hence cannot be informative about adrenarche. Adrenarche is of interest evolutionarily because it appears to be a recent innovation, physiologically because the function (if any) of adrenal C19 steroids is controversial, endocrinologically because its regulatory mechanisms are unknown, and biochemically because it represents an unusual example of post-translational differential regulation of the two enzymatic activities.
The traditional questions concerning adrenarche have concerned its regulation, its intracellular mechanisms, and its role in human physiology. A number of searches for a hypothetical, specific adrenal androgen-stimulating hormone that would regulate C19 steroid synthesis by the adrenal zona reticularis, analogous to angiotensin II acting on the zona glomerulosa or ACTH acting on the zona fasciculata, have failed. The PCOS is a complex disorder of unknown cause(s) characterized by adrenal and ovarian hyperandrogenism, insulin resistance, and obesity, although many affected women have only some of these features (42). Adrenarche that begins earlier and that results in higher concentrations of C19 steroids frequently precedes PCOS, and many now regard premature exaggerated adrenarche as an early form of PCOS (83–85). Because of such connections between adrenal C19 steroid production and metabolic regulation, factors not specific to the adrenal including nutrition (86), insulin (87), insulin-like factors (50, 88, 89), leptin (38), and fibroblast growth factor (90) have been considered as potential triggers of adrenarche. However, the degree to which P450c17 is phosphorylated in different androgen-producing cell types is unknown, and all such “top down” approaches have yielded results of uncertain significance largely because a cellular model of adrenarche has not been developed.
It has been suggested that the increased adrenal and ovarian C19 steroid production and insulin resistance in PCOS are connected by an unidentified signal transduction pathway that ultimately increases the Ser/Thr phosphorylation of both P450c17 and either the insulin receptor or its substrate (37, 52). However, despite numerous studies (55, 57, 58), a relevant kinase has not been identified. We have now shown that P450c17 can be phosphorylated by p38α in a fashion that selectively augments its 17,20 lyase activity, thus providing a proof of principle for the regulation of 17,20 lyase activity by P450c17 phosphorylation. This discovery now permits a new “bottom up” approach to the study of adrenarche. Because p38α and other MAPKs are typically terminal components of a three-kinase cascade (91, 92), one can now apply similar approaches to identifying the kinase (presumably an MKK) that phosphorylates p38α in turn permitting identification of the initial MAP3K and ultimately the factor(s) that triggers that kinase. Fig. 6 outlines our current speculation about the nature of this pathway. Hormones (e.g. insulin, insulin-like growth factors, leptin, etc.), environmental agents, and dietary factors would activate a pathway that may involve ROCK1, which has previously been implicated in P450c17 phosphorylation (55). ROCK1 might act as an upstream scaffolding protein in a MAPK pathway (93) or might activate one of a large number of mitogen-activated protein kinase kinase kinases (M3Ks), which in turn would activate a mitogen-activated protein kinase kinase, most likely MKK3, -4, or -6, which are known to activate p38α and which are found in NCI-H295A cells (54). As shown herein, p38α can then phosphorylate P450c17 in a fashion that confers 17,20 lyase activity. We have shown previously that P450c17 is specifically dephosphorylated by PP2A, which in turn may be inhibited by the phosphoprotein SET (39). This SET/PP2A pathway has recently been confirmed in the mouse ovary (94) and may participate in cross-talk with other second messenger pathways. Although our approach may or may not identify a triggering event for adrenarche, it will illuminate the intracellular pathways regulating adrenal C19 steroid synthesis and may reveal links to insulin signal transduction, permitting better understanding of PCOS and potentially identifying new treatment targets. However, the identification of p38α as a kinase that augments 17,20 lyase activity by phosphorylating P450c17 does not exclude the possibility that other kinases may also exert this action on P450c17.
FIGURE 6.
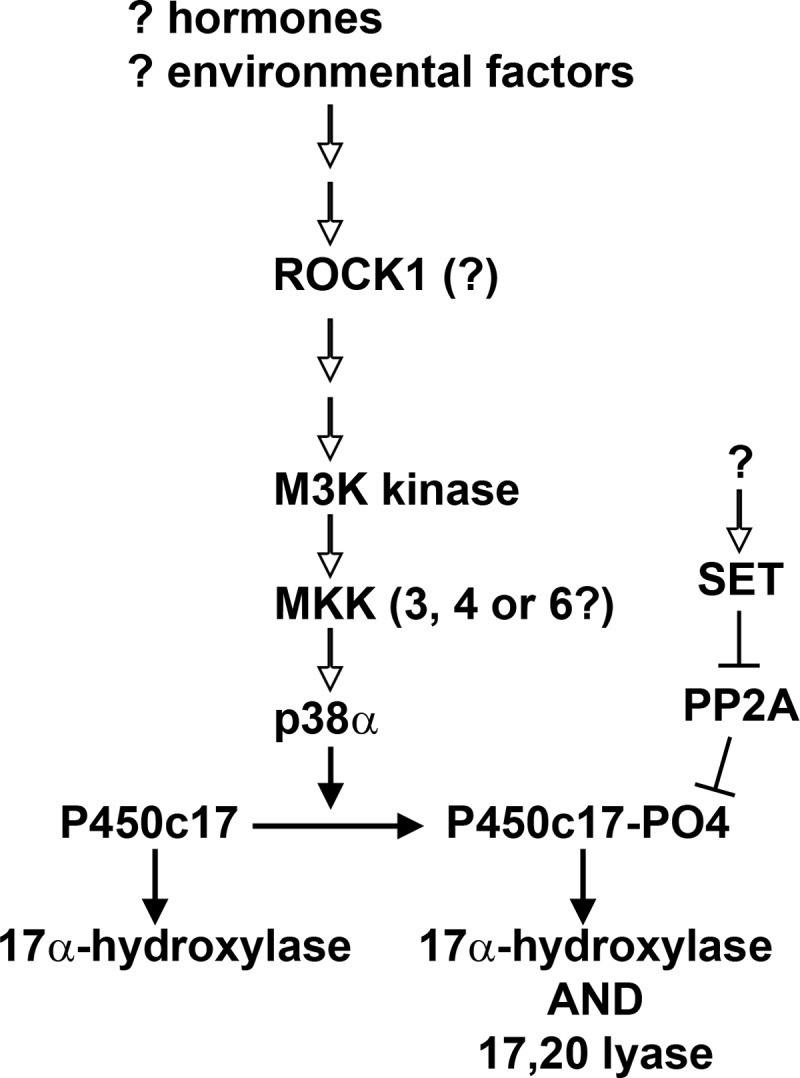
Simplified hypothetical model of signaling leading to P450c17 phosphorylation. We suggest that hormones and other factors activate an intracellular pathway that may include ROCK1, eventually leading to an M3K; the number of steps involved and potential sites of cross-talk remain undetermined. An activated M3K would activate a mitogen-activated protein kinase kinase, such as MKK3, -4, or -6, which would then activate p38α, permitting phosphorylation of P450c17 and the acquisition of 17,20 lyase activity. P450c17 is dephosphorylated by PP2A, which in turn can be inhibited by the phosphoprotein SET. Potential cross-talk with other second messenger pathways is not shown. Steps that have been established experimentally are shown with closed arrowheads, and hypothetical steps are shown with open arrowheads.
Acknowledgment
We thank Izabella Damm for excellent technical assistance.
This work was supported in part by National Institutes of Health Grants R01DK37922 and R01GM73020. This work was also supported by a grant from the University of California San Francisco Academic Senate and a private donation.
- DHEA
- dehydroepiandrosterone
- POR
- P450 oxidoreductase
- Preg
- pregnenolone
- PCOS
- polycystic ovary syndrome
- ROCK1
- Rho-associated, coiled coil-containing protein kinase 1
- 8-Br-cAMP
- 8-bromoadenosine 3′,5′-cyclic monophosphate
- Ni-NTA
- nickel-nitrilotriacetic acid
- M3K
- mitogen-activated protein kinase kinase kinase
- PP
- protein phosphatase
- MKK
- mitogen-activated protein kinase kinase.
REFERENCES
- 1. Miller W. L., Auchus R. J. (2011) The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nakajin S., Shively J. E., Yuan P. M., Hall P. F. (1981) Microsomal cytochrome P-450 from neonatal pig testis: two enzymatic activities (17α-hydroxylase and C17,20-lyase) associated with one protein. Biochemistry 20, 4037–4042 [DOI] [PubMed] [Google Scholar]
- 3. Nakajin S., Hall P. F. (1981) Microsomal cytochrome P-450 from neonatal pig testis. Purification and properties of a C21 steroid side-chain cleavage system (17α-hydroxylase-C17,20 lyase). J. Biol. Chem. 256, 3871–3876 [PubMed] [Google Scholar]
- 4. Yanagibashi K., Hall P. F. (1986) Role of electron transport in the regulation of the lyase activity of C21 side-chain cleavage P-450 from porcine adrenal and testicular microsomes. J. Biol. Chem. 261, 8429–8433 [PubMed] [Google Scholar]
- 5. Sakai N., Tanaka M., Adachi S., Miller W. L., Nagahama Y. (1992) Rainbow trout cytochrome P-450c17 (17α-hydroxylase/17,20-lyase). cDNA cloning, enzymatic properties and temporal pattern of ovarian P-450c17 mRNA expression during oogenesis. FEBS Lett. 301, 60–64 [DOI] [PubMed] [Google Scholar]
- 6. Lutz L. B., Cole L. M., Gupta M. K., Kwist K. W., Auchus R. J., Hammes S. R. (2001) Evidence that androgens are the primary steroids produced by Xenopus laevis ovaries and may signal through the classical androgen receptor to promote oocyte maturation. Proc. Natl. Acad. Sci. U.S.A. 98, 13728–13733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zuber M. X., Simpson E. R., Waterman M. R. (1986) Expression of bovine 17α-hydroxylase cytochrome P-450 cDNA in nonsteroidogenic (COS 1) cells. Science 234, 1258–1261 [DOI] [PubMed] [Google Scholar]
- 8. Fevold H. R., Lorence M. C., McCarthy J. L., Trant J. M., Kagimoto M., Waterman M. R., Mason J. I. (1989) Rat P45017α from testis: characterization of a full-length cDNA encoding a unique steroid hydroxylase capable of catalyzing both Δ4- and Δ5-steroid-17,20-lyase reactions. Mol. Endocrinol. 3, 968–975 [DOI] [PubMed] [Google Scholar]
- 9. Mathieu A. P., Auchus R. J., LeHoux J. G. (2002) Comparison of the hamster and human adrenal P450c17 (17α-hydroxylase/17,20-lyase) using site-directed mutagenesis and molecular modeling. J. Steroid Biochem. Mol. Biol. 80, 99–107 [DOI] [PubMed] [Google Scholar]
- 10. Tremblay Y., Fleury A., Beaudoin C., Vallée M., Bélanger A. (1994) Molecular cloning and expression of guinea pig cytochrome P450c17 cDNA (steroid 17α-hydroxylase/17,20 lyase): tissue distribution, regulation, and substrate specificity of the expressed enzyme. DNA Cell Biol. 13, 1199–1212 [DOI] [PubMed] [Google Scholar]
- 11. Lin D., Black S. M., Nagahama Y., Miller W. L. (1993) Steroid 17α-hydroxylase and 17,20-lyase activities of P450c17: contributions of serine106 and P450 reductase. Endocrinology 132, 2498–2506 [DOI] [PubMed] [Google Scholar]
- 12. Auchus R. J., Lee T. C., Miller W. L. (1998) Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J. Biol. Chem. 273, 3158–3165 [DOI] [PubMed] [Google Scholar]
- 13. Flück C. E., Miller W. L., Auchus R. J. (2003) The 17, 20-lyase activity of cytochrome P450c17 from human fetal testis favors the Δ5 steroidogenic pathway. J. Clin. Endocrinol. Metab. 88, 3762–3766 [DOI] [PubMed] [Google Scholar]
- 14. Picado-Leonard J., Miller W. L. (1987) Cloning and sequence of the human gene for P450c17 (steroid 17α-hydroxylase/17,20 lyase): similarity with the gene for P450c21. DNA 6, 439–448 [DOI] [PubMed] [Google Scholar]
- 15. Chung B. C., Picado-Leonard J., Haniu M., Bienkowski M., Hall P. F., Shively J. E., Miller W. L. (1987) Cytochrome P450c17 (steroid 17α-hydroxylase/17,20 lyase): cloning of human adrenal and testis cDNAs indicates the same gene is expressed in both tissues. Proc. Natl. Acad. Sci. U.S.A. 84, 407–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Auchus R. J., Miller W. L. (1999) Molecular modeling of human P450c17 (17α-hydroxylase/17,20-lyase): insights into reaction mechanisms and effects of mutations. Mol. Endocrinol. 13, 1169–1182 [DOI] [PubMed] [Google Scholar]
- 17. Orentreich N., Brind J. L., Rizer R. L., Vogelman J. H. (1984) Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J. Clin. Endocrinol. Metab. 59, 551–555 [DOI] [PubMed] [Google Scholar]
- 18. Apter D., Pakarinen A., Hammond G. L., Vihko R. (1979) Adrenocortical function in puberty. Serum ACTH, cortisol and dehydroepiandrosterone in girls and boys. Acta Paediatr. Scand. 68, 599–604 [DOI] [PubMed] [Google Scholar]
- 19. Sklar C. A., Kaplan S. L., Grumbach M. M. (1980) Evidence for dissociation between adrenarche and gonadarche: studies in patients with idiopathic precocious puberty, gonadal dysgenesis, isolated gonadotropin deficiency, and constitutionally delayed growth and adolescence. J. Clin. Endocrinol. Metab. 51, 548–556 [DOI] [PubMed] [Google Scholar]
- 20. Arlt W., Martens J. W., Song M., Wang J. T., Auchus R. J., Miller W. L. (2002) Molecular evolution of adrenarche: structural and functional analysis of P450c17 from four primate species. Endocrinology 143, 4665–4672 [DOI] [PubMed] [Google Scholar]
- 21. Wang M., Roberts D. L., Paschke R., Shea T. M., Masters B. S. S., Kim J. J. (1997) Three-dimensional structure of NADPH-cytochrome P450 reductase: prototype for FMN- and FAD-containing enzymes. Proc. Natl. Acad. Sci. U.S.A. 94, 8411–8416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ellis J., Gutierrez A., Barsukov I. L., Huang W. C., Grossmann J. G., Roberts G. C. (2009) Domain motion in cytochrome P450 reductase: conformational equilibria revealed by NMR and small-angle x-ray scattering. J. Biol. Chem. 284, 36628–36637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xia C., Panda S. P., Marohnic C. C., Martásek P., Masters B. S., Kim J. J. (2011) Structural basis for human NADPH-cytochrome P450 oxidoreductase deficiency. Proc. Natl. Acad. Sci. U.S.A. 108, 13486–13491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jenner M., Ellis J., Huang W. C., Lloyd Raven E., Roberts G. C., Oldham N. J. (2011) Detection of a protein conformational equilibrium by electrospray ionisation-ion mobility-mass spectrometry. Angew. Chem. Int. Ed. Engl. 50, 8291–8294 [DOI] [PubMed] [Google Scholar]
- 25. Miller W. L. (2009) Androgen synthesis in adrenarche. Rev. Endocr. Metab. Disord. 10, 3–17 [DOI] [PubMed] [Google Scholar]
- 26. DeVore N. M., Scott E. E. (2012) Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001. Nature 482, 116–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller W. L., Auchus R. J., Geller D. H. (1997) The regulation of 17,20 lyase activity. Steroids 62, 133–142 [DOI] [PubMed] [Google Scholar]
- 28. Miller W. L. (2005) Minireview: regulation of steroidogenesis by electron transfer. Endocrinology 146, 2544–2550 [DOI] [PubMed] [Google Scholar]
- 29. Onoda M., Hall P. F. (1982) Cytochrome b5 stimulates purified testicular microsomal cytochrome P-450 (C21 side-chain cleavage). Biochem. Biophys. Res. Commun. 108, 454–460 [DOI] [PubMed] [Google Scholar]
- 30. Kominami S., Ogawa N., Morimune R., De-Ying H., Takemori S. (1992) The role of cytochrome b5 in adrenal microsomal steroidogenesis. J. Steroid Biochem. Mol. Biol. 42, 57–64 [DOI] [PubMed] [Google Scholar]
- 31. Katagiri M., Kagawa N., Waterman M. R. (1995) The role of cytochrome b5 in the biosynthesis of androgens by human P450c17. Arch. Biochem. Biophys. 317, 343–347 [DOI] [PubMed] [Google Scholar]
- 32. Lee-Robichaud P., Wright J. N., Akhtar M. E., Akhtar M. (1995) Modulation of the activity of human 17α-hydroxylase-17,20-lyase (CYP17) by cytochrome b5: endocrinological and mechanistic implications. Biochem. J. 308, 901–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee-Robichaud P., Kaderbhai M. A., Kaderbhai N., Wright J. N., Akhtar M. (1997) Interaction of human CYP17 (P-45017α, 17α-hydroxylase-17,20-lyase) with cytochrome b5: importance of the orientation of the hydrophobic domain of cytochrome b5. Biochem. J. 321, 857–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pandey A. V., Miller W. L. (2005) Regulation of 17,20 lyase activity by cytochrome b5 and by serine phosphorylation of P450c17. J. Biol. Chem. 280, 13265–13271 [DOI] [PubMed] [Google Scholar]
- 35. Akhtar M. K., Kelly S. L., Kaderbhai M. A. (2005) Cytochrome b5 modulation of 17α hydroxylase and 17–20 lyase (CYP17) activities in steroidogenesis. J. Endocrinol. 187, 267–274 [DOI] [PubMed] [Google Scholar]
- 36. Estrada D. F., Laurence J. S., Scott E. E. (2013) Substrate-modulated cytochrome P450 17A1 and cytochrome b5 interactions revealed by NMR. J. Biol. Chem. 288, 17008–17018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang L. H., Rodriguez H., Ohno S., Miller W. L. (1995) Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and the polycystic ovary syndrome. Proc. Natl. Acad. Sci. U.S.A. 92, 10619–10623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Biason-Lauber A., Zachmann M., Schoenle E. J. (2000) Effect of leptin on CYP17 enzymatic activities in human adrenal cells: new insight in the onset of adrenarche. Endocrinology 141, 1446–1454 [DOI] [PubMed] [Google Scholar]
- 39. Pandey A. V., Mellon S. H., Miller W. L. (2003) Protein phosphatase 2A and phosphoprotein SET regulate androgen production by P450c17. J. Biol. Chem. 278, 2837–2844 [DOI] [PubMed] [Google Scholar]
- 40. Kempná P., Hirsch A., Hofer G., Mullis P. E., Flück C. E. (2010) Impact of differential P450c17 phosphorylation by cAMP stimulation and by starvation conditions on enzyme activities and androgen production in NCI-H295R cells. Endocrinology 151, 3686–3696 [DOI] [PubMed] [Google Scholar]
- 41. Azziz R., Woods K. S., Reyna R., Key T. J., Knochenhauer E. S., Yildiz B. O. (2004) The prevalence and features of the polycystic ovary syndrome in an unselected population. J. Clin. Endocrinol. Metab. 89, 2745–2749 [DOI] [PubMed] [Google Scholar]
- 42. Ehrmann D. A. (2005) Polycystic ovary syndrome. N. Engl. J. Med. 352, 1223–1236 [DOI] [PubMed] [Google Scholar]
- 43. Legro R. S., Driscoll D., Strauss J. F., 3rd, Fox J., Dunaif A. (1998) Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc. Natl. Acad. Sci. U.S.A. 95, 14956–14960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Govind A., Obhrai M. S., Clayton R. N. (1999) Polycystic ovaries are inherited as an autosomal dominant trait: analysis of 29 polycystic ovary syndrome and 10 control families. J. Clin. Endocrinol. Metab. 84, 38–43 [DOI] [PubMed] [Google Scholar]
- 45. Diamanti-Kandarakis E., Piperi C. (2005) Genetics of polycystic ovary syndrome: searching for the way out of the labyrinth. Hum. Reprod. Update 11, 631–643 [DOI] [PubMed] [Google Scholar]
- 46. Bollag G. E., Roth R. A., Beaudoin J., Mochly-Rosen D., Koshland D. E., Jr. (1986) Protein kinase C directly phosphorylates the insulin receptor in vitro and reduces its protein-tyrosine kinase activity. Proc. Natl. Acad. Sci. U.S.A. 83, 5822–5824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stadtmauer L., Rosen O. M. (1986) Increasing the cAMP content of IM-9 cells alters the phosphorylation state and protein kinase activity of the insulin receptor. J. Biol. Chem. 261, 3402–3407 [PubMed] [Google Scholar]
- 48. Takayama S., White M. F., Kahn C. R. (1988) Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J. Biol. Chem. 263, 3440–3447 [PubMed] [Google Scholar]
- 49. Chin J. E., Dickens M., Tavare J. M., Roth R. A. (1993) Overexpression of protein kinase C isoenzymes α, βI, γ, and ϵ in cells overexpressing the insulin receptor. Effects on receptor phosphorylation and signaling. J. Biol. Chem. 268, 6338–6347 [PubMed] [Google Scholar]
- 50. Miller W. L. (1999) The molecular basis of premature adrenarche: an hypothesis. Acta Paediatr. Suppl. 88, 60–66 [DOI] [PubMed] [Google Scholar]
- 51. Bremer A. A., Miller W. L. (2008) The serine phosphorylation hypothesis of polycystic ovary syndrome: a unifying mechanism for hyperandrogenemia and insulin resistance. Fertil. Steril. 89, 1039–1048 [DOI] [PubMed] [Google Scholar]
- 52. Dunaif A., Xia J., Book C. B., Schenker E., Tang Z. (1995) Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. A potential mechanism for insulin resistance in the polycystic ovary syndrome. J. Clin. Investig. 96, 801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Diamanti-Kandarakis E., Dunaif A. (2012) Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr. Rev. 33, 981–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rui L., Aguirre V., Kim J. K., Shulman G. I., Lee A., Corbould A., Dunaif A., White M. F. (2001) Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J. Clin. Investig. 107, 181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tee M. K., Dong Q., Miller W. L. (2008) Pathways leading to phosphorylation of P450c17 and to the posttranslational regulation of androgen biosynthesis. Endocrinology 149, 2667–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S. (2002) The protein kinase complement of the human genome. Science 298, 1912–1934 [DOI] [PubMed] [Google Scholar]
- 57. Corbould A., Kim Y. B., Youngren J. F., Pender C., Kahn B. B., Lee A., Dunaif A. (2005) Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am. J. Physiol. Endocrinol. Metab. 288, E1047–E1054 [DOI] [PubMed] [Google Scholar]
- 58. Corbould A., Zhao H., Mirzoeva S., Aird F., Dunaif A. (2006) Enhanced mitogenic signaling in skeletal muscle of women with polycystic ovary syndrome. Diabetes 55, 751–759 [DOI] [PubMed] [Google Scholar]
- 59. Efimova T., Broome A. M., Eckert R. L. (2003) A regulatory role for p38δ MAPK in keratinocyte differentiation. Evidence for p38δ-ERK1/2 complex formation. J. Biol. Chem. 278, 34277–34285 [DOI] [PubMed] [Google Scholar]
- 60. Cargnello M., Roux P. P. (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 75, 50–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wickenheisser J. K., Nelson-DeGrave V. L., McAllister J. M. (2006) Human ovarian theca cells in culture. Trends Endocrinol. Metab. 17, 65–71 [DOI] [PubMed] [Google Scholar]
- 62. Inagaki K., Otsuka F., Miyoshi T., Yamashita M., Takahashi M., Goto J., Suzuki J., Makino H. (2009) p38-mitogen-activated protein kinase stimulated steroidogenesis in granulosa cell-oocyte cocultures: role of bone morphogenetic proteins 2 and 4. Endocrinology 150, 1921–1930 [DOI] [PubMed] [Google Scholar]
- 63. Manna P. R., Stocco D. M. (2011) The role of specific mitogen-activated protein kinase signaling cascades in the regulation of steroidogenesis. J. Signal Transduct. 2011, 821615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ge B., Gram H., Di Padova F., Huang B., New L., Ulevitch R. J., Luo Y., Han J. (2002) MAPKK-independent activation of p38α mediated by TAB1-dependent autophosphorylation of p38α. Science 295, 1291–1294 [DOI] [PubMed] [Google Scholar]
- 65. Rodriguez H., Hum D. W., Staels B., Miller W. L. (1997) Transcription of the human genes for cytochrome P450scc and P450c17 is regulated differently in human adrenal NCI-H295 cells than in mouse adrenal Y1 cells. J. Clin. Endocrinol. Metab. 82, 365–371 [DOI] [PubMed] [Google Scholar]
- 66. Gazdar A. F., Oie H. K., Shackleton C. H., Chen T. R., Triche T. J., Myers C. E., Chrousos G. P., Brennan M. F., Stein C. A., La Rocca R. V. (1990) Establishment and characterization of a human adrenocortical carcinoma cell line that expresses multiple pathways of steroid biosynthesis. Cancer Res. 50, 5488–5496 [PubMed] [Google Scholar]
- 67. Staels B., Hum D. W., Miller W. L. (1993) Regulation of steroidogenesis in NCI-H295 cells: a cellular model of the human fetal adrenal. Mol. Endocrinol. 7, 423–433 [DOI] [PubMed] [Google Scholar]
- 68. Imai T., Globerman H., Gertner J. M., Kagawa N., Waterman M. R. (1993) Expression and purification of functional human 17α-hydroxylase/17,20-lyase (P450c17) in Escherichia coli. Use of this system for study of a novel form of combined 17α-hydroxylase/17,20-lyase deficiency. J. Biol. Chem. 268, 19681–19689 [PubMed] [Google Scholar]
- 69. Wang Y. H., Tee M. K., Miller W. L. (2010) Human cytochrome P450c17: single step purification and phosphorylation of serine 258 by protein kinase A. Endocrinology 151, 1677–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sandee D., Miller W. L. (2011) High-yield expression of a catalytically active membrane-bound protein: human P450 oxidoreductase. Endocrinology 152, 2904–2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Omura T., Sato R. (1962) A new cytochrome in liver microsomes. J. Biol. Chem. 237, 1375–1376 [PubMed] [Google Scholar]
- 72. Rubinson D. A., Dillon C. P., Kwiatkowski A. V., Sievers C., Yang L., Kopinja J., Rooney D. L., Zhang M., Ihrig M. M., McManus M. T., Gertler F. B., Scott M. L., Van Parijs L. (2003) A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat. Genet. 33, 401–406 [DOI] [PubMed] [Google Scholar]
- 73. Peng H. M., Auchus R. J. (2013) The action of cytochrome b5 on CYP2E1 and CYP2C19 activities requires anionic residues D58 and D65. Biochemistry 52, 210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lin D., Harikrishna J. A., Moore C. C. D., Jones K. L., Miller W. L. (1991) Missense mutation serine106 → proline causes 17α-hydroxylase deficiency. J. Biol. Chem. 266, 15992–15998 [PubMed] [Google Scholar]
- 75. Arlt W., Auchus R. J., Miller W. L. (2001) Thiazolidinediones but not metformin directly inhibit the steroidogenic enzymes P450c17 and 3β-hydroxysteroid dehydrogenase. J. Biol. Chem. 276, 16767–16771 [DOI] [PubMed] [Google Scholar]
- 76. Cuadrado A., Nebreda A. R. (2010) Mechanisms and functions of p38 MAPK signalling. Biochem. J. 429, 403–417 [DOI] [PubMed] [Google Scholar]
- 77. Sharrocks A. D., Yang S. H., Galanis A. (2000) Docking domains and substrate-specificity determination for MAP kinases. Trends Biochem. Sci. 25, 448–453 [DOI] [PubMed] [Google Scholar]
- 78. Reményi A., Good M. C., Bhattacharyya R. P., Lim W. A. (2005) The role of docking interactions in mediating signaling input, output, and discrimination in the yeast MAPK network. Mol. Cell 20, 951–962 [DOI] [PubMed] [Google Scholar]
- 79. Auchus R. J., Rainey W. E. (2004) Adrenarche—physiology, biochemistry and human disease. Clin. Endocrinol. 60, 288–296 [DOI] [PubMed] [Google Scholar]
- 80. Cutler G. B., Jr., Glenn M., Bush M., Hodgen G. D., Graham C. E., Loriaux D. L. (1978) Adrenarche: a survey of rodents, domestic animals, and primates. Endocrinology 103, 2112–2118 [DOI] [PubMed] [Google Scholar]
- 81. Smail P. J., Faiman C., Hobson W. C., Fuller G. B., Winter J. S. D. (1982) Further studies on adrenarche in nonhuman primates. Endocrinology 111, 844–848 [DOI] [PubMed] [Google Scholar]
- 82. Voutilainen R., Tapanainen J., Chung B. C., Matteson K. J., Miller W. L. (1986) Hormonal regulation of P450scc (20,22-desmolase) and P450c17 (17α-hydroxylase/17,20-lyase) in cultured human granulosa cells. J. Clin. Endocrinol. Metab. 63, 202–207 [DOI] [PubMed] [Google Scholar]
- 83. Oppenheimer E., Linder B., DiMartino-Nardi J. (1995) Decreased insulin sensitivity in prepubertal girls with premature adrenarche and acanthosis nigricans. J. Clin. Endocrinol. Metab. 80, 614–618 [DOI] [PubMed] [Google Scholar]
- 84. Ibáñez L., Dimartino-Nardi J., Potau N., Saenger P. (2000) Premature adrenarche—normal variant or forerunner of adult disease? Endocr. Rev. 21, 671–696 [DOI] [PubMed] [Google Scholar]
- 85. Idkowiak J., Lavery G. G., Dhir V., Barrett T. G., Stewart P. M., Krone N., Arlt W. (2011) Premature adrenarche: novel lessons from early onset androgen excess. Eur. J. Endocrinol. 165, 189–207 [DOI] [PubMed] [Google Scholar]
- 86. Remer T., Manz F. (1999) Role of nutritional status in the regulation of adrenarche. J. Clin. Endocrinol. Metab. 84, 3936–3944 [DOI] [PubMed] [Google Scholar]
- 87. Ibáñez L., Potau N., Francois I., de Zegher F. (1998) Precocious pubarche, hyperinsulinism, and ovarian hyperandrogenism in girls: relation to reduced fetal growth. J. Clin. Endocrinol. Metab. 83, 3558–3562 [DOI] [PubMed] [Google Scholar]
- 88. Voutilainen R., Miller W. L. (1987) Coordinate tropic hormone regulation of mRNAs for insulin-like growth factor II and the cholesterol side-chain-cleavage enzyme, P450scc in human steroidogenic tissues. Proc. Natl. Acad. Sci. U.S.A. 84, 1590–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mesiano S., Katz S. L., Lee J. Y., Jaffe R. B. (1997) Insulin-like growth factors augment steroid production and expression of steroidogenic enzymes in human fetal adrenal cortical cells: implications for adrenal androgen regulation. J. Clin. Endocrinol. Metab. 82, 1390–1396 [DOI] [PubMed] [Google Scholar]
- 90. Guasti L., Sze W. C., McKay T., Grose R., King P. J. (2013) FGF signalling through Fgfr2 isoform IIIb regulates adrenal cortex development. Mol. Cell. Endocrinol. 371, 182–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Widmann C., Gibson S., Jarpe M. B., Johnson G. L. (1999) Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 79, 143–180 [DOI] [PubMed] [Google Scholar]
- 92. Kyriakis J. M., Avruch J. (2001) Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81, 807–869 [DOI] [PubMed] [Google Scholar]
- 93. Pullikuth A. K., Catling A. D. (2007) Scaffold mediated regulation of MAPK signaling and cytoskeletal dynamics: a perspective. Cell. Signal. 19, 1621–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gao L.-L., Liu X.-Q., Xu B.-Q., Jiang S.-W., Cui Y.-G., Liu J. Y. (2013) SET/PP2A system regulates androgen production in ovarian follicles in vitro. Mol. Cell. Endocrinol. 374, 108–116 [DOI] [PubMed] [Google Scholar]